Abstract
Investigation of the antitumor and immunomodulatory activities of interferon (IFN) began shortly after the cytokine was discovered in 1957. Early work showed a direct correlation between administration of IFN and inhibition of symptoms associated with virally induced leukemia in mice as well as an increase in their survival time. Subsequent studies with purified IFNs confirmed the direct and indirect stimulation of immune cells, resulting in antitumor activities of IFN. Clinically, IFN-alphas (αs) have been shown to have activity against a variety of tumors. Initially, the U.S. Food and Drug Administration licensed 2 recombinant IFN-αs for the treatment of hairy-cell leukemia and then later for several other cancers. The success rate seen with IFNs and certain tumors has been varied. Unfortunately, some neoplasms show no response to IFN. Monocytes/macrophages play an important role in cancer progression. Monocytes in combination with IFN may be an important therapy for several cancers. This article focuses on the role of IFN and monocytes alone or in combination in affecting malignancies.
Introduction
Interferon (IFN) was defined as an antiviral agent by Isaacs and Lindenmann (1957) and by Paucker and others (1962) for its antigrowth activity. Intron-A (IFN-α2b, recombinant; Schering-Plough) and Roferon-A (IFN-α2a, recombinant; Hoffmann-La Roche, Nutley, N.J.) were the first IFNs licensed by the U.S. Food and Drug Administration (USFDA) for the treatment of hairy-cell leukemia. This event was indeed a milestone and served to introduce biotechnology-derived products for the treatment of cancer. Later, the USFDA licensed Intron-A and Roferon-A for AIDS-related Kaposi's sarcoma (1988). Subsequently, Intron-A was licensed by the USFDA for malignant melanoma (1995) and follicular lymphoma (1997), and Roferon-A was licensed for chronic myelogenous leukemia (1995). IFN-γ1b (Actimmune) was licensed by the USFDA in 1991 for the treatment of chronic granulomatous disease and in 2000 for the treatment of malignant osteopetrosis. The primary functions associated with IFN-γ are related to host defense and immunomodulation (e.g., antiviral defense and MHC class I upregulation), but its antitumor effects have been widely examined. Studies show that IFN-γ plays an important role in tumor surveillance. In addition to antitumor effects associated with the immune system, IFN-γ affects tumors directly by virtue of its antiangiogenic and antiproliferative properties (Miller and others 2009). IFN-γ has been used for the treatment of various malignancies, including ovarian and colorectal cancers, as well as in combination with IFN-α in the treatment of chronic myelogenous leukemia, although with mixed results (Zaidi and Merlino 2011). Interestingly, IFN-γ has not been approved as a single agent by the USFDA for any malignancy.
Although Type I and II IFNs were used with varying success with certain neoplasms, exploration and possible enhancement of the components that contribute to these effects are, therefore, of great interest. This review will focus on some of the mechanisms of IFN signaling related to the antitumor and immunomodulatory activities associated with Type I and II IFNs that lead to cell death in vitro and in vivo. The role of immune cells, especially monocytes, and their interaction with IFN and their resultant antitumor activity will be discussed.
IFN-Induced Mechanism of Antitumor Activity
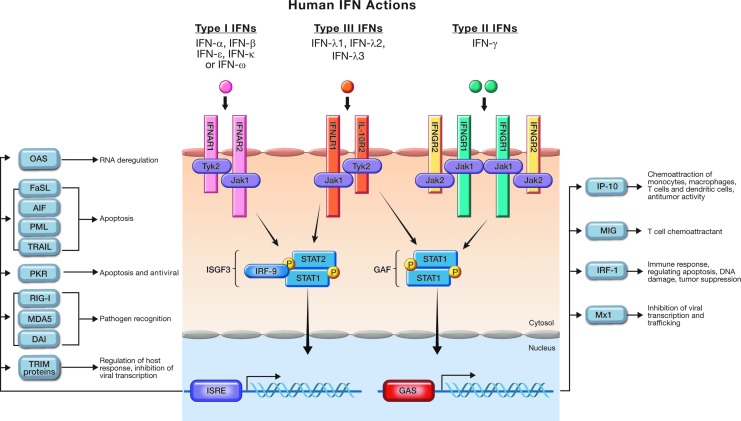
IFNs are classified as Type I (IFN-α, β, and ω), Type II (IFN-γ) or Type III (IFN-λ). Each signals through different receptors. The receptor of Type I IFNs, known as IFNAR, is made up of 2 components, IFNAR1 and IFNAR2. Engagement of Type I IFNs with the IFNAR receptor leads to activation of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, which results in formation of the ISGF3 (IFN-stimulated gene factor 3) complex, which is composed of STAT1, STAT2, and IRF-9. This complex then translocates to the nucleus, binds to the IFN-stimulated response element (ISRE), and results in the transcription of IFN-stimulated genes (ISGs), the products of which are responsible for antiviral, antiproliferative, and antitumor activities (Fig. 1). In its biologically active form, IFN-γ is a noncovalent homodimer that binds to the IFNGR1 component of the Type II IFN receptor complex (IFNGR1 and IFNGR2) in a 2:2 binding stoichiometry. Signaling then proceeds through a phosphorylated STAT1 homodimer that dissociates before entering the nucleus, where it binds DNA at the IFN-γ activated (GAS) sites, followed by the transcription of IFN-gamma-induced genes. IFN-γ elicits factors that biologically lead to antiviral and immunomodulatory activities (Fig. 1). As with Type I IFNs, binding of Type III IFN (IFN-λ) to its receptor results in activation of the JAKs, Jak1 and Tyk2, followed by activation of STAT1 and STAT2. It should be noted that in some cell lines, STATs 3, 4, and 5 may also be activated. The 3 isoforms of the IFN-λ family (IFN-λ1, 2, and 3) employ a heterodimeric receptor complex, one component of which (IFNLR1) is specific for IFN-λ, whereas the other, interleukin (IL)-10R2, is also used by IL-10, IL-22, and IL-26. After activation, the STATs are phosphorylated and form homo- and heterodimers, translocate to the nucleus, and initiate transcription. Like Type I IFNs, Type III IFNs phosphorylate STAT 1 and 2 and associate with IRF-9 to form the ISGF3 complex, which binds to the ISRE in the nucleus, which also results in transcription of a number of factors that lead to antiviral and antitumor activities (Fig. 1) (Schroder and others 2004; Uddin and Platanias 2004; Li and others 2009).
