Abstract
MicroRNA (miRNA) regulation is a novel approach to manipulating the fate of mesenchymal stem cells, but an easy, safe, and highly efficient method of transfection is required. In this study, we developed an miRNA reverse transfection formulation by lyophilizing Lipofectamine 2000-miRNA lipoplexes on a tissue culture plate. The lipoplexes can be immobilized on a tissue culture plate with an intact pseudospherical structure and lyophilization without any lyoprotectant. In this study, reverse transfection resulted in highly efficient cellular uptake of miRNA and enabled significant manipulation of the intracellular target miRNA level. Reverse transfection formulations containing Lipofectamine 2000 1 μL per well generated much higher transfection efficiency without obvious cytotoxicity compared with conventional and other transfection methods. Further, the transfection efficiency of the reverse transfection formulations did not deteriorate during 90 days of storage at 4°C and −20°C. We then assessed the efficiency of the miRNA reverse transfection formulation in promoting osteogenic differentiation of mesenchymal stem cells. We found that transfection with anti-miR-138 and miR-148b was efficient for enhancing osteogenic differentiation, as indicated by enhanced osteogenesis-related gene expression, amount of alkaline phosphatase present, production of collagen, and matrix mineralization. Overall, the miRNA reverse transfection formulation developed in this study is a promising approach for miRNA transfection which can control stem cell fate and is suitable for loading miRNAs onto various biomaterials.
Keywords: microRNAs, lyophilization, tissue culture plate, mesenchymal stem cells, osteogenic differentiation
Introduction
The discovery of microRNAs (miRNAs) has led to considerable interest in their use as signatures in tissue development and for potential diagnostics and therapeutics in human disease.1,2 miRNAs are a class of noncoding RNAs that inhibit translation of mRNA via imperfect base pairing with the 3′-untranslated region of target mRNAs,3 and are involved in various aspects of cell function, including proliferation, differentiation, apoptosis, and other metabolic processes.4 miRNAs are able to enhance the differentiation of mesenchymal stem cells into many lineages including bone,5–8 cartilage,9 fat,10,11 and muscle and nerve cells,12–14 and therefore hold great promise for promoting healing and regeneration of various tissues.
Compared with small interfering RNAs (siRNAs), which specifically target the mRNAs of their complementary sequence, miRNAs simultaneously silence multiple genes with variable efficiency, and one specific gene target may respond to different miRNAs. The knockdown effect of miRNAs might be more comparable with the innate transcriptional regulation mechanism because of their endogenous origin, so their application in vivo might better mimic the natural regulatory pathway.15,16
One important aspect in research and therapeutic application of miRNAs is simple and highly efficient transfection. Vectors are normally required for stabilization and efficient delivery of oligonucleotides, especially for in vivo application, and nonviral vectors are better choices than viral ones in terms of safety.17,18 However, nonviral vectors have the drawback of relatively low transfection efficiency. In addition, the instability of transfection complexes in aqueous solution necessitates their preparation immediately before use, which makes them laborious to manufacture and, importantly, makes it difficult to control the reproducibility and quality of transfection.19 Therefore, there is considerable interest in developing stable, storable, easily handled, and efficient transfection approaches. Reverse transfection, also known as surface-mediated transfection, first reported by Ziauddin and Sabatini,20 refers to immobilizing transfection agents on a solid surface and delivering them locally into attached cells.21 Compared with conventional transfection, reverse transfection enables direct and sufficient contact between cells and the transfection agents, thereby improving transfection efficiency.22 Reverse transfection is usually mediated by physical adsorption of complexes onto surfaces, multilayered thin films on surfaces, or contact printing.21,22 Lyophilization enabling long-term storage of nucleic acid formulations has recently been demonstrated to be able to load nucleic acid complexes onto surfaces.23–27 Hence, reverse transfection formulations on a surface formed by lyophilization may provide an easy and efficient transfection approach for miRNA delivery. To our knowledge, although DNA and siRNAs have been plated on tissue culture plates for reverse transfection, there has been no such attempt using miRNAs.21,28,29
We freeze-dried Lipofectamine 2000-miRNA lipoplexes on tissue culture plates to develop reverse transfection miRNA formulations. Lyoprotectant is normally needed to protect the integrity of the complex during lyophilization,25 but normal lyoprotectants such as glucose are reported to interfere with cell differentiation.30 Of interest, it has been reported that siRNA lipoplexes lyophilized without a lyoprotectant have good storability and high transfection efficiency.25 Hence, we have developed a lyophilized miRNA reverse transfection formulation without any lyoprotectant and demonstrated high miRNA transfection efficiency and stability when stored for up to 90 days. We have also successfully used this system for reverse transfection of anti-miR-138 and miR-148b in mesenchymal stem cells and observed significant osteogenic differentiation. This study provides beneficial experience in the process of loading miRNA onto biomaterials, with enhanced transfection efficiency which may have relevance for clinical application.
Materials and methods
Preparation and morphological characterization of reverse transfection formulations
All the miRNAs (pre-miRNAs), anti-miRNAs (miRNA inhibitors), and their respective controls in this study were synthesized by Guangzhou RiboBio Co, Ltd (Guangzhou, People’s Republic of China). Two groups of miRNA reverse transfection formulations were prepared. The first group was prepared using Lipofectamine™ 2000 (Invitrogen, Carlsbad CA, USA). Briefly, both Lipofectamine 2000 (1 μL) and 20 μM miRNAs (0.3125, 0.625, 1.25, and 2.5 μL) were diluted using Opti-MEM® (Invitrogen, Carlsbad, CA, USA) to a final volume of 50 μL, and then mixed together following incubation at room temperature for 20 minutes. The miRNA lipoplexes were put on tissue culture plates and kept on dry ice for 5 minutes to snap-freeze then lyophilized at −40°C for 24 hours. In this way, we produced miRNA reverse transfection formulations containing the same amount of Lipofectamine 2000 but different amounts of miRNA (12.5, 25, 50, and 100 nM). When referring to the amount of plated miRNAs, we used concentration (nM) of the miRNA when the sample is added to 500 μL of medium. The second group of reverse transfection formulations had a Lipofectamine 2000 to miRNA volume ratio of 1. Different volumes of Lipofectamine 2000 (0.3125, 0.625, 1.25, and 2.5 μL) and 20 μM miRNAs at different volumes (0.3125, 0.625, 1.25, and 2.5 μL) were diluted using Opti-MEM to a final volume of 50 μL, and then mixed together. The lipoplexes were then plated and lyophilized to generate a series of miRNA formulations with a constant Lipofectamine 2000 to miRNA ratio but containing different amounts of miRNA (12.5, 25, 50, and 100 nM). For conventional aqueous transfection, the miRNA lipoplexes were fabricated in the same manner as that used for the first group. Conventional aqueous transfection for 6 hours and 24 hours was used as the control. It has been reported that adding transfection complexes immediately after cell plating can improve transfection efficiency,31 so such a control group was also included. The surface morphology of the reverse transfection formulations was investigated using a field-emission scanning electron microscope (S-4800 Hitachi, Tokyo, Japan).
