Abstract
Objective
Estimate the mortality impact of delay in antiretroviral therapy (ART) initiation from the time of entry-into-care.
Design
A state-transition Markov process model. This technique allows for assessing mortality before and after ART initiation associated with delays in ART initiation among a general population of ART eligible patients without conducting a randomized trial.
Methods
We used patient-level data from three South African cohorts to determine transition probabilities for pre-ART CD4 count changes and pre-ART and on-ART mortality. For each parameter we generated probabilities and distributions for Monte Carlo simulations with one week cycles to estimate mortality 52 weeks from clinic entry.
Results
We estimated an increase in mortality from 11.0% to 14.7% (relative increase of 34%) with a 10 week delay in ART for patients entering care with our pre-ART cohort CD4 distribution. When we examined low CD4 ranges, the relative increase in mortality delays remained similar; however, the absolute increase in mortality rose. For example, among patients entering with CD4 count 50–99 cells/mm3, 12 month mortality increased from 13.3% with no delay compared to 17.0% with a 10 week delay and 22.9% with a 6 month delay.
Conclusions
Delays in ART initiation, common in routine HIV programs, can lead to important increases in mortality. Prompt ART initiation for patients entering clinical care and eligible for ART, especially those with lower CD4 counts, could be a relatively low cost approach with a potential marked impact on mortality.
Keywords: ART delay, Africa, CD4 count, mortality, state-transition model
Introduction
Use of combination antiretroviral therapy (ART) dramatically reduces mortality among people living with HIV 1;2 with maximal benefit when ART is started at higher CD4 counts.3–5 However, in many sub-Saharan African clinical settings, patients meeting local ART initiation guidelines experience delays of several weeks to several months in initiating treatment.6–9 These delays may occur as a result of health system, provider, and patient-level factors. For example, ART agent shortages and ART waiting lists are health-system factors leading to delay.10–13 Pre-ART counseling sessions and investigations for TB and other opportunistic diseases are clinic or provider-level factors that may lead to delay, while failure to return for timely clinic visits or declining ART due to fears of ART side effects are patient-level factors.8;14–17 Hesitation by a patient to accept ART may reflect a combination of patient-level and provider-level factors if the patient has not received an accurate and consistent message on the value of ART.
Several recent clinical trials have demonstrated reduced survival with delays in ART initiation in patients with an opportunistic illness or tuberculosis (TB).18–22 These trials highlight the benefit of prompt ART initiation in HIV infected patients with lower CD4 counts (<50 cells/mm3) and raise a question regarding whether ART delay causes a similar increase in mortality among ART-eligible people who do not present with opportunistic disease. If so, then accelerating ART initiation may be a key priority for reducing HIV-associated mortality. To address this question, using patient-level mortality and CD4 count data from three cohorts in South Africa, we developed a Markov state-transition model to simulate one-year mortality from time from entry into HIV care under different scenarios for time to ART initiation.
Methods
Model Structure
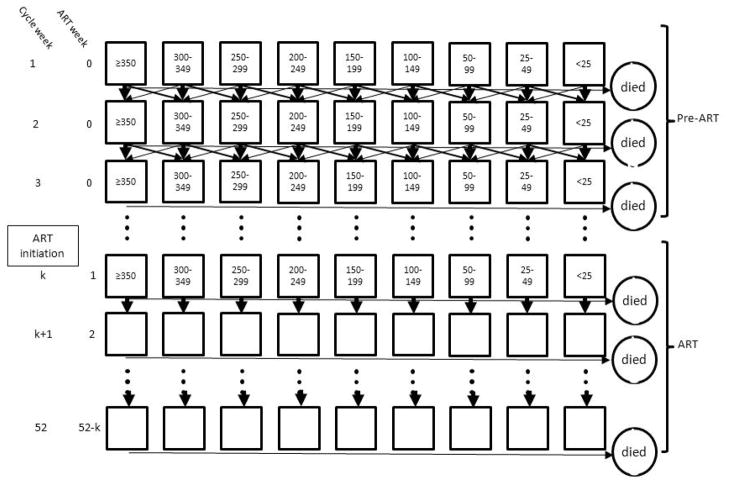
We constructed a Markov state-transition model to simulate mortality while allowing for variation in timing of ART initiation (0 to 52 weeks after clinic entry) and variation in CD4 count state at clinic entry. By simulating thousands of patients, this model can provide an estimate of mortality based on ART delay and CD4 count state at clinic entry. The model allows for transitions between CD4 count states prior to ART initiation and transition between alive and dead, prior to ART initiation and on-ART (Figure 1).
Figure 1.
Markov state transition model with CD4 states and arrows indicating the allowed transitions between states for a cohort followed for 52 weeks from clinic entry and initiating ART at week “k”. Mortality occurs from each state each cycle.
Before ART initiation, transitions can occur between adjacent CD4 count states. After ART initiation, mortality is estimated as a function of CD4 count state at ART initiation and weeks since ART initiation, and thus there are no transitions between CD4 count states and the boxes are blank because individual patients may have a wide range of CD4 counts.
Patient-level data from three cohorts were used to calculate parameters and parameter variance for the model. These cohorts were (i) a study of isoniazid preventive therapy among mine workers with HIV conducted prior to the availability of ART,23 (ii) data from a large cohort of patients who were provided free HIV care services through a network of community general practitioner HIV providers,24 and (iii) data from a centrally managed ART program in multiple workplace and community clinics.25 Isoniazid preventive study patient-level data were used to calculate CD4 count state transition probabilities, the community general practitioner cohort (the pre-ART cohort) was used to calculate pre-ART mortality, and the ART program cohort (the ART cohort) was used to calculate on-ART mortality. In developing transition probabilities for the state-transition model, we described uncertainty in the CD4 count state transition probabilities using a beta distribution, based on the mean and standard deviation. We also used a beta distribution to describe pre-ART mortality uncertainty generated from the means and standard errors from the primary data. For on-ART mortality, we generated mortality uncertainty distributions following a Normal distribution with a mean centered at the estimated mortality probability and standard deviation of 0.2 (greater detail on the model construction and transition probabilities are provided in an on-line Technical Appendix).