FIG. 1.
Signal transduction pathways of Type I, II, and III interferons.
It is known than IFN-α and -β can induce inhibition of cell growth and/or apoptosis in some tumor cell lines while not having the same effect in others (Hu and others 1999; Murata and others 2006). A number of apoptotic proteins have been identified, including tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), Fas ligand (FasL), apoptosis-inducing factor (AIF), Protein Kinase R (PKR), and promyelocytic leukemia protein (Chawla-Sarkar and others 2003) (Fig. 1). The contrast between apoptosis (actual cell death) and antiproliferation (cessation of cell growth) was addressed in a study (Scarzello and others 2007) in which it was shown that mutation of a conserved motif in the SH2 domain of STAT2 (which is also shared by STAT1 and 3) conferred an apoptotic, rather than just an antiproliferative, effect in response to Type I IFN treatment. Specifically, the tyrosine at position 631 in STAT2 was mutated to a phenylalanine (Y631F) after which IFN acted in a more apoptotic fashion, a mode of action apparently contributed to by the Y631F mutation resulting in sustained tyrosine phosphorylation of STAT1 and 2. TRAIL, also known as Apo-2L, is a death-inducing member of the tumor necrosis factor (TNF) superfamily, which has been shown to specifically lead to apoptosis (a form of programmed cell death) in certain tumor cells while having a minimal effect on normal cells. This can be explained by the fact that cancer cells generally express TRAIL's death receptors (TRAIL-R1 and TRAIL-R2), which can lead to apoptosis, whereas normal cells express its decoy receptors (TRAIL-R3, R4, and R5) allowing survival after death ligand binding (LeBlanc and Ashkenazi 2003). Due to these phenomena, there has been a great amount of consideration focused on the TNF receptors and ligands as possible cancer agents in the past few years (Newsom-Davis and others 2009; Wu 2009). It has been observed in IFN-α-induced ovarian adenocarcinoma (OVCAR3) cells that interferon regulatory factor-9 (IRF-9) siRNA inhibits transcription of TRAIL, an effect not seen with STAT1 siRNA (Tsuno and others 2009). This result suggests that IRF-9 is required for the IFN-α-induced transcription of TRAIL. In addition, TRAIL-R2 siRNA was seen to inhibit the antiproliferative activities of both IFN-α and TRAIL, which points to the likelihood that TRAIL-R2 is important for the mediation of the signals associated with the antiproliferative activities of TRAIL and IFN-α in OVCAR3 cells. Further evidence of IRF-9's role in eliciting antiproliferative activity is evident when IRF-9 is overexpressed in T98G (human glioblastoma multiforme) and human A549 (lung adenocarcinoma) cells, which are resistant to IFN-α. After overexpression, an IFN-α-induced antiproliferative response is observed. The above suggests that TRAIL may be one of the potential mediators for inhibiting cell viability after IFN-α treatment in which the induced apoptosis is facilitated by IRF-9 (Tsuno and others 2009). Upregulation of TRAIL has also been shown to mediate IFN-α-induced apoptosis in Daudi B-lymphoma and U266 myeloma cells (Yanase and others 2007). Interestingly, cells from another human Burkitt's lymphoma (RAMOS) and human myeloma (RPMI 8226) tested in the above study performed by Yanase were seen to undergo apoptosis with no apparent TRAIL involvement. Such findings question the essential nature of TRAIL upregulation in the induction of apoptosis and the variability of TRAIL induction in different cells.
In addition to TRAIL, another member of the death ligand family associated with apoptosis is the Fas ligand (FasL or C95L). Like TRAIL, it is a member of the TNF family and induces apoptosis by binding to its death receptor (FasR). In the work done to better understand the pathways used in which TRAIL and FasL induce apoptosis (Juo and others 1999; Knight and others 2001), it was observed that both Caspase-8 and Fas-associated protein with death domain (FADD) are necessary for FAS-mediated apoptosis in malignant glioma cells. When the 2 ligands were compared using OVCAR3 cells, it was shown that TRAIL is more effective than FasL in inhibiting cell viability in response to IFN-α2c as evidenced by IC50s for TRAIL of 0.9 ng/ml and of >100 ng/ml for FasL. In OVCAR3 cells, gene expression appears to be in agreement with cell viability inhibition in response to TRAIL and FasL. Expression of FasL was not significantly inhibited by siRNA targeted against IFNAR1, IFNAR2, or IRF-9, whereas the opposite was seen with TRAIL expression. Thus, in this study, TRAIL is a potential mediator for inhibiting cell viability after IFN-α treatment. Further, significant increases of TRAIL were observed in the supernatant and on the membrane of OVCAR3 cells, but not in the cytosol after IFN-α2c treatment (Tsuno and others 2012).