Cell culture and transfection
The animal procedures used in this study were approved by the research ethics committee of The Fourth Military Medical University. Primary rat bone marrow-derived mesenchymal stem cells were obtained from two-week-old Sprague-Dawley rats. The cells were cultured in Alpha Minimal Essential Medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin, and incubated in a humidified atmosphere of 5% CO2 at 37°C. The medium was replaced twice a week. Passages 2–4 were used in the experiment. For conventional aqueous transfection, 2.5 × 104 cells/cm2 were cultured in 24-well plates for 24 hours. Next, 100 μL of Lipofectamine 2000-miRNA complexes and 400 μL of culture medium were added to the cell culture, and after 6 or 24 hours of transfection, the medium was replaced with 500 μL of fresh medium. For reverse transfection, 2.5 × 104 cells/cm2 were seeded into the wells with lyophilized miRNAs in 500 μL of culture medium. After 24 hours of transfection, the spent medium was replaced with 500 μL of fresh medium. For osteogenic induction, osteogenic medium containing 10 mM β-glycerophosphate, 50 μg/mL ascorbic acid and 10−7 M dexamethasone (all obtained from Sigma, St Louis, MO, USA) was added 48 hours after cell seeding.
Cellular uptake of miRNA and transfection efficiency mediated by reverse transfection
To observe the internalization of miRNAs by cells as clearly as possible, Cy3-labeled miRNAs were used to construct reverse transfection miRNA formulations, with cell transfection performed as described above. Immediately after transfection, the cells were fixed with 4% paraformaldehyde and washed in phosphate-buffered saline. The cell membrane was highlighted with 3,3’-dioctadecyloxacarbocyanine perchlorate (DIO) (Beyotime Institute of Biotechnology, Jiangsu, People’s Republic of China) for 15 minutes and washed again with phosphate-buffered saline. The DIO and Cy3 fluorescence signals were then observed using laser scanning confocal microscopy (Fluo View, Olympus FV1000).
In order to compare the transfection efficiency of the different formulations, the transfected cells were harvested using trypsin, washed with phosphate-buffered saline, and fixed in 1% paraformaldehyde in phosphate-buffered saline. Cy3 fluorescence emitted by the cells was measured using a flow cytometer (FACScalibur, BD Biosciences, Franklin Lakes, NJ, USA). Each sample was performed in triplicate.
Manipulating efficiency of reverse transfection at target intracellular miRNA level
The amount of miR-138 produced by the reverse transfection formulations was measured after transfection with anti-miR-138 (an miR-138 inhibitor) to assess the efficiency achieved in reaching the target intracellular miRNA level by manipulation of reverse transfection. For detection of mature miR-138, RNA was reverse-transcribed using a PrimeScript RT reagent kit (TaKaRa Bio Inc, Shiga, Japan) and a specific reverse transcription primer (Guangzhou RiboBio Co, Ltd) according to the manufacturer’s recommendations. The primer sequence was designed according to the corresponding miR-138 sequence (GCCGGACUAAGUGUUGUGGUCGA). The miR-138 level was quantified using a real-time polymerase chain reaction (PCR) system (CFX96™, Bio-Rad Hercules, CA, USA) with SYBR Premix Ex Taq™ II (TaKaRa). U6 small nuclear RNA was used as an endogenous normalization control.
Cell viability
Cell viability was evaluated quantitatively using a cell count kit-8 (CCK-8, Beyotime).32 Briefly, 48 hours after cell seeding, the culture medium was removed and the cultures were washed with phosphate-buffered saline. Next, 360 μL of serum-free Alpha Minimal Essential Medium and 40 μL of CCK-8 solution were added to each well, followed by incubation at 37°C for 3 hours. The supernatant was transferred to a 96-well plate and optical density was determined at 450 nm using a spectrophotometer (Bio-Tek Instruments Inc, Winooski, VT, USA). The viability of cells cultured on tissue culture plates without the transfection formulation was set as the blank control, in which viability of transfected cells was normal (n = 3).
Stability of miRNA reverse transfection formulations on storage
The 100 nM sample in the reverse transfection group contained a representative quantity of Lipofectamine 2000 to assess the stability of the miRNA reverse transfection formulation during storage. After fabrication, the plates were stored under three different conditions, ie, at room temperature, 40°C, or −20°C in a dark environment for a total period of up to 3 months. Mesenchymal stem cell transfection was performed on the stored plates at designated time points (10, 20, and 90 days) and transfection efficiency was determined as described above.
Osteogenesis-related gene expression
To investigate the effects of the anti-miR-138 and miR-148b reverse transfection formulations on osteogenesis, we evaluated osteogenesis-related gene expression using real-time reverse transcription PCR. An anti-miR-control (a negative miRNA inhibitor control) reverse transfection formulation, a pre-miRNA negative control (miR-control) reverse transfection formulation, and control cells on blank tissue culture plates were prepared. The cells were then cultured for 14 days in the osteogenic medium. Total RNA was isolated using the TRIzol reagent (Invitrogen). Next, the RNA contained in 2 μg from each sample was reversed-transcribed into complementary DNA using the PrimeScript RT reagent kit. Expression of osteogenesis-related genes, including collagen type 1 α1, runt-related transcription factor 2, alkaline phosphatase, bone morphogenetic protein-2, osterix, and osteocalcin was quantified using the CFX96™ real time reverse transcription PCR system with SYBR Premix Ex Taq™ II. The relative expression levels for each gene of interest were normalized to that of the GAPDH housekeeping gene. The PCR primers were synthesized as shown in Table 1.
Table 1.
Primers used for real time reverse transcription polymerase chain reaction
| Gene | Forward primer sequence (5′–3′) | Reverse primer sequence (5′–3′) |
|---|---|---|
| COL1α1 | GCCTCCCAGAACATCACCTA | GCAGGGACTTCTTGAGGTTG |
| ALP | AACGTGGCCAAGAACATCATCA | TGTCCATCTCCAGCCGTGTC |
| OCN | GGTGCAGACCTAGCAGACACCA | AGGTAGCGCCGGAGTCTATTCA |
| OSX | AAGGCAGTTGGCAATAGTGG | TGAATGGGCTTCTTCCTCAG |
| BMP-2 | CAACACCGTGCTCAGCTTCC | TTCCCACTCATTTCTGAAAGTTCC |
| RUNX2 | CCATAACGGTCTTCACAAATCCT | TCTGTCTGTGCCTTCTTGGTTC |
| GAPDH | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; COL1α1, collagen type 1 α1; ALP, alkaline phosphatase; OCN, osteocalcin; OSX, osterix; BMP-2, bone morphogenetic protein-2; RUNX2, runt-related transcription factor 2.
Production of alkaline phosphatase
After 7 days of culture in the osteogenic medium, the cells were washed with phosphate-buffered saline and fixed. Alkaline phosphatase production was then identified by staining with the BCIP/NBT alkaline phosphatase color development kit (Beyotime) for 15 minutes. The samples were washed thoroughly with phosphate-buffered saline, followed by taking of images.
