Abstract
Objective
To review the world’s (English-language) publications related to depression following stroke.
Method
The databases from MEDLINE and PubMed were reviewed for articles related to poststroke depression (PSD), depression and cerebral vascular accident, depression and cerebral vascular disease, and depression and cerebral infarction.
Results
Most studies examined prevalence rates of depression and the clinical correlates of depression. Based on pooled data, the overall prevalence of major depression was 21.7% and minor depression was 19.5%. The strongest single correlate of depression was severity of impairment in activities of daily living. However, the existence of depression at baseline was found to be associated with greater impairment at follow-up, ranging from 6 weeks to 2 years in 83% of studies. Further, depression following acute stroke was also associated with greater cognitive impairment and increased mortality. PSD has been shown in 6 double-blind controlled studies to be effectively treated with antidepressants, and 1 study has recently shown that PSD can be effectively prevented.
Conclusions
During the past 20 years, significant progress has been made in the identification and treatment of depression following stroke. In the future, antidepressant treatment will likely play an increasing role in the management of patients with acute stroke. Further research is needed to identify the mechanisms of depression and why antidepressants lead to improved physical and cognitive recovery and decreased mortality.
Keywords: poststroke depression, stroke-related impairments, prevalence, diagnosis, mortality, mechanism of depression
The group of cerebrovascular diseases, which includes both sudden-onset ischemia of the brain, owing to thrombosis or emboli and hemorrhagic bleeds within the brain parenchyma or subdural or epidural regions, usually owing to aneurysms or trauma, constitute some of the most common life-threatening problems among the elderly population. Stroke, defined as a sudden loss of blood supply to the brain leading to permanent tissue damage caused by thrombotic, embolic, or hemorrhagic events, ranks as the third leading cause of death (behind only heart disease and cancer) in patients aged 50 years and older. The psychiatric complications of stroke lesions, although recognized for more than 100 years,1 have never received the attention that has been devoted to poststroke motor deficits, language problems, or intellectual disturbances. Some of these psychiatric complications of stroke, such as depression, have been a focus of research, whereas other complications, such as anxiety or emotional lability, have received relatively little attention. Our paper reviews the world’s literature on PSD.
Method
A detailed search of the medical literature was conducted. Search methods included MEDLINE and PubMed databases for articles in English using search words of PSD, depression and cerebral vascular accident, depression and cerebral vascular disease, depression, and cerebral infarction. A total of 2905 articles were screened for inclusion. In addition, the bibliographies of all relevant articles were searched for further publications. All articles in this review were evaluated for relevance and methodology.
Results
Diagnosis
The method for diagnosis of depression among patients with stroke is to conduct a structured or semi-structured MMSE using instruments such as the SCID2 or the CIDI.3 The type of symptoms, their severity, and their duration are applied to DSM-IV4 diagnostic criteria for “mood disorder due to a general medical condition with major depressive-like episode. Although there is a second subtype, entitled “mood disorder due to a general medical condition with depressive features,” this does not require any specific diagnostic criteria and we have therefore chosen not to use this very nonspecific diagnostic category.
To diagnose patients with less severe depressive disorders, we have used the research criteria from DSM-IV for minor depression. This is a subsyndromal form of major depression that requires at least 2 but less than 5 symptoms of major depression as well as the other duration and functional impairment diagnostic criteria. The duration of symptoms must be at least 2 weeks and at least 1 of the symptoms is either depressed mood or loss of interest or pleasure. Spalletta et al5 compared the frequency of specific depressive symptoms among 50 patients with major depression, 62 with minor depression, and 88 nondepressed patients. Symptoms were elicited using the SCID–Psychiatrist version. Results showed that the 3 groups differed significantly in the frequency of every symptom. However, after correction for multiple comparisons, symptoms of self-blame and guilt failed to distinguish the 3 groups, although all other symptoms did distinguish all 3 groups. Further, patients with minor depression had significantly higher frequency of depressed mood, loss of energy, insomnia, and psychomotor disturbance, com- pared with nondepressed patients with stroke. Paradiso et al6 examined the specificity of psychiatric disorders during 2 years among 205 patients following acute stroke. Patients were categorized by the presence or absence of depressed mood. The gold standard for major depression was the existence of 5 or more symptoms that were significantly more frequent in depressed, compared with nondepresssed, patients. Using this gold standard, the sensitivity of standard DSM-IV criteria was 100% during the acute stroke period as well as at 3-, 6-, 12-, and 24-month follow-up. The specificity was 98%, 97%, 95%, and 96%, respectively.
In summary, mood disorder owing to stroke with major depressivelike episode and mood disorder owing to stroke with minor depression, we believe, are the 2 most useful categories for diagnosing PSDs.
Prevalence
Most studies throughout the world that have studied PSD (that is, major and minor depression) have primarily examined prevalence rates of depression in patients examined in acute or rehabilitation hospital settings, community settings, or in outpatient clinics. Based on the world’s literature, the current number of patients who were examined for PSD exceeds 7000. However, there are several factors that have complicated the assessment of prevalence rates. The first is the setting in which patients were examined. Community studies have generally reported somewhat lower prevalence rates than studies done in rehabilitation hospitals, outpatient settings, or acute hospital settings. Second, patients with comprehension deficits owing to fluent or global aphasias have been excluded from some or all studies of PSD. Further patients with hemorrhagic rather than ischemic stroke, decreased level of consciousness, fatigue, atypical strokes, or other systemic illnesses have often been excluded. Although some investigators have tried to estimate the frequency of depression in some of these patients, such as those with com- prehension deficits owing to fluent or global aphasia,7 no reliable method has been devised to examine patients who are unable to reliably respond to a verbal interview. Examiners’ judgments about whether a patient is depressed, depending on observations of behaviour, such as difficulty falling asleep, waking up early in the morning, not eating, losing weight, frequent tearfulness, social withdrawal, or acts of self-harm, are often unreliable and have not been validated as a method of diagnosing depression. The third factor that has led to variability in reported prevalence rates for PSD is the use of cut-off scores on depression rating scales to determine the existence of depression. However, the widely accepted method for diagnosis of depression is to conduct a structured interview and apply the findings to established diagnostic criteria such as DSM-IV-TR. The prevalence rates from studies reported in the literature divided by the setting in which the patient was examined are shown in online eTable 1. The lowest prevalence rates were depressive disorder found in patients studied in community settings where 14% of the patients were found to have major depression, while 9% had minor depression. In acute or rehabilitation hospitals, the mean prevalence rate for major depression was 21.6% and for minor depression was 20.0%. In outpatient settings, which vary between 3 months and 3 or more years following stroke, the prevalence rate for major depression was 24.0%, and for minor depression was 23.9%. Based on prevalence estimates by the American Heart Association, there are 5 million stroke survivors in the United States, suggesting that there may be 2.4 million patients presently in the United States with PSD, among whom almost one-half having major depression. It is likely that more than 3 million of these patients would have been depressed at some time since their initial stroke. The enormity of the problem is obvious from these findings.
