Abstract
Cleft formation is the initial step of branching morphogenesis in many organs. We previously demonstrated that ROCK 1 regulates a non-muscle myosin II-dependent mechanochemical checkpoint to transition initiated clefts to progressing clefts in developing submandibular salivary glands. Here, we report that ROCK-mediated integrin activation and subsequent formation of focal adhesion complexes comprise this mechanochemical checkpoint. Inhibition of ROCK1 and non-muscle myosin II activity decreased integrin β1 activation in the cleft region and interfered with localization and activation of focal adhesion complex proteins, such as focal adhesion kinase (FAK). Inhibition of FAK activity also prevented cleft progression, by disrupting recruitment of the focal adhesion proteins talin and vinculin and subsequent fibronectin assembly in the cleft region while decreasing ERK1/2 activation. These results demonstrate that inside-out integrin signaling leading to a localized recruitment of active FAK-containing focal adhesion protein complexes generates a mechanochemical checkpoint that facilitates progression of branching morphogenesis.
Keywords: branching morphogenesis, ROCK, MLC2, fibronectin, focal adhesions, integrin activation
INTRODUCTION
During morphogenesis, the cells comprising epithelial tissues must be dynamically remodeled to build a functional epithelial architecture. In the developing submandibular salivary gland (SMG), remodeling occurs through the formation of clefts, or indentations, in the basement membrane surrounding the periphery of an epithelial bud, which then progress, or extend, to separate the epithelium into distinct epithelial lobules. While many hypotheses have been proposed to explain cleft formation (Spooner and Wessells, 1972; Nakanishi et al., 1987; Nakanishi et al., 1988; Wan et al., 2008), recent evidence suggests that this process is driven by the focal loss of epithelial cell-cell adhesions and their subsequent replacement by cell-matrix adhesions (Harunaga et al., 2011). However, the mechanisms by which such cell-matrix adhesions can promote the physical changes leading to cleft formation remain unknown.
The ECM protein fibronectin (FN) is required for cleft formation during SMG branching morphogenesis (Sakai et al., 2003; Larsen et al., 2006; Liu et al., 2010). FN is focally expressed by epithelial cells adjacent to a forming cleft, and siRNA knockdown of FN prevents cleft formation in embryonic day 12 (E12) SMG organ cultures, while the addition of exogenous FN enhances epithelial clefting (Sakai et al., 2003). Subsequent experiments with salivary epithelial cells in culture suggested a mechanism by which FN might promote cleft formation; when exogenous FN was added to epithelial cell monolayers, E-cadherin-mediated cell-cell adhesions were rapidly depleted and replaced by FN-mediated cell-matrix adhesions (Sakai et al., 2003). Live imaging of GFP-labeled salivary gland organ explants cultured with fluorescently labeled FN subsequently revealed that FN is directionally translocated inward into progressing clefts (Larsen et al., 2006). These studies led to a model in which FN serves as an ECM “wedge” that promotes the separation of SMG epithelial cells, which, due to the dynamic and plastic nature of the SMG epithelium, reinforces and stabilizes such separation.
We recently demonstrated that Rho kinase (ROCK 1)-mediated actomyosin contraction is the upstream signal that drives FN assembly during SMG cleft progression (Daley et al., 2009). ROCK promotes cytoskeletal contraction by enhancing the activation of myosin light chain 2 (MLC2), both directly through phosphorylation on Ser19 (Amano et al., 1996; Totsukawa et al., 2000) and indirectly by phosphorylating and inactivating the myosin binding subunit of myosin light chain phosphatase (Kimura et al., 1996; Feng et al., 1999; Kawano et al., 1999). Such cytoskeletal contraction regulates FN matrix assembly, which requires the unfolding of cell surface-bound FN to expose a cryptic self-assembly site via cytoskeletal-mediated forces transmitted through integrin cytoplasmic domains (Zhong et al., 1998; Baneyx et al., 2002; Yoneda et al., 2007).
Since ROCK and myosin inhibition prevented cleft progression, but not cleft initiation, our previous results suggested that ROCK-stimulated myosin activity is required for the transition of initiated clefts to a progression-competent state (Daley et al., 2009). Such a finding is consistent with a role for ROCK and myosin in the stabilization of already initiated clefts, and is supported by earlier observations demonstrating that cleft initiation in the salivary gland is a dynamic event, with some clefts becoming stabilized at the epithelial surface, while others rapidly disappear (Nogawa, 1983; Larsen et al., 2006). We proposed a model for cleft progression in which a localized increase in ROCK-mediated actomyosin contraction reaches a threshold at the epithelial surface that triggers the assembly of FN fibrils in the cleft region, thereby stabilizing the initiated cleft. FN, in turn, promotes epithelial cell proliferation in the regions adjacent to the stabilized cleft and generates an outward-directed expansion force which results in cleft progression. We designated the state at which a sufficient threshold of contractility is reached to trigger FN assembly within an initiated cleft and facilitate its transition to a progression-competent state as a mechanochemical checkpoint (Daley et al., 2009). Although the biochemical composition of such a checkpoint was unknown, the requirement for FN assembly in the basement membrane, which is a cell-mediated process (Zhong et al., 1998; Mao and Schwarzbauer, 2005), as well as FN’s role in the downregulation of cell-cell adhesions and their replacement by cell-extracellular matrix adhesions (Sakai et al., 2003), suggests that such a checkpoint likely involves an inside-out integrin activation signal that enhances cell-matrix interactions. Previous studies have demonstrated that the initiation of fibrillogenesis requires high-affinity binding of FN to integrins in the activated state prior to fibril assembly (Wu et al., 1995), in further support of an inside-out signal as a key activator of the mechanochemical checkpoint.
One class of cell-matrix adhesion complexes, known as focal adhesions (FAs), are large cytoplasmic signaling complexes that mechanically link cells to the ECM via cell surface integrin receptors. FAs form upon integrin ligation to the ECM, followed by subsequent integrin clustering at the cell surface to activate the integrin. Such clustering rapidly recruits many signaling and scaffolding proteins, examples of which include the adaptor proteins vinculin, talin, and paxillin, that link integrin cytoplasmic domains to the actin cytoskeleton (Geiger and Bershadsky, 2001; Zaidel-Bar et al., 2003; Wozniak et al., 2004; Bershadsky et al., 2006). A growing body of evidence clearly demonstrates that FAs can form and mature only if they experience forces transmitted through the actin cytoskeleton. Indeed, the FA protein talin, which binds directly to integrin cytoplasmic tails and promotes their inside-out activation (Tadokoro et al., 2003; Tanentzapf and Brown, 2006; Wegener et al., 2007), has recently emerged as a potential force sensor that, upon its unfolding by cytoskeletal-mediated forces, promotes FA enlargement by recruiting vinculin to stabilize the adhesion (del Rio et al., 2009). Thus, FA formation is dependent upon non-muscle myosin type II activity (Galbraith et al., 2002; Choi et al., 2008), and Rho-mediated contractility has been directly implicated in the formation and maturation of such cell-matrix contacts (Ridley and Hall, 1992; Chrzanowska-Wodnicka and Burridge, 1996), acting through the Rho-associated kinase, ROCK.
In the current study, we identify molecular components of the mechanochemical checkpoint. We identify FAK as a downstream mediator of a ROCK-mediated actomyosin contraction inside-out integrin activation signal. Importantly, signaling through FAK is required for cleft progression, but not cleft initiation, and failure of clefts to progress in the presence of FAK inhibition is due to decreased FN assembly and focal adhesion protein localization. FAK is also required for activation of ERK1/2 to control cell proliferation during cleft progression. These results identify inside-out integrin signaling leading to a localized recruitment of active FAK-containing focal adhesion protein complexes as the molecular components driving progression through the mechanochemical checkpoint to promote FN assembly and cell proliferation to transition initiated clefts to progressing clefts. Finally, we demonstrate that FN assembly itself induces FA protein accumulation and FAK activation in the cleft region, suggesting that outside-in signaling triggered by FN assembly then acts a signal to stimulate continued cleft propagation downstream of the mechanochemical checkpoint.