Collagen secretion
After culture for 7 days in the osteogenic medium, the cells were washed with phosphate-buffered saline, fixed in 4% paraformaldehyde, and stained with 0.1 wt% Sirius red (Sigma) in saturated picric acid for 18 hours, in order to show the amount of collagen secreted. Images were taken after removing the unbound stain by thorough washing in 0.1 M acetic acid. For quantitative analysis, staining was eluted by 500 μL of destaining solution (0.2 M NaOH/methanol 1:1) and optical density was measured at 540 nm using the spectrophotometer.
Mineralized nodule staining in the extracellular matrix
After culture for 14 days and 21 days in the osteogenic medium, the cells were washed with phosphate-buffered saline and then fixed in 4% paraformaldehyde for 10 minutes. The mineralized nodules formed in the extracellular matrix were stained with 1 wt% Alizarin red (Sigma) for 10 minutes. Images were taken after thorough washing with distilled water. For quantitative analysis, the stain was dissolved using 10% cetylpyridinium chloride in 10 mM sodium phosphate (pH 7), and absorbance values were measured at 620 nm.
Statistical analysis
One-way analysis of variance and Tukey’s post hoc tests were used to determine the level of statistical significance of differences between groups. P values < 0.05, 0.01, and 0.001 were considered to be statistically significant.
Results
Characterization of lipoplexes in miRNA reverse transfection formulations
The morphology, size, and distribution of the lipoplexes in the miRNA reverse transfection formulations were observed using field emission scanning electron microscopy (Figure 1). Because the two types of reverse transfection formulations had similar morphology, only results for a given quantity of Lipofectamine 2000 are shown. The reverse transfection formulations containing different amounts of miRNA showed similar morphology, size, and distribution. The lipoplexes distributed evenly and formed a monolayer on the tissue culture plate surfaces with an intact pseudospherical shape. The diameters ranged from 40 nm to 200 nm.
Figure 1.
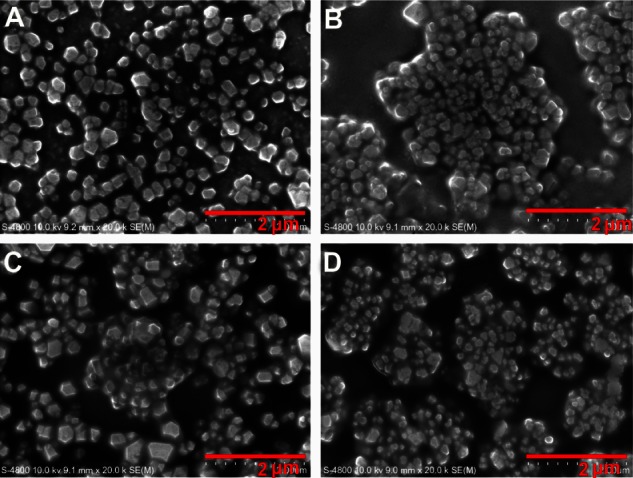
Morphological characterization of microRNA reverse transfection formulations. Scanning electron microscopic images (20,000 × magnification) demonstrate the morphology of lipoplexes in microRNA reverse transfection formulations containing a given quantity of Lipofectamine 2000 but different microRNA amounts of 12.5 nM (A), 25 nM (B), 50 nM (C), and 100 nM (D).
Cellular uptake and transfection efficiency of miRNA reverse transfection formulations
The reverse transfection formulation containing 100 nM Cy3-miRNA in the group containing a given quantity of Lipofectamine 2000 was chosen as representative to investigate internalization of miRNAs to cells. Immediately after transfection, the mesenchymal stem cells were washed, fixed, and stained using DIO. Cy3-labeled miRNAs (red) and the entire cell (green) could be visualized by microscopy (Figure 2). We observed abundant miRNAs over almost all the cells, indicating high cellular uptake of the lipoplexes and good transfection efficiency. The miRNAs were found to be either surrounding the nucleus or sparsely distributed in cytoplasm (Figure 2A and C), and it is at this location that their functions are believed to take place.
Figure 2.
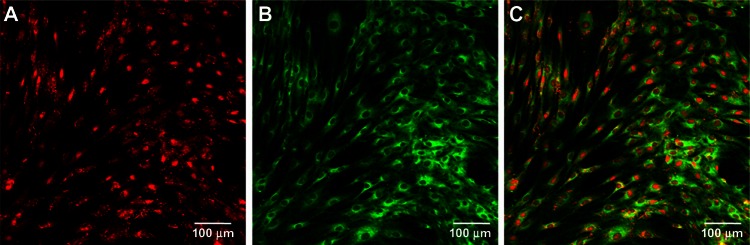
Uptake of microRNA by cells. Fluorescence images showing cellular microRNA uptake after 24 hours of transfection in the 100 nM reverse transfection formulation containing a given quantity of Lipofectamine 2000. (A) Cy3-labeled microRNA (red), (B) cell body stained with 3,3′-dioctadecyloxacarbocyanine perchlorate (green), and (C) the merged images.
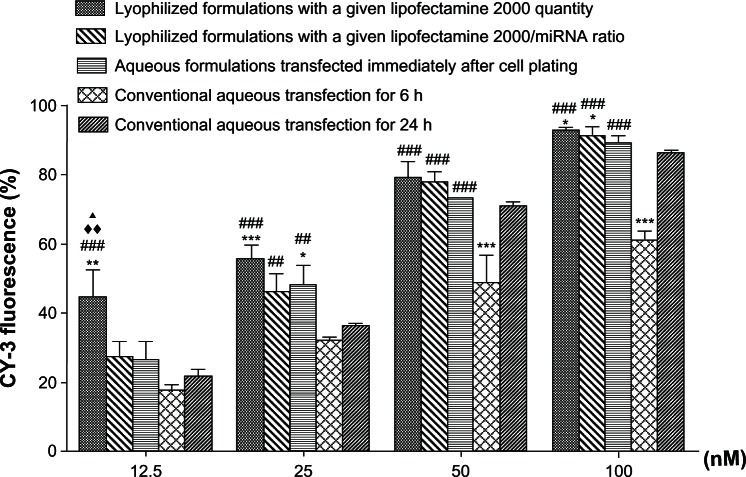
We compared the transfection efficiency of the reverse transfection formulations, conventional aqueous formulations, and aqueous formulations transfected immediately after cell plating by flow cytometry (Figure 3). The transfection efficiencies of all the formulations correlated positively with the amount of miRNA in the formulations (from 12.5 nM to 100 nM). Generally, at the lower miRNA concentration, the transfection efficiency for the different formulations followed the order of the reverse transfection formulations, with a given Lipofectamine 2000 quantity > reverse transfection formulations with a given Lipofectamine 2000/miRNA ratio > aqueous formulations transfected immediately after cell plating > conventional aqueous formulations for 24 hours > conventional aqueous formulations for 6 hours. However, at 12.5 nM, the reverse transfection formulation containing the given Lipofectamine 2000 quantity showed the highest transfection efficiency, with higher miRNA amounts showing similar transfection efficiency.
Figure 3.
Comparison of transfection efficiency between reverse transfection and other methods.