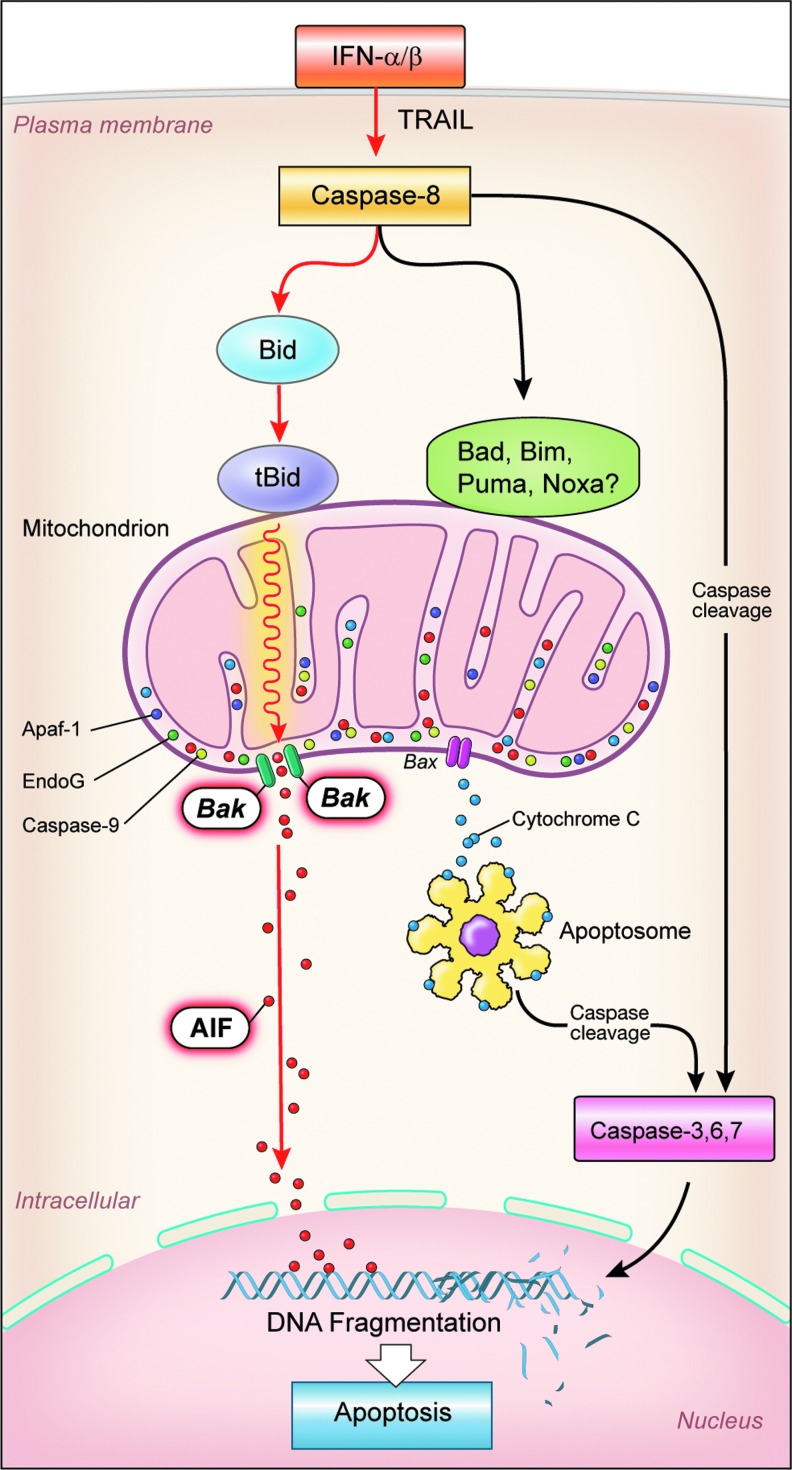
BH3-interacting domain death agonist (BID) has a major role in apoptosis due to its interaction with caspase-8 and its effect on the mitochondrial membrane potential (MMP), the collapse of which is a significant factor and can be considered the point of no return in many forms of programmed cell death (Galluzzi and others 2007) (Fig. 2). Permeabilization of the outer mitochondrial membrane leads to release of proteins such as cytochrome c and AIF, both of which are found in the space between the outer and inner membranes. Subsequent loss of the inner membrane potential (Δψm) hastens cell death due to consequent release of caspases directly associated with downstream apoptosis, factors related to the caspase-independent death of the cell, as well as loss of mitochondrial function (Green and Kroemer 2004). The relationship of BID to cell viability is shown by the fact that BID siRNA can avert the collapse of the MMP by both IFN-α and TRAIL in OVCAR3 cells, thus restoring cell viability, whereas BID overexpression resulted in a substantial loss of cell viability after IFN-α and TRAIL treatments of A549 (lung adenocarcinoma) cells (Miyake and others 2012; Tsuno and others 2012). The relationship among BID, TRAIL, and the protein kinase, Akt, which is known to play a role in several cell processes, including apoptosis and cell proliferation, is shown by a reduction in TRAIL-induced apoptosis in ovarian cancer cells due to BID expression inhibition by Akt. This resistance to TRAIL was studied in epithelial ovarian cancer cells that are known to show increased activity of the PI3K/Akt pathway and are often TRAIL resistant. Akt is thought to decrease TRAIL-induced apoptosis by leading to a reduction in the levels of BID protein (Goncharenko-Khaider and others 2010). The importance of the role of Akt in cancer treatment is evidenced by the existence of ongoing clinical trials using Akt inhibitors for treatment of melanoma and multiple myeloma.
FIG. 2.
Type I interferon-induced apoptosis in the pathway to the left signifying the proposed apoptotic pathway in OVCAR3 cells (Caspase 8→ BID→ tBID).