Relation to Lesion Variables
The relation between depressive disorder and lesion location has been perhaps the most controversial area of PSD research.8 Although establishing an association between specific clinical symptoms and lesion location is one of the fundamental goals in neurology, this has rarely been the case in psychiatric disorders. Numerous studies, particularly those conducted between 3 and 12 months following stroke, have failed to show a difference in prevalence rates of depression among patients with left- or right-hemisphere stroke.8 However, several studies have found a significant association between lesion location and the development of PSD, particularly during the first few months following stroke.9–11
Robinson12 conducted a meta-analysis of studies done within 2 months following stroke comparing the frequency of major depression among patients with left anterior, compared with left posterior, lesions, and left anterior, compared with right anterior, lesions. Among 128 patients in the left-anterior and left-posterior comparison, the odds ratio for depression was 2.29 times greater for left anterior than left posterior (95% CI 1.6 to 3.4) using the fixed model assumptions and odds ratio of 2.29 (95% CI 1.5 to 3.4) using the random model assumptions. Similarly, the comparison of left- and right- anterior lesions had an odds ratio of 2.18 times greater for left anterior than right anterior for the fixed model (95% CI 1.4 to 3.3) and 2.16 for the random model (95% CI 1.3 to 3.6). In summary, the time since stroke appears to be a crucial variable in determining whether there is an increased frequency of mood disorders among patients with injury or dysfunction to frontal regions of the left hemisphere.
In addition to the association of left-anterior lesion location with depression, there is a significant inverse correlation between the severity of depression and the distance of the anterior border of the lesion from the frontal pole in the left hemisphere.13,14 A meta-analysis by Narushima et al15 found 8 independent studies of severity of depression and proximity of lesion to the left or right frontal pole conducted within 6 months following stroke. A total of 163 patients were included and the combined correlation coefficient (r) was −0.53 using fixed and −0.59 using random model assumptions (P < 0.001). However, in the right hemi- sphere, a total of 106 patients were included and the com- bined correlation between severity of depression and distance of the lesion from the right frontal pole was nonsignificant (that is, r = −0.20 fixed model, P = 0.14; r = −0.23 random model, P = 0.17).
Association With Physical Impairment
Besides the examination of prevalence rates for PSD, the most frequently reported association of PSD has been severity of impairment in ADL. Among 18 studies involving 3281 patients, 15 (83%) found a statistically significant relation between the severity or existence of PSD and severity of impairment in ADL12 (online eTable 2). Whether this association is construed as the severe functional impairment producing depression or alternatively as the severe depression producing greater severity of functional impairment was not examined in most of these studies. Sinyor et al16 were the first to report that depression influenced recovery in ADL. Nondepressed stroke patients were found to show either a slight improvement or no change in functional status from the acute poststroke period to 1-month follow-up, while depressed patients showed a significant decline in ADL during the first month.
In another study, Parikh et al17 compared a consecutive series of 33 patients with major or minor depression during the acute stroke period with 30 nondepressed stroke patients and examined their recovery at 2-year follow-up. Although both groups had similar impairments in ADL during the time they were in hospital, the depressed patients had significantly less improvement at the 2-year follow-up than the nondepressed patients. This finding was independent of type and amount of in-hospital rehabilitation therapy, size or location of the lesion, patients’ demographic characteristics, the nature of the stroke, the occurrence of another stroke during the follow-up period, and the patient’s medical history. Similar results were reported by Pohjasvaara et al18 who found that among 256 patients, depression at 3 months was associated with a Rankin Scale score of greater than 2 (that is, significant residual motor impairment) at 15 months, compared with nondepressed patients (OR 2.5; P < 0.01). A recent review by Hackett and Anderson19 reported that 9 of 11 studies that assessed physical disability found greater physical impairment was significantly associated with a greater frequency of depression, compared with no depression, following stroke.
The effect of treatment on recovery in ADL was examined by Narushima and Robinson20 where 34 patients who received antidepressant treatment with either nortriptyline (100 mg/day) or fluoxetine (40 mg/day) for 12 weeks beginning 19 days (SD 25) poststroke, compared with 28 patients who received the same antidepressant but began at 140 days (SD 28) poststroke. There were no significant differences between the groups in age, education, lesion volume, lesion location, or amount of rehabilitation services. During the period from 6 to 24 months following stroke, when the 2 groups were matched for the time since stroke, there was a significant Group × Time interaction using either ITT or efficacy analysis (Figure 1). The early treatment group continued to show gradual recovery in ADL during 2 years, whereas the late-treatment group showed gradual deterioration between 12- and 24-month follow-up. Logistic regression analysis examining the effects of diagnosis (that is, depressed or nondepressed), medication (that is, fluoxetine or nortriptyline), presence of severe motor impairment (that is, a National Institutes of Health Stroke Scale rating of more or less than 15), presence or absence of prior psychiatric history, use of antidepressants beyond the 12-week study period, and use of early, compared with late, antidepressant treatment showed that only the use of early, compared with late, antidepressant treatment was an independent prediction of ADL scores at 2-year follow-up. Thus antidepressant treatment of depression appears to significantly influence recovery in ADL; however, early treatment within the first month poststroke appears to be significantly more effective than delayed treatment. It should also be noted that this effect was independent of the existence of depression. Thus both depressed and nondepressed patients given antidepressants within the first month poststroke improved in their ADL more than patients given antidepressants after the first month poststroke.
Figure 1.

ADL improvement in Functional Independence Measure (FIM)
Association With Cognitive Impairment
Numerous investigators have reported that elderly patients with functional (that is, no brain injury) major depression have cognitive deficits that improve with treatment of depression (that is, dementia of depression).21 Among patients with PSD, this issue was examined by Robinson et al,22 whereby patients with major depression following a left-hemisphere stroke were found to have significantly more impairment on the MMSE than a comparable group of nondepressed patients. Both the size of the patient’s lesion and the severity of their depression correlated independently with severity of cognitive impairment. In a follow-up study, Starkstein et al23 reported that stroke patients with major depression—who were matched with nondepressed patients, with comparable levels of education, for both lesion location and lesion volume—had significantly lower (that is, more impaired) MMSE scores.
Morris et al,3 Downhill et al,24 and Spalletta et al25 also reported a similar phenomenon. In the Spalletta et al study,25 among 153 patients with first-ever stroke lesions of the left (n = 87) or right (n = 66) hemisphere who were less than 1-year poststroke, patients with left-hemisphere lesions and major depression (n = 30) showed significantly more impairment on the MMSE than nondepressed patients with left-hemisphere lesions (n = 27).