We used probabilistic modeling of CD4 state transitions and mortality by sampling from transition distributions in a Monte Carlo micro-simulation (Figure 1). For the purposes of estimating the “whole cohort” impact of reducing time to ART initiation, we evaluated mortality in a hypothetical cohort of 1000 patients whose CD4 distribution at clinic entry was set to be the same as our patient level data in our pre-ART cohort. We further analyzed a sub-group of the whole cohort, limiting to patients entering care with CD4 counts <150 cells/mm3. In order to allow for comparisons of impact of ART delay by CD4 count at clinic entry we also separately modeled outcomes for each CD4 count state.
We developed the state-transition model using TreeAge Pro 2011 software (TreeAge Software Inc., Williamstown, MA, USA). Monte Carlo micro-simulation of 10,000 cohorts each with 1000 patients was used to estimate mortality probabilities and 95% uncertainty ranges. We performed the following uncertainty analyses: (1) excluding patients with TB disease diagnosed at the time of entry into pre-ART care and (2) separately adjusting CD4 state transition, pre-ART mortality, and on-ART mortality by a relative increase or decrease of 25% while repeating the whole cohort analysis for 0, 10 and 26 week delays in ART initiation.
Data analysis used to populate model
For parameter estimation from each of the three cohorts (CD4 transition, pre-ART, and on-ART) we included all adults (age ≥18 years) who were ART naïve and had WHO clinical stage I, II, or III conditions at cohort entry. We excluded patients with WHO clinical stage IV conditions as disease specific management strategies may influence both timing of ART initiation and mortality. For the pre-ART and on-ART cohorts, we maximized ascertainment of deaths using clinic reports and linkage of national civil identification numbers to the South African Department of Home Affairs vital status registry. This linkage provided the date of death for deceased patients among the 65% (pre-ART cohort) to 80% (ART cohort) of patients with valid national ID numbers. Linkage was performed one month after our cohort closure date. We estimated mortality among those lost to follow-up but without identification numbers through inverse probability weighting.26;27 To calculate CD4 transitions pre-ART, we stratified all longitudinal CD4 count values into one of 9 states: <25, 25–49, 50–99, 100–149, 150–199, 200–249, 250–299, 300–349, and ≥350 cells/mm3. For each of the nine CD4 states, we calculated the Nelson-Aalen hazards of transition to another CD4 state and expressed these as one-week probabilities. To determine pre-ART mortality, we used survival analysis with cohort entry defined as the first CD4 count. We used time of first CD4 count as time of clinic entry because CD4 count enumeration generally occurs at this time in the South African context. Patients exited at the time of death or were censored at ART initiation; because we could ascertain vital status through national registry linkage, we did not censor based on loss from care. Mortality hazard, stratified by CD4 count, was relatively constant over follow-up time. Thus we converted the six-month Nelson-Aalen survival probability into a one-week mortality probability. We calculated mortality hazard in the on-ART cohort using Nelson-Aalen hazards based on the CD4 count state at ART initiation and time on ART. Because the decline in mortality, by time on ART, approximated the initial slope of an exponential decline, we used an exponential distribution to estimate weekly mortality probabilities by duration on ART.
Results
Data analysis to populate model
The CD4 transition cohort had 1,413 ART naïve individuals with a median of 3 (interquartile range; IQR: 2, 4) CD4 assays and median interval between measurements of 196 (IQR: 175, 308) days. The pre-ART cohort consisted of 31,017 individuals contributing 41,724 years of observation. The median CD4 count at care entry was 216 (IQR: 96–376) with none of the 9 CD4 count strata containing less than 9% or more than 15% of the population. Among those meeting an ART eligibility threshold of <350 cells/mm3, the median CD4 count was 143 cells/mm3 and 56% were men. The on-ART cohort consisted of 23,940 individuals contributing 61,374 person years of observation. The median CD4 at ART initiation was 147 cells/mm3 (IQR: 66, 228) and 58% were men.
Markov state-transition model – CD4 count changes
We estimated that patients with CD4 counts of 350 and 75 cells/mm3 would have CD4 cell declines of 48 and 71 cells/mm3/year, respectively. Using the entry CD4 distribution of the pre-ART cohort, we estimated a 10% pre-ART mortality 6 months from clinic entry without ART initiation. Among the survivors at 6 months, 52% remained in the CD4 state in which they entered care, 7.8% increased by one or more states, 22% decreased by one state, and 6.0% decreased by two or more states. During the subsequent 26 weeks of ART, 9% of the pre-ART survivors died for a total 52 week mortality of 19%.
Markov state-transition model - Mortality by delay in ART initiation
Using a hypothetical whole cohort with the same CD4 count distribution as seen in our pre-ART cohort, 11.1% of the population (95% CI: 7.2–15%) were projected to die within 52 weeks, if ART was started immediately on clinic entry. Deaths increased to 13.2% (95% CI: 9.5–17%) with a 6 week delay, 14.7% (95% CI: 11–18%) with a 10 week delay, 19.6% (95% CI: 16–23%) with a 26 week delay, and 24% (95% CI: 20–29%) with a 52 week delay (Table 1). Thus, as compared with immediate ART initiation, delaying ART initiation by 10 weeks resulted in 37 additional deaths (34% increase in one-year mortality) and delaying by 52 weeks resulted in 130 additional deaths (118% increase), per 1,000 patients.