In the process of apoptosis, a large protein structure known as the apoptosome is sometimes formed, and this is triggered by the release of cytochrome c from the mitochondria (Fig. 2). It has been shown that apoptosome formation is not required for IFN-α2a-induced BID-mediated cell death. A gene-silencing approach was used to examine the potential role of AIF downstream of BID-mediated mitochondrial depolarization through Bak oligomerization. AIF siRNA significantly attenuated IFN-α2a-induced cell death in OVCAR3 cells, while cytochrome c-siRNA and EndoG-siRNA did not, thus suggesting that AIF, but not cytochrome c and EndoG, is responsible for IFN-α2a-induced BID-mediated caspase-dependent execution of cell death. BID inhibition prevents the release of AIF from the mitochondria by IFN-α2a (Miyake and others 2012) (Fig. 2).
Monocytes and Cancer
Monocytes originate in the bone marrow and play multiple roles in immune functions, including replenishment of resident macrophages and dendritic cells under normal states. In response to inflammation, monocytes can move quickly (∼8–12 h) to the sites of infection and divide/differentiate into macrophages and dendritic cells to elicit an immune response and remove necrotic debris or pathogens. Monocytes and macrophages are also critical effectors and regulators of inflammation and the innate immune response by producing inflammatory cytokines and removing cells and toxic molecules (Gordon and Taylor 2005). Macrophages are one of the major populations of tumor-infiltrating immune cells. In most solid tumors, however, the existence of macrophages is advantageous for tumor growth and metastasis.
Macrophages can be phenotypically polarized by the microenvironment to mount specific M1 (classical) or M2 (regulatory) programs. In the presence of IFN-γ, lipopolysaccharide (LPS), or TNF-α and other microbial products, monocytes differentiate into classical M1 macrophages. Alternatively, in the presence of colony-stimulating factor, IL-4, IL-13, IL-10, and immunocomplexes in association with either IL-1 or TLR-ligands, monocytes differentiate into M2 macrophages. M1 macrophages have high microbicidal activity, immunostimulatory functions, and tumor cytotoxicity, whereas M2 cells have high scavenging ability, promote tissue repair and angiogenesis, and favor tumor progression. The plasticity function of macrophages plays a crucial role in both the onset and progression of malignant tumors. Tumorigenesis appears to involve a proinflammatory phenotype that through the chronic release of inflammatory mediators drives damaged epithelial cells toward neoplastic transformation. Once the tumor is established, M2 macrophages assume an immunosuppressive phenotype and carry out a number of potent tumor-promoting functions (Biswas and others 2008). Clinical and experimental studies have reported that tumors with large infiltration of M2 tumor-associated macrophages (TAMs) show tumor progression.
Macrophages express inducible nitric oxide synthase (iNOS) enzyme, which is induced by cytokines such as IFN-γ and microbial products, and LPS, and typically generate high levels of nitric oxide (NO) over a prolonged period of time. iNOS-derived NO exerts numerous effector and immunoregulatory functions under physiological and pathological conditions, such as the control or killing of infectious pathogens, the inhibition or promotion of tumor growth, the protection or damage of tissue in autoimmune disease, and the modulation of cytokine production and T cell development. High iNOS-producing M1 macrophages with tumoricidal activity have been described in numerous tumors. iNOS tumoricidal cytotoxicity is attributed to the conversion of arginine and oxygen to NO. M1 macrophages retain high levels of the substrate arginine by producing less arginase, hence preventing arginine degradation to account for greater amounts of substrate for the conversion to NO. In contrast, M2 macrophages are characterized by decreased levels of iNOS and higher arginase-1 expression (O'Brien and others 2010). Arginase induction in M2 macrophages can enhance tumor cell growth by reducing NO production. Thus, it appears that l-arginine metabolism through the arginase and iNOS pathways in macrophages can have very different influences on the growth of nearby tumor cells, depending on which pathway is prevailing (Chang and others 2001).
Monocytes and macrophages have powerful antineoplastic properties even in the absence of specific immunity (Gaurnier-Hausser and others 2008). They are able to distinguish malignancy and attack neoplastic cells through a contact-dependent mechanism in which the precise molecular events leading to target cell death still remain elusive. It has been reported that IL-12 and NO take part in the antitumor activity of macrophages in a melanoma model (Kozlowska and others 2006). The relationship between vascular factors and tumoricidal activity of monocytes has also been reported. TNF-α caused tumor-infiltrating macrophage-derived iNOS-mediated tumor NO rise and sustained permanent tumor blood flow shutdown, resulting in tumor ulceration (Menon and others 2008). Recent evidence has shown that large numbers of M2 TAMs are attracted to and are retained in the avascular and necrotic areas, where they are exposed to tumor hypoxia. Since hypoxia is a hallmark feature of malignant tumors and hypoxic tumor cells are relatively resistant to radio- and chemotherapy, these areas have become a target for novel forms of anticancer therapy. The responses of macrophages to tumor hypoxia can be exploited to deliver potent antitumor agents to these poorly vascularized and otherwise largely inaccessible areas of tumors (Murdoch and Lewis 2005).
Macrophages when activated through different activators demonstrate a potent antitumor activity. αCD40 plus CpG treatment of macrophages induces tumor B-cell apoptosis in vitro and markedly retards tumor growth in immunodeficient mice after transplantation of tumor B-cells, in a primary tumor model that accurately simulates human chronic lymphocytic leukemia (Wu and others 2009). BCG-activated macrophages have also been shown to have surprisingly high tumoricidal activities through the cell–cell contact. In this process, membrane proteins play an important role for macrophages to express cytotoxicity against sarcoma cells (Zhao and others 2012).
