Abstract
Objective
To determine the role of Toll-like receptor 3 in cardiac dysfunction during polymicrobial sepsis.
Design
controlled animal study
Setting
University Research Laboratory
Subjects
Male C57BL/6, Wild type, Toll-like receptor 3−/−
Intervention
Myocardial dysfunction is a major consequence of septic shock and contributes to the high mortality of sepsis. Toll-like receptors (TLRs) play a critical role in the pathophysiology of sepsis/septic shock. TLR3 is located in intracellular endosomes and recognizes double stranded RNA. This study examined the role of TLR3 in cardiac dysfunction following cecal ligation and puncture (CLP)-induced sepsis. TLR3 knockout (TLR3−/−, n=12) and age-matched wild type (WT, n=12) mice were subjected to CLP. Cardiac function was measured by echocardiography before and 6 hrs after CLP.
Measurements and results
CLP resulted in significant cardiac dysfunction as evidenced by decreased ejection fraction by 25.7% and fractional shortening by 29.8%, respectively. However, TLR3−/− mice showed a maintenance of cardiac function at pre-CLP levels. Wild type mice showed 50% mortality at 58 hrs and 100% mortality at 154 hrs after CLP. In striking contrast, 70% of TLR3−/− mice survive indefinitely, i.e. >200 hrs. TLR3 deficiency significantly decreased CLP-induced cardiac myocyte apoptosis and attenuated CLP-induced Fas and FasL expression in the myocardium. CLP-activation of TLR4-meidated NF-κB and TRIF-dependent IFN signaling pathways was prevented by TLR3 deficiency. In addition, CLP-increased VCAM-1 and ICAM-1 expression and neutrophil and macrophage sequestration in the myocardium were also attenuated in septic TLR3−/− mice. More significantly, adoptive transfer of WT bone marrow stromal cells to TLR3−/− mice abolished the cardioprotective effect in sepsis.
Conclusions
These data indicate that TLR3 plays a deleterious role in mediating cardiac dysfunction in sepsis. Thus, modulation of TLR3 activity may be useful in preventing cardiac dysfunction in sepsis.
Keywords: Cardiac function, sepsis, TLR3, apoptosis, neutrophils
Introduction
Cardiovascular dysfunction is a major consequence of septic shock and contributes to the high morbidity and mortality of sepsis(1). Increasing evidence suggests that innate immune and inflammatory responses are involved in the pathophysiology of sepsis, shock, and multiple organ failure which ultimately leads to death(2). However, the mechanisms by which innate immune and inflammatory responses are involved in myocardial dysfunction during septic shock have remained elusive.
Toll-like receptors (TLRs) play a critical role in the induction of innate immune and inflammatory responses(3). TLRs recognize pathogen associated molecular patterns (PAMPs) and transduce a signal into the cell(3). For example, TLRs (TLR1, TLR2, TLR4, TLR5 and TLR6) which are expressed on the cell surface, are involved in the recognition of structures unique to bacteria or fungi, while TLRs that are localized in intracellular compartments (TLR3, TLR7, TLR8, and TLR9) recognize viral or bacterial nucleic acids(4). TLRs have been implicated in cardiac dysfunction in several important disease states, including ischemia/reperfusion (I/R) injury(5), congestive heart failure(6) and septic shock(7,8). TLR-mediated signaling predominately activates NFκB which is an important transcription factor controlling the expression of inflammatory cytokine genes. We and other investigators have reported that TLR4-mediated NF-κB activation plays an important role in mediating the pathophysiology of cecal ligation and puncture (CLP)-induced sepsis/septic shock(8,9,10).
TLR3 is located in intracellular endosomes and recognizes double-stranded RNA (dsRNA) and polyinosinic-polycytidylic acid (Poly I:C, a synthetic analog of dsRNA), resulting in induction of antiviral immune responses(11). TLR3 also recognized byproducts from apoptotic and necrotic cells(12,13,14). More significantly, a recent study by Cavassani et al demonstrated that TLR3 deficient (TLR3−/−) mice showed an increased survival rate in CLP-septic mice(12). Administration of anti-TLR3 antibody to wild type (WT) mice increased the survival rate in CLP-septic mice(12). This study suggests that TLR3 contributes to the pathophysiology of sepsis/septic shock. However, the role of TLR3 in cardiac dysfunction during the development of sepsis/septic shock has not been investigated.