Table 1.
Total mortality and excess mortality by delay in ART initiation, stratified by CD4 count at care entry
| CD4, cells/mm3 | Total: One year mortality risk (%) according to delay from clinic entry to ART initiation (95% uncertainty range) | |||||
|---|---|---|---|---|---|---|
| Excess: attributable mortality associated with delay (95% uncertainty range)
| ||||||
| 0 weeks | 6 weeks | 10 weeks | 26 weeks | 52 weeks | ||
| State-transition model for pre-ART cohort CD4 distribution | ||||||
|
| ||||||
| Whole Cohort (median CD4: 143) | Total | 11.0 (7.2, 15) | 13.2 (9.5, 17) | 14.7 (11, 18) | 19.7 (16, 23) | 24.2 (19, 29) |
| Excess | 0 | 2.2 (−3.2, 7.7) | 3.7 (−1.8, 9.1) | 8.6 (3.4, 14) | 13.0 (7.2, 19) | |
|
| ||||||
| Restricted to CD4 <150 cell/mm3 | Total | 15.5 (10, 21) | 18.8 (13, 24) | 20.8 (16, 26) | 27.7 (23, 32) | 34.2 (28, 40) |
| Excess | 0 | 3.3 (−4.3, 1.1) | 5.2 (−2.2, 13) | 12.1 (4.8, 19) | 18.6 (10, 27) | |
|
| ||||||
| State transition model for pre-ART CD4 strata at entry into clinic | ||||||
|
| ||||||
| 300–350 | Total | 2.8 (1.7, 3.9) | 3.2 (1.9, 4.9) | 3.5 (2.0, 6.0) | 4.3 (2.1, 8.8) | 5.1 (1.5, 12) |
| Excess | 0 | 0.4 (−1.4, 2.3) | 0.7 (−1.6, 3.0) | 1.5 (−2.1, 5.2) | 2.3 (−3.7, 8.3) | |
|
| ||||||
| 250–299 | Total | 3.5 (2.2, 4.9) | 4.1 (2.6, 6.1) | 4.6 (2.7, 7.4) | 6.0 (3.2, 11) | 7.1 (2.3, 16) |
| Excess | 0 | 0.6 (−1.4, 2.3) | 1.1 (−1.5, 3.8) | 2.5 (−1.8, 6.8) | 3.6 (−3.2, 10.5) | |
|
| ||||||
| 200–249 | Total | 4.7 (2.8, 6.6) | 5.6 (3.7, 7.6) | 6.2 (4.2, 8.7) | 8.4 (5.2, 13) | 10.0 (5.0, 17) |
| Excess | 0 | 0.9 (−1.8, 3.6) | 1.5 (−1.4, 4.5) | 3.7 (−0.7, 8.1) | 5.2 (−1.2, 12) | |
|
| ||||||
| 150–199 | Total | 6.6 (4.1, 9.1) | 7.9 (5.3, 10) | 8.6 (5.9, 11) | 11.6 (8.1, 16) | 13.9 (9.0, 21) |
| Excess | 0 | 1.3 (−2.4, 4.9) | 2.0 (−1.8, 5.9) | 5.0 (0.37, 10) | 7.3 (1.0, 14) | |
|
| ||||||
| 100–149 | Total | 9.4 (5.5, 14) | 11.0 (7.6, 14) | 12.2 (8.8, 16) | 16.3 (12, 21) | 20.0 (14, 27) |
| Excess | 0 | 1.6 (−3.7, 6.9) | 2.8 (−2.6, 8.3) | 6.9 (1.0, 13) | 10.6 (2.9, 19) | |
|
| ||||||
| 50–99 | Total | 13.3 (8.4, 18) | 15.6 (10, 21) | 17.0 (12, 22) | 22.9 (17, 31) | 29.2 (21, 40) |
| Excess | 0 | 2.2 (−4.9, 9.3) | 3.7 (−3.3, 11) | 9.6 (1.1, 18) | 15.8 (5.2, 26) | |
|
| ||||||
| <50 | Total | 21.3 (14, 29) | 26.2 (19, 33) | 29.2 (22, 36) | 38.8 (33, 44) | 47.4 (39, 55) |
| Excess | 0 | 4.8 (−5.3, 15) | 7.8 (−2.1, 18) | 17.4 (7.9, 27) | 26.1 (15, 37) | |
Limiting the simulation to patients entering with CD4 <150 cells/mm3, we observed an increase in mortality from 160 deaths per 1000 patients with no delay, to 210 deaths with a 10 week delay (31% increase) and 340 deaths (112% increase) with a 52 week delay.
The effect of ART delay on mortality within specific CD4 count states at clinic entry is presented in Table 1 and graphically presented in Figure 2. The effect of a 10 week delay among patients with a CD4 count of 50–99 cells/mm3 was an increase of 37 deaths in a cohort of 1000 patients (31% increase). A 26 week delay was associated with an increase of 96 deaths (77% increase).