Immunomodulatory and Antitumor Activity of IFN and Immune Cells
Monocytes/macrophages have been shown to play a crucial role in cancer progression and disease prognosis in the human and murine cancer models, and the role of IFN in the enhancement of monocyte-mediated tumoricidal activity is well known. Twenty-one components purified from lymphoblastoid IFN-α were analyzed for their role in monocyte-mediated lysis of the human melanoma cell line, A375 (Zoon and others 1986; Webb and others 1989). Generally, tumoricidal activity was seen to be in direct correlation with antiviral activity, and many of the components were as or more tumoricidal than IFN-γ. Interestingly, immunomodulatory assays done on these IFN-α components (e.g., NK activity, IL-1 induction, and Class I antigen induction) showed that each had its own immunomodulatory fingerprint with no one being exactly like any other (Zoon and others 1986). Work done to examine the effects of IFN-α and IFN-γ on IL-1 secretion by monocytes (Gerrard and others 1987) showed that IFN-α directly induced IL-1 secretion by monocytes, whereas IFN-γ did not; however, IFN-γ, but not IFN-α, significantly enhanced IL-1 secretion when monocytes were stimulated by agents such as LPS or Staphylococcus aureus. It should be noted that this enhancement effect was not observed with other agents tested such as phorbol myristate acetate, which has been shown to induce secretion of IL-1 from human monocytes (Jessop and Hoffman 1993). Tumoricidal activity of IFN-α and IFN-γ was studied using the human melanoma cell line, A375, and the human colon cancer line, HT-29 (Webb and Gerrard 1990). It was shown that both IFNs enhanced monocyte-mediated cytotoxity by acting on both the tumor cells and the monocytes. Although both IFNs enhanced monocyte-mediated cell lysis, IFN-α could induce more such toxicity on the A375 cells, whereas IFN-γ induced more on the HT-29 cells. In pretreatment of the 2 tumor lines with these IFNs, both IFN-α and γ were shown to enhance the lysis of A375 cells, but only IFN-γ pretreatment could enhance cell lysis of HT-29 cells by untreated monocytes. Addition of IFN-γ and either A375 or HT-29 cells to monocytes resulted in TNF release, whereas addition of IFN-α did not. Even though the presence of TNF was not seen to play a major role in the killing of either tumor cell line, both IFN-α- and IFN-γ-induced cytotoxicity of HT-29 cells was suppressed when inhibitors of the hydrogen peroxide–myeloperoxidase (MPO) system were used. This MPO system has been known for some time to be a possible mediator of the killing of tumor cells by human neutrophils (Clark and Szot 1981). The above-mentioned work with IFN, monocytes, and tumor cells led to further in vitro and in vivo studies to examine the possible role of IFN-activated monocytes in the cytotoxicity of human tumors.
It has been previously reported that a low concentration of IFN-activated monocytes (105 cells/well) exerts a near-eradicative cytocidal activity against low concentration of several human tumor cells (103–4 cells/well). In vitro culture of HOS osteosarcoma, LOX melanoma, and A529 lung tumor with IFN-activated human monocytic cells inhibited cell growth, which reached an eradicative level (95%–100%). On the other hand, diploid cells (WI-38 and MRC-5) did not show growth inhibition (Baron and others 2007). To model larger in vivo tumors, the target tumor cell concentration was increased to 10-fold to 100-fold (105 cells/well). This larger tumor required an increase of cytocidal concentrations of IFN-α2a by over 100-fold (10 ng/mL) and monocytes by 10-fold (106 cells/well) to cause near eradication of OVCAR3 (ovarian), HOS osteosarcoma, A549 lung, and LOX melanoma tumor cells. In some cases, high concentrations of monocytes could kill high concentrations of tumor cells in the absence of IFN. Out of 2 diploid cell lines that were insensitive with the low concentration of monocytes, one became sensitive to the cytocidal activity of monocytes, but activation by IFN was required. These results suggest that many unidentified variables are involved in killing of tumor cells by monocytes (Baron and others 2011).
IFN-activated monocytes inhibit growth of established tumors and prolong survival of host
Encouraged by in vitro studies, it was important to determine whether a combination of IFNs and human monocytes can mediate antitumor effects against established human tumors in animal models. Subcutaneous human ovarian and melanoma tumors in athymic nude mice were established. Intratumoral injection of human monocytes and IFN-α2 and IFN-γ into established tumor models reduced tumor burden. Mice receiving combination treatment 15 days after ovarian tumor implantation resulted in significant inhibition of tumor growth and complete regression in 40% of the mice. Tumor volumes in these mice were significantly smaller than those of mice treated with IFNs alone, monocytes alone, or untreated controls. A significant tumor inhibition was also seen in treatment of much larger ovarian tumors (day 30 post-tumor implantation). Similar intratumoral treatment of LOX melanoma tumors by monocytes and both IFNs could inhibit tumor growth compared to PBS-treated control tumors. Treatment of mice with IFNs alone or monocytes alone showed little or no impact on tumor growth. Monocytes when combined with either IFN-α2a or IFN-γ mediated little or no inhibition of melanoma tumors. The presence of both Type I and II IFNs and monocytes was required to significantly enhance their antitumor efficacy (Nakashima and others 2012).
The combination treatment of monocytes and IFNs significantly prolonged the overall survival in both early and advanced tumor models. Prolonged survival time in the mice with the combination therapy group in the early tumor model was more than double that of the untreated control group. Mice treated with either IFN or monocytes did not significantly increase survival. Combination of both IFNs and monocytes was more effective in increasing the overall survival of tumor-bearing mice (Nakashima and others 2012).
Mechanism of antitumor activity of IFN-activated macrophages
Immunohistochemistry studies revealed that a large number of activated macrophages infiltrated into ovarian tumors treated with combination therapy compared to the controls. These monocytes displayed both the CD31+ and CD68+ phenotypes indicating their activated status. They were classically activated macrophages (M1) by exhibiting a high IL-12/low IL-10 profile and also staining positive for the M1 biomarkers such as CXCL-10 (IP-10) and NOS2. Immunosuppressive (regulatory) M2 macrophages, characterized by high IL-10 and low IL-12 phenotypes, were not detected in the treated tumor bed. It is interesting to note that the induced M1 macrophages survive in the surrounding tumors for a long time (at least 10 days post-treatment) (Nakashima and others 2012).