Notes: Transfection efficiency of the different microRNA formulations are assessed by flow cytometry and evaluated by one-way analysis of variance and Tukey’s post hoc tests. *P < 0.05; **P < 0.01; and ***P < 0.001 versus conventional aqueous transfection for 24 hours; #P < 0.05; ##P < 0.01; and ###P < 0.001 versus conventional aqueous transfection for 6 hours; ♦♦P < 0.01 versus aqueous formulations transfected immediately after cell plating; ▲P < 0.05 versus lyophilized formulations with a given Lipofectamine 2000 to microRNA ratio.
Regulation of intracellular miR-138 by anti-miR-138 reverse transfection formulations
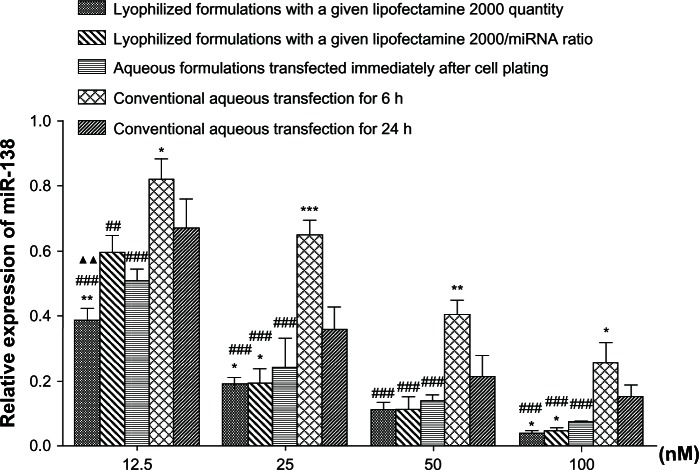
To assess the regulatory effect of miRNA reverse transfection on intracellular target miRNA levels, the amount of miR-138 was measured after transfecting with anti-miR-138 formulations (Figure 4). miR-138 levels in the mesenchymal stem cells were successfully downregulated by anti-miR-138 reverse transfection and inversely correlated with the anti-miR-138 concentration in formulations from 12.5 to 100 nM. miR-138 expression was demonstrated to follow the trend of reverse transfection formulations < aqueous formulations transfected immediately after cell plating < conventional aqueous formulations for 24 hours < conventional aqueous formulations for 6 hours, which was in good agreement with the transfection efficiency shown above.
Figure 4.
Downregulation of microRNA expression by reverse transfection formulations.
Notes: Expression of miR-138 was assessed by real time reverse transcription polymerase chain reaction after transfecting mesenchymal stem cells with the anti-miR-138 reverse transfection formulations. *P < 0.05; **P < 0.01; and ***P < 0.001 versus conventional aqueous transfection for 24 hours; #P < 0.05; ##P < 0.01; and ###P < 0.001 versus conventional aqueous transfection for 6 hours; ▲▲P < 0.01 versus lyophilized formulations with a given Lipofectamine 2000 to microRNA ratio.
Effect of transfection on cell viability
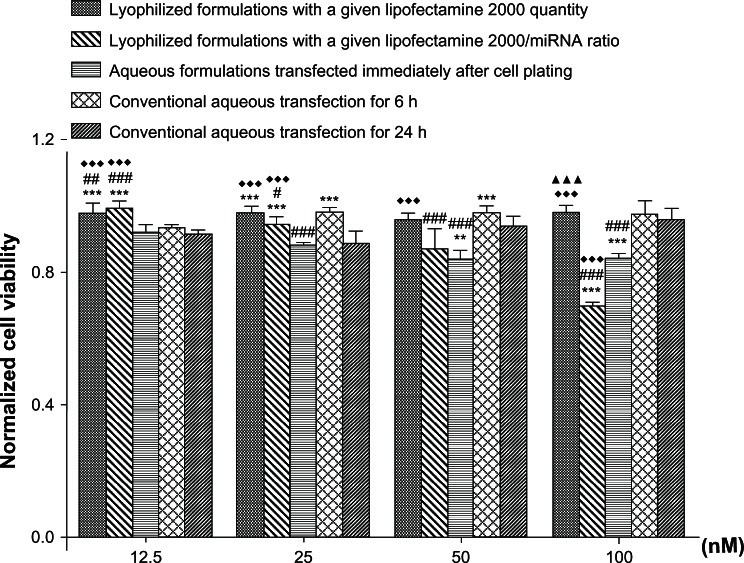
The influence of the different transfection formulations on cell viability was assessed using the CCK-8 kit (Figure 5). Comparable cell viability was seen with the conventional aqueous transfection formulations and the reverse transfection formulations containing different amounts of miRNA and a given quantity of Lipofectamine 2000. Cell viability for the reverse transfection formulations containing a given Lipofectamine 2000 to miRNA ratio and the aqueous formulations transfected immediately after cell plating showed a negative relationship with the amounts of miRNA in the formulations.
Figure 5.
Cell viability affected by reverse transfection formulation.
Notes: Cell viability was measured by CCK-8 at 24 hours after transfection, and normalized to the viability of blank control tissue culture plates. **P < 0.01 and ***P < 0.001 versus conventional aqueous transfection for 24 hours; #P < 0.05; ##P < 0.01; and ###P < 0.001 versus conventional aqueous transfection for 6 hours; ♦♦♦P < 0.001 versus aqueous formulations transfected immediately after cell plating; ▲▲▲P < 0.001 versus lyophilized formulations with a given Lipofectamine 2000 to microRNA ratio.
Stability of miRNA reverse transfection formulations during storage
The influence of storage on the transfection efficiency of the miRNA reverse transfection formulations was investigated over a 3-month period using the 100 nM miRNA sample in the reverse transfection formulations with a given Lipofectamine 2000 quantity as representative (Figure 6). We found that the stability of the formulations depended on the storage temperature. Excellent stability with no obvious decrease in transfection efficiency was seen when samples were stored at 4°C and −20°C. However, when stored at room temperature, a time-dependent decrease in transfection efficiency was observed over 3 months, suggesting gradual degradation of miRNAs during storage at room temperature.
Figure 6.
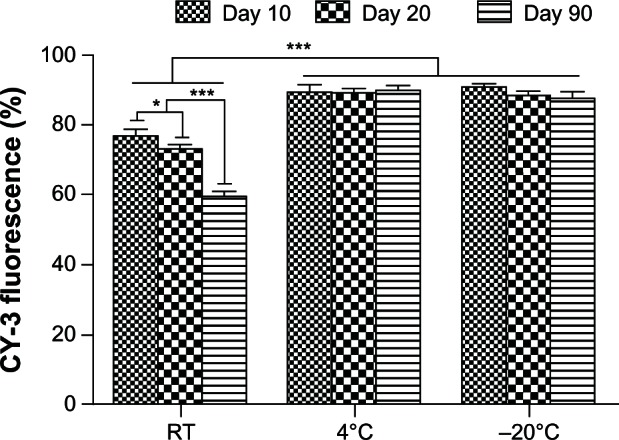
Storable stability of reverse-transfected formulations of microRNA.