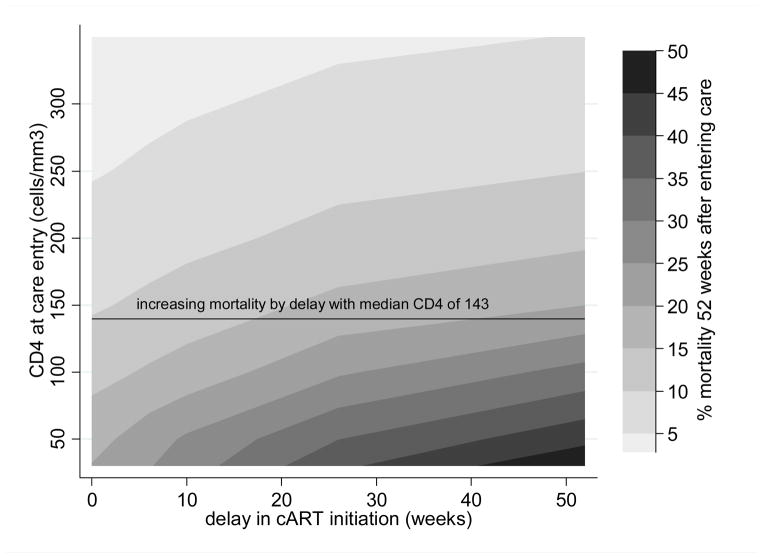
Figure 2.
Contour plot of total mortality risk 52 weeks after entering HIV care by duration of delay in ART initiation (x-axis) and CD4 at care entry (y-axis).
CD4 at the time of entry into care is on the y-axis, delay in ART initiation on the x-axis, and contours represent mortality bands by percent of the cohort who died 52 weeks after entering care. The close contour lines at lower entry CD4 represent the more rapid increase in mortality with delays among such patients. The black line represents the increasing mortality with the whole cohort analysis (median CD4 count 143 cells/mm3) in which mortality increases from 11.0% with no delay to 19.7% with a 26 week delay and 24.2% with a 52 week delay.
Excluding patients with TB at clinic entry (5.6% of all patients) gave similar results (results not shown). To assess the impact of uncertainty on state change probabilities, we performed serial one-way analyses with a relative 25% increase or decrease in probability for each parameter for CD4 state transitions, pre-ART mortality, and on-ART mortality (Figure 3). The ranges for the 52-week mortality for 0, 10, and 26 week delays in ART initiation after these one-way uncertainty analyses all fell within our simulated uncertainty ranges (Table 1).
Figure 3.
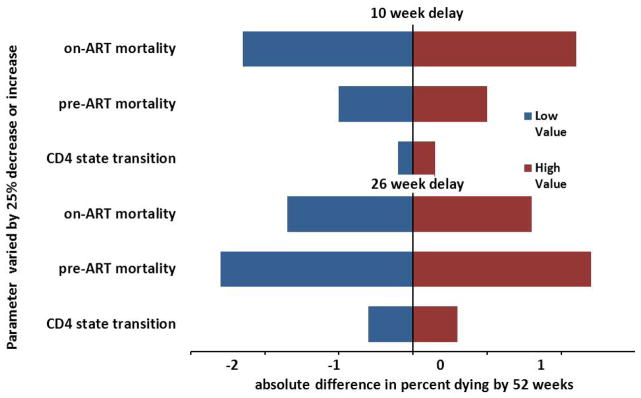
Tornado diagram of absolute change in percent mortality at 10 weeks and 26 weeks with a 25% decrease or 25% increase in parameter for CD4 transition, pre-ART mortality, or on-ART mortality.
Discussion
Using data from South African cohorts we modeled a relative 34% increase in 12 month mortality attributable to a 10 week delay in ART initiation. A 10 week delay is consistent with the range reported in the literature of 9 to 125 days 8;10;28–32 as well as our experience in a hospital-based ART program.33 Notably, the absolute increase in mortality was highest with lower CD4 count states. For example, a 10-week delay in ART initiation would be expected to result in 15 excess deaths per 1000 patients with CD4 counts at clinic entry between 200–249 cells/mm3, compared to 37 excess deaths among patients entering with CD4 counts between 50–99 cells/mm3. Thus potential gain in survival, if a 10 week delay in ART initiation could be eliminated, is only slightly less than the estimated 40% relative reduction in mortality during the first year of ART through universal use of cotrimoxazole prophylaxis.34–36
While there are no empirical studies of ART delay and mortality from clinic entry and after ART initiation in an unselected population, we can compare estimates from each of our parameters with prior parameter specific reports. Our estimates of pre-ART mortality are similar to prior reports from Africa (Table 2).37–39 In this regard, the similarity of our estimates with observational data supports the validity of the pre-ART parameters of our model. In contrast, our parameter-based estimates of on-ART mortality are somewhat higher than previously reported (Table 2) 40–43. This discrepancy in on-ART mortality may be a result of deriving our parameters from data linked to a robust national vital status registry leading to greater ascertainment of deaths. Such registers are not widely available in sub-Saharan Africa outside of South Africa. Failure to completely ascertain deaths among patients lost to follow-up can markedly reduce mortality estimates.27;44–47 Thus, we believe that the on-ART mortality for our cohort is likely reflective of the true mortality experience of other similar settings. Of note, an overestimate of on-ART mortality would lead to an underestimate of the mortality impact of delays in ART initiation. Programs with lower overall pre-ART and on-ART mortality will not achieve as large an absolute reduction in mortality. Nevertheless, the relative reductions in mortality achievable by reducing delays in ART initiation may be similar.
Table 2.