Treatment studies of PSD have consistently failed to show an improvement in cognitive function, even when poststroke mood disorders responded to antidepressant therapy.26 However, Kimura et al27 examined this issue in a study of patients with major (n = 33) or minor (n = 14) PSD, comparing nortriptyline- and placebo-treated patients using double-blind methodology. When patients were divided into those who responded to treatment (that is, greater than 50% decline in HDRS score) and those who did not respond, there is a significantly greater improvement in MMSEs among patients who responded to treatment (n = 24), compared with patients who did not respond to treatment (n = 23). A repeated measures ANOVA demonstrated a significant Group × Time interaction, with responders having significantly less impaired MMSE scores than nonresponders at nortriptyline doses of 75 mg (P = 0.04) and 100 mg (P = 0.03). A follow-up of patients treated for cognitive impairment associated with PSD showed that the improved cognitive function lasts for 2 years or more, even after the antidepressant is stopped.28
Earlier treatment studies did not show a significant effect of treatment of depression on cognitive function because of effect size. When nortriptyline-treated patients, some of whom responded to treatment and some of whom did not, were compared with placebo treatment, some of whom responded and some of whom did not, the effect size was only 0.16. When patients were divided into those who responded and those who did not respond, the effect size increased to 0.96, thus allowing a significant difference to be demonstrated with a much smaller group size.
Association With Mortality
Increased mortality is perhaps the ultimate validation of the importance of depression in the prognosis following stroke. The first study to examine mortality associated with PSD was reported by Morris et al.29 At 10-year follow-up, mortality status was obtained for 91 of 103 patients who were initially examined following acute stroke. The 48 (53%) patients who had died were compared with those who had survived. Patients with acute PSD were 3.4 times more likely to have died during 10 years of follow- up, compared with patients who were nondepressed after the acute stroke (OR 3.4; P = 0.007). Further, the frequency of death among patients with a diagnosis of major, compared with minor, depression at the time of the acute stroke was identical (that is, 70%). A multiple logistic regression was carried out examining factors of age, marital status, sex, social class, social ties, social functioning, MMSE score, ADL, alcohol use, medical comorbidity, type of stroke, hemispheric and cortical-subcortical lesion location, volume of lesion, severity of impairment, and severity of depression on the likelihood of survival during 10 years. After controlling for these variables, depression severity was independently associated with mortality (AOR 3.7; P = 0 .03). House et al30 examined 2 4 8 hospitalized patients 1 month following acute stroke, with follow- up at 12 and 24 months. Mood symptoms were assessed using the semi-structured Present State Examination and the GHQ-28. Patients scoring 1 or more on the depression subscale of the GHQ (that is, 3 items examining depressive symptoms) had an odds ratio of mortality 2.4 times greater than those who scored zero on the depression subscale at 1-year follow-up. At 24 months, a multiple logistic regression analysis found a GHQ–Depression subscale score of 1 or greater, older age, and MMSE scores of less than 24 were independently associated with increased mortality. The largest study was a retrospective study conducted by Williams et al31 in which the records of 151 119 veterans hospitalized for ischemic stroke who survived more than 30 days were reviewed. Within 3 years poststroke, 2405 veterans had a diagnosis of depression and 2257 had other psychiatric diagnoses (primarily substance abuse or anxiety disorder). Among the depressed patients, 59.0% were alive at 6 years, while 58.7% of the patients with substance abuse and anxiety disorders and 63% of the patients with no depression or substance abuse diagnosis were alive (HR 1.13; P < 0.01 for depression). The major shortcoming of this retrospective review is the low rate of depression (5%), which suggests many depressions were missed.
Thus PSD appears to be a significant risk factor for increased death as early as 1 year and as late as 7 years following stroke. The mechanism that produces these increased death rates is an important issue, which has, thus far, not been examined except for one recent study32 showing that PSD is associated with decreased HRV. Tokgozoglu et a133 reported that patients with decreased HRV, as a result of stroke lesions of the insular cortex, are at risk for sudden death, and Makikallio et a134 found that decreased long-term HRV was the only multivariate predictor of death (HR 4.5; P < 0.001) after adjusting for age.
Mechanism of Depression
Experiments assessing the association of focal lesions with depressive disorder were the first to identify strategic brain regions whose dysfunction was associated with depression. Robinson et al35 found that lesions involving the left frontal region of the brain were associated with a significantly higher frequency of depression during the first 2 months following acute stroke than comparable lesions of the right hemisphere or posterior lesions of the left hemisphere. Subsequently the work of other investigators36–39 identified that left-lateral frontal lobe, caudate, or putamen lesions were significantly more likely to produce depression during the acute stroke period than com- parable lesions of the right hemisphere. In addition to this anatomic basis of depression, there is growing evidence that abnormality of proinflammatory protein release provoked by brain injury or regional dysfunction may play a crucial role in the physiological and neurochemical dysfunctions that may underlie the pathophysiology of depression. There is a great deal of human and animal experimentation documenting that cerebral ischemia leads to the perturbation of – proinflammatory cytokines such as IL-1_, IL-6, and IL-18, and tumour necrosis factor-alpha 20–22.40 These cytokines have also been shown to produce widespread activation of indolamine 2, 3-dioxygenase, which metabolizes tryptophan to kynurenin, thus depleting serotonin.40 Further, antidepressant drugs have been shown to have an antiinflammatory effect.41 We have recently described that IL-18 peripheral levels are negatively correlated with depression severity in acute stroke survivors.42 This may indicate that the inflammatory processes leading to PSD, just as laterality of lesion location, are time dependent. Thus mechanisms of depression in patients with acute and chronic stroke must be considered different and this may impact on future treatment interventions.
Other mechanisms of PSD have also been proposed. For example, Kohen et al43 recently showed that among 75 depressed and 76 nondepressed patients attending an outpatient stroke clinic that people with serotonin transporter protein promoter polymorphism/s were 3.1 times more likely to have PSD than patients with the 1/1 or 1/xl geno- type. Further, patients with the serontonin transporter geno- type STin2 (variable number tandem repeats) 9/12 or 12/12 were 4.1 times more likely to be depressed than the patients with the STin2 (tandem repeats) 10/10 type. However, the mechanisms by which these genes act to increase the probability or decrease the probability of depression are not known.
Treatment of PSD
Currently, there are 10 placebo-controlled, randomized double-blind treatment studies on the efficacy of single antidepressant treatment of PSD (online eTable 3). The first treatment study of PSD was conducted by Lipsey et al44 in which 11 patients treated with nortriptyline who completed the 6-week study with doses increasing from 25 to 100 mg showed significantly greater improvement in their HDRS scores than did the 15 placebo-treated patients who completed the study (P < 0.01). The first study45 to use a selective serotonin reuptake inhibitor drug found that patients receiving citalopram (20 mg for patients aged 64 years and younger; 10 mg for patients aged 65 years and older [n = 27]) showed a significantly greater reduction in HDRS scores both at 3 and at 6 weeks, administered identically with fluoxetine. ITT analysis46 demonstrated a significant Time × Treatment interaction, with nortriptyline-treated patients showing a significantly greater decline in HDRS scores than either the placebo- or fluoxetine-treated patients at 12 weeks of treatment. ITT response rates were 10 of 16 (62% for nortriptyline), 2 of 23 (9% for fluoxetine), and 4 of 17 (24% for placebo). There were no significant differences between the fluoxetine and placebo groups.