RESULTS
ROCK-mediated actomyosin contraction is an inside-out signal that promotes β1 integrin activation and FA formation in the cleft region of E13 SMGs
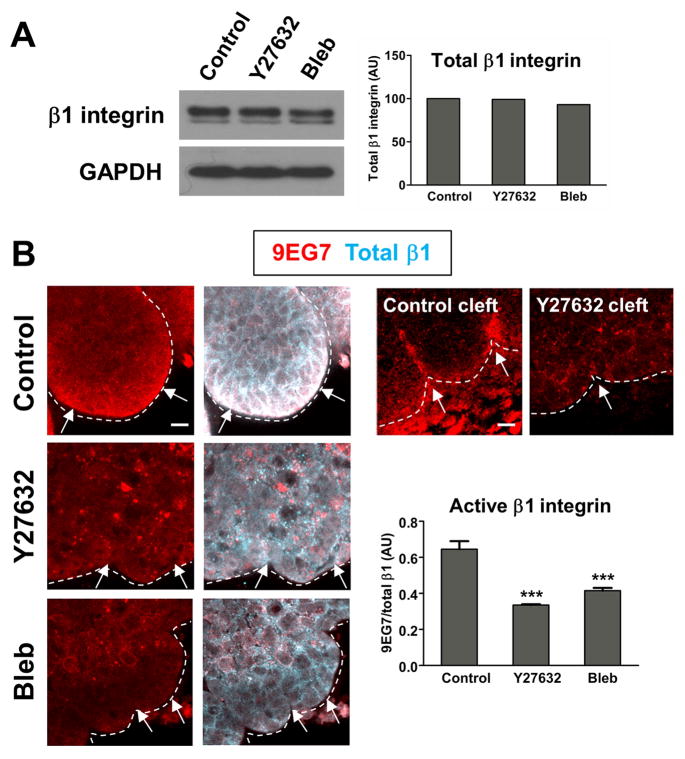
To specifically examine a role for ROCK-mediated actomyosin contraction in the inside-out activation of β1 integrin in SMG epithelium, we treated mesenchyme-free epithelial rudiments with pharmacological inhibitors of ROCK or non-muscle myosin II and immunostained them to detect active and total β1 integrin. The monoclonal antibody 9EG7 has been reported to selectively bind β1 integrins only when they are in their extended, or active, conformation (Supplementary Fig. 1A). 9EG7 binding is induced by Mn2+, a strong stimulator of β1 function, as well as by FN and RGD peptides (Bazzoni et al., 1995). The ratio of 9EG7 staining intensity to total β1 serves as a measure of the overall level of β1 integrins in the activated conformation. To ensure that 9EG7 does indeed bind activated β1 integrin in SMG organ cultures, we treated E13 SMGs with increasing concentrations of MnCl2. As expected, we observed a dose-dependent increase in the ratio of active to total β1 with increasing concentrations of MnCl2. MgCl2, which does not activate β1 integrin (Bazzoni et al., 1995), did not produce these effects (Supplementary Fig. 1B). Thus, as previously reported for cells in two dimensional culture, Mn2+ treatment induces the 9EG7 epitope and results in an increase in activated β1 integrin in SMG ex vivo organ cultures. There was no change in total β1 integrin expression in ROCK and myosin-inhibited SMGs, as determined by immunoblot analysis (Fig. 1A); however, we observed significant changes in 9EG7 immunostaining. In vehicle control-treated rudiments, 9EG7 staining was restricted primarily to the periphery of the epithelium (Fig. 1B), while total β1 was present throughout the epithelium but with preferential localization at the epithelial basal periphery. In contrast, ROCK and non-muscle myosin II inhibition resulted in a significant reduction in the ratio of 9EG7 to total β1, with treated SMGs exhibiting only remnant puncta of 9EG7 staining (Fig 1B). While 9EG7 staining was particularly pronounced in the progressing clefts of control intact SMGs, such staining was absent from the immature initiated clefts of ROCK-inhibited SMGs (Fig. 1B). We conclude that ROCK and myosin-mediated cytoskeletal contraction at the epithelial surface may constitute an inside-out signal that promotes β1 integrin activation during SMG branching morphogenesis.
Figure 1. ROCK-mediated actomyosin contraction constitutes an inside-out β1 integrin activation signal.
(A) Immunoblotting of SMG cell lysates treated with vehicle control, Y27632, or blebbistatin reveals no change in overall levels of total β1 integrin among treatments. Quantification of β1 integrin is normalized to a GAPDH loading control. (B) E13 SMG mesenchyme-free epithelial rudiments were cultured for 24 hours in the presence of vehicle control, Y27632, or blebbistatin prior to co-staining with mAb9EG7 (active integrin β1) and a polyclonal antibody recognizing total β1 integrin. 9EG7 staining (red) localizes most intensely to the periphery of epithelial buds (white arrows), while total β1 (cyan) is present throughout the bud but with preferential localization at the epithelial periphery. 9EG7 staining at the periphery of the epithelium is greatly reduced in ROCK and myosin-inhibited epithelial rudiments. 9EG7 localization is particularly intense in the progressing clefts of vehicle control-treated intact SMGs, but greatly reduced in the immature initiated clefts present when ROCK is inhibited (Control Cleft, Y27632 Cleft; compare white arrows). ANOVA, ***p<0.001. Scale bars,
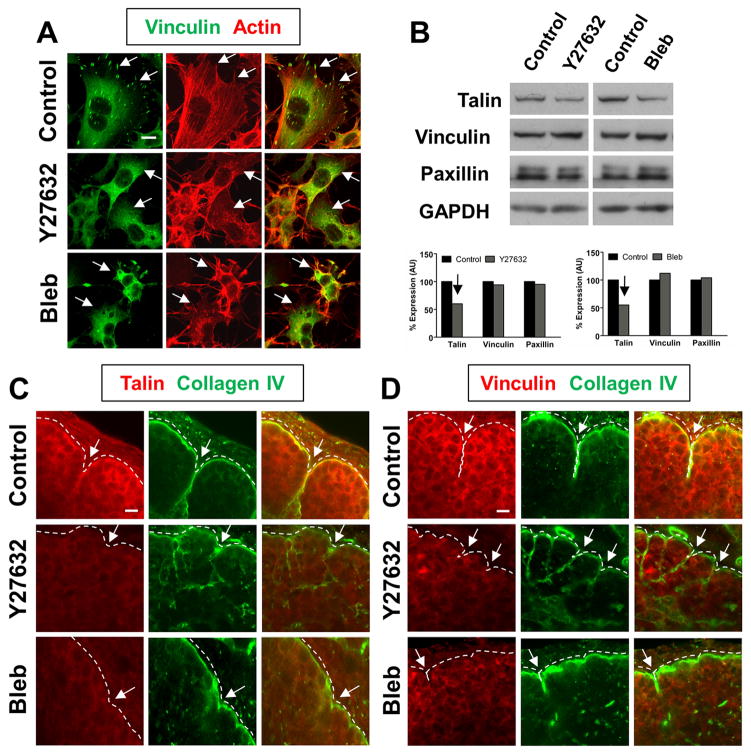
Given that ROCK-mediated contractility is capable of activating β1 integrins at the epithelial surface, which is a prerequisite for focal adhesion (FA) complex formation, we next questioned whether a critical component of the mechanochemical checkpoint might be a ROCK-induced formation of FAs in the cleft region of embryonic SMGs. Since FAs were first reported in 2D cell cultures grown on tissue culture plastic, we examined FAs in the salivary gland epithelial cell line, SCA-9. In vehicle control-treated SCA-9 cell cultures, short stitches of vinculin were present in focal accumulations across the basal surface, where they co-aligned with actin stress fibers (Fig. 2A). Accumulations of talin and paxillin, two other components of focal adhesions, were also observed to associate with these structures (data not shown), suggesting that they are FAs. As previously reported in cultured cells (Galbraith et al., 2002), ROCK and myosin inhibition prevented the formation of such cell-matrix adhesions and disrupted F-actin organization (Fig. 2A). We observed similar results when we stained for paxillin and talin, in that FAs were not observed (data not shown), indicating that ROCK and myosin are required for FA formation in salivary epithelial cells in 2D cell culture.
Figure 2. ROCK-mediated actomyosin contraction is required for focal adhesion protein localization in the cleft region of branching SMGs.
(A) SCA-9 salivary gland cell cultures treated with vehicle control media alone form vinculincontaining focal adhesions (green, white arrows) that co-align with actin stress fibers (red). In contrast, SCA-9 cells cultured in the presence of Y27632 or blebbistatin fail to form focal adhesions (compare white arrows) and exhibit a disorganized actin cytoskeleton. (B) Immunoblot analysis was performed to examine the expression of the focal adhesion components talin, vinculin, and paxillin in the presence of the ROCK and myosin inhibitors. Quantification normalized to a GAPDH loading control reveals an approximately 50% decrease in talin levels with Y27632 and blebbistatin treatment, but no change in vinculin or paxillin at the protein level. (C and D) Both talin (red, C) and vinculin (red, D) localize to the basal periphery of control SMG epithelial buds, in close proximity to the basement membrane (collagen IV, green). Talin and vinculin staining are alsoparticularly prominent along the side walls of progressing clefts (C and D, white arrows). Incontrast, such localization is greatly reduced at the basal periphery and in immature initiated clefts of Y27632 and blebbistatin-treated SMGs (compare white arrows). Scale bars, 10 μm (A, C, and D).
We next examined FA protein levels and localization in E13 organ explants cultured in the presence of the ROCK and myosin inhibitors. While we observed no significant difference in the levels of the FA proteins, vinculin and paxillin, there was an approximately 50% reduction in the levels of talin protein in the presence of ROCK and myosin inhibitors (Fig. 2B). Immunocytochemistry for FA proteins in intact E13 SMGs revealed that in vehicle control SMGs, both vinculin and talin were detectable at low levels within the interior of the epithelium at cell borders, but they localized much more intensely at sites of cell-matrix contacts adjacent to the basement membrane, as indicated by co-staining with an antibody detecting the basement membrane protein, collagen IV (Fig. 2C, D). Moreover, such staining was particularly intense in the distal cleft regions (Fig. 2C, D). In contrast, vinculin and talin staining were greatly reduced adjacent to the basement membrane and in the cleft regions of ROCK and myosin-inhibited SMGs. These results demonstrate that when ROCK-mediated actomyosin contraction is perturbed, FA protein localization within cleft regions is compromised. Since these clefts are also blocked at the initiation stage, these results also suggest that such FA proteins are components of the mechanochemical checkpoint that stabilizes initiated clefts and promotes their transition to a progression-competent state.
Focal adhesion kinase (FAK) activation occurs downstream of ROCK-mediated actomyosin contraction during SMG branching morphogenesis
Focal adhesion kinase (FAK) is a non-receptor protein tyrosine kinase that localizes to FAs in a variety of tissues and is a crucial mediator of downstream signals from cell-matrix adhesions (Parsons et al., 2000; Tilghman and Parsons, 2008; Zhao and Guan, 2009). The clustering of integrins upon adhesion to the ECM leads to the rapid recruitment and activation of FAK at FAs, primarily due to phosphorylation on Tyr397 by adjacent FAK molecules, with subsequent phosphorylation on Tyr576/577 required for maximal FAK activation (Lipfert et al., 1992; Schaller et al., 1994; Calalb et al., 1995). Active FAK maintains and further promotes the generation of cellular tension in response to adhesion to the ECM through maturation and strengthening of FAs (Michael et al., 2009). Furthermore, Rho-mediated contractility through its downstream effector ROCK has been implicated in the stretch-induced activation of FAK at Tyr397 (Seufferlein and Rozengurt, 1994; Torsoni et al., 2005).