Notes: After storage for different durations (10, 20 and 90 days), the transfection efficiency of the 100 nM microRNA lyophilized formulation in the group containing a given quantity of Lipofectamine 2000 was measured to assess the stability of the lyophilized formulations during storage. *P < 0.05; and ***P < 0.001.
Efficacy of miRNA reverse transfection in promoting osteogenesis
The effects of miR-148b reverse transfection and anti-miR-138 reverse transfection on osteogenic differentiation of mesenchymal stem cells were assessed in terms of osteogenesis-related gene expression, production of alkaline phosphatase, secretion of collagen, and mineralization in the extracellular matrix.
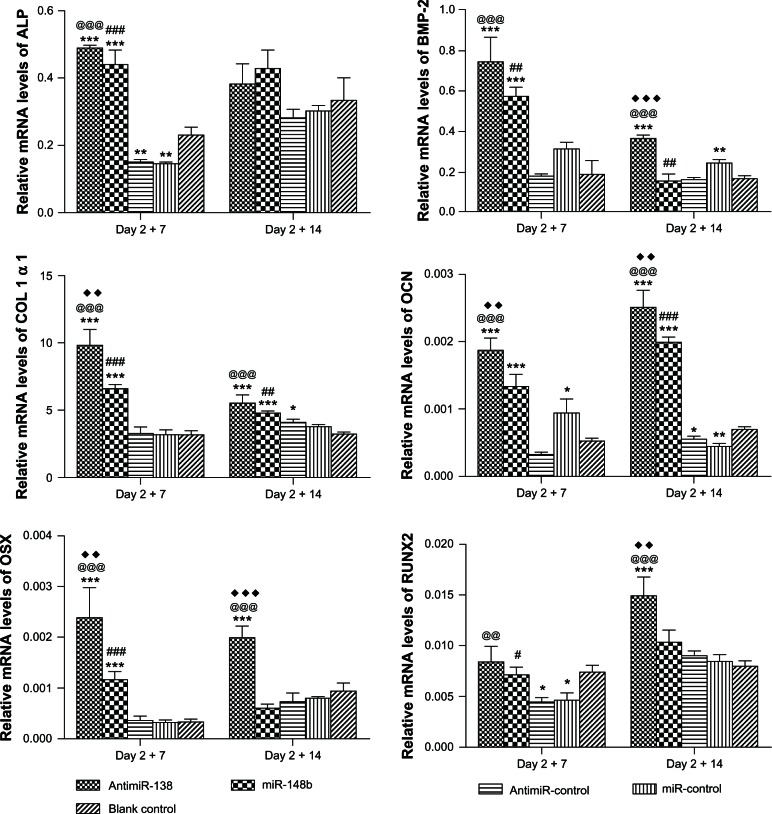
Osteogenesis-related gene expression was determined by real time reverse transcription PCR (Figure 7). For all the genes involved, including collagen type 1 α1, runt-related transcription factor 2, alkaline phosphatase, bone morphogenetic protein-2, osterix, and osteocalcin, miR-148b and anti-miR-138 reverse transfection gave rise to obviously higher expression compared with the blank control, miR-control, and anti-miR-control. The effect of anti-miR-138 reverse transfection was slightly stronger than that of miR-148b.
Figure 7.
Osteogenesis-related gene expression mediated by reverse transfection of anti-miR-138 and miR-148b, ie, relative expression of alkaline phosphatase, bone morphogenetic protein-2, collagen type 1 α1, osteocalcin, osterix, and runt-related transcription factor 2, was assessed by real time reverse transcription polymerase chain reaction for mesenchymal stem cells transfected with lyophilized formulations carrying anti-miR-138, miR-148b, anti-miR-control, and miR-control after culturing in osteogenic medium for 7 days and 14 days.
Notes: The tissue culture plate is set as the blank control. *P < 0.05; **P < 0.01; and ***P < 0.001 versus blank control tissue culture plate; @@P < 0.01 and @@@P < 0.001 versus reverse transfection formulation of anti-miR-control; #P < 0.05; ##P < 0.01; and ###P < 0.001 versus reverse transfection formulation of miR-control; ♦P < 0.05, ♦♦P < 0.01, and ♦♦♦P < 0.001 versus reverse transfection formulation of miR-148b.
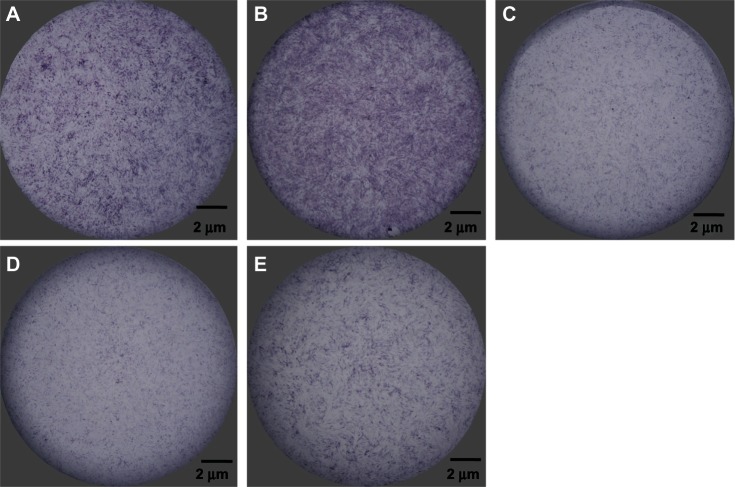
The amount of alkaline phosphatase present in cells after 7 days of culture in the osteogenic medium was assessed by BCIP/NBT staining (Figure 8). anti-miR-138 reverse transfection resulted in markedly more alkaline phosphatase than in the blank control tissue culture plate, while miR-148b reverse transfection gave rise to the most production of alkaline phosphatase among the groups.
Figure 8.
Production of alkaline phosphatase induced by anti-miR-138 and miR-148b. Alkaline phosphatase production was measured after 7 days of culture. (A) Reverse transfection formulation of anti-miR-138, (B) reverse transfection formulation of miR-148b, (C) reverse transfection formulation of anti-miR-control, (D) reverse transfection formulation of miR-control, and (E) blank control tissue culture plate.
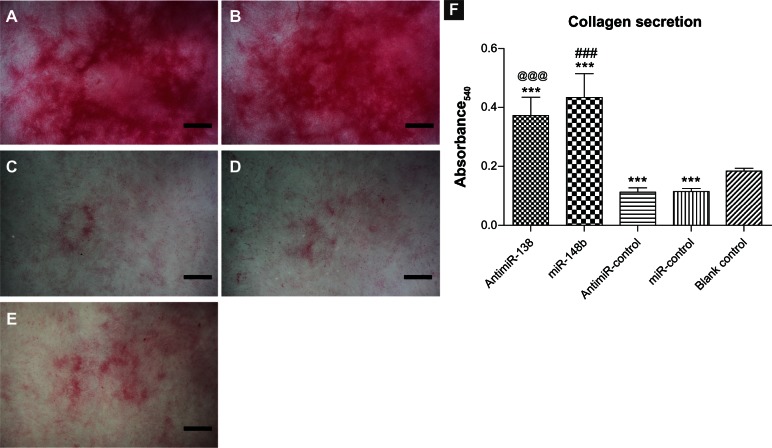
Collagen secreted by the cells after 7 days in culture was stained using Sirius red (Figure 9). The optical images show that the anti-miR-138 and miR-148b reverse transfection groups contained much denser collagen layers than the blank control, anti-miR-control, and miR-control tissue culture plates. The quantitative results in Figure 9F indicate that the amounts of collagen yielded in the anti-miR-138 and miR-148b reverse transfection groups were approximately double those for the blank control tissue culture plate.