In the present study, we examined the role of TLR3 in cardiac function during CLP-induced sepsis/septic mice. We observed that TLR3−/− mice exhibited protection against CLP-induced cardiac dysfunction. TLR3 deficiency prevented CLP-activated Fas/FasL mediated apoptotic signaling and attenuated neutrophil and macrophage infiltration into the myocardium. Our data indicate that TLR3 plays a major role in the pathophysiology of cardiac dysfunction during sepsis and could be a promising target for preservation of cardiac function in patients with sepsis/septic shock.
Materials and Methods
Experimental animals
TLR3 knockout mice (TLR3−/−), that were crossbreed with C57BL/6, and age-and weight-matched male C57BL/6 mice were obtained from Jackson Laboratory (Indianapolis, IN).The mice were maintained in the Division of Laboratory Animal Resources at East Tennessee State University. The experiments outlined in this article conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No.85-23, Revised 1996). All aspects of the animal care and experimental protocols were approved by the East Tennessee State University Committee on Animal Care.
CLP polymicrobial sepsis model
Cecal ligation and puncture (CLP) was performed to induce sepsis in mice as previously described(7,8,15,16). Briefly, the mice were anesthetized by 5.0% Isoflurane. A midline incision was made on the anterior abdomen and the cecum was exposed and ligated with a 4-0 suture. Two punctures were made through the cecum with an 18-gauge needle and feces were extruded from the holes. The abdomen was then closed in two layers. Sham surgically operated mice served as the surgery control group. Immediately following surgery, a single dose of resuscitative fluid (lactated Ringer’s solution, 50 ml/kg body weight) was administered by subcutaneous injection(15).
Echocardiography
Transthoracic two-dimensional M-mode echocardiogram and pulsed wave Doppler spectral tracings were obtained using a Toshiba Aplio 80 Imaging System (Toshiba Medical Systems, Tochigi, Japan) equipped with a 12-MHz linear transducer as described previously(8,15). M-mode tracings were used to measure left ventricular (LV) wall thickness, LV end-systolic diameter (LVESD), and LV end-diastolic diameter (LVEDD). Percent fractional shortening (%FS) and percent ejection fraction (%EF) were calculated as described previously(8,15). All measurements were made by one observer who was blinded with respect to the identity of the tracings. All data were collected from 10 cardiac cycles.
In vitro experiments
Thioglycollate elicited peritoneal macrophages were collected from WT and TLR3−/− mice, respectively, and suspended in RPMI 1640 medium supplemented with 10% fetal calf serum, 0.1 mg/ml streptomycin, and 100 U/ml penicillin. The cells (2×106/ml) were cultured in 6-well tissue culture plates (Corning, Inc, Corning, NY) for 2 hrs at 37°C in a humidified incubator with 5% CO2. After washing with PBS, adherent macrophages were incubated at 37°C with 5% CO2 overnight. The cells were treated with lipopolysaccharide (LPS, 1 μg/ml), peptidoglycan (PGN, 10 μg/ml), or Poly I:C (10 μg/ml), respectively, for 24 hrs. Untreated cells served as control. The cells were harvested and the supernatants were collected for analysis of chemokines. There were 4 replicates in each group.
Western blot
Western blots were performed as described previously(7,8,15,16). Briefly, the cellular proteins were separated by SDS-polyacrylamide gel electrophoresis, transferred onto Hybond ECL membranes (Amersham Pharmacia, Piscataway, NJ). The membranes were incubated with appropriate primary antibody [anti-Fas (CD95), anti-FasL, anti-Bax, anti-vascular cell adhesion molecule-1 (VCAM-1), anti-intercellular adhesion molecule-1 (ICAM-1), anti-IFNβ, anti-IκBα, and anti-TLR4, (Santa Cruz Biotech, Santa Cruz, CA), anti-phospho-IκBα, (Cell Signaling Technology, Inc., Danvers, MA), anti-TRIF (Imgenex, Corporation)], respectively, followed by incubation with peroxidase-conjugated second antibodies (Cell Signaling Technology) and analysis by the ECL system (Amersham Pharmacia, Piscataway, NJ). The signals were quantified using the G:Box gel imaging system by Syngene (Syngene, USA, Fredrick, MD).