Pre-and on-ART 52 week mortality estimates (per 100-person-years) from the model simulation and the published literature
| CD4 strata (cells/mm3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mortality per 100 person-years | <25 | 25–49 | 50–99 | 100–149 | 150–199 | 200–249 | 250–299 | 300–349 | ≥350 |
|
| |||||||||
| Pre-ART | |||||||||
| Model | 72 | 56 | 34 | 22 | 15 | 11 | 7.7 | 7.6 | 3.8 |
| Anglaret | 69 | 32 | 17 | 4.2 | 0.6 | ||||
| Fielding | 43 | 11 | 3.3 | 0.6 | |||||
| Badri | 27 | 8 | NR | ||||||
| Amuron* | 80% | 50% | 31% | NR | |||||
|
| |||||||||
| On-ART | |||||||||
| Model | 29 | 20 | 14 | 9.9 | 6.9 | 4.8 | 3.6 | 2.8 | 2.8 |
| May | 24 | 16 | 8.7 | 5.5 | 5.1 | ||||
| Brinkhof | 17 | 11 | 7.3 | 4.8 | 3.8 | ||||
| Mills | 13 | 6.4 | 4.4 | 3 | 3.3 | 2.2 | 2.8 | ||
mortality risk over a 12 month time period; NR not reported
We also compared our findings to three randomized trials of timing of ART initiation for TB patients.19–22 It is notable that although our overall (pre and on-ART mortality combined) mortality predictions were higher than each of the three trials, the absolute increases in mortality were similar when comparing similar entry CD4 counts and durations of delay. The similarity in absolute increases in mortality provides added support to the validity of our model. Our higher overall mortality may reflect the differences in routine clinical practice versus clinical trials, especially clinical trials of TB treatment as a large proportion of mortality in routine care occurs from undiagnosed and untreated TB.48
The mortality benefit of accelerating ART initiation should be balanced against potential harms. For example, people started on ART sooner may be less likely to be correctly diagnosed with concurrent opportunistic illnesses (e.g., TB) or adequately prepared for ART initiation. This may increase the risk of immune reconstitution inflammatory reaction (IRIS) and future non-adherence, respectively. However, IRIS is rarely fatal 21;22;49, and limited available evidence suggests that adherence counseling during early ART may equal pre-ART counseling.50
As with any model-based analysis, our model has several limitations. First, due to limitations of available data, we used primary data with variable duration between CD4 counts to estimate CD4 transition probabilities. Although this limitation likely introduces the greatest imprecision in our transition probabilities, the findings from our uncertainty analyses are reassuring because even a relatively large change in the CD4 count transition probability (25% increase or decrease) had a small effect on model outcome (Figure 3). The CD4 transition cohort may show a healthy worker bias with a slower decline in CD4 count. However, a calculated decline of 71 cells/mm3/year is consistent with several other studies in which CD4 count decline is 34 to 97 cells/mm3/year.51–55 The pre-ART cohort also may not be representative of the natural history of untreated HIV disease, as unmeasured factors may have affected delays in ART initiation (e.g., ill-appearing patients started on ART sooner). However, our analysis is designed to reflect the impact of delays in routine care, not in the abstract situation of untreated natural history. Importantly, we may have underestimated the effect of delay by using the date of the first CD4 test as the date of clinic entry, as delays may occur between clinic entry and CD4 enumeration. However, in our clinical setting, CD4 measurement on the first clinic visit was routine, thereby minimizing the likely effect of this bias. Finally, we did not address delays in diagnosis of HIV or entry into care following diagnosis because our intent was to focus on delays in ART initiation among patients who had already been linked to HIV-specific care. To provide a full spectrum of the impact of delays on HIV-associated morbidity and mortality, future models could also address delays in HIV diagnosis and entry-into-care, as well as modeling the impact of such delays on HIV transmission.
Our findings highlight the need to develop approaches to more promptly initiate ART after patients present to care. These approaches must address both clinic-level and patient-level factors. Clinic-level reduction in delays may be achieved through improved turn-around times in obtaining laboratory results, fewer “ART preparation visits”, and streamlined pre-ART assessment protocols. Interventions may include changes in pre-ART counseling, educating staff on the risks of ART delay, and improving access to CD4 count testing, including the use of point-of-care CD4 count assays.28 Patient-level barriers to prompt ART initiation may include barriers to reaching the clinic as well as concerns of ART side effects, stigma, and coping.9;16;41;56–59 Proactive patient-centered approaches (e.g., financial enablers, community-based educational campaigns, home-based care) to overcome these barriers must be advanced if we are to maximize the number of lives saved with ART. Such programs must also address issues of adherence and retention in order to minimize any potential negative consequences of more rapid initiation of ART. Failure to retain patients starting ART would undermine short-term survival benefits.
Our model, based on data from routine programs in South Africa, suggests that the delays in ART initiation which are typical of some existing clinics, may cause substantial excess mortality. Among the most immunosuppressed patients, this absolute mortality increase is substantially higher. Reducing delays to ART initiation for all eligible patients in high-burden settings, especially those with lower CD4 counts and not just those with opportunistic infections, could save thousands of lives every year and should be prioritized if the promise of ART in these populations is to be fully realized.