Figure 9.
Collagen secretion induced by anti-miR-138 and miR-148b. Collagen secreted by mesenchymal stem cells was investigated in the osteogenic medium after 7 days of culture. (A) Reverse transfection formulation of anti-miR-138, (B) reverse transfection formulation of miR-148b, (C) reverse transfection formulation of anti-miR-control, (D) reverse transfection formulation of miR-control, and (E) blank control tissue culture plate. Scale bar in micrographs represents 1 mm. (F) Quantitative colorimetric results.
Notes: ***P < 0.001 versus blank control tissue culture plate; ###P < 0.001 versus miR-control group; @@@P < 0.001 versus anti-miR-control group.
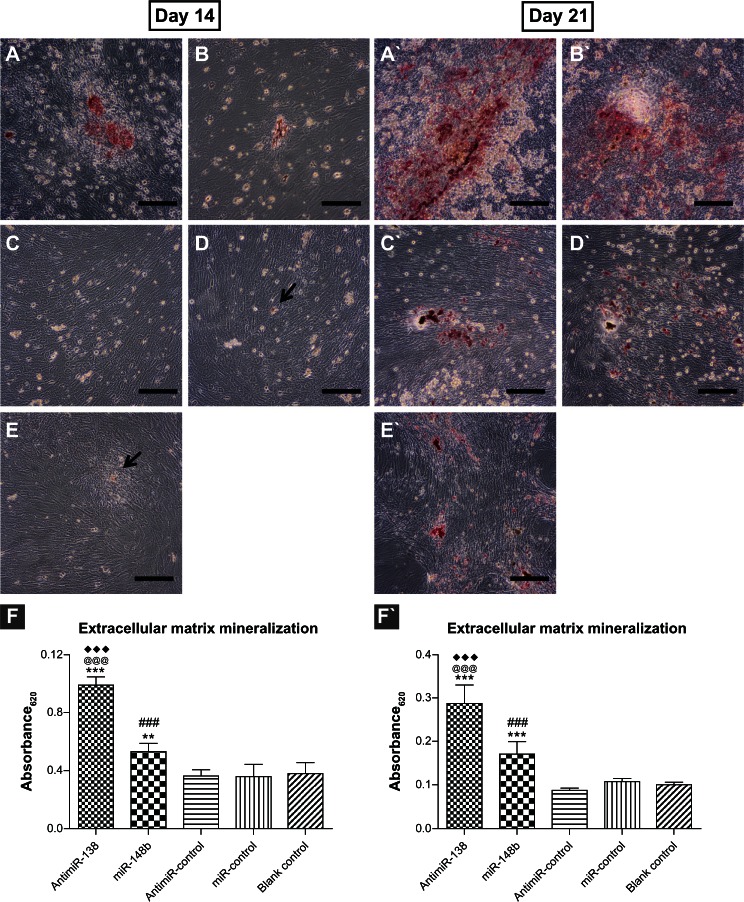
Mineralized nodule formation in the extracellular matrix was investigated by Alizarin red staining (Figure 10). At day 14, there are very small mineralized dots on the blank control and miR-control tissue culture plate surfaces, as indicated by the black arrows. miR-148b reverse transfection resulted in slightly larger mineralized nodules, and anti- miR-138 reverse transfection gave rise to the most abundant and largest mineralized nodules. After 21 days in culture, there were markedly more and much larger mineralized nodules formed via miR-148b and anti-miR-138 reverse transfection.
Figure 10.
Extracellular matrix mineralization mediated by anti-miR-138 and miR-148b. Transfected mesenchymal stem cells were stained using Alizarin red after 14 and 21 days of culture in osteogenic medium. (A and A`) Reverse transfection formulation of anti-miR-138, (B and B`) reverse transfection formulation of miR-148b, (C and C`) reverse transfection formulation of anti-miR-control, (D and D`) reverse transfection formulation of anti-miR-control and (E and E`) blank control tissue culture plate. The black arrows in (D and E) indicate tiny mineralized dots. Scale bar in micrographs represents 100 μm. (F and F`) Quantitative colorimetric results.
Notes: **P < 0.01 and ***P < 0.001 versus blank control tissue culture plate; ###P < 0.001 versus miR-control group; @@@P < 0.001 versus anti-miR-control group; ♦♦♦P < 0.001 versus miR-148b group.
Discussion
In this study, we developed a miRNA reverse transfection formulation by freeze-drying Lipofectamine 2000-miRNA lipoplexes onto tissue culture plates. The lipoplexes maintained intact pseudospherical forms after lyophilization. Reverse transfection resulted in high miRNA transfection efficiency. In particular, the formulations containing a given quantity of Lipofectamine 2000 showed much higher transfection efficiency and had no discernible cytotoxicity. They showed good long-term storability at 4°C and −20°C. On functional assessment, we found that anti-miR-138 and miR-148b were both effective in promoting osteogenic differentiation of mesenchymal stem cells when reverse-transfected. These findings may advance the miRNA transfection procedure in addition to providing beneficial experience in loading miRNAs onto various kinds of biomaterial, eg, dental implants.
miRNAs have emerged as an exciting method for manipulating the fate of mesenchymal stem cells, but lack of storability, easy handling, and safe and highly efficient transfection are obstacles to expanding their application in basic research and ultimate clinical therapy. Reverse transfection refers to delivery of transfection agents that are initially immobilized on a solid surface with cultured cells adherent on it,21 and has been demonstrated to have improved transfection efficiency compared with conventional transfection.22 Hitherto, reverse transfection has been used widely to transfect DNA and siRNAs,21,28,29 but has not been attempted with miRNAs.