A 7-year follow-up of the patients in this study47 found that treatment with either fluoxetine or nortriptyline was associated with a significantly lower mortality rate than placebo.47 This finding is consistent with the hypothesis that antidepressants block the effect of cytokines and other inflammatory agents in producing decreased HRV. The only study to examine a norepinephrine uptake inhibitor drug was conducted by Rampello et al,48 comparing reboxetine and placebo in PSD with psychomotor retardation. Among the 16 patients given reboxetine (4 mg/day) there was a significantly greater decline in HDRS and BDI49 (HDRS, mean 24.1, SD 1.5, to mean 9.3, SD 2.1; BDI, mean 20.6, SD 2.2, to mean 8.1, SD 3.4), compared with placebo during the 16-week trial (HDRS, mean 24.0, SD 1.3, to mean 22.7, SD 2.4; BDI, mean 19.9, SD 1.5, to mean 18.4, SD 3.3). The number of completers and the relative rates of response and remission were not given.
Based on the available data, if there were no contraindications to nortriptyline, such as heart block, cardiac arrythmia, narrow angle glaucoma, sedation, or orthostatic hypotension, nortriptyline remains the most extensively investigated treatment for PSD. Doses of nortriptyline should be increased slowly and blood levels should be monitored with a goal of achieving serum concentrations between 50 and 150 ng/mL. If there are contraindications to the use of nortriptyline, citalopram (20 mg for patients aged 65 years and younger and 10 mg for those aged 66 years and older), or reboxetine (2 mg twice per day) would be alternative choices.
Although it has not been used in a randomized control trial, electroconvulsive therapy has also been reported to be effective in treating PSD.50 It caused few side effects and no neurological deterioration. Psychostimulants have also been reported in open-label trials to be effective for the treatment of PSD. Finally, psychological treatment using CBT in 123 stroke patients has been found by Lincoln et al51 to be no more effective than attention (that is, placebo) treatment (CBT completers, n = 39; placebo completers, n = 43).
Prevention of PSD
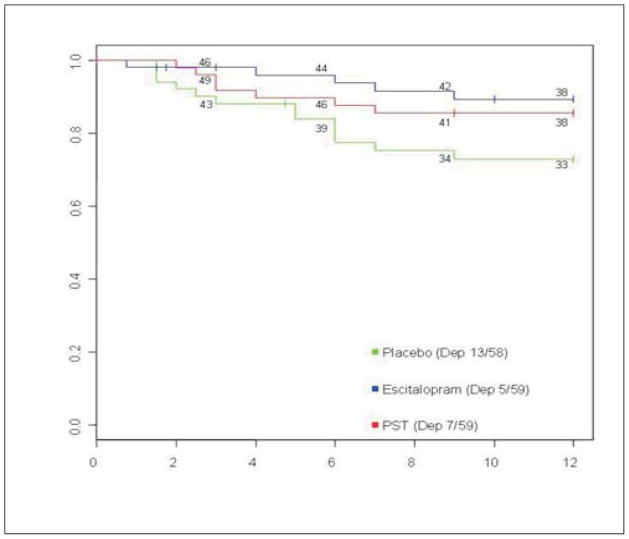
Based on prevalence rates of depression following stroke, patients who survive the acute stroke may constitute a particularly good population for preventive intervention for depression. Four groups of investigators have attempted to prevent the development of PSD.52–54 The first was reported by Palomäki et al,52 who conducted a randomized double-blind, placebo-controlled study of 100 patients aged 70 years and younger admitted to hospital for acute ischemic stroke. Patients received either 60 mg/day of mianserin or placebo for 1 year and were examined at 2-, 6-, 12-, and 18-month follow-up. At no time during the 18 months did the preva- lence rate for DSM-III-R major depression differ between treatment groups. Rasmussen et al,54 using double-blind methods and random assignment, treated 137 nondepressed patients, during 12 months following acute stroke, with sertraline (mean dose 63 mg/day; n = 70) or placebo (n = 67). The depression occurrence rate during the 12 months, based on an HDRS-17 score of more than 18, was 8.2% (90% CI 2.4% to 13.9%) (that is, 3 of 35 completers) for sertraline compared with 2 2 .8 % (90 % C 13 . 7 % to 32 . 0 % ) ( that is, 7 of 32 completers) for placebo. Although the placebo rate of depression was higher than the sertraline rate, the difference between groups was not significant, owing to the relatively small number of completers (that is, only 49% completed the study). Almeida et a153 also conducted a prevention trial in which 111 patients within 2 weeks following stroke were randomized to treatment with sertralin e (5 0 mg/day) (n = 55) or placebo (n = 56) in a 24-week double- blind clinical trial. Results demonstrated no significant inter- group difference in the prevalence of Hospital Depression and Anxiety Scale scores of 8 or more during the 24 weeks of treatment. Among sertraline-treated patients, 8 out of 48 (16.7%) developed depression, compared with 11 out of 51 (21.6%) placebo-treated patients (incidence rate ratio 0.8; 95% CI 0.3 to 2.1). The most recent prevention trial was reported by Robinson et al5 in which 176 nondepressed patients within 3 months following acute stroke were treated in a double-blind randomized trial of escitalopram (n = 59) and placebo (n = 58) and a nonblinded arm of problem-solving therapy (n = 59). Treatment failure was determined by developing either major or minor depression using DSM-IV criteria. Based on an ITT analysis, patients who received placebo were significantly more likely to develop depression than those who received escitalopram; (that is, 11 with major and 2 with minor depressions [22.4%], compared with 3 with major and 2 with minor depressions [8.5%], adjusted HR 4.5; P < 0.001); and also more likely than people who received problem-solving therapy (5 major and 2 minor depression [11.9%]) (adjusted HR 2.2; P < 0.001) (Figure 2). Findings were adjusted for history of mood disorders, age, sex, treatment site, and severity of impairment. The vast majority of depressions that were prevented were first-episode depressions. This was the first study to demonstrate, using double-blind methodology, that first-episode depressions can be prevented by antidepressants.
Figure 2.
Kaplan-Meier curves for each treatment arm
Conclusion
There has been a steadily increasing interest during the past 20 years in PSD. Effective treatments have been demonstrated both for acute PSD and for prevention of depression. Given the adverse effect of PSD on recovery in ADL and cognitive function, as well as the increased mortality rate, prevention of PSD using antidepressant medications may play an increasingly prominent role in the management of patients following acute stroke.
Clinical Implications
Depression occurs in about 40% of acute stroke patients.
Depression is an independent factor leading to poor recovery and mortality.
Depression may be effectively treated and prevented.
Limitations
There is significant variability between studies in method of diagnosis.
The time since stroke is crucial in assessing the course of depression.
Further research on the mechanism of depression and morality is urgently needed.
