To the Editor: Q fever is a zoonosis caused by the bacterium Coxiella burnetii. The Q fever outbreak in the Netherlands affected ≈4,000 humans during 2007–2010 (1). In this outbreak, 1 genotype of C. burnetii appeared to be responsible for abortions in small ruminants and for clinical disease in humans (2,3). However, little is known about the outbreak genotype and the prevalence of C. burnetii in possible additional reservoirs for human Q fever (i.e., cats, dogs, horses, sheep, and cattle) in the Netherlands.
We aimed to search for possible additional reservoirs for human Q fever in the Netherlands. Placentas from 15 cats, 54 dogs, and 31 horses were collected in 2011 at 5 veterinary practices. Placentas were collected by targeted sampling at breeding facilities and during parturition with veterinary assistance. In addition, 27 ovine, 11 caprine, 16 porcine, 8 equine, and 139 bovine placentas (originating from aborting animals from throughout the Netherlands that were submitted in 2011 to investigate the abortion cause) were included in the study. Samples were stored at −20°C before testing.
DNA was extracted from the allantochorion of the placenta and analyzed as described (2). Samples with sufficient DNA load (cycle threshold [Ct] value <32) were typed by using 2 multilocus variable-number tandem-repeat analyses (MLVA) genotyping methods (MLVA-12 and MLVA-6), and the multispacer sequence typing method (3–5). Two C. burnetii strains from the Netherlands representing the outbreak genotype (X09003262, 3345937) and the Nine Mile RSA 493 were included as reference. For prevalence calculations, the Netherlands was divided in a southern part, comprising the Q fever hot spot area of notified cases in humans and small ruminants during the 2007–2010 epidemic (1,6), and a northern part, comprising the rest of the country.
C. burnetii DNA was not detected in placentas from cats, goats, or pigs. C. burnetii DNA was detected in 4 (7% [95% CI 0.4–14.4]) of 54 canine placentas; 3 from the north and 1 from the south of the Netherlands. C. burnetii DNA was detected in 3 (8% [95% CI 0.0–16.1]) of 39 equine placentas, all from the north of the country, without known abortion history. C. burnetii DNA was detected in 7 (26% [95% CI 9.4–42.5]) of 27 ovine and in 33 (24% [95% CI 16.7–30.8]) of 139 bovine placentas. The prevalence of C. burnetii DNA–positive ovine and bovine placentas from the north and the south did not differ significantly.
The C. burnetii DNA load in the placentas from dogs (Ct value 37.4–38.0) and horses (Ct value 35.4–37.4) was too low to be suitable for genotyping. Typing of 1 positive sheep sample resulted in an incomplete genotype, which is related to the outbreak genotype (sheep 192, Figure). Seven of the 33 C. burnetii DNA–positive bovine placentas were suitable for typing. One sample had a genotype similar to the outbreak genotype (2,3). Six other samples revealed a (partial) genotype related to bovine genotypes from the Netherlands (2,5,7), including a novel one. MLVA-6 and multispacer sequence typing results were consistent with the MLVA-12 results (Figure).
Figure.
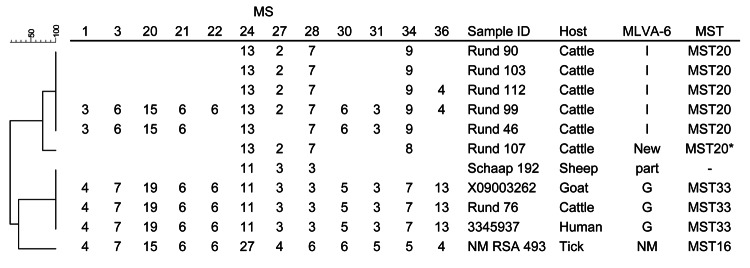
Phylogenetic tree of the genotypes of Coxiella burnetii from the samples of this study based on multilocus variable-number tandem-repeat analyses (MLVA) including 12 loci (MLVA-12). Repeats per locus are shown, and open spots indicate missing values. MLVA-6 are results of the analysis with 6 MLVA loci (3). MST are results of the analysis with multispacer sequence typing (MST) (5). MLVA 6 loca (MLVA-6) and MST revealed full genotypes unless stated otherwise. Two strains representing the outbreak genotype of C. burnetii (X09003262, 3345937) in the Netherlands and the Nine Mile (NM) RSA 493 are included as reference. MS, mini satellite; G and I, MLVA-6 genotypes of C. burnetii as published (3,7); MSTxx, MST genotypes as published (5). *Based on partial genotype; part, partial genotype. – (in MST column) indicates no results obtained. Scale bar indicates percentage similarity.