To our knowledge, our results demonstrate for the first time that reverse transfection is also feasible with miRNAs. Reverse transfection is usually mediated by physical adsorption of transfection complexes onto surfaces,22 by contact printing, or by multilayered thin films on surfaces.21,22 In the present study, we choose lyophilization for fabricating the miRNA reverse transfection formulations and demonstrated that this is able to load nucleic acid complexes onto a surface effectively and to make long-term storage of nucleic acid formulations possible.23–27 Encouragingly, we found that the miRNA lipoplexes could be loaded onto the tissue culture plate surface evenly by lyophilization and that they could retain their intact structure. Lyoprotectants are generally used for lipoplex lyophilization, but it has been reported that ordinary lyoprotectants, such as glucose, can interfere with cell differentiation.30 To avoid the unwanted side effects of lyoprotectants, lipoplexes were lyophilized in the present study in the absence of any lyoprotectant, and functioned well after being freeze-dried on surfaces, as previously reported by Andersen et al.25,27
To assess transfection efficiency, a 100 nM sample in the group of miRNA reverse transfection formulations containing a given Lipofectamine 2000 quantity was selected. We demonstrated that reverse transfection achieved good internalization of miRNAs by the attached cells, indicating high transfection efficiency, and in agreement with the observations of Fujita et al in their study using reverse-transfected siRNA.29 Using flow cytometry, we also found that reverse transfection resulted in much higher transfection efficiency than that using conventional aqueous transfection.29 It has been suggested that transfection immediately after cell plating can improve transfection efficiency compared with conventional transfection.31
Our results are in line with that, and demonstrate that reverse transfection performs even better. The high transfection efficiency of about 90% generated by the 100 nM sample in the group of miRNA reverse transfection formulations containing a given quantity of Lipofectamine 2000 strongly validates the methods used in our study. Reverse transfection potentially improves transfection efficiency for the following reasons. First, it enables direct contact between cells and the transfection agents, thereby facilitating endocytosis and internalization of the transfection agent.22 Second, it gives rise to higher local concentrations of transfection agents on the tissue culture plate surface where the cells are located compared with conventional transfection. Enhanced concentration of transfection agents on the plate surface where cells are attached is reported to result in higher transfection efficiency.33 The high silencing efficiency of miRNA reverse transfection was observed using anti-miR-138 as an example. miR-138 was successfully downregulated by anti-miR-138 transfection in a dose-dependent manner, which is consistent with reports of reverse transfection with DNA and siRNA.28,29 Our data on miRNAs provide more evidence in support of the generally high manipulating effect of reverse transfection on target level.
Cytocompatibility is a key concern with transfection, especially in the clinical setting. The reverse transfection formulations in this study that contained a given quantity of Lipofectamine 2000 had no apparent cytotoxicity, while those containing a given Lipofectamine 2000 to miRNA ratio showed cytotoxicity at higher miRNA doses of 50 nM and 100 nM, which may have resulted from the extra Lipofectamine 2000 content. Cationic lipids, including Lipofectamine 2000, are thought to be cytotoxic because they inhibit important proteins like protein kinase C,34 so controlling the amount of Lipofectamine 2000 in the transfection formulation is important for achieving satisfactory cytocompatibility. Importantly, the reverse transfection formulations containing a given quantity of Lipofectamine 2000 also performed better in terms of transfection efficiency than those with a given Lipofectamine 2000 to miRNA ratio. Specifically, the 100 nM sample containing a given quantity of Lipofectamine 2000 generated the most transfection without showing toxicity, so is considered optimal in the current context.
Good storability of a transfection formulation is crucial with regard to industrial production, transportation, and use. At lower temperatures of 4°C and −20°C, the reverse transfection formulations show consistent transfection efficiency with increasing duration of storage. There is abundant evidence demonstrating that storage at 4°C does not reduce the transfection efficiency of lyophilized DNA-polyethylenimine reverse transfection or even that of reverse transfection formulations produced by simple vacuum-drying.28,29 However, when stored at room temperature, we found that the transfection efficiency of the reverse transfection formulations decreased markedly with time. The transfection ability of the lyophilized lipoplexes depended on storage temperature. A steady decline in gene transfection efficiency and an increase in lipoplex degradation was observed as the storage temperature of the lipoplexes increased from −20°C to 50°C–60°C.35,36 For other vectors, such as nanoparticles of calcium phosphate, a slightly greater decrease in transfection efficiency of plasmid DNA is also observed for lyophilized samples stored at 25°C compared with those stored at 4°C. Overall, we suggest that 4°C is the optimal storage temperature for lyophilized reverse transfection formulations.
A major area of interest in mesenchymal stem cell research lies in steering their differentiation into osteoblasts to enhance bone regeneration and osseointegration of implants. Therefore, we assessed the efficacy of the miRNA reverse transfection formulations in this regard. miR-148b and anti-miR-138 were transfected to mesenchymal stem cells using reverse transfection to promote osteogenic differentiation. Encouragingly, we observed that when reverse-transfected, miR-148b and anti-miR-138 resulted in enhanced osteogenic activity, as represented by increased osteogenesis-related gene expression, production of alkaline phosphatase, collagen secretion, and mineralized nodule formation in the extracellular matrix. It has been reported that miR-138 inhibits osteogenic differentiation of mesenchymal stem cells in vitro and formation of bone in vivo, and that inhibition of miR-138 by anti-miR-138 promotes osteogenesis.7 miR-148b has been found to play a critical role in osteogenesis of human mesenchymal stem cells, potentially by regulating multiple genes related to skeletal development.8 Our results are in good agreement with these previous reports. Of note is that the control miRNA interfered with osteogenic differentiation of the mesenchymal stem cells when compared with the tissue culture plate control. The decreasing effect of the control miRNA on osteogenic differentiation of mesenchymal stem cells may have been due to possible off-target activity of RNA inhibition. It has been reported that nonspecific siRNA controls decrease osteogenic differentiation and increase adipogenic differentiation,27,37 and such an effect may also exist with nonspecific miRNA controls, although the possibility of interference from liposomes found between the tissue culture plate surface and the cells cannot be excluded. Improved bioactivity may be envisaged when other vectors, such as chitosan, are used.25 The present study confirms the feasibility of fabricating reverse transfection miRNA formulations by lyophilization and demonstrates their application for transfecting miRNAs and anti-miRNAs when steering the fate of mesenchymal stem cells.
Conclusion
We have shown that Lipofectamine 2000-miRNA lipoplexes can be lyophilized onto a tissue culture plate surface with an intact structure, thereby providing easy to use and storable miRNA for reverse transfection. Reverse transfection gives rise to high miRNA transfection and silencing efficiency of the intracellular target miRNA without inducing discernible cytotoxicity. With regard to clinical relevance, reverse transfection of anti-miR-138 and miR-148b promotes significant osteogenic differentiation of mesenchymal stem cell and provides us with a useful strategy in future miRNA therapeutics.
Acknowledgments
The authors are appreciative of grants received from the National Natural Science Foundation of China (31200716 to LZZ, and 81070862 and 31170915 to YMZ) and from The Fourth Military Medical University and School of Stomatology, The Fourth Military Medical University to LZZ.
Footnotes
Disclosure
The authors report no conflicts of interest relevant to this work.