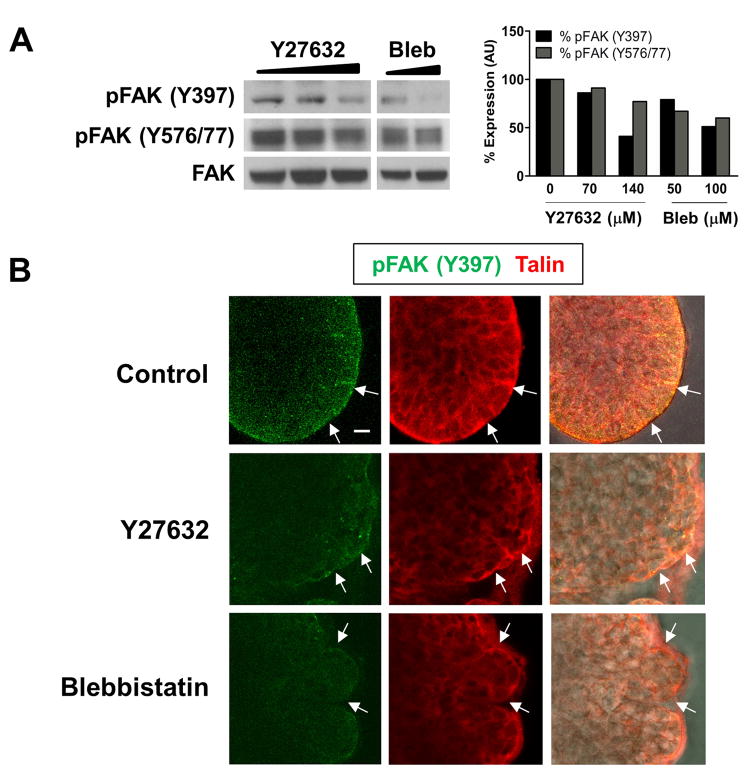
Since signaling through FAK is involved in FA strengthening and maturation, we questioned whether FAK might function as part of the mechanochemical checkpoint downstream of ROCK and myosin. To this end, we performed Western analysis on ROCK and myosin-inhibited SMG cell lysates with antibodies specific for the phosphorylated forms of FAK, and observed a dose-dependent decrease in FAK phosphorylation on Tyr397 (pFAK Y397) and on Tyr576/577 (pFAK Y576/77) (Fig. 3A). We performed immunocytochemistry to examine the localization of pFAK Y397 in SMG epithelial rudiments. In vehicle control-treated rudiments, pFAK Y397 localized to the extreme periphery of epithelial buds and co-localized with the FA protein talin (Fig. 3B). Such staining and localization were significantly decreased in ROCK and myosin-inhibited rudiments (Fig. 3B). These results demonstrate that ROCK-mediated actomyosin contraction is required for the phosphorylation and activation of FAK in SMGs undergoing branching morphogenesis. Since ROCK signaling also promotes β1 integrin activation and FA formation at the periphery of epithelial buds, it is likely that these effects on FAK activation are caused by ROCK-mediated integrin clustering at the epithelial surface and in cleft regions.
Figure 3. FAK is activated downstream of ROCK-mediated actomyosin contraction during SMG branching morphogenesis.
(A) E13 SMGs were cultured for 24 hours with increasing concentrations of Y27632 or blebbistatin added to the culture media, and cell lysates were immunoblotted with antibodies specific for FAK phosphorylated on Tyr 397 or Tyr 576/577. We observed a dose-dependent decrease in FAK phosphorylation at both of these sites, with quantification shown relative to total FAK protein.
(B) E13 mesenchyme-free epithelial rudiments cultured with vehicle control, Y27632, or blebbistatin were immunostained for FAK phosphorylated on Tyr 397 (green) and the focal adhesion protein talin (red). In vehicle control rudiments, pFAK Y397 localized to the periphery of epithelial buds (white arrows) with the focal adhesion protein talin. Both pFAK Y397 and talin localization and staining were reduced with the ROCK and myosin inhibitors. Scale bar, 10 μm (B).
Inhibition of FAK blocks SMG branching morphogenesis at the cleft initiation stage and promotes additional cleft initiations
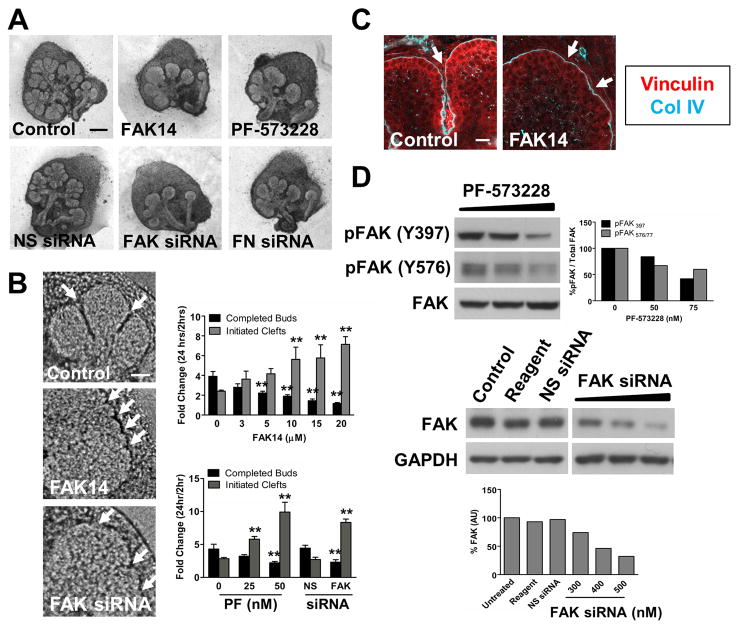
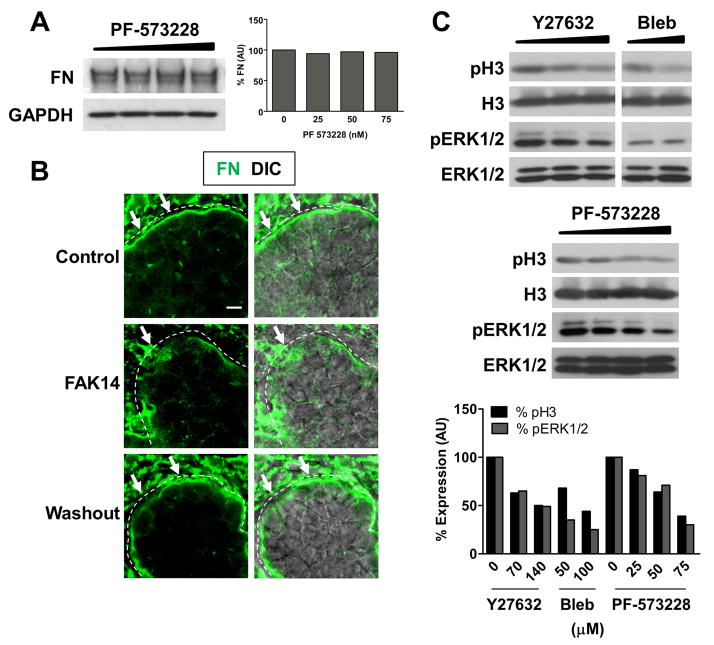
We investigated a function for FAK in cleft progression by culturing E13 SMGs in the presence of two structurally distinct pharmacological inhibitors of FAK, FAK14 and PF-573228, as well as FAK siRNAs. With both the FAK inhibitors and with FAK siRNAs, we observed a dose-dependent inhibition of branching (Fig. 4A). Furthermore, the morphology of SMGs lacking FAK function strongly resembled that of SMGs that had been treated with FN siRNA, suggesting a functional link between FN and FAK signaling. Interestingly, FAK inhibition also resulted in an increase in the number of small initiated, but not elongated, clefts at the surface of SMG epithelial buds, much like the immature initiated clefts resulting from treatment with ROCK and myosin inhibitors (Fig. 4B) previously reported (Daley et al., 2009). Morphometric analysis of SMGs treated with FAK14, PF-573228, and FAK siRNA revealed a dose-dependent decrease in the number of completed epithelial buds and an increase in such small initiated clefts (Fig. 4B). Consistent with these results, FA protein accumulation in the cleft region was likewise impaired in FAK-inhibited SMGs, suggestive of a role for FAK in the stabilization of initiated clefts via the formation of cell-matrix adhesions (Fig. 4C). To confirm the effectiveness of FAK inhibition and knock-down, we performed Western analysis on SMG cell lysates treated with PF-573228 and FAK siRNA. We observed a dose-dependent decrease in both pFAK Y397 and pFAK Y576/577 with increasing concentrations of PF-573228 and a decrease in total FAK protein levels with FAK siRNA (Fig. 4D). Taken together, these results indicate that FAK activation, downstream of ROCK and non-muscle myosin II activation, is a critical regulator of cleft progression during SMG branching morphogenesis and is required for the transition of initiated clefts to progressing clefts.
Figure 4. Inhibition of FAK, like inhibition of ROCK and myosin, blocks SMG branching morphogenesis at the cleft initiation stage.