Much higher accumulation of apoptotic cells in tumors indicated that macrophages activated by IFNs induce apoptosis. Because in vitro results showed that IFN-activated monocytes directly kill the tumor cells by cell-to-cell contact, it can be assumed that macrophages are perhaps in part involved in direct killing of tumor cells. Tumor cell killing may be also be caused by NO-induced necrosis and release of cytokines such as TNF and chemokines by macrophages (Mosser and Edwards 2008; Nakashima and others 2012).
The activated monocytes did not cause histological changes in vital organs, including the liver, kidney, lung, and spleen of treated mice, which is consistent with the clinical trials where low toxicity was observed in patients treated with autologous monocytes (Faradji and others 1991a).
Clinical studies with activated monocytes
Several clinical trials have been conducted defining the scope of specific immunotherapy strategies to overcome tumor-induced immunosuppression and elicit a monocyte-/macrophage-mediated antitumor response. In phase 1 studies, monocyte-derived IFN-γ-activated killer (MAK) cells were safely administered to cancer patients with variable clinical diagnoses and minimal residual disease. Tolerance and preliminary antitumoral activity of intrapleural infusion of MAK cells were limited (Monnet and others 2002). Intraperitoneal (I.P.) administration of a large number of MAK cells (up to 2 billion cells per infusion) in patients with metastatic ovarian carcinoma could be achieved without significant toxicity (de Gramont and others 2002). In another phase 1 study, the transurethral instillation of MAK cells into the bladder of patients with superficial papillary bladder cancer was well tolerated. A significant decrease in the number of relapses was observed when compared with the events before treatment (Thiounn and others 2002). Adoptive immunotherapy of autologous macrophages generated in vitro from blood monocytes after stimulating with IFN-γ was well tolerated with side effects of low-grade fever in patients with various tumors of advanced stage. An indication of a possible therapeutic effect was observed in I.P.-treated patients, which consisted of disappearance of malignant ascites in some patients (Andreesen and others 1990). Gamma-imaging of labeled autologous macrophages showed an immediate, but transient, lung uptake and a progressive uptake of radioactivity in the liver and spleen postinfusion (Faradji and others 1991b). This phase 1 study showed that preparation of high numbers of autologous MAK cells is feasible, and that I.V or I.P infusion is safe for patients. It appears that to harness the therapeutic potential of macrophages, a large number of these cells need to be produced. Baron-Bodo and others (2005) developed a process for ex vivo production of large numbers of IFN-γ-activated monocyte-derived macrophages and characterized them by studying their phenotype and functions. Their safe administration was demonstrated in phase I/II clinical studies. MAK cells were shown to exert antitumor activity by killing tumor cells and inhibiting their proliferation. These activities were enhanced by activation with IFN-γ and addition of antitumor antibodies. TNF-α was one of the mediators used by MAK cells to inhibit tumor proliferation. To facilitate logistics of clinical trials, a process for MAK cell cryopreservation has been developed. Finally, to evaluate the efficacy of MAK cells, phase III clinical studies are necessary.
Conclusions
Studies demonstrated that both Type I and Type II IFNs with monocytes are needed for optimal antitumor effects. Further studies of their effect in advanced-stage human tumor models in mice are needed. Since systemic administration of a high number of macrophages has been accomplished safely in subjects with various cancers, it will be of interest to test the safety of both Type I and Type II IFN-activated monocytes in additional preclinical tumor models and in clinical trials. Further, since cell-to-cell contact is critical for macrophage-induced tumor cell death, local intratumoral, I.P., or intravenous administration of activated macrophages in advanced metastatic ovarian cancer may be an appropriate strategy.
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- Andreesen R. Scheibenbogen C. Brugger W. Krause S. Meerpohl HG. Leser HG. Engler H. Lohr GW. Adoptive transfer of tumor cytotoxic macrophages generated in vitro from circulating blood monocytes: a new approach to cancer immunotherapy. Cancer Res. 1990;50(23):7450–7456. [PubMed] [Google Scholar]
- Baron-Bodo V. Doceur P. Lefebvre ML. Labroquere K. Defaye C. Cambouris C. Prigent D. Salcedo M. Boyer A. Nardin A. Anti-tumor properties of human-activated macrophages produced in large scale for clinical application. Immunobiology. 2005;210(2–4):267–277. doi: 10.1016/j.imbio.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Baron S. Finbloom J. Horowitz J. Bekisz J. Morrow A. Zhao T. Fey S. Schmeisser H. Balinsky C. Miyake K. Clark C. Zoon K. Near eradication of clinically relevant concentrations of human tumor cells by interferon-activated monocytes in vitro. J Interferon Cytokine Res. 2011;31(7):569–573. doi: 10.1089/jir.2010.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron S. Hernandez J. Bekisz J. Poast J. Goldman N. Clouse K. Fields K. Bacot S. Wang J. Zoon K. Clinical model: interferons activate human monocytes to an eradicative tumor cell level in vitro. J Interferon Cytokine Res. 2007;27(2):157–163. doi: 10.1089/jir.2006.0083. [DOI] [PubMed] [Google Scholar]