References
- 1.Kota J, Chivukula RR, O’Donnell KA, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137(6):1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132(21):4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Martignani E, Miretti S, Accornero P, Baratta M. miRNAs highlights in stem and cancer cells. Mini Rev Med Chem. 2011;11(13):1165–1182. doi: 10.2174/138955711797655371. [DOI] [PubMed] [Google Scholar]
- 5.Kim YJ, Bae SW, Yu SS, Bae YC, Jung JS. miR-196a regulates proliferation and osteogenic differentiation in mesenchymal stem cells derived from human adipose tissue. J Bone Miner Res. 2009;24(5):816–825. doi: 10.1359/jbmr.081230. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Xie H, Liu W, et al. A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J Clin Invest. 2009;119(12):3666–3677. doi: 10.1172/JCI39832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eskildsen T, Taipaleenmaki H, Stenvang J, et al. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc Natl Acad Sci USA. 2011;108(15):6139–6144. doi: 10.1073/pnas.1016758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoolmeesters A, Eklund T, Leake D, et al. Functional profiling reveals critical role for miRNA in differentiation of human mesenchymal stem cells. PLoS One. 2009;4(5):e5605. doi: 10.1371/journal.pone.0005605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuddenham L, Wheeler G, Ntounia-Fousara S, et al. The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett. 2006;580(17):4214–4217. doi: 10.1016/j.febslet.2006.06.080. [DOI] [PubMed] [Google Scholar]
- 10.Esau C, Kang X, Peralta E, et al. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem. 2004;279(50):52361–52365. doi: 10.1074/jbc.C400438200. [DOI] [PubMed] [Google Scholar]
- 11.Sun F, Wang J, Pan Q, et al. Characterization of function and regulation of miR-24-21 and miR-31. Biochem Biophys Res Commun. 2009;380(3):660–665. doi: 10.1016/j.bbrc.2009.01.161. [DOI] [PubMed] [Google Scholar]
- 12.Chen JF, Mandel EM, Thomson JM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38(2):228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Q, Zhang Y, Yang G, et al. Transforming growth factor-beta-regulated miR-24 promotes skeletal muscle differentiation. Nucleic Acids Res. 2008;36(8):2690–2699. doi: 10.1093/nar/gkn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim PK, Patel SA, Gregory LA, Rameshwar P. Neurogenesis: role for microRNAs and mesenchymal stem cells in pathological states. Curr Med Chem. 2010;17(20):2159–2167. doi: 10.2174/092986710791299894. [DOI] [PubMed] [Google Scholar]
- 15.Yau WW, Rujitanaroj PO, Lam L, Chew SY. Directing stem cell fate by controlled RNA interference. Biomaterials. 2012;33(9):2608–2628. doi: 10.1016/j.biomaterials.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merdan T, Kopecek J, Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv Drug Deliv Rev. 2002;54(5):715–758. doi: 10.1016/s0169-409x(02)00046-7. [DOI] [PubMed] [Google Scholar]
- 18.Elsabahy M, Nazarali A, Foldvari M. Non-viral nucleic acid delivery: key challenges and future directions. Curr Drug Deliv. 2011;8(3):235–244. doi: 10.2174/156720111795256174. [DOI] [PubMed] [Google Scholar]
- 19.Anchordoquy TJ, Koe GS. Physical stability of nonviral plasmid-based therapeutics. J Pharm Sci. 2000;89(3):289–296. doi: 10.1002/(SICI)1520-6017(200003)89:3<289::AID-JPS1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 20.Ziauddin J, Sabatini DM. Microarrays of cells expressing defined cDNAs. Nature. 2001;411(6833):107–110. doi: 10.1038/35075114. [DOI] [PubMed] [Google Scholar]
- 21.Erfle H, Neumann B, Liebel U, et al. Reverse transfection on cell arrays for high content screening microscopy. Nat Protoc. 2007;2(2):392–399. doi: 10.1038/nprot.2006.483. [DOI] [PubMed] [Google Scholar]
- 22.Jewell CM, Lynn DM. Surface-mediated delivery of DNA: cationic polymers take charge. Curr Opin Colloid Interface Sci. 2008;13(6):395–402. doi: 10.1016/j.cocis.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yadava P, Gibbs M, Castro C, Hughes JA. Effect of lyophilization and freeze-thawing on the stability of siRNA-liposome complexes. AAPS Pharm Sci Tech. 2008;9(2):335–341. doi: 10.1208/s12249-007-9000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allison SD, Molina MC, Anchordoquy TJ. Stabilization of lipid/DNA complexes during the freezing step of the lyophilization process: the particle isolation hypothesis. Biochim Biophys Acta. 2000;1468(1–2):127–138. doi: 10.1016/s0005-2736(00)00251-0. [DOI] [PubMed] [Google Scholar]
- 25.Andersen MO, Howard KA, Paludan SR, Besenbacher F, Kjems J. Delivery of siRNA from lyophilized polymeric surfaces. Biomaterials. 2008;29(4):506–512. doi: 10.1016/j.biomaterials.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Kundu AK, Chandra PK, Hazari S, et al. Stability of lyophilized siRNA nanosome formulations. Int J Pharm. 2012;423(2):525–534. doi: 10.1016/j.ijpharm.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersen MO, Nygaard JV, Burns JS, et al. siRNA nanoparticle functionalization of nanostructured scaffolds enables controlled multilineage differentiation of stem cells. Mol Ther. 2010;18(11):2018–2027. doi: 10.1038/mt.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinisalo M, Urtti A, Honkakoski P. Freeze-drying of cationic polymer DNA complexes enables their long-term storage and reverse transfection of post-mitotic cells. J Control Release. 2006;110(2):437–443. doi: 10.1016/j.jconrel.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Fujita S, Ota E, Sasaki C, Takano K, Miyake M, Miyake J. Highly efficient reverse transfection with siRNA in multiple wells of microtiter plates. J Biosci Bioeng. 2007;104(4):329–333. doi: 10.1263/jbb.104.329. [DOI] [PubMed] [Google Scholar]
- 30.Aguiari P, Leo S, Zavan B, et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci USA. 2008;105(4):1226–1231. doi: 10.1073/pnas.0711402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escobedo J, Koh TJ. Improved transfection technique for adherent cells using a commercial lipid reagent. BioTechniques. 2003;35(5):936–940. doi: 10.2144/03355bm06. [DOI] [PubMed] [Google Scholar]
- 32.Cheng ST, Chen ZF, Chen GQ. The expression of cross-linked elastin by rabbit blood vessel smooth muscle cells cultured in polyhydroxyal-kanoate scaffolds. Biomaterials. 2008;29(31):4187–4194. doi: 10.1016/j.biomaterials.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Luo D, Saltzman WM. Enhancement of transfection by physical concentration of DNA at the cell surface. Nat Biotechnol. 2000;18(8):893–895. doi: 10.1038/78523. [DOI] [PubMed] [Google Scholar]
- 34.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114(1):100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Maitani Y, Aso Y, Yamada A, Yoshioka S. Effect of sugars on storage stability of lyophilized liposome/DNA complexes with high transfection efficiency. Int J Pharm. 2008;356(1–2):69–75. doi: 10.1016/j.ijpharm.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 36.Molina MC, Armstrong TK, Zhang Y, Patel MM, Lentz YK, Anchordoquy TJ. The stability of lyophilized lipid/DNA complexes during prolonged storage. J Pharm Sci. 2004;93(9):2259–2273. doi: 10.1002/jps.20138. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Mirmalek-Sani SH, Lin F, Zhang J, Oreffo RO. Adipocyte differentiation induced using nonspecific siRNA controls in cultured human mesenchymal stem cells. RNA. 2007;13(8):1179–1183. doi: 10.1261/rna.527207. [DOI] [PMC free article] [PubMed] [Google Scholar]