(A) E13 SMGs were cultured for 24 hours with the FAK inhibitors, FAK 14 and PF-573228, or transfected for 24–48 hours with FN or FAK siRNAs. In all cases, FAK inhibition or reduction reduced SMG branching morphogenesis, with FAK-inhibited SMGs exhibiting an overall morphology similar to that of SMGs transfected with FN siRNA. (B) Pharmacological inhibition of FAK and reduction of FAK protein levels with siRNA, like ROCK and myosin inhibition, result in an increased number of small initiated, but not elongated, clefts in the surface of epithelial buds (white arrows). Morphometric analysis revealed a dose-dependent decrease in the number of completed epithelial buds and an increase in the number of initiated clefts. (C) FAK inhibition likewise decreased focal adhesion protein localization in SMG cleft regions, as demonstrated by immunostaining for vinculin (red) to mark focal adhesions and collagen IV(cyan) to delineate the basement membrane. (D) Immunoblot analysis reveals that pharmacological inhibitors of FAK result in a dose-dependent decrease in bothpFAK Y397 and pFAK Y576/577, with quantification normalized to total FAK protein. Immunoblot analysis also reveals a dose-dependent decrease in total FAK protein levels with increasing concentrations of FAK siRNA, but not non-targeting negative control siRNA (NS siRNA) or transfection reagent alone. Total FAK protein quantification is normalized to a GAPDH loading control. ANOVA, **p<0.01. Scale bars, 250 μm (A) and 50 μm (B)..
Inhibition of FAK activity disrupts FN assembly in the basement membrane of SMG organ cultures and decreases cell proliferation
Since we previously demonstrated that FN assembly drives subsequent cleft progression once the mechanochemical checkpoint has been reached, we questioned whether FAK activation might also be required for FN assembly in SMG basement membranes. We therefore compared total endogenous FN expression by immunoblotting in the presence of increasing concentrations of FAK inhibitor and observed no significant difference in the level of total FN relative to vehicle control-treated SMGs (Fig. 5A). We performed immunocytochemistry to detect any changes in FN localization within the basement membrane of FAK-inhibited SMGs. FAK inhibition resulted in disorganized FN assembly in the basement membrane, with accumulation of punctate stitches of FN and entire regions lacking FN at the periphery of epithelial buds (Fig. 5B). Washout experiments, in which SMGs were treated for 24 hours with FAK inhibitor and then cultured in the presence of vehicle control media alone, showed that FN was once again present in a continuous manner around the periphery of epithelial buds, much like in vehicle control-treated samples (Fig. 5B). Thus FAK signaling is required for organized FN assembly in the basement membrane during SMG branching morphogenesis.
Figure 5. FAK activity is also required for FN assembly and cell proliferation in branching SMGs.
(A) Immunoblotting for total endogenous FN reveals no difference between vehicle control and PF-573228-treated SMGs. Quantification is shown normalized to GAPDH loading control. (B) Immunocytochemistry reveals that FN (green) is present in a continuous manner throughout the basement membrane of vehicle control-treated SMGs. In contrast, FN is disorganized in the basement membrane of SMGs treated with FAK inhibitor, instead exhibiting punctate stitches of FN and entire regions lacking FN (compare white arrows). Washout experiments revealed that FN assembly in the basement membrane was able to recover after the FAK inhibitor was replaced with vehicle control media. (C) Like SMGs treated with ROCK and myosin inhibitors, FAK-inhibited SMGs exhibit decreased levels of phosphorylated histone H3 and phosphorylated ERK1/2, indicative of a decrease in cell proliferation. Quantification is normalized to total histone H3 and total ERK1/2 for pH3 and pERK1/2, respectively. Scale bars, 5 and 20 μm (B).
We previously demonstrated that the inhibition of ROCK and myosin activity results in a decrease in SMG epithelial cell proliferation, which contributes to the inhibitor-induced failure of cleft progression (Daley et al., 2009). Thus, we next questioned whether this proliferative effect might be mediated by FAK in the developing SMG. In the presence of FAK inhibitors, we observed a decrease in the mitotic marker phospho-histone H3, similar to both the ROCK and myosin inhibitors (Fig. 5C). We also detected a dose-dependent decrease in the amount of phosphorylated ERK1/2, which can promote G1/S progression, in the presence of all three inhibitors (Fig. 5C). These results suggest that the proliferative effects of the ROCK, non-muscle myosin II, and FN-mediated pathway during cleft progression may be primarily due to downstream FAK signaling converging on the Ras/MAPK pathway (Schlaepfer et al., 1994). Taken together, these data further support the notion of a FAK-mediated signaling pathway functioning downstream of ROCK signaling that is required for transition of an initiated cleft to a stabilized, progression-competent state in the developing SMG, thereby promoting subsequent cleft progression.
FN may function as an outside-in signal that induces the continued formation of cell-matrix adhesions and downstream cleft propagation
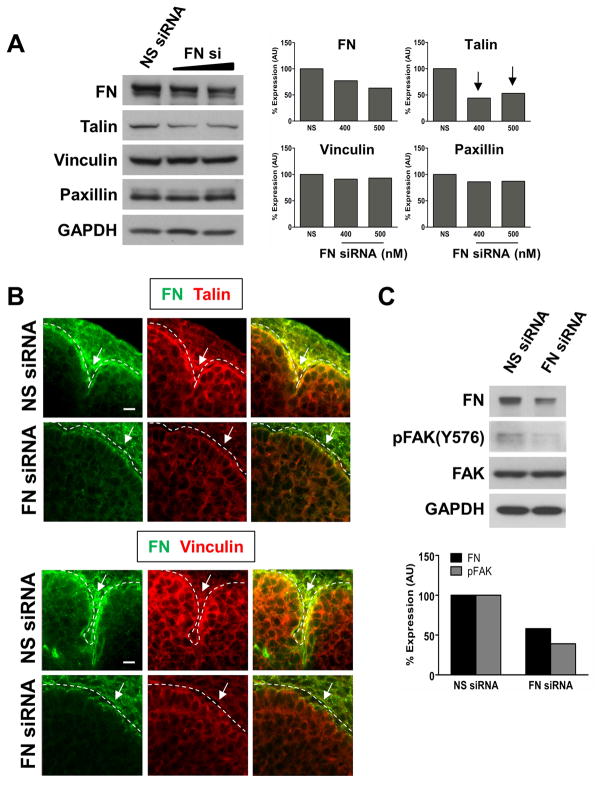
Previous reports have indicated that FN induces the formation of FAs in salivary epithelial cells in culture (Sakai et al., 2003). Thus, we next questioned whether FN, which functions downstream of ROCK and myosin in cleft progression (Daley et al., 2009), might constitute an outside-in signal that further promotes FA protein localization in the clefts of intact E13 SMGs, thereby inducing continued cleft propagation once the mechanochemical checkpoint has been successfully passed. As shown in Figure 6A, when FN protein levels were reduced in SMG organ cultures with FN siRNA, there was no significant change in the levels of the FA proteins, vinculin and paxillin, but as in the presence of the ROCK and myosin inhibitors, there was an approximately 50% decrease in talin protein levels (Fig. 6A). We examined FA protein localization in the cleft regions of SMGs transfected with either FN or random sequence non-targeting siRNAs, and found that when FN protein levels were reduced, talin and vinculin accumulation at the basal periphery of epithelial buds and in immature initiated clefts failed to occur (Fig. 6B). These data indicate that ROCK-mediated assembly of FN into the basement membrane stimulates an outside-in signal potentiated by FN that is required for focal adhesion protein localization in the cleft region of branching SMGs.
Figure 6. Fibronectin induces an outside-in signal that promotes focal adhesion protein accumulation in branching SMGs.
SMGs transfected for 24 hours with FN siRNA, but not a non-targeting negative control siRNA (NS siRNA), exhibit an approximately 50% reduction in talin, but no change in vinculin or paxillin protein levels. Quantification of immunoblots is expressed relative to a GAPDH loading control. (B) Localization of talin and vinculin (red) at the basal periphery of FN siRNA-transfected SMG epithelial buds and in immature initiated clefts is greatly reduced relative to the progressing clefts of SMGs transfected with NS siRNA (compare white arrows). Immunocytochemistry for FN (green) was performed to confirm FN knockdown. (C) Western blot analysis reveals that FAK phosphorylation is also decreased in SMGs transfected with FN, but not non-targeting negative control (NS), siRNAs. Quantification of FN and pFAK Y576/577 are normalized to GAPDH and total FAK, respectively. Scale bar, 10 μm (B).
ROCK-stimulated myosin activity promotes FN assembly in branching SMGs (Daley et al., 2009), and FN induces FA formation in salivary epithelial cells. Because this would be expected to cluster multiple FAK molecules in close proximity to one another to promote their autophosphorylation, we next questioned whether FAK activation also occurs downstream of FN-induced cell-matrix adhesion formation in the developing SMG. To this end, we transfected E13 SMGs with FN siRNA and examined FAK activation by immunoblotting. We observed that in the presence of FN siRNA, but not non-targeting negative control siRNA, FAK phosphorylation was reduced by approximately 50% (Fig. 6C). These results indicate that outside-in signaling mediated by FN can also result in FAK activation in branching SMGs.