- Biswas SK. Sica A. Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008;180(4):2011–2017. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- Chang CI. Liao JC. Kuo L. Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity. Cancer Res. 2001;61(3):1100–1106. [PubMed] [Google Scholar]
- Chawla-Sarkar M. Lindner DJ. Liu YF. Williams BR. Sen GC. Silverman RH. Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8(3):237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- Clark RA. Szot S. The myeloperoxidase-hydrogen peroxide-halide system as effector of neutrophil-mediated tumor cell cytotoxicity. J Immunol. 1981;126(4):1295–1301. [PubMed] [Google Scholar]
- de Gramont A. Gangji D. Louvet C. Garcia ML. Tardy D. Romet-Lemonne JL. Adoptive immunotherapy of ovarian carcinoma. Gynecol Oncol. 2002;86(1):102–103. doi: 10.1006/gyno.2002.6667. [DOI] [PubMed] [Google Scholar]
- Faradji A. Bohbot A. Frost H. Schmitt-Goguel M. Siffert JC. Dufour P. Eber M. Lallot C. Wiesel ML. Bergerat JP, et al. Phase I study of liposomal MTP-PE-activated autologous monocytes administered intraperitoneally to patients with peritoneal carcinomatosis. J Clin Oncol. 1991a;9(7):1251–1260. doi: 10.1200/JCO.1991.9.7.1251. [DOI] [PubMed] [Google Scholar]
- Faradji A. Bohbot A. Schmitt-Goguel M. Roeslin N. Dumont S. Wiesel ML. Lallot C. Eber M. Bartholeyns J. Poindron P, et al. Phase I trial of intravenous infusion of ex-vivo-activated autologous blood-derived macrophages in patients with non-small-cell lung cancer: toxicity and immunomodulatory effects. Cancer Immunol Immunother. 1991b;33(5):319–326. doi: 10.1007/BF01756597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L. Zamzami N. de La Motte Rouge T. Lemaire C. Brenner C. Kroemer G. Methods for the assessment of mitochondrial membrane permeabilization in apoptosis. Apoptosis. 2007;12(5):803–813. doi: 10.1007/s10495-007-0720-1. [DOI] [PubMed] [Google Scholar]
- Gaurnier-Hausser A. Rothman VL. Dimitrov S. Tuszynski GP. The novel angiogenic inhibitor, angiocidin, induces differentiation of monocytes to macrophages. Cancer Res. 2008;68(14):5905–5914. doi: 10.1158/0008-5472.CAN-07-6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard TL. Siegel JP. Dyer DR. Zoon KC. Differential effects of interferon-alpha and interferon-gamma on interleukin 1 secretion by monocytes. J Immunol. 1987;138(8):2535–2540. [PubMed] [Google Scholar]
- Goncharenko-Khaider N. Lane D. Matte I. Rancourt C. Piche A. The inhibition of Bid expression by Akt leads to resistance to TRAIL-induced apoptosis in ovarian cancer cells. Oncogene. 2010;29(40):5523–5536. doi: 10.1038/onc.2010.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Green DR. Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305(5684):626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Hu R. Bekisz J. Hayes M. Audet S. Beeler J. Petricoin E. Zoon K. Divergence of binding, signaling, and biological responses to recombinant human hybrid IFN. J Immunol. 1999;163(2):854–860. [PubMed] [Google Scholar]
- Isaacs A. Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147(927):258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- Jessop JJ. Hoffman T. Production and release of IL-1 beta by human peripheral blood monocytes in response to diverse stimuli: possible role of “microdamage” to account for unregulated release. Lymphokine Cytokine Res. 1993;12(1):51–58. [PubMed] [Google Scholar]
- Juo P. Woo MS. Kuo CJ. Signorelli P. Biemann HP. Hannun YA. Blenis J. FADD is required for multiple signaling events downstream of the receptor Fas. Cell Growth Differ. 1999;10(12):797–804. [PubMed] [Google Scholar]
- Knight MJ. Riffkin CD. Muscat AM. Ashley DM. Hawkins CJ. Analysis of FasL and TRAIL induced apoptosis pathways in glioma cells. Oncogene. 2001;20(41):5789–5798. doi: 10.1038/sj.onc.1204810. [DOI] [PubMed] [Google Scholar]
- Kozlowska K. Cichorek M. Wachulska M. Bautembach I. Role of interleukins and nitric oxide secretion by peritoneal macrophages in differential tumoricidal effect to transplantable melanomas as regarding their biological properties. Immunopharmacol Immunotoxicol. 2006;28(2):305–317. doi: 10.1080/08923970600809413. [DOI] [PubMed] [Google Scholar]
- LeBlanc HN. Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10(1):66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- Li M. Liu X. Zhou Y. Su SB. Interferon-lambdas: the modulators of antivirus, antitumor, and immune responses. J Leukoc Biol. 2009;86(1):23–32. doi: 10.1189/jlb.1208761. [DOI] [PubMed] [Google Scholar]
- Menon C. Bauer TW. Kelley ST. Raz DJ. Bleier JI. Patel K. Steele K. Prabakaran I. Shifrin A. Buerk DG. Sehgal CM. Fraker DL. Tumoricidal activity of high-dose tumor necrosis factor-alpha is mediated by macrophage-derived nitric oxide burst and permanent blood flow shutdown. Int J Cancer. 2008;123(2):464–475. doi: 10.1002/ijc.23499. [DOI] [PubMed] [Google Scholar]