Acknowledgments
This work was supported by National Institute of Mental Health grant R01-MH065134. The Canadian Psychiatric Association proudly supports the In Review series by providing an honorarium to the authors.
References
- 1.Kraepelin E. Manic depressive insanity and paranoia. Edinburgh (GB): E & S Livingstone; 1921. [Google Scholar]
- 2.Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) New York (NY): Biometric Research, New York State Psychiatric Institute; 1995. [Google Scholar]
- 3.Morris PLP, Robinson RG, Raphael B. Prevalence and course of depressive disorders in hospitalized stroke patients. Intl J Psychiatr Med. 1990;20(4):349–364. doi: 10.2190/N8VU-6LWU-FLJN-XQKV. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington (DC): APA; 1994. [Google Scholar]
- 5.Spalletta G, Ripa A, Caltagirone C. Symptom profile of DSM-IV major and minor depressive disorders in first-ever stroke patients. Am J Geriatr Psychiatry. 2005;13(2):108–115. doi: 10.1176/appi.ajgp.13.2.108. [DOI] [PubMed] [Google Scholar]
- 6.Paradiso S, Ohkubo T, Robinson RG. Vegetative and psychological symptoms associated with depressed mood over the first two years after stroke. Int J Psychiatry Med. 1997;27(2):137–157. doi: 10.2190/BWJA-KQP3-7VUY-D06T. [DOI] [PubMed] [Google Scholar]
- 7.Damecour CL, Caplan D. The relationship of depression to symptomatology and lesion site in aphasic patients. Cortex. 1991;27:385– 401. doi: 10.1016/s0010-9452(13)80034-2. [DOI] [PubMed] [Google Scholar]
- 8.Carson AJ, MacHale S, Allen K, et al. Depression after stroke and lesion location: a systematic review. Lancet. 2000;356(9224):122–126. doi: 10.1016/S0140-6736(00)02448-X. [DOI] [PubMed] [Google Scholar]
- 9.Bhogal SK, Teasell R, Foley N, et al. Lesion location and poststroke depression. Systematic review of the methodological limitations in the literature. Stroke. 2004;35:794– 802. doi: 10.1161/01.STR.0000117237.98749.26. [DOI] [PubMed] [Google Scholar]
- 10.Astrom M, Adolfsson R, Asplund K. Major depression in stroke patients: a 3-year longitudinal study. Stroke. 1993;24(7):976–982. doi: 10.1161/01.str.24.7.976. [DOI] [PubMed] [Google Scholar]
- 11.Morris PLP, Robinson RG, Raphael B, et al. Lesion location and post-stroke depression. J Neuropsychiatry Clin Neurosci. 1996;8:399–403. doi: 10.1176/jnp.8.4.399. [DOI] [PubMed] [Google Scholar]
- 12.Robinson RG. The clinical neuropsychiatry of stroke. 2. Cambridge (MA): Cambridge University Press; 2006. [Google Scholar]
- 13.Robinson RG, Szetela B. Mood change following left hemispheric brain injury. Ann Neurol. 1981;9:447–453. doi: 10.1002/ana.410090506. [DOI] [PubMed] [Google Scholar]
- 14.Eastwood MR, Rifat SL, Nobbs H, et al. Mood disorder following cerebrovascular accident. Br J Psychiatry. 1989;154:195–200. doi: 10.1192/bjp.154.2.195. [DOI] [PubMed] [Google Scholar]
- 15.Narushima K, Kosier JT, Robinson RG. A reappraisal of poststroke depression, intra- and inter-hemispheric lesion location using meta-analysis. J Neuropsychiatry Clin Neurosci. 2003;15(4):422–430. doi: 10.1176/jnp.15.4.422. [DOI] [PubMed] [Google Scholar]
- 16.Sinyor D, Amato P, Kaloupek P. Post-stroke depression: relationship to functional impairment, coping strategies, and rehabilitation outcome. Stroke. 1986;17:112–117. doi: 10.1161/01.str.17.6.1102. [DOI] [PubMed] [Google Scholar]
- 17.Parikh RM, Robinson RG, Lipsey JR, et al. The impact of poststroke depression on recovery in activities of daily living over two-year follow-up. Arch Neurol. 1990;47:785–789. doi: 10.1001/archneur.1990.00530070083014. [DOI] [PubMed] [Google Scholar]
- 18.Pohjasvaara T, Vataja R, Leppavuori A, et al. Depression is an independent predictor of poor long-term functional outcome post-stroke. Eur J Neurol. 2001;8(4):315–319. doi: 10.1046/j.1468-1331.2001.00182.x. [DOI] [PubMed] [Google Scholar]
- 19.Hackett ML, Anderson CS. Predictors of depression after stroke: a systematic review of observational studies. Stroke. 2005;36(10):2296–2301. doi: 10.1161/01.STR.0000183622.75135.a4. [DOI] [PubMed] [Google Scholar]
- 20.Narushima K, Robinson RG. The effect of early versus late antidepressant treatment on physical impairment associated with poststroke depression: is there a time-related therapeutic window? J Nerv Ment Dis. 2003;191(10):645–652. doi: 10.1097/01.nmd.0000092197.97693.d2. [DOI] [PubMed] [Google Scholar]
- 21.Wells CE. Pseudodementia. Am J Psychiatry. 1979;136:895–900. doi: 10.1176/ajp.136.7.895. [DOI] [PubMed] [Google Scholar]
- 22.Robinson RG, Bolla-Wilson K, Kaplan E, et al. Depression influences intellectual impairment in stroke patients. Br J Psychiatry. 1986;148:541–547. doi: 10.1192/bjp.148.5.541. [DOI] [PubMed] [Google Scholar]
- 23.Starkstein SE, Robinson RG, Price TR. Comparison of patients with and without post-stroke major depression matched for size and location of lesion. Arch Gen Psychiatry. 1988;45:247–252. doi: 10.1001/archpsyc.1988.01800270061007. [DOI] [PubMed] [Google Scholar]
- 24.Downhill JE, Jr, Robinson RG. Longitudinal assessment of depression and cognitive impairment following stroke. J Nerv Ment Dis. 1994;182:425–431. doi: 10.1097/00005053-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Spalletta G, Guida G, De Angelis D, et al. Predictors of cognitive level and depression severity are different in patients with left and right hemispheric stroke within the first year of illness. J Neurol. 2002;249(11):1541–1551. doi: 10.1007/s00415-002-0885-z. [DOI] [PubMed] [Google Scholar]
- 26.Andersen G, Vestergaard K, Riis JO, et al. Intellectual impairment in the first year following stroke, compared to an age-matched population sample. Cerebrovasc Dis. 1996;6(6):363–369. [Google Scholar]
- 27.Kimura M, Robinson RG, Kosier T. Treatment of cognitive impairment after poststroke depression. Stroke. 2000;31(7):1482–1486. doi: 10.1161/01.str.31.7.1482. [DOI] [PubMed] [Google Scholar]