Results give no indication for major reservoirs of C. burnetii in cats, goats, and pigs in the Netherlands in 2011. However, the low numbers of placentas may have biased the results. Dogs and horses should be considered as reservoirs for C. burnetii. The detection of C. burnetii DNA–positive placentas in dogs and horses in the northern part of the country indicates the presence of a true reservoir rather than a spillover effect from the contaminated environment in the south. This observation is consistent with a reported seroprevalence of 13% in dogs in the Netherlands in 1992 (1). Until now, horses had been discussed as a risk factor in the Q fever outbreak in the Netherlands (8).
Prevalence data from sheep and cattle suggest that C. burnetii is present in placentas in 25% of the abortion cases in these species. Presence of the outbreak genotype of C. burnetii in sheep has been observed (2,5), indicating sheep are a reservoir for Q fever in humans. Genotyping data show a distinct genotype in 6 of the 7 cattle samples in accordance with previous work (2,5,7). However, the outbreak genotype was detected in 1 sample from a cow. Whether this is an incidental finding or the first observation of the outbreak genotype being transferred to the cattle population is not clear. If the latter, exposure to cattle also possibly might become a risk factor for human Q fever, in addition to goats and sheep.
Acknowledgments
We thank D. Frangoulidis for providing the Nine Mile RSA 493 and the MedVetNet WP 25 for support in setting up the MLVA typing method. We thank Arie Hoogendoorn, Betty van Gelderen, Robin Ruuls, and Jeanet van der Goot for their technical assistance. We also thank veterinarians from veterinary practices in the south of the Netherlands for the submission of placentas.
This study received financial support from the Dutch Ministry of Economic Affairs, Agriculture and Innovation (project number 1640038500) and from DG Sanco of the European Commission (Directive 2011/89/EU).
Footnotes
Suggested citation for this article: Roest HIJ, van Solt CB, Tilburg JJHC, Klaassen CHW, Hovius EK, Roest TF, et al. Search for possible additional reservoirs for human Q fever, the Netherlands [letter]. Emerg Infect Dis [Internet]. 2013 May [date cited]. http://dx.doi.org/10.3201/eid1905.121489
References
- 1.Roest HI, Tilburg JJ, van der Hoek W, Vellema P, van Zijderveld FG, Klaassen CH, et al. The Q fever epidemic in the Netherlands: history, onset, response and reflection. Epidemiol Infect. 2011;139:1–12 . 10.1017/S0950268810002268 [DOI] [PubMed] [Google Scholar]
- 2.Roest HI, Ruuls RC, Tilburg JJ, Nabuurs-Franssen MH, Klaassen CH, Vellema P, et al. Molecular epidemiology of Coxiella burnetii from ruminants in Q fever outbreak, the Netherlands. Emerg Infect Dis. 2011;17:668–75 . 10.3201/eid1704.101562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilburg JJ, Rossen JW, van Hannen EJ, Melchers WJ, Hermans MH, van de Bovenkamp J, et al. Genotypic diversity of Coxiella burnetii in the 2007–2010 Q fever outbreak episodes in the Netherlands. J Clin Microbiol. 2012;50:1076–8 . 10.1128/JCM.05497-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arricau-Bouvery N, Hauck Y, Bejaoui A, Frangoulidis D, Bodier CC, Souriau A, et al. Molecular characterization of Coxiella burnetii isolates by infrequent restriction site-PCR and MLVA typing. BMC Microbiol. 2006;6:38 . 10.1186/1471-2180-6-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilburg JJ, Roest HJ, Buffet S, Nabuurs-Franssen MH, Horrevorts AM, Raoult D, et al. Epidemic genotype of Coxiella burnetii among goats, sheep, and humans in the Netherlands. Emerg Infect Dis. 2012;18:887–9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dijkstra F, van der Hoek W, Wijers N, Schimmer B, Rietveld A, Wijkmans CJ, et al. The 2007–2010 Q fever epidemic in the Netherlands: characteristics of notified acute Q fever patients and the association with dairy goat farming. FEMS Immunol Med Microbiol. 2012;64:3–12 . 10.1111/j.1574-695X.2011.00876.x [DOI] [PubMed] [Google Scholar]
- 7.Tilburg JJ, Roest HJ, Nabuurs-Franssen MH, Horrevorts AM, Klaassen CH. Genotyping reveals the presence of a predominant genotype of Coxiella burnetii in consumer milk products. J Clin Microbiol. 2012;50:2156–8. 10.1128/JCM.06831-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karagiannis I, Schimmer B, Van Lier A, Timen A, Schneeberger P, Van Rotterdam B, et al. Investigation of a Q fever outbreak in a rural area of the Netherlands. Epidemiol Infect. 2009;137:1283–94 . 10.1017/S0950268808001908 [DOI] [PubMed] [Google Scholar]