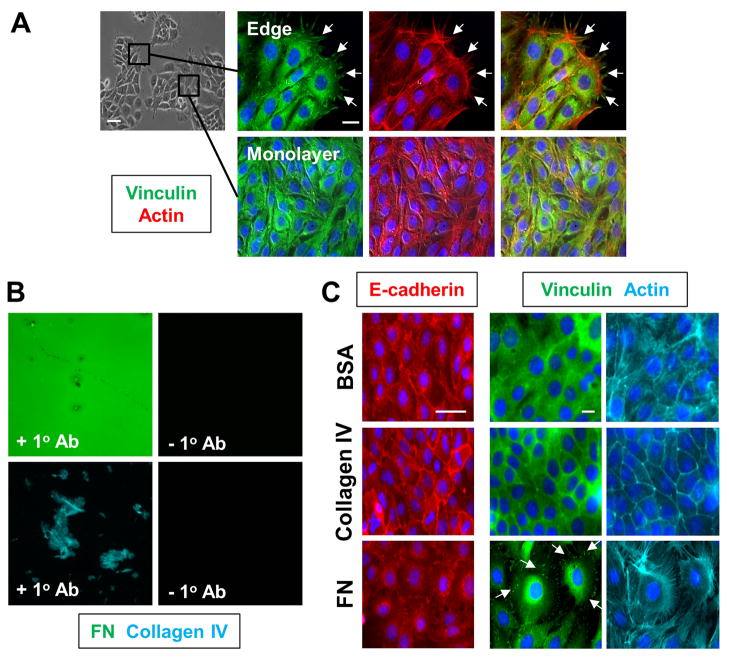
Finally, to determine whether FN specifically induces the formation of cell-matrix adhesions, we utilized the SIMS salivary gland epithelial cell line. SIMS cells, unlike SCA-9 cells, express E-cadherin and predominately form epithelial monolayers in 2D culture (Laoide et al., 1996; Laoide et al., 1999). Immunostaining for vinculin revealed that FAs and actin stress fibers are relatively abundant at the edges of SIMS monolayers, where cells contact the tissue culture plastic rather than neighboring cells. In contrast, at the center of SIMS monolayers where cells are surrounded by neighboring cells, vinculin is predominately cytoplasmic with few FAs evident, and actin is primarily cortical (Fig. 7A). We hypothesized that FN, but not other matrix proteins, might induce the formation of cell-matrix adhesions within the center of a SIMS monolayer, where cell-cell adhesions are normally abundant. To this end, we cultured SIMS cells on coverslips coated with FN, collagen IV, or BSA, and confirmed matrix protein coating by immunocytochemistry (Fig. 7B). We first confirmed that FN downregulates salivary epithelial cell-cell adhesions in mouse salivary epithelial cells, as previously reported for a human salivary gland cell line (Sakai et al., 2003). As expected, FN, but not BSA or collagen IV, resulted in a reduction in E-cadherin in SIMS monolayers (Fig. 7C). Furthermore, SIMS cells on FN exhibited increased spreading relative to cells cultured on BSA or collagen IV, evident at high magnification (Fig. 7C). Importantly, we also observed that cells at the center of SIMS monolayers cultured on FN exhibited vinculin-containing FAs that co-aligned with actin stress fibers, rather than the cytoplasmic vinculin distribution and cortical actin exhibited on BSA and collagen IV (Fig. 7C). Taken together, these data indicate that FN, but not collagen IV, is not only required for the formation of FAs in salivary gland epithelial cells, but it specifically induces the down-regulation and/or disassembly of cell-cell adhesions and their subsequent replacement by cell-matrix adhesions. Furthermore, these results indicate that FN functions as an outside-in signal that promotes the formation of cell-matrix interactions by salivary epithelial cells, thereby suggesting a mechanism for dynamic cleft propagation downstream of cleft stabilization via the mechanochemical checkpoint.
Figure 7. Fibronectin, but not other ECM proteins, specifically induces focal adhesion formation and cell spreading in a salivary gland epithelial cell line.
(A) SIMS cells, a salivary gland epithelial cell line, form monolayers in 2D culture, with E-cadherinmediated cell-cell adhesions forming between adjacent cells. Immunocytochemistry for the FA protein vinculin (green) with actin (red) demonstrates that FAs and stress fibers form at the periphery of SIMS monolayers where neighboring cells are absent (white arrows), but not in the interior of cell monolayers where cell-cell adhesions predominate. (B) SIMS cells were cultured for 18 hours on glass coverslips coated with fibronectin, collagen IV, or BSA and cellular morphology was compared. Coverslip coating with ECM proteins was confirmed by immunocytochemistry for FN (green) or collagen IV (cyan). (C) SIMS cells were cultured for 18 hours on glass coverslips coated with either FN, collagen IV, or BSA, and cellular morphology was compared by labeling with the nuclear marker DAPI (blue), Alexa546-phalloidin (actin, cyan), and immunostaining for E-cadherin (red). SIMS cells on FN, but not collagen IV or BSA, are significantly more spread and downregulateE-cadherin, as previously reported. Immunocytochemistry for vinculin (green) within the center of SIMS cell monolayers reveals that FN, but not collagen IV or BSA, induces the formation of vinculincontaining FAs (compare white arrows). In contrast, vinculin is diffusely cytoplasmic in cells on BSA and collagen IV. Cells on FN also form robust actin stress fibers (cyan), while cortical actin is instead present in cells on collagen IV or BSA. Scale bars, 50 and 20 μm (A and C). 254×338mm (300 × 300 DPI)
DISCUSSION
Cleft formation is the primary morphogenetic event that changes tissue shape during salivary gland branching morphogenesis. While previous studies have indicated a crucial role for the ECM protein FN in the process of cleft formation (Sakai et al., 2003; Larsen et al., 2006), which induces the replacement of E-cadherin-mediated cell-cell adhesions with FN-mediated cell-matrix adhesions, little is known regarding the composition of and the potential signaling function of such adhesions in the mechanism of cleft propagation. Here, we demonstrate that ROCK-stimulated non-muscle myosin type II activity constitutes an inside-out integrin activation signal that promotes FN assembly and the formation of cell-matrix adhesions (focal adhesions, FAs) in the cleft region. FAK is activated downstream of such adhesions, and requires ROCK-mediated actomyosin contraction. Furthermore, FAK signaling is required for the transition of clefts from an initiated to a progression-competent state. These data indicate that FAK activation is a molecular component of the mechanochemical checkpoint that we previously identified as a regulator of the transition of initiated clefts to a stabilized, progression-competent state (Daley et al., 2009).
Based on these results, we propose the following working model for the mechanochemical checkpoint (Fig. 8). As localized ROCK-mediated actomyosin contraction at the epithelial surface reaches a certain threshold, this triggers the inside-out activation of β1 integrins in an initiated cleft and promotes the rapid recruitment of FA components such as vinculin, talin, and FAK to β1 integrin cytoplasmic tails. The clustering of multiple FAK molecules results in autophosphorylation on Tyr397, leading to FAK activation and signaling. This ROCK-mediated inside-out signal also supports the initiation of FN fibrillogenesis within the cleft, which requires myosin-mediated contraction to drive the unfolding of cell surface-bound FN into an assembly-competent state. We refer to the threshold of contractility that must be reached to trigger all of these events as the mechanochemical checkpoint that regulates the transition of an initiated cleft to a progression-competent state.
Figure 8. Model for a focal adhesion protein-based mechanochemical checkpoint regulating cleft progression during SMG branching morphogenesis.

ROCK-mediated actomyosin contraction regulates a mechanochemical checkpoint at the epithelial surface that promotes the transition of dynamically unstable initiated clefts to a stabilized state competent to undergo progression. Such a checkpoint entails a focal increase in ROCK-mediated myosin activity within an initiated cleft which promotes the inside-out activation of β1 integrins, initiation of FN fibrillogenesis, and FN-mediated downregulation of epithelial cell-cell adhesions and their replacement by cell-matrix adhesions (focal adhesions). Focal adhesion formation and integrin clustering bring multiple FAK molecules into close proximity to one another, which trigger FAK autophosphorylation and further focal adhesion strengthening and maturation. We refer to the threshold of cytoskeletal contraction that must be reached within an initiated cleft to trigger these events as a mechanochemical checkpoint. Once the checkpoint has been successfully passed and the cleft stabilized, we propose that downstream signals (possibly through FAK-mediated ERK1/2activation) stimulate localized epithelial cell proliferation leading to cleft progression.
The major FN receptor, integrin α5β1, is required for SMG cleft formation (Sakai et al., 2003), and early studies indicated that FN binding to cell surface integrin receptors requires them to be in a high affinity, “activated” conformation (Wu et al., 1995). Integrin activation or priming is a major cellular mechanism by which cells regulate their binding affinity to a variety of ECM ligands; this process involves conformational changes in integrin α and β cytoplasmic and extracellular domains that result in their separation and subsequent unfolding into an extended conformation (Takagi et al., 2002; Kim et al., 2003). While this can occur through either an “outside-in” or “inside-out” mechanism, our results demonstrate that ROCK-mediated myosin activity promotes β1 integrin activation at the SMG epithelial surface through the latter mechanism. This conclusion is supported by the fact that ROCK and myosin activity reduce 9EG7 binding to β1 integrins at the periphery of epithelial buds and in the cleft region, as well as by our previous finding that while exogenous FN added to the culture media can stimulate cleft formation, it requires ROCK and non-myosin type II activity to do so (Daley et al., 2009). Although reports indicate that the particular α subunit partnered with β1 integrin affects 9EG7’s ability to recognize activated β1 integrin, it has been demonstrated that 9EG7 binds with the highest affinity to integrin α5β1, the major FN receptor required for SMG branching morphogenesis (Bazzoni et al., 1998).