- 28.Narushima K, Chan KL, Kosier JT, et al. Does cognitive recovery after treatment of poststroke depression last? A 2-year follow-up of cognitive function associated with poststroke depression. Am J Psychiatry. 2003;160(6):1157–1162. doi: 10.1176/appi.ajp.160.6.1157. [DOI] [PubMed] [Google Scholar]
- 29.Morris PLP, Robinson RG, Samuels J. Depression, introversion and mortality following stroke. Aust N Z J Psychiatry. 1993;27(3):443–449. doi: 10.3109/00048679309075801. [DOI] [PubMed] [Google Scholar]
- 30.House A, Knapp P, Bamford J, et al. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke. 2001;32(3):696–701. doi: 10.1161/01.str.32.3.696. [DOI] [PubMed] [Google Scholar]
- 31.Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am J Psychiatry. 2004;161(6):1090–1095. doi: 10.1176/appi.ajp.161.6.1090. [DOI] [PubMed] [Google Scholar]
- 32.Robinson RG, Spalletta G, Jorge RE, et al. Decreased heart rate variability is associated with poststroke depression. Am J Geriatr Psychiatry. 2008;16(11):867–873. doi: 10.1097/JGP.0b013e318180057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tokgozoglu SL, Batur MK, Topcuoglu MA, et al. Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke. 1999;30(7):1307–1311. doi: 10.1161/01.str.30.7.1307. [DOI] [PubMed] [Google Scholar]
- 34.Makikallio AM, Makikallio TH, Korpelainen JT, et al. Heart rate dynamics predict poststroke mortality. Neurology. 2004;62(10):1822–1826. doi: 10.1212/01.wnl.0000125190.10967.d5. [DOI] [PubMed] [Google Scholar]
- 35.Robinson RG, Kubos KL, Starr LB, et al. Mood disorders in stroke patients: importance of location of lesion. Brain. 1984;107(Pt 1):81–93. doi: 10.1093/brain/107.1.81. [DOI] [PubMed] [Google Scholar]
- 36.Starkstein SE, Robinson RG, Price TR. Comparison of cortical and subcortical lesions in the production of post-stroke mood disorders. Brain. 1987;110:1045–1059. doi: 10.1093/brain/110.4.1045. [DOI] [PubMed] [Google Scholar]
- 37.Astrom M, Olsson T, Asplund K. Different linkage of depression to hypercortisolism early versus late after stroke: a 3-year longitudinal study. Stroke. 1993;24:52–57. doi: 10.1161/01.str.24.1.52. [DOI] [PubMed] [Google Scholar]
- 38.Herrmann N, Black SE, Lawrence J, et al. The Sunnybrook stroke study. A prospective study of depressive symptoms and functional outcome. Stroke. 1998;29:618–624. doi: 10.1161/01.str.29.3.618. [DOI] [PubMed] [Google Scholar]
- 39.Morris PL, Robinson RG, de Carvalho ML, et al. Lesion characteristics and depressed mood in the stroke data bank study. J Neuropsychiatry Clin Neurosci. 1996;8:153–159. doi: 10.1176/jnp.8.2.153. [DOI] [PubMed] [Google Scholar]
- 40.Spalletta G, Bossu P, Ciaramella A, et al. The etiology of poststroke depression: a review of the literature and a new hypothesis involving inflammatory cytokines. Mol Psychiatry. 2006;11(11):984–991. doi: 10.1038/sj.mp.4001879. [DOI] [PubMed] [Google Scholar]
- 41.O’Brien SM, Scott LV, Dinan TG. Antidepressant therapy and C-reactive protein levels. Br J Psychiatry. 2006;188:449–452. doi: 10.1192/bjp.bp.105.011015. [DOI] [PubMed] [Google Scholar]
- 42.Bossù P, Salani F, Cacciari C, et al. Disease outcome, alexithymia and depression are differently associated with serum IL-18 levels in acute stroke. Curr Neurovasc Res. 2009;6(3):163–170. doi: 10.2174/156720209788970036. [DOI] [PubMed] [Google Scholar]
- 43.Kohen R, Cain KC, Mitchell PH, et al. Association of serotonin transporter gene polymorphisms with poststroke depression. Arch Gen Psychiatry. 2008;65(11):1296–1302. doi: 10.1001/archpsyc.65.11.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lipsey JR, Robinson RG, Pearlson GD, et al. Nortriptyline treatment of post-stroke depression: a double-blind study. Lancet. 1984;1(8372):297–300. doi: 10.1016/s0140-6736(84)90356-8. [DOI] [PubMed] [Google Scholar]
- 45.Andersen G, Vestergaard K, Lauritzen L. Effective treatment of poststroke depression with the selective serotonin reuptake inhibitor citalopram. Stroke. 1994;25:1099–1104. doi: 10.1161/01.str.25.6.1099. [DOI] [PubMed] [Google Scholar]
- 46.Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry. 2000;157(3):351–359. doi: 10.1176/appi.ajp.157.3.351. [DOI] [PubMed] [Google Scholar]
- 47.Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823–1829. doi: 10.1176/appi.ajp.160.10.1823. [DOI] [PubMed] [Google Scholar]
- 48.Rampello L, Alvano A, Chiechio S, et al. An evaluation of efficacy and safety of reboxetine in elderly patients affected by “retarded” post-stroke depression. A random, placebo-controlled study. Arch Gerontol Geriatr. 2005;40(3):275–285. doi: 10.1016/j.archger.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:551–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 50.Murray GB, Shea V, Conn DK. Electroconvulsive therapy for poststroke depression. J Clin Psychiatry. 1986;47(5):258–260. [PubMed] [Google Scholar]
- 51.Lincoln NB, Nicholl CR, Flannaghan T, et al. The validity of questionnaire measures for assessing depression after stroke. Clin Rehabil. 2003;17(8):840–846. doi: 10.1191/0269215503cr687oa. [DOI] [PubMed] [Google Scholar]
- 52.Palomäki H, Kaste M, Berg A, et al. Prevention of poststroke depression: 1 year randomised placebo controlled double blind trial of mainserin with 6 month follow up after therapy. J Neurol Neurosurg Psychiatry. 1999;66(4):490–494. doi: 10.1136/jnnp.66.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Almeida OP, Waterreus A, Hankey GJ. Preventing depression after stroke: results from a randomized placebo-controlled trial. J Clin Psychiatry. 2006;67(7):1104–1109. doi: 10.4088/jcp.v67n0713. [DOI] [PubMed] [Google Scholar]
- 54.Rasmussen A, Lunde M, Poulsen DL, et al. A double-blind, placebo-controlled study of sertraline in the prevention of depression in stroke patients. Psychosomatics. 2003;44(3):216–221. doi: 10.1176/appi.psy.44.3.216. [DOI] [PubMed] [Google Scholar]