- Miller CH. Maher SG. Young HA. Clinical Use of Interferon-gamma. Ann N Y Acad Sci. 2009;1182:69–79. doi: 10.1111/j.1749-6632.2009.05069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K. Bekisz J. Zhao T. Clark CR. Zoon KC. Apoptosis-inducing factor (AIF) is targeted in IFN-alpha2a-induced Bid-mediated apoptosis through Bak activation in ovarian cancer cells. Biochim Biophys Acta. 2012;1823(8):1378–1388. doi: 10.1016/j.bbamcr.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet I. Breau JL. Moro D. Lena H. Eymard JC. Menard O. Vuillez JP. Chokri M. Romet-Lemonne JL. Lopez M. Intrapleural infusion of activated macrophages and gamma-interferon in malignant pleural mesothelioma: a phase II study. Chest. 2002;121(6):1921–1927. doi: 10.1378/chest.121.6.1921. [DOI] [PubMed] [Google Scholar]
- Mosser DM. Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M. Nabeshima S. Kikuchi K. Yamaji K. Furusyo N. Hayashi J. A comparison of the antitumor effects of interferon-alpha and beta on human hepatocellular carcinoma cell lines. Cytokine. 2006;33(3):121–128. doi: 10.1016/j.cyto.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Murdoch C. Lewis CE. Macrophage migration and gene expression in response to tumor hypoxia. Int J Cancer. 2005;117(5):701–708. doi: 10.1002/ijc.21422. [DOI] [PubMed] [Google Scholar]
- Nakashima H. Miyake K. Clark CR. Bekisz J. Finbloom J. Husain SR. Baron S. Puri RK. Zoon KC. Potent antitumor effects of combination therapy with IFNs and monocytes in mouse models of established human ovarian and melanoma tumors. Cancer Immunol Immunother. 2012;61(7):1081–1092. doi: 10.1007/s00262-011-1152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsom-Davis T. Prieske S. Walczak H. Is TRAIL the holy grail of cancer therapy? Apoptosis. 2009;14(4):607–623. doi: 10.1007/s10495-009-0321-2. [DOI] [PubMed] [Google Scholar]
- O'Brien J. Lyons T. Monks J. Lucia MS. Wilson RS. Hines L. Man YG. Borges V. Schedin P. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol. 2010;176(3):1241–1255. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucker K. Cantell K. Henle W. Quantitative studies on viral interference in suspended L cells. III. Effect of interfering viruses and interferon on the growth rate of cells. Virology. 1962;17:324–334. doi: 10.1016/0042-6822(62)90123-x. [DOI] [PubMed] [Google Scholar]
- Scarzello AJ. Romero-Weaver AL. Maher SG. Veenstra TD. Zhou M. Qin A. Donnelly RP. Sheikh F. Gamero AM. A Mutation in the SH2 domain of STAT2 prolongs tyrosine phosphorylation of STAT1 and promotes type I IFN-induced apoptosis. Mol Biol Cell. 2007;18(7):2455–2462. doi: 10.1091/mbc.E06-09-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K. Hertzog PJ. Ravasi T. Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Thiounn N. Pages F. Mejean A. Descotes JL. Fridman WH. Romet-Lemonne JL. Adoptive immunotherapy for superficial bladder cancer with autologous macrophage activated killer cells. J Urol. 2002;168(6):2373–2376. doi: 10.1016/S0022-5347(05)64148-1. [DOI] [PubMed] [Google Scholar]
- Tsuno T. Mejido J. Zhao T. Phillips T. Myers TG. Bekisz J. Zoon KC. BID is a critical factor controlling cell viability regulated by IFN-alpha. J Immunother. 2012;35(1):23–31. doi: 10.1097/CJI.0b013e3182372dcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuno T. Mejido J. Zhao T. Schmeisser H. Morrow A. Zoon KC. IRF9 is a key factor for eliciting the antiproliferative activity of IFN-alpha. J Immunother. 2009;32(8):803–816. doi: 10.1097/CJI.0b013e3181ad4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin S. Platanias LC. Mechanisms of type-I interferon signal transduction. J Biochem Mol Biol. 2004;37(6):635–641. doi: 10.5483/bmbrep.2004.37.6.635. [DOI] [PubMed] [Google Scholar]
- Webb DS. Gerrard TL. IFN-alpha and IFN-gamma can affect both monocytes and tumor cells to modulate monocyte-mediated cytotoxicity. J Immunol. 1990;144(9):3643–3648. [PubMed] [Google Scholar]
- Webb DS. Zur Nedden D. Miller DM. Zoon KC. Gerrard TL. Enhancement of monocyte-mediated tumoricidal activity by multiple interferon-alpha species. Cell Immunol. 1989;124(1):158–167. doi: 10.1016/0008-8749(89)90119-6. [DOI] [PubMed] [Google Scholar]
- Wu GS. TRAIL as a target in anti-cancer therapy. Cancer Lett. 2009;285(1):1–5. doi: 10.1016/j.canlet.2009.02.029. [DOI] [PubMed] [Google Scholar]
- Wu QL. Buhtoiarov IN. Sondel PM. Rakhmilevich AL. Ranheim EA. Tumoricidal effects of activated macrophages in a mouse model of chronic lymphocytic leukemia. J Immunol. 2009;182(11):6771–6778. doi: 10.4049/jimmunol.0801847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanase N. Kanetaka Y. Mizuguchi J. Interferon-alpha-induced apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-dependent and -independent manner. Oncol Rep. 2007;18(4):1031–1038. [PubMed] [Google Scholar]
- Zaidi MR. Merlino G. The two faces of interferon-gamma in cancer. Clin Cancer Res. 2011;17(19):6118–6124. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. Liu Q. Du B. Li P. Cui Q. Han X. Yan D. Zhu X. A novel accessory molecule Trim59 involved in cytotoxicity of BCG-activated macrophages. Mol Cells. 2012;34(3):263–270. doi: 10.1007/s10059-012-0089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoon KC. ZurNedden DL. Enterline JC. Manischewitz JF. Dyer DR. Boykins RA. Bekisz J. Gerrard TL. Chemical and biological characterization of natural human lymphoblastoid interferon alphas. In: Cantell K, editor; Schellekens H, editor. The Biology of the Interferon System. Dordecht: Martinus Nijhoff Pub.; 1986. pp. 567–569. [Google Scholar]