There is currently some controversy surrounding the existence of true focal adhesions in 3D tissues. On two dimensional cell culture surfaces, FAs exist as distinct multi-protein structures closely associated with cell surface integrin receptors and the actin cytoskeleton (Geiger et al., 2009; Kanchanawong et al., 2010). In contrast, FA organization has been shown to change significantly when cells are cultured in 3D; while FAs form as distinct complexes in 2D, such FA components appear to be diffusely localized throughout cells in 3D (Fraley et al., 2010). Despite the controversy surrounding the existence of true focal adhesions in 3D tissues (Kubow and Horwitz, 2011), we demonstrate here that focal adhesion kinase (FAK), a major component of FAs, plays an important developmental role in the branching submandibular salivary gland. Interestingly, recent ultrastructural studies of SMG cleft regions have revealed the presence of a small cytoplasmic protrusion, referred to as a “shelf”, composed of a densely packed core of actin microfilaments at the base of progressing clefts (Kadoya and Yamashina, 2010). Transmission electron micrographs revealed that the basement membrane appears to invade the groove formed by this shelf and the sidewall of the cleft. Taken together with our results demonstrating the requirement for β1 integrin activation and cell-matrix adhesion formation for the transition of clefts to a stabilized state, it is entirely possible that such a cytoplasmic shelf serves as the initial attachment point for cleft stabilization. ROCK-mediated actomyosin contraction pulling against this bundle of microfilaments could provide the forces necessary to form this initial attachment point and enhance cell-matrix interactions at the shelf. An interesting future investigation would be to examine immunolocalization of activated β1 integrins and FA components at the shelf during cleft progression.
The results presented here also indicate a crucial role for ROCK-mediated focal adhesion kinase (FAK) activation in the transition of clefts from an initiated to progression-competent state. FAK is a ubiquitously expressed non-receptor protein tyrosine kinase that interacts with a number of other FA components and has been shown to modulate adhesion strengthening (Hanks et al., 1992; Schaller et al., 1992). FAK activation is initiated by proximity-induced autophosphorylation on Tyr397 upon clustering (Parsons et al., 2000; Tilghman and Parsons, 2008; Zhao and Guan, 2009). Here, we demonstrate that ROCK-mediated actomyosin contraction is required for FAK phosphorylation on Tyr397 in SMGs undergoing branching morphogenesis. This is consistent with other recent results indicating a similar role for ROCK in the stimulation of FAK activation in other systems, namely in cardiac myocytes and mammary epithelial cells (Torsoni et al., 2005; Provenzano et al., 2009). We propose that the primary requirement for ROCK and myosin in FAK activation is most likely due to their effects on β1 integrin activation and cell-matrix adhesion formation, since such an event would be expected to cluster activated integrins within the cleft, thereby clustering FAK molecules recruited to the activated integrins.
How might FAK activation contribute to cleft stabilization? A primary role for FAK has been suggested to be the modulation of cell-matrix adhesion strength. In some cases, this has been shown to occur through FAK-mediated inside-out activation of β1 integrin after initial adhesion to the ECM, which serves to strengthen the initial adhesion and promote further integrin clustering (Michael et al., 2009). Other studies have demonstrated a role for FAK in the enhanced recruitment of other FA adaptors, such as vinculin, which also enhanced integrin clustering and promoted further FA strengthening (Pasapera et al., 2010). In the context of SMG cleft formation and the mechanochemical checkpoint, FAK activation downstream of a ROCK-mediated inside-out integrin activation signal that triggers adhesion to the ECM may serve to strengthen and reinforce such early adhesions, thereby promoting cleft stabilization and the transition to a progression-competent state.
Our results also demonstrate that the ROCK and FN-mediated effects on SMG epithelial cell proliferation that we previously demonstrated are required for cleft progression are most likely mediated by downstream signaling through FAK and ERK1/2. This finding is consistent with studies of epithelial cells in 2D culture demonstrating that FAK activation enables its interaction with the adaptor protein Grb2, which in turn leads to the activation of the Ras/ERK/MAPK signaling pathway (Schlaepfer et al., 1994; Schlaepfer et al., 1998). Moreover, FAK has been shown to increase expression of cyclin D1 and decrease the Cdk inhibitor p21, respectively, thereby promoting G1/S cell cycle progression (Zhao et al., 1998; Zhao et al., 2001). Interestingly, these same effects have also been attributed to ROCK, although a downstream requirement for FAK activation was not investigated in those studies (Huang et al., 1998; Croft and Olson, 2006).
A significant outstanding question remains as to how the tension comprising the mechanochemical checkpoint might be sensed within the cleft region. Interestingly, a number of recent studies have demonstrated that the FA adaptor protein talin is not only capable of activating integrins in an inside-out manner, but may also be responsive to cytoskeletal forces, thus acting as a cellular mechanosensor required for cell-matrix adhesion formation. Talin binding to β1 integrin cytoplasmic tails results in their separation from the alpha subunit and subsequent inside-out activation (Tadokoro et al., 2003; Tanentzapf and Brown, 2006; Wegener et al., 2007). This appears to be one of the earliest steps that occurs during cell-matrix adhesion formation, since talin is not only present in mature, stabilized FAs, but also in their precursors, the immature, unstabilized focal contacts that exist prior to integrin receptor clustering (Zaidel-Bar et al., 2003; Ballestrem et al., 2006; Zaidel-Bar et al., 2007a; Zaidel-Bar et al., 2007b). While the mechanisms by which cytoskeletal forces drive FA strengthening and maturation remain incompletely understood, recent studies suggest that such forces can promote conformational changes in a number of proteins, thereby triggering protein unfolding to expose cryptic protein-protein interaction sites (Vogel, 2006; Johnson et al., 2007). In particular, molecular simulations of the interaction between vinculin and talin at nascent FAs suggest that the application of mechanical force may enhance the recruitment of vinculin by driving the unfolding of talin to expose vinculin binding sites (Gingras et al., 2006; Patel et al., 2006; Lee et al., 2007; Hytonen and Vogel, 2008); such modeling has recently been corroborated experimentally, since the mechanical stretching of talin in vitro was shown to stimulate vinculin binding (del Rio et al., 2009). Because talin binds directly to β integrin cytoplasmic tails and stabilizes them in the activated conformation (Tadokoro et al., 2003; Tanentzapf and Brown, 2006; Wegener et al., 2007), and vinculin binding to talin crosslinks multiple talin molecules and is required for integrin clustering (Humphries et al., 2007), it is apparent how such a force-mediated event could lead to the stabilization and enlargement of FAs. Given this potential force-induced activation of talin, it is interesting to speculate that a focal increase in ROCK-stimulated myosin activity at an initiated cleft may drive the unfolding of talin to promote α5β1 inside-out activation, vinculin binding to talin, and subsequent integrin clustering to initiate FA protein accumulation, cleft stabilization, and FN fibrillogenesis. Interestingly, of the FA components whose expression we examined in the presence of the ROCK, myosin, and FN inhibitors, only talin was observed to decrease at the protein level, suggesting that tension-mediated cell matrix adhesion may prime cells for morphogenetic and homeostatic responses by increasing expression of this critical FA component.
Once the mechanochemical checkpoint has been passed and a cleft stabilized, a progressing cleft must continue to propagate. Our results suggest that such propagation is likely driven by a FN-induced outside-in signal that promotes continued FA protein accumulation and FAK activation. We demonstrate that not only is FN required for the accumulation of such proteins in the cleft region, but that it also specifically induces the formation of cell matrix adhesions by salivary epithelial cells. These results are consistent with recent studies in mammary tumor biology, which have revealed that one of the key hallmarks of epithelial malignancy in this tissue is a stiffening of the stromal microenvironment caused by the increased deposition of ECM components such as stromal FN (Paszek et al., 2005; Williams et al., 2008; Provenzano et al., 2009). Such ECM stiffening promotes integrin activation, FA formation, and downstream activation of FAK and ERK1/2 to drive hyperproliferation and the acquisition of a malignant phenotype. Overall, such studies have led to the idea of a constitutively elevated signaling circuit involving Rho/ROCK-mediated adhesion to the ECM, and downstream signaling through FAK/MAPK which feeds forward to promote further ECM remodeling, FA formation, and cellular contractility (Paszek et al., 2005; Provenzano et al., 2009). Because it is often the case that malignant transformation involves normal morphogenetic events recapitulated without regulation, it is conceivable that these molecular pathways occur normally in the cleft region during SMG branching morphogenesis. That is, assembly of FN in the cleft region could drive localized basement membrane stiffening, increased cell-matix adhesion, and FA formation that in turn feeds forward to promote increased contraction and cell-matrix adhesion in adjacent cells to propagate the cleft. Indeed, live imaging of SMG organ explants cultured in the presence of fluorescently labeled FN revealed that FN is assembled in a directional manner within a cleft as it progresses, with older FN assembled at the base of a cleft and newer FN assembled behind it (Larsen et al., 2006), and that the cells at the base of a cleft undergo dynamic wiggling movements as the cleft progresses (Kadoya and Yamashina, 2010) involving the epithelial-mesenchyme transition (EMT) regulators Snail and Slug in response to FN assembly (Onodera et al., 2010). This dynamic wiggling might also pull against adjacent cells and the ECM to promote continued FN assembly and the transmission of force from cell to cell along a propagating cleft.
In summary, our data suggest a mechanism by which actomyosin-mediated formation of cell-matrix adhesions in the cleft region can promote the physical changes required for dynamic cleft propagation during SMG branching morphogenesis. We propose that such adhesions are required for the stabilization of initiated clefts and their subsequent transition to a progression-competent state, thereby promoting salivary gland branching morphogenesis.
EXPERIMENTAL PROCEDURES
Ex vivo organ culture and inhibition assays
Mouse SMGs were dissected from timed-pregnant female mice (strain CD-1, Charles River Laboratories) at embryonic day 13 (E13), following protocols approved by the University at Albany IACUC committee. The day of plug discovery was designated as E0. Embryonic SMGs were microdissected as previously described (Sakai and Onodera, 2008; Daley et al., 2009) and cultured at the air/media interface on Nuclepore Track-Etch membrane filters (Whatman) floating on DMEM/Ham’s F12 medium (F12) (Invitrogen). SMG epithelial rudiments without mesenchyme were prepared and cultured as previously described (Daley et al., 2009; Rebustini and Hoffman, 2009). Briefly, dissected SMGs were incubated in 1.6 U/mL dispase prepared in DMEM/F12 media (Invitrogen) for 15 minutes at 37°C. Dispase was neutralized with 5% BSA, and the mesenchyme was physically separated from the epithelium with sterile forceps. Epithelial rudiments were cultured in 6 mg/mL Matrigel (BD Biosciences) diluted 1:1 in DMEM/F12, with media supplemented with 20 ng/mL EGF and 200 ng/mL FGF7 (R&D Systems). Five intact SMGs or three epithelial rudiments were used for each condition with all experiments repeated at least three times.