- 55.Robinson RG, Jorge RE, Moser DJ, et al. Escitalopram and problem-solving therapy for prevention of poststroke depression: a randomized controlled trial. JAMA. 2008;299(20):2391– 2400. doi: 10.1001/jama.299.20.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wade DT, Legh-Smith J, Hewer RA. Depressed mood after stroke, a community study of its frequency. Br J Psychiatry. 1987;151:200–205. doi: 10.1192/bjp.151.2.200. [DOI] [PubMed] [Google Scholar]
- 57.House A, Dennis M, Mogridge L, et al. Mood disorders in the year after first stroke. Br J Psychiatry. 1991;158:83–92. doi: 10.1192/bjp.158.1.83. [DOI] [PubMed] [Google Scholar]
- 58.Burvill PW, Johnson GA, Jamrozik KD, et al. Prevalence of depression after stroke: the Perth Community Stroke Study. Br J Psychiatry. 1995;166(3):320–327. doi: 10.1192/bjp.166.3.320. [DOI] [PubMed] [Google Scholar]
- 59.Kotila M, Numminen H, Waltimo O, et al. Depression after stroke: results of the FINNSTROKE Study. Stroke. 1998;29:368–372. doi: 10.1161/01.str.29.2.368. [DOI] [PubMed] [Google Scholar]
- 60.Hayee MA, Akhtar N, Haque A, et al. Depression after stroke-analysis of 297 stroke patients. Bangladesh Med Res Counc Bull. 2001;27(3):96–102. [PubMed] [Google Scholar]
- 61.Stewart R, Prince M, Richards M, et al. Stroke, vascular risk factors and depression—cross-sectional study in a UK Caribbean-born population. Br J Psychiatry. 2001;178:23–28. doi: 10.1192/bjp.178.1.23. [DOI] [PubMed] [Google Scholar]
- 62.Desmond DW, Remien RH, Moroney JT, et al. Ischemic stroke and depression. J Int Neuropsychol Soc. 2003;9(3):429–439. doi: 10.1017/S1355617703930086. [DOI] [PubMed] [Google Scholar]
- 63.Ebrahim S, Barer D, Nouri F. Affective illness after stroke. Br J Psychiatry. 1987;151:52–56. doi: 10.1192/bjp.151.1.52. [DOI] [PubMed] [Google Scholar]
- 64.Shima S, Kitagawa Y, Kitamura T, et al. Poststroke depression. Gen Hosp Psychiatry. 1994;16(4):286–289. doi: 10.1016/0163-8343(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez-Torrecillas JL, Mendlewicz J, Lobo A. Effects of early treatment of poststroke depression on neuropsychological rehabilitation. Int Psychogeriatr. 1995;7(4):547–560. doi: 10.1017/s1041610295002286. [DOI] [PubMed] [Google Scholar]
- 66.Herrmann M, Bartles C, Wallesch CW. Depression in acute and chronic aphasia: symptoms, pathoanatomical-clinical correlations and functional implications. J Neurol Neurosurg Psychiatry. 1993;56:672–678. doi: 10.1136/jnnp.56.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andersen G, Vestergaard K, Riis JO, et al. Incidence of post-stroke depression during the first year in a large unselected stroke population determined using a valid standardized rating scale. Acta Psychiatr Scand. 1994;90(8875):190–195. doi: 10.1111/j.1600-0447.1994.tb01576.x. [DOI] [PubMed] [Google Scholar]
- 68.Kauhanen M, Korpelainen JT, Hiltunen P, et al. Poststroke depression correlates with cognitive impairment and neurological deficits. Stroke. 1999;30(9):1875–1880. doi: 10.1161/01.str.30.9.1875. [DOI] [PubMed] [Google Scholar]
- 69.Gainotti G, Azzoni A, Marra C. Frequency, phenomenology and anatomical-clinical correlates of major post-stroke depression. Br J Psychiatry. 1999;175:163–167. doi: 10.1192/bjp.175.2.163. [DOI] [PubMed] [Google Scholar]
- 70.Aben I, Verhey F, Lousberg R, et al. Validity of the Beck depression inventory, hospital anxiety and depression scale, SCL-90, and Hamilton depression rating scale as screening instruments for depression in stroke patients. Psychosomatics. 2002;43(5):386–393. doi: 10.1176/appi.psy.43.5.386. [DOI] [PubMed] [Google Scholar]
- 71.Singh A, Black SE, Herrmann N, et al. Functional and neuroanatomic correlations in poststroke depression: the Sunnybrook Stroke Study. Stroke. 2000;31:637–644. doi: 10.1161/01.str.31.3.637. [DOI] [PubMed] [Google Scholar]
- 72.Berg A, Palomäki H, Lehtihalmes M, et al. Poststroke depression: an 18-month follow-up. Stroke. 2003;34(1):138–143. doi: 10.1161/01.str.0000048149.84268.07. [DOI] [PubMed] [Google Scholar]
- 73.Folstein MF, Maiberger R, McHugh PR. Mood disorder as a specific complication of stroke. J Neurol Neurosurg Psychiatry. 1977;40:1018–1020. doi: 10.1136/jnnp.40.10.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Finklestein S, Benowitz LI, Baldessarini RJ, et al. Mood, vegetative disturbance, and dexamethasone suppression test after stroke. Ann Neurol. 1982;12:463–468. doi: 10.1002/ana.410120509. [DOI] [PubMed] [Google Scholar]
- 75.Finset A, Goffeng L, Landro NI, et al. Depressed mood and intra-hemispheric location of lesion in right hemisphere stroke patients. Scand J Rehabil Med. 1989;21:1–6. [PubMed] [Google Scholar]
- 76.Schubert DSP, Taylor C, Lee S, et al. Physical consequences of depression in the stroke patient. Gen Hosp Psychiatry. 1992;14:69–76. doi: 10.1016/0163-8343(92)90028-9. [DOI] [PubMed] [Google Scholar]
- 77.Schwartz JA, Speed NM, Brunberg JA, et al. Depression in stroke rehabilitation. Biol Psychiatry. 1993;33:694–699. doi: 10.1016/0006-3223(93)90118-w. [DOI] [PubMed] [Google Scholar]
- 78.Robinson RG. Stroke. In: Lauterbach EC, editor. Psychiatric management in neurological disease. Washington (DC): American Psychiatric Association; 2000. pp. 219–247. [Google Scholar]
- 79.Cassidy E, O’Connor R, O’Keane V. Prevalence of post-stroke depression in an Irish sample and its relationship with disability and outcome following inpatient rehabilitation. Disabil Rehabil. 2004;26(2):71–77. doi: 10.1080/09638280410001650142. [DOI] [PubMed] [Google Scholar]
- 80.Pohjasvaara T, Leppavuori A, Siira I, et al. Frequency and clinical determinants of poststroke depression. Stroke. 1998;29:2311–2317. doi: 10.1161/01.str.29.11.2311. [DOI] [PubMed] [Google Scholar]