Supplementary Material
Acknowledgments
Support: C.J.H. was supported by National Institutes of Health AI083099; J.J.L was fully funded and K.L.F. partially funded by the Bill and Melinda Gates Foundation, through the Biostatistics Core of CREATE; R.E.C. by National Institutes of Health grants AI5535901 and HL090312 and the Bill and Melinda Gates Foundation; A.D.G. by Global Health Trials and the Bill and Melinda Gates Foundation
Footnotes
Conflicts of interest: All authors, no conflicts
Reference List
- 1.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, Vaeth M, Obel N. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 2.Hogg RS, Heath KV, Yip B, Craib KJ, O’Shaughnessy MV, Schechter MT, Montaner JS. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279:450–454. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 3.El-Sadr W, Neaton J The SMART Study Investigators. Episodic CD4-guided use of antiretroviral therapy is inferior to continuous therapy: results of the SMART study. Program and abstracts of the 13th Conference on Retroviruses and Opportunistic Infections; 2006. [Google Scholar]
- 4.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, Hogg RS, Deeks SG, Eron JJ, Brooks JT, Rourke SB, Gill MJ, Bosch RJ, Martin JN, Klein MB, Jacobson LP, Rodriguez B, Sterling TR, Kirk GD, Napravnik S, Rachlis AR, Calzavara LM, Horberg MA, Silverberg MJ, Gebo KA, Goedert JJ, Benson CA, Collier AC, Van Rompaey SE, Crane HM, McKaig RG, Lau B, Freeman AM, Moore RD. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sterne JA, May M, Costagliola D, de WF, Phillips AN, Harris R, Funk MJ, Geskus RB, Gill J, Dabis F, Miro JM, Justice AC, Ledergerber B, Fatkenheuer G, Hogg RS, Monforte AD, Saag M, Smith C, Staszewski S, Egger M, Cole SR. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368:1254–59. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 7.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–48. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 8.Parkes-Ratanshi R, Bufumbo L, Nyanzi-Wakholi B, Levin J, Grosskurth H, Lalloo DG, Kamali A. Barriers to starting ART and how they can be overcome: individual and operational factors associated with early and late start of treatment. Trop Med Int Health. 2010;15:1347–56. doi: 10.1111/j.1365-3156.2010.02620.x. [DOI] [PubMed] [Google Scholar]
- 9.Zachariah R, Tayler-Smith K, Manzi M, Massaquoi M, Mwagomba B, van GJ, van EI, Arnould L, Schouten EJ, Chimbwandira FM, Harries AD. Retention and attrition during the preparation phase and after start of antiretroviral treatment in Thyolo, Malawi, and Kibera, Kenya: implications for programmes? Trans R Soc Trop Med Hyg. 2011;105:421–30. doi: 10.1016/j.trstmh.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Ingle SM, May M, Uebel K, Timmerman V, Kotze E, Bachmann M, Sterne JA, Egger M, Fairall L. Outcomes in patients waiting for antiretroviral treatment in the Free State Province, South Africa: prospective linkage study. AIDS. 2010;24:2717–25. doi: 10.1097/QAD.0b013e32833fb71f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon JG, Gibson S, McPake B, Maleta K. Antiretroviral therapy (ART) rationing and access mechanisms and their impact on youth ART utilization in Malawi. Malawi Med J. 2011;23:48–54. [PMC free article] [PubMed] [Google Scholar]
- 12.Muhamadi L, Nsabagasani X, Tumwesigye MN, Wabwire-Mangen F, Ekstrom AM, Peterson S, Pariyo G. Inadequate pre-antiretroviral care, stock-out of antiretroviral drugs and stigma: policy challenges/bottlenecks to the new WHO recommendations for earlier initiation of antiretroviral therapy (CD<350 cells/microL) in eastern Uganda. Health Policy. 2010;97:187–94. doi: 10.1016/j.healthpol.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Stein J, Lewin S, Fairall L. Hope is the pillar of the universe: health-care providers’ experiences of delivering anti-retroviral therapy in primary health-care clinics in the Free State province of South Africa. Soc Sci Med. 2007;64:954–64. doi: 10.1016/j.socscimed.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Wringe A, Roura M, Urassa M, Busza J, Athanas V, Zaba B. Doubts, denial and divine intervention: understanding delayed attendance and poor retention rates at a HIV treatment programme in rural Tanzania. AIDS Care. 2009;21:632–37. doi: 10.1080/09540120802385629. [DOI] [PubMed] [Google Scholar]
- 15.Lubega M, Nsabagasani X, Tumwesigye NM, Wabwire-Mangen F, Ekstrom AM, Pariyo G, Peterson S. Policy and practice, lost in transition: Reasons for high drop-out from pre-antiretroviral care in a resource-poor setting of eastern Uganda. Health Policy. 2010;95:153–58. doi: 10.1016/j.healthpol.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Marcellin F, Abe C, Loubiere S, Boyer S, Blanche J, Koulla-Shiro S, Ongolo-Zogo P, Moatti JP, Spire B, Carrieri MP. Delayed first consultation after diagnosis of HIV infection in Cameroon. AIDS. 2009;23:1015–19. doi: 10.1097/QAD.0b013e32832a5996. [DOI] [PubMed] [Google Scholar]