The pharmacological inhibitors Y27632 and (−)-blebbistatin (Calbiochem) were used at concentrations ranging from 0–140 μM for Y27632 and 0–100 μM for blebbistatin, as previously described (Daley et al., 2009). The FAK inhibitors FAK14 and PF-573228 were obtained from Tocris Bioscience and used at concentrations ranging from 0–20 μM for FAK 14 and 0–75 nM for PF-573228. Inhibitor containing media was replaced every 24 hours, and brightfield images were acquired on a Nikon Eclipse TS100 microscope equipped with a Canon EOS 450D digital camera.
Validated duplexed siRNAs (Silencer Select) were obtained from Applied Biosytems: FN (s66182 and s66183), Ptk2 (s65838 and s65840), Negative Control siRNA #1, and Cy3-conjugated Negative Control siRNA #1. These negative control siRNAs consist of random sequences having no sequence similarity to mouse, rat, or human gene sequences. SMGs were transfected with 100–500 nM siRNA using RNAiFect transfection reagent (Qiagen) according to the manufacturer’s protocol and as previously described (Daley et al., 2009). SMGs on Nuclepore filters were cultured on siRNA-containing media for 24–48 hours. siRNA uptake was monitored using a Cy3-conjugated non-targeting siRNA, with target knockdown confirmed by immunoblot analysis or immunocytochemistry. Each experiment was repeated 2–3 times for each siRNA with eight SMGs per group.
Morphometric analysis was performed using MetaVue (Version 6.2r6, Molecular Devices) and fold change was calculated by normalizing to the number of clefts or buds present at the initial time point (2 hours) and displayed using Prism (GraphPad, Version 5.01). For statistical analysis, one-way analysis of variance (ANOVA) was performed with a Bonferroni post-test (GraphPad Prism).
Whole mount immunocytochemistry and confocal microscopy
Immunostaining of SMG organ cultures or epithelial rudiments was performed as previously described (Daley et al., 2009), with all antibody incubations performed overnight at 4°C. Specimens were imaged on a Zeiss LSM510 confocal microscope equipped with Argon, HeNe1, and HeNe2 lasers at 10× (Fluor 10×/0.50 NA), 20× (Plan Apo/0.75 NA), or 63× (Plan Apo/1.4 NA) magnification. Antibodies used with dilutions are as follows: monoclonal antibody 9EG7 (BD Pharmingen, 1:100), anti-β1 polyclonal antibody (a kind gift of Dr. Mary Ann Stepp, 1:100), talin (Clone 8d4, Sigma, 1:100), vinculin (Clone hVIN-1, Sigma, 1:100), Collagen IV (AB769, AB756P, Millipore, both 1:100), anti-fibronectin polyclonal antibody (R5386, a kind gift of Dr. Kenneth Yamada, 1:100), E-cadherin (Clone 36, BD Biosciences, 1:250), and phosphorylated FAK (Tyr 397) (Cell Signaling, 1:100). Alexa546-phalloidin (Invitrogen, 1:350) was also used. For secondary antibodies, cyanine dye-conjugated AffiniPure F(ab’)2 fragments were used (Jackson ImmunoResearch Laboratories, 1:100). For quantification of 9EG7 staining, 9EG7 and total β1 pixel intensity were quantified in MetaVue (Molecular Devices) from confocal stacks and expressed as a ratio of 9EG7 to total β1 pixel intensity.
Immunoblot analysis
Protein assays and Western blot analysis were performed as previously described (Larsen et al., 2003; Daley et al., 2009). Blots were imaged using X-ray film (ECL Hyperfilm) and densitometric analysis was performed using Quantity One (Version 4.6.1, BioRad) with normalization to either GAPDH, total FAK for phosphorylated FAK, total histone H3 for phosphorylated histone H3, and total ERK1/2 for phosphorylated ERK1/2. Blots were repeated at least three times, but only one representative blot and its quantification are shown. Antibodies used with dilutions are as follows: rabbit anti-β1 integrin polyclonal antibody (a kind gift of Dr. Mary Ann Stepp, 1:1,000), talin (Clone 8d4, Sigma, 1:1,000), vinculin (Clone hVIN-1, Sigma, 1:1,000), paxillin (2542, Cell Signaling Technology, 1:1,000), anti-fibronectin rabbit polyclonal antibody (R5386, a kind gift of Dr. Kenneth Yamada, 1:1,000), phosphorylated FAK (Tyr 397) (3283, Cell Signaling, 1:1,000), phosphorylated FAK (Tyr 576/577) (3281, Cell Signaling, 1:1,000), total FAK (3285, Cell Signaling, 1:1,000), phosphorylated histone H3 (Ser 10) (9701, Cell Signaling, 1:1,000), total histone H3 (9712, Cell Signaling, 1:1,000), phosphorylated ERK1/2 (4370, Cell Signaling, 1:1,000), total ERK1/2 (Cell Signaling, 1:1,000), and GAPDH (Fitzgerald, 1:10,000).
Salivary gland cell culture and immunocytochemistry
SIMS cells, a salivary gland epithelial cell line derived from the ducts of adult SMG (Laoide et al., 1996; Laoide et al., 1999), were maintained in DMEM containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen). For experiments to examine cellular morphology and focal adhesion formation in response to different ECM proteins, SIMS cells were seeded at identical density on coverslips coated with BSA, fibronectin, or collagen IV. Cells were cultured overnight for 18 hours in standard DMEM, and fixed and processed for immunocytochemistry as previously described. To detect focal adhesions at the edges and within the middle of SIMS monolayers, a primary antibody to vinculin was used (Sigma, 1:100). Alexa546-phalloidin (Invitrogen, 1:350) was used to label the actin cytoskeleton, and an E-cadherin monoclonal antibody (Clone 36, BD Biosciences, 1:250) was used to detect E-cadherin-containing cell-cell adhesions. SCA-9 cell culture and immunocytochemistry was performed as previously described (Daley et al., 2009).
For coating reactions, coverslips were incubated with 10 μg/mL human plasma FN (courtesy of Dr. Ken Yamada), collagen IV, or BSA in sterile 1× PBS for either 2 hours at 37°C or overnight at 4°C. Coverslips were then blocked for 1 hour at room temperature with 1% heat denatured BSA in sterile 1× PBS. Coverslips were washed three times with 1× PBS, and cells were seeded either immediately or after the coverslips had been left to dry overnight at 4°C. ECM protein coating was confirmed by immunocytochemistry with a polyclonal anti-FN antibody (R5386, 1:100) or a collagen IV antibody (Millipore, 1:100).
Supplementary Material
Monoclonal antibody 9EG7 preferentially binds β1 integrin in the activated conformation.
(A) Integrin binding affinity for various ECM ligands is regulated by conformational changes that promote an extended, or activated, conformation. This can occur through either an outside-in or inside-out mechanism, depending on the source of the signal that induces the conformational change. Divalent cations such as Mn2+ bind to integrin extracellular domains and mediate outside-in conformational changes, while cytoplasmic adaptors such as talin mediate inside-out signaling. Conformation specific antibodies such as 9EG7 bind β1 integrin in the extended conformation.
(B) E13 SMGs were cultured for 24 hours in the presence of increasing concentrations of MnCl2 or MgCl2 and co-stained with mAb 9EG7 (cyan), which recognizes β1 integrin in the activated conformation, and a polyclonal antibody for total integrin s1 (red). 9EG7 staining is increased in the presence of MnCl2 but not MgCl2. XZ projections of confocal stacks reveal increased 9EG7 staining within the interior of epithelial buds with Mn2+ treatment. Quantification of 9EG7staining is normalized to total β1 integrin. ANOVA, ***p<0.001. Scale bar, 100 μm (B).
Acknowledgments
The authors acknowledge the NIH (R01 DE019244, R21DE019197, and RC1 DE020402 to M.L.) and the University at Albany, SUNY start-up funds for supporting this research. Drs. Kenneth Yamada and Mary Ann Stepp are thanked for supplying antibodies. The authors thank other members of the Larsen Lab, especially Drs. Deirdre Nelson and Sharon Sequeira, for helpful discussions, and Dr. Nelson for critical reading of the manuscript.