- 81.Feibel JH, Springer CJ. Depression and failure to resume social activities after stroke. Arch Phys Med Rehabil. 1982;63:276–278. [PubMed] [Google Scholar]
- 82.Robinson RG, Price TR. Post-stroke depressive disorders: a follow-up study of 103 outpatients. Stroke. 1982;13:635–641. doi: 10.1161/01.str.13.5.635. [DOI] [PubMed] [Google Scholar]
- 83.Kim JS, Choi-Kwon S. Poststroke depression and emotional incontinence: correlation with lesion location. Neurology. 2000;54(9):1805–1810. doi: 10.1212/wnl.54.9.1805. [DOI] [PubMed] [Google Scholar]
- 84.Collin SJ, Tinson D, Lincoln NB. Depression after stroke. Clin Rehabil. 1987;1:27–32. [Google Scholar]
- 85.Castillo CS, Schultz SK, Robinson RG. Clinical correlates of early-onset and late-onset poststroke generalized anxiety. Am J Psychiatry. 1995;152:1174–1179. doi: 10.1176/ajp.152.8.1174. [DOI] [PubMed] [Google Scholar]
- 86.Robinson RG, Starr LB, Kubos KL, et al. A two-year longitudinal study of post-stroke mood disorders: findings during the initial evaluation. Stroke. 1983;14:736–744. doi: 10.1161/01.str.14.5.736. [DOI] [PubMed] [Google Scholar]
- 87.Angeleri F, Angeleri VA, Foschi N, et al. The influence of depression, social activity, and family stress on functional outcome after stroke. Stroke. 1993;24(20):1478–1483. doi: 10.1161/01.str.24.10.1478. [DOI] [PubMed] [Google Scholar]
- 88.Shima S. The efficacy of antidepressants in post-stroke depression. Keio J Med. 1997;46(1):25–26. doi: 10.2302/kjm.46.25. [DOI] [PubMed] [Google Scholar]
- 89.Ramasubbu R, Robinson RG, Flint AJ, et al. Functional impairment associated with acute poststroke depression: the Stroke Data Bank Study. J Neuropsychiatry Clin Neurosci. 1998;10(1):26–33. doi: 10.1176/jnp.10.1.26. [DOI] [PubMed] [Google Scholar]
- 90.Kauhanen ML, Korpelainen JT, Hiltunen P, et al. Domains and determinants of of life after stroke caused by brain infarction. Arch Phys Med Rehabil. 2000;81(12):1541–1546. doi: 10.1053/apmr.2000.9391. [DOI] [PubMed] [Google Scholar]
- 91.Gainotti G, Antonucci G, Marra C, et al. Relation between depression after stroke, antidepressant therapy, and functional recovery. J Neurol Neurosurg Psychiatry. 2001;71(2):258–261. doi: 10.1136/jnnp.71.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van de Weg FB, Kuik DJ, Lankhorst GJ. Post-stroke depression and functional outcome: a cohort study investigating the influence of depression on functional recovery from stroke. Clin Rehabil. 1999;13:268–272. doi: 10.1191/026921599672495022. [DOI] [PubMed] [Google Scholar]
- 93.Paolucci S, Antonucci G, Grasso MG, et al. Early versus delayed inpatient stroke rehabilitation: a matched comparison conducted in Italy. Arch Phys Med Rehabil. 2000;81(6):695–700. doi: 10.1016/s0003-9993(00)90095-9. [DOI] [PubMed] [Google Scholar]
- 94.Paolucci S, Antonucci G, Grasso MG, et al. Post-stroke depression, antidepressant treatment and rehabilitation results. A case-control study. Cerebrovasc Dis. 2001;12(3):264–271. doi: 10.1159/000047714. [DOI] [PubMed] [Google Scholar]
- 95.Paolucci S, Antonucci G, Pratesi L, et al. Poststroke depression and its role in rehabilitation of inpatients. Arch Phys Med Rehabil. 1999;80:985–990. doi: 10.1016/s0003-9993(99)90048-5. [DOI] [PubMed] [Google Scholar]
- 96.Paolucci S, Grasso MG, Antonucci G, et al. One-year follow-up in stroke patients discharged from rehabilitation hospital. Cerebrovasc Dis. 2000;10(1):25–32. doi: 10.1159/000016021. [DOI] [PubMed] [Google Scholar]
- 97.Sturm JW, Donnan GA, Dewey HM, et al. Determinants of handicap after stroke: the North East Melbourne Stroke Incidence Study (NEMESIS) Stroke. 2004;35:715–720. doi: 10.1161/01.STR.0000117573.19022.66. [DOI] [PubMed] [Google Scholar]
- 98.Reding MJ, Orto LA, Winter SW, et al. Antidepressant therapy after stroke: a double-blind trial. Arch Neurol. 1986;43:763–765. doi: 10.1001/archneur.1986.00520080011011. [DOI] [PubMed] [Google Scholar]
- 99.Dam M, Tonin P, De Boni A, et al. Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy. Stroke. 1996;27(7):1211–1214. doi: 10.1161/01.str.27.7.1211. [DOI] [PubMed] [Google Scholar]
- 100.Chemerinski E, Robinson RG. The neuropsychiatry of stroke. Psychosomatics. 2000;41(1):5–14. doi: 10.1016/S0033-3182(00)71168-6. [DOI] [PubMed] [Google Scholar]
- 101.Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31:1829–1832. doi: 10.1161/01.str.31.8.1829. [DOI] [PubMed] [Google Scholar]
- 102.Chemerinski E, Robinson RG, Arndt S, et al. The effect of remission of poststroke depression on activities of daily living in a double-blind randomized treatment study. J Nerv Ment Dis. 2001;189(7):421–425. doi: 10.1097/00005053-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 103.Grade C, Redford B, Chrostowski J, et al. Methylphenidate in early poststroke recovery: a double-blind, placebo-controlled study. Arch Phys Med Rehabil. 1998;79(9):1047–1050. doi: 10.1016/s0003-9993(98)90169-1. [DOI] [PubMed] [Google Scholar]
- 104.Fruehwald S, Gatterbauer E, Rehak P, et al. Early fluoxetine treatment of post-stroke depression—a three-month double-blind placebo-controlled study with an open-label long-term follow up. J Neurol. 2003;250(3):347–351. doi: 10.1007/s00415-003-1014-3. [DOI] [PubMed] [Google Scholar]
- 105.Murray V, von Arbin M, Bartfai A, et al. Double-blind comparison of sertraline and placebo in stroke patients with minor depression and less severe major depression. J Clin Psychiatry. 2005;66(6):708–716. doi: 10.4088/jcp.v66n0606. [DOI] [PubMed] [Google Scholar]
- 106.Choi-Kwon S, Han SW, Kwon SU, et al. Fluoxetine treatment in poststroke depression, emotional incontinence, and anger proneness: a double-blind, placebo-controlled study. Stroke. 2006;37(1):156–161. doi: 10.1161/01.STR.0000190892.93663.e2. [DOI] [PubMed] [Google Scholar]