- 17.Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, Lehe JD, Peter TF. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378:1572–79. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 18.Zolopa A, Andersen J, Powderly W, Sanchez A, Sanne I, Suckow C, Hogg E, Komarow L. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009;4:e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray AL, Gengiah T, Gengiah S, Naidoo A, Jithoo N, Nair G, El-Sadr WM, Friedland G, Abdool KQ. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365:1492–501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, Gengiah T, Nair G, Bamber S, Singh A, Khan M, Pienaar J, El-Sadr W, Friedland G, Abdool KQ. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, Luetkemeyer AF, Hogg E, Rooney JF, Wu X, Hosseinipour MC, Lalloo U, Veloso VG, Some FF, Kumarasamy N, Padayatchi N, Santos BR, Reid S, Hakim J, Mohapi L, Mugyenyi P, Sanchez J, Lama JR, Pape JW, Sanchez A, Asmelash A, Moko E, Sawe F, Andersen J, Sanne I. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–91. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, Madec Y, Marcy O, Chan S, Prak N, Kim C, Lak KK, Hak C, Dim B, Sin CI, Sun S, Guillard B, Sar B, Vong S, Fernandez M, Fox L, Delfraissy JF, Goldfeld AE. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–81. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant AD, Charalambous S, Fielding KL, Day JH, Corbett EL, Chaisson RE, De Cock KM, Hayes RJ, Churchyard GJ. Effect of routine isoniazid preventive therapy on tuberculosis incidence among HIV-infected men in South Africa. JAMA. 2005;293:2719–25. doi: 10.1001/jama.293.22.2719. [DOI] [PubMed] [Google Scholar]
- 24.Innes C, Hamilton R, Hoffmann CJ, Hippner P, Fielding K, Grant AD, Churchyard GJ, Charalambous S. A novel HIV treatment model using private practitioners in South Africa. Sex Transm Infect. 2012;88:136–40. doi: 10.1136/sextrans-2011-050194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann CJ, Fielding KL, Johnston V, Charalambous S, Innes C, Moore RD, Chaisson RE, Grant AD, Churchyard GJ. Changing predictors of mortality over time from cART start: implications for care. J Acquir Immune Defic Syndr. 2011;58:269–76. doi: 10.1097/QAI.0b013e31823219d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox MP, Brennan A, Maskew M, MacPhail P, Sanne I. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2010;15:405–13. doi: 10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann CJ, Katherine FL, Victoria J, Salome C, Craig I, Moore RD, Chaisson RE, Grant AD, Churchyard GJ. Changing predictors of mortality over time from cART start: implications for care. J Acquir Immune Defic Syndr. 2011 doi: 10.1097/QAI.0b013e31823219d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, Lehe JD, Peter TF. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378:1572–79. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 29.Bassett IV, Regan S, Chetty S, Giddy J, Uhler LM, Holst H, Ross D, Katz JN, Walensky RP, Freedberg KA, Losina E. Who starts antiretroviral therapy in Durban, South Africa?.. not everyone who should. AIDS. 2010;24 (Suppl 1):S37–S44. doi: 10.1097/01.aids.0000366081.91192.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Micek MA, Gimbel-Sherr K, Baptista AJ, Matediana E, Montoya P, Pfeiffer J, Melo A, Gimbel-Sherr S, Johnson W, Gloyd S. Loss to follow-up of adults in public HIV care systems in central Mozambique: identifying obstacles to treatment. J Acquir Immune Defic Syndr. 2009;52:397–405. doi: 10.1097/QAI.0b013e3181ab73e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulissa Z, Jerene D, Lindtjorn B. Patients present earlier and survival has improved, but pre-ART attrition is high in a six-year HIV cohort data from Ethiopia. PLoS One. 2010;5:e13268. doi: 10.1371/journal.pone.0013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guthrie BL, Choi RY, Liu AY, Mackelprang RD, Rositch AF, Bosire R, Manyara L, Gatuguta A, Kiarie JN, Farquhar C. Barriers to antiretroviral initiation in HIV-1-discordant couples. J Acquir Immune Defic Syndr. 2011;58:e87–e93. doi: 10.1097/QAI.0b013e31822f064e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanifa Y, Fielding KL, Charalambous S, Variava E, Luke B, Churchyard GJ, Grant AD. Tuberculosis among adults starting antiretroviral therapy in South Africa: the need for routine case finding. Int J Tuberc Lung Dis. 2012;16:1252–59. doi: 10.5588/ijtld.11.0733. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann CJ, Fielding KL, Charalambous S, Innes C, Chaisson RE, Grant AD, Churchyard GJ. Reducing mortality with cotrimoxazole preventive therapy at initiation of antiretroviral therapy in South Africa. AIDS. 2010;24:1709–16. doi: 10.1097/QAD.0b013e32833ac6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiktor SZ, Sassan-Morokro M, Grant AD, Abouya L, Karon JM, Maurice C, Djomand G, Ackah A, Domoua K, Kadio A, Yapi A, Combe P, Tossou O, Roels TH, Lackritz EM, Coulibaly D, De Cock KM, Coulibaly IM, Greenberg AE. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d’Ivoire: a randomised controlled trial. Lancet. 1999;353:1469–75. doi: 10.1016/s0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 36.Lowrance D, Makombe S, Harries A, Yu J, Aberle-Grasse J, Eiger O, Shiraishi R, Marston B, Ellerbrock T, Libamba E. Lower early mortality rates among patients receiving antiretroviral treatment at clinics offering cotrimoxazole prophylaxis in Malawi. J Acquir Immune Defic Syndr. 2007;46:56–61. [PubMed] [Google Scholar]
- 37.May M, Boulle A, Phiri S, Messou E, Myer L, Wood R, Keiser O, Sterne JA, Dabis F, Egger M. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet. 2010;376:449–57. doi: 10.1016/S0140-6736(10)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brinkhof MW, Boulle A, Weigel R, Messou E, Mathers C, Orrell C, Dabis F, Pascoe M, Egger M. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009;6:e1000066. doi: 10.1371/journal.pmed.1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mills EJ, Bakanda C, Birungi J, Mwesigwa R, Chan K, Ford N, Hogg RS, Cooper C. Mortality by baseline CD4 cell count among HIV patients initiating antiretroviral therapy: evidence from a large cohort in Uganda. AIDS. 2011;25:851–55. doi: 10.1097/QAD.0b013e32834564e9. [DOI] [PubMed] [Google Scholar]