References
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Ballestrem C, Erez N, Kirchner J, Kam Z, Bershadsky A, Geiger B. Molecular mapping of tyrosine-phosphorylated proteins in focal adhesions using fluorescence resonance energy transfer. J Cell Sci. 2006;119:866–875. doi: 10.1242/jcs.02794. [DOI] [PubMed] [Google Scholar]
- Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci U S A. 2002;99:5139–5143. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–25577. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Ma L, Blue ML, Hemler ME. Divalent cations and ligands induce conformational changes that are highly divergent among beta 1 integrins. J Biol Chem. 1998;273:6670–6678. doi: 10.1074/jbc.273.12.6670. [DOI] [PubMed] [Google Scholar]
- Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, Kozlov MM. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur J Cell Biol. 2006;85:165–173. doi: 10.1016/j.ejcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008 doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft DR, Olson MF. The Rho GTPase effector ROCK regulates cyclin A, cyclin D1, and p27Kip1 levels by distinct mechanisms. Mol Cell Biol. 2006;26:4612–4627. doi: 10.1128/MCB.02061-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley WP, Gulfo KM, Sequeira SJ, Larsen M. Identification of a mechanochemical checkpoint and negative feedback loop regulating branching morphogenesis. Dev Biol. 2009;336:169–182. doi: 10.1016/j.ydbio.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- Fraley SI, Feng Y, Krishnamurthy R, Kim DH, Celedon A, Longmore GD, Wirtz D. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol. 2010;12:598–604. doi: 10.1038/ncb2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr Opin Cell Biol. 2001;13:584–592. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- Gingras AR, Vogel KP, Steinhoff HJ, Ziegler WH, Patel B, Emsley J, Critchley DR, Roberts GC, Barsukov IL. Structural and dynamic characterization of a vinculin binding site in the talin rod. Biochemistry. 2006;45:1805–1817. doi: 10.1021/bi052136l. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci U S A. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harunaga J, Hsu JC, Yamada KM. Dynamics of Salivary Gland Morphogenesis. J Dent Res. 2011;90:1070–7. doi: 10.1177/0022034511405330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Chen CS, Ingber DE. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol Biol Cell. 1998;9:3179–3193. doi: 10.1091/mbc.9.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol. 2007;179:1043–1057. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hytonen VP, Vogel V. How force might activate talin’s vinculin binding sites: SMD reveals a structural mechanism. PLoS Comput Biol. 2008;4:e24. doi: 10.1371/journal.pcbi.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CP, Tang HY, Carag C, Speicher DW, Discher DE. Forced unfolding of proteins within cells. Science. 2007;317:663–666. doi: 10.1126/science.1139857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya Y, Yamashina S. Cellular dynamics of epithelial clefting during branching morphogenesis of the mouse submandibular gland. Dev Dyn. 2010;239:1739–1747. doi: 10.1002/dvdy.22312. [DOI] [PubMed] [Google Scholar]
- Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301:1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kubow KE, Horwitz AR. Reducing background fluorescence reveals adhesions in 3D matrices. Nat Cell Biol. 2011;13:3–5. doi: 10.1038/ncb0111-3. author reply 5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoide BM, Courty Y, Gastinne I, Thibaut C, Kellermann O, Rougeon F. Immortalised mouse submandibular epithelial cell lines retain polarised structural and functional properties. J Cell Sci. 1996;109 ( Pt 12):2789–2800. doi: 10.1242/jcs.109.12.2789. [DOI] [PubMed] [Google Scholar]
- Laoide BM, Gastinne I, Rougeon F. Tubular morphogenesis and mesenchymal interactions affect renin expression and secretion in SIMS mouse submandibular cells. Exp Cell Res. 1999;248:172–185. doi: 10.1006/excr.1999.4404. [DOI] [PubMed] [Google Scholar]
- Larsen M, Hoffman MP, Sakai T, Neibaur JC, Mitchell JM, Yamada KM. Role of PI 3-kinase and PIP3 in submandibular gland branching morphogenesis. Dev Biol. 2003;255:178–191. doi: 10.1016/S0012-1606(02)00047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M, Wei C, Yamada KM. Cell and fibronectin dynamics during branching morphogenesis. J Cell Sci. 2006;119:3376–3384. doi: 10.1242/jcs.03079. [DOI] [PubMed] [Google Scholar]
- Lee SE, Kamm RD, Mofrad MR. Force-induced activation of talin and its possible role in focal adhesion mechanotransduction. J Biomech. 2007;40:2096–2106. doi: 10.1016/j.jbiomech.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Lipfert L, Haimovich B, Schaller MD, Cobb BS, Parsons JT, Brugge JS. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Cheng L, Flesken-Nikitin A, Huang L, Nikitin AY, Pauli BU. Conditional knockout of fibronectin abrogates mouse mammary gland lobuloalveolar differentiation. Dev Biol. 2010;346:11–24. doi: 10.1016/j.ydbio.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–399. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Michael KE, Dumbauld DW, Burns KL, Hanks SK, Garcia AJ. Focal adhesion kinase modulates cell adhesion strengthening via integrin activation. Mol Biol Cell. 2009;20:2508–2519. doi: 10.1091/mbc.E08-01-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y, Morita T, Nogawa H. Cell proliferation is not required for the initiation of early cleft formation in mouse embryonic submandibular epithelium in vitro. Development. 1987;99:429–437. doi: 10.1242/dev.99.3.429. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Nogawa H, Hashimoto Y, Kishi J, Hayakawa T. Accumulation of collagen III at the cleft points of developing mouse submandibular epithelium. Development. 1988;104:51–59. doi: 10.1242/dev.104.1.51. [DOI] [PubMed] [Google Scholar]
- Nogawa H. Determination of the curvature of epithelial cell mass by mesenchyme in branching morphogenesis of mouse salivary gland. J Embryol Exp Morphol. 1983;73:221–232. [PubMed] [Google Scholar]
- Onodera T, Sakai T, Hsu JC, Matsumoto K, Chiorini JA, Yamada KM. Btbd7 regulates epithelial cell dynamics and branching morphogenesis. Science. 2010;329:562–565. doi: 10.1126/science.1191880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606–5613. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol. 2010;188:877–890. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Patel B, Gingras AR, Bobkov AA, Fujimoto LM, Zhang M, Liddington RC, Mazzeo D, Emsley J, Roberts GC, Barsukov IL, Critchley DR. The activity of the vinculin binding sites in talin is influenced by the stability of the helical bundles that make up the talin rod. J Biol Chem. 2006;281:7458–7467. doi: 10.1074/jbc.M508058200. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebustini IT, Hoffman MP. ECM and FGF-dependent assay of embryonic SMG epithelial morphogenesis: investigating growth factor/matrix regulation of gene expression during submandibular gland development. Methods Mol Biol. 2009;522:319–330. doi: 10.1007/978-1-59745-413-1_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- Sakai T, Onodera T. Embryonic organ culture. Curr Protoc Cell Biol. 2008;Chapter 19(Unit 19):18. doi: 10.1002/0471143030.cb1908s41. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Jones KC, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18:2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufferlein T, Rozengurt E. Lysophosphatidic acid stimulates tyrosine phosphorylation of focal adhesion kinase, paxillin, and p130. Signaling pathways and cross-talk with platelet-derived growth factor. J Biol Chem. 1994;269:9345–9351. [PubMed] [Google Scholar]
- Spooner BS, Wessells NK. An analysis of salivary gland morphogenesis: role of cytoplasmic microfilaments and microtubules. Dev Biol. 1972;27:38–54. doi: 10.1016/0012-1606(72)90111-x. [DOI] [PubMed] [Google Scholar]
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–511. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- Tanentzapf G, Brown NH. An interaction between integrin and the talin FERM domain mediates integrin activation but not linkage to the cytoskeleton. Nat Cell Biol. 2006;8:601–606. doi: 10.1038/ncb1411. [DOI] [PubMed] [Google Scholar]
- Tilghman RW, Parsons JT. Focal adhesion kinase as a regulator of cell tension in the progression of cancer. Semin Cancer Biol. 2008;18:45–52. doi: 10.1016/j.semcancer.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsoni AS, Marin TM, Velloso LA, Franchini KG. RhoA/ROCK signaling is critical to FAK activation by cyclic stretch in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2005;289:H1488–1496. doi: 10.1152/ajpheart.00692.2004. [DOI] [PubMed] [Google Scholar]
- Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu Rev Biophys Biomol Struct. 2006;35:459–488. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- Wan X, Li Z, Lubkin SR. Mechanics of mesenchymal contribution to clefting force in branching morphogenesis. Biomech Model Mechanobiol. 2008;7:417–426. doi: 10.1007/s10237-007-0105-y. [DOI] [PubMed] [Google Scholar]
- Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Williams CM, Engler AJ, Slone RD, Galante LL, Schwarzbauer JE. Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer Res. 2008;68:3185–3192. doi: 10.1158/0008-5472.CAN-07-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Wu C, Keivens VM, O’Toole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995;83:715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- Yoneda A, Ushakov D, Multhaupt HA, Couchman JR. Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Mol Biol Cell. 2007;18:66–75. doi: 10.1091/mbc.E06-08-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116:4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci. 2007;120:137–148. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009;28:35–49. doi: 10.1007/s10555-008-9165-4. [DOI] [PubMed] [Google Scholar]
- Zhao J, Pestell R, Guan JL. Transcriptional activation of cyclin D1 promoter by FAK contributes to cell cycle progression. Mol Biol Cell. 2001;12:4066–4077. doi: 10.1091/mbc.12.12.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JH, Reiske H, Guan JL. Regulation of the cell cycle by focal adhesion kinase. J Cell Biol. 1998;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Monoclonal antibody 9EG7 preferentially binds β1 integrin in the activated conformation.
(A) Integrin binding affinity for various ECM ligands is regulated by conformational changes that promote an extended, or activated, conformation. This can occur through either an outside-in or inside-out mechanism, depending on the source of the signal that induces the conformational change. Divalent cations such as Mn2+ bind to integrin extracellular domains and mediate outside-in conformational changes, while cytoplasmic adaptors such as talin mediate inside-out signaling. Conformation specific antibodies such as 9EG7 bind β1 integrin in the extended conformation.
(B) E13 SMGs were cultured for 24 hours in the presence of increasing concentrations of MnCl2 or MgCl2 and co-stained with mAb 9EG7 (cyan), which recognizes β1 integrin in the activated conformation, and a polyclonal antibody for total integrin s1 (red). 9EG7 staining is increased in the presence of MnCl2 but not MgCl2. XZ projections of confocal stacks reveal increased 9EG7 staining within the interior of epithelial buds with Mn2+ treatment. Quantification of 9EG7staining is normalized to total β1 integrin. ANOVA, ***p<0.001. Scale bar, 100 μm (B).