- 40.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368:1254–59. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 41.Amuron B, Namara G, Birungi J, Nabiryo C, Levin J, Grosskurth H, Coutinho A, Jaffar S. Mortality and loss-to-follow-up during the pre-treatment period in an antiretroviral therapy programme under normal health service conditions in Uganda. BMC Public Health. 2009;9:290. doi: 10.1186/1471-2458-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anglaret X, Minga A, Gabillard D, Ouassa T, Messou E, Morris B, Traore M, Coulibaly A, Freedberg KA, Lewden C, Menan H, Abo Y, Dakoury-Dogbo N, Toure S, Seyler C. AIDS and Non-AIDS Morbidity and Mortality Across the Spectrum of CD4 Cell Counts in HIV-Infected Adults Before Starting Antiretroviral Therapy in Cote d’Ivoire. Clin Infect Dis. 2012;54:714–23. doi: 10.1093/cid/cir898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fielding K, Koba A, Grant AD, Charalambous S, Day J, Spak C, Wald A, Huang ML, Corey L, Churchyard GJ. Cytomegalovirus viremia as a risk factor for mortality prior to antiretroviral therapy among HIV-infected gold miners in South Africa. PLoS One. 2011;6:e25571. doi: 10.1371/journal.pone.0025571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geng EH, Emenyonu N, Bwana MB, Glidden DV, Martin JN. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA. 2008;300:506–7. doi: 10.1001/jama.300.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, Wood R, Laurent C, Sprinz E, Seyler C, Bangsberg DR, Balestre E, Sterne JA, May M, Egger M. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 46.Bisson GP, Gaolathe T, Gross R, Rollins C, Bellamy S, Mogorosi M, Avalos A, Friedman H, Dickinson D, Frank I, Ndwapi N. Overestimates of survival after HAART: implications for global scale-up efforts. PLoS One. 2008;3:e1725. doi: 10.1371/journal.pone.0001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fox MP, Brennan A, Maskew M, MacPhail P, Sanne I. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2010;15:405–13. doi: 10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinson NA, Karstaedt A, Venter WD, Omar T, King P, Mbengo T, Marais E, McIntyre J, Chaisson RE, Hale M. Causes of death in hospitalized adults with a premortem diagnosis of tuberculosis: an autopsy study. AIDS. 2007;21:2043–50. doi: 10.1097/QAD.0b013e3282eea47f. [DOI] [PubMed] [Google Scholar]
- 49.Murdoch DM, Venter WD, Feldman C, Van RA. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22:601–10. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 50.Siedner MJ, Lankowski A, Haberer JE, Kembabazi A, Emenyonu N, Tsai AC, Muzoora C, Geng E, Martin JN, Bangsberg DR. Rethinking the “pre” in pre-therapy counseling: no benefit of additional visits prior to therapy on adherence or viremia in Ugandans initiating ARVs. PLoS One. 2012;7:e39894. doi: 10.1371/journal.pone.0039894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, Kingsley LA, Todd JA, Saah AJ, Detels R, Phair JP, Rinaldo CR., Jr Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 52.Kiwanuka N, Robb M, Laeyendecker O, Kigozi G, Wabwire-Mangen F, Makumbi FE, Nalugoda F, Kagaayi J, Eller M, Eller LA, Serwadda D, Sewankambo NK, Reynolds SJ, Quinn TC, Gray RH, Wawer MJ, Whalen CC. HIV-1 viral subtype differences in the rate of CD4+ T-cell decline among HIV seroincident antiretroviral naive persons in Rakai district, Uganda. J Acquir Immune Defic Syndr. 2010;54:180–184. doi: 10.1097/QAI.0b013e3181c98fc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mussini C, Cossarizza A, Sabin C, Babiker A, De LA, Bucher HC, Fisher M, Rezza G, Porter K, Dorrucci M. Decline of CD4(+) T-cell count before start of therapy and immunological response to treatment in antiretroviral-naive individuals. AIDS. 2011;25:1041–49. doi: 10.1097/QAD.0b013e3283463ec5. [DOI] [PubMed] [Google Scholar]
- 54.Holmes CB, Wood R, Badri M, Zilber S, Wang B, Maartens G, Zheng H, Lu Z, Freedberg KA, Losina E. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42:464–69. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 55.Ding M, Tarwater P, Rodriguez M, Chatterjee R, Ratner D, Yamamura Y, Roy P, Mellors J, Neogi D, Chen Y, Gupta P. Estimation of the predictive role of plasma viral load on CD4 decline in HIV-1 subtype C-infected subjects in India. J Acquir Immune Defic Syndr. 2009;50:119–25. doi: 10.1097/QAI.0b013e3181911991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karcher H, Omondi A, Odera J, Kunz A, Harms G. Risk factors for treatment denial and loss to follow-up in an antiretroviral treatment cohort in Kenya. Trop Med Int Health. 2007;12:687–94. doi: 10.1111/j.1365-3156.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 57.Ochieng-Ooko V, Ochieng D, Sidle JE, Holdsworth M, Wools-Kaloustian K, Siika AM, Yiannoutsos CT, Owiti M, Kimaiyo S, Braitstein P. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bull World Health Organ. 2010;88:681–88. doi: 10.2471/BLT.09.064329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moon TD, Burlison JR, Blevins M, Shepherd BE, Baptista A, Sidat M, Vergara AE, Vermund SH. Enrolment and programmatic trends and predictors of antiretroviral therapy initiation from president’s emergency plan for AIDS Relief (PEPFAR)-supported public HIV care and treatment sites in rural Mozambique. Int J STD AIDS. 2011;22:621–27. doi: 10.1258/ijsa.2011.010442. [DOI] [PubMed] [Google Scholar]
- 59.Katz IT, Essien T, Marinda ET, Gray GE, Bangsberg DR, Martinson NA, de BG. Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. AIDS. 2011;25:2177–81. doi: 10.1097/QAD.0b013e32834b6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.