Electrophoretic mobility shift assay (EMSA)
Nuclear proteins were isolated from heart samples as previously described(7,8,15,16) and NF-κB binding activity was measured using a LightShift Chemiluminescent EMSA kit (Thermo Fisher Scientific, Waltham, MA) according to the instructions of manufacturer.
Immunohistochemistry staining
Immunohistochemistry was performed as described previously(17). Briefly, heart tissues were immersion-fixed in 4% buffered paraformaldehyde, embedded in paraffin, and cut at 5 um sections. The sections were stained with specific goat anti-ICAM-1 (1:50 dilution, Santa Cruz Biotechnology) and rabbit anti-VCAM-1 (1:50 dilution, Santa Cruz Biotechnology), respectively, and treated with the ABC staining system (Santa Cruz Biotechnology) according to the instructions of the manufacturer. Three slides from each block were evaluated, counterstained with hematoxylin, and examined brightfield microscopy. Four different areas of each section were evaluated.
Accumulation of neutrophils and macrophages
Neutrophil accumulation in the heart tissues was examined by staining with naphtol AS-D Chloroacetate Esterase (Sigma-Aldrich, St. Louis, MO) as described previously(18). Macrophages in the myocardium were examined with the macrophage specific antibody F4/80 (1:50 dilution, Santa Cruz, CA). Three slides from each block were evaluated, counterstained with hematoxylin, and examined with brightfield microscopy. Four different areas of each section were evaluated. The results are expressed as the numbers of macrophages/field (40×).
In situ apoptosis assay
Cardiac myocyte apoptosis was examined by the TUNEL assay (Roche Applied Science, Indianapolis, IN) in the heart sections according to the instructions provided by the manufacturer as described previously(8,15,16). Three slides from each block were evaluated for percentage of apoptotic cells. Four fields of each slide were randomly examined using a defined rectangular field area with a magnification of 40×.
Myeloperoxidase (MPO) activity assay
MPO activity was measured using a MPO fluorometric Detection kit (Assay Designs Inc., Ann Arbor, MI) according to the manufacturers’ instructions (17).
ELISA
The levels of monocyte chemoattractant protein-1 (MCP-1), interferon gamma-induced protein-10 (IP-10), and the mouse analog to interleukin 8, keratinocyte chemoattractant (KC), were measured using commercially available ELISA kits (PeproTech, Rocky Hill, NJ) The levels of inflammatory cytokines (TNFα, IL-1β, and IL-6) in the peritoneal fluid, plasma, and the heart and liver tissues were assessed by ELISA (PeproTech, Rocky Hill, NJ) according to the instructions provided by the manufacturer.
Transfer bone marrow stromal cells
Bone marrow stromal cells were isolated from femurs and tibias of WT mice with complete medium constituted of EMEM-LG (Sigma, St., Louis, MO), 10% fetal calf serum (HyClone, ThermoFisher Scientific Waltham, MA), glutamine (2 mM) and penicillin/streptomycin (50 U/ml and 50 mg/ml, Sigma), respectively, supplemented with heparin at a final concentration of 5 U/ml. Cells were then washed twice in a medium without heparin, plated in a Peri dish at a density of 2 × 106 cells/cm2 and incubated at 37°C with 5% CO2. After three days, non-adherent cells were removed by two to three washes with PBS and adherent cells further cultured in complete medium at 37°C with 5% CO2. The medium was changed every other days. Two weeks after incubation, the cells were harvested, washed three times with PBS and transplanted to WT and TLR3−/− mice (1×107) by intravenous injection.
Statistical analysis
Survival trends were compared with the Cox regression proportional hazards procedures. All other data were expressed as mean ± SE. Comparisons of data between groups were made using one-way analysis of variance (ANOVA) and Tukey’s procedure for multiple range tests was performed. P< 0.05 was considered to be significant.
Results
TLR3 deficiency attenuated cardiac dysfunction and increased survival outcome following CLP-induced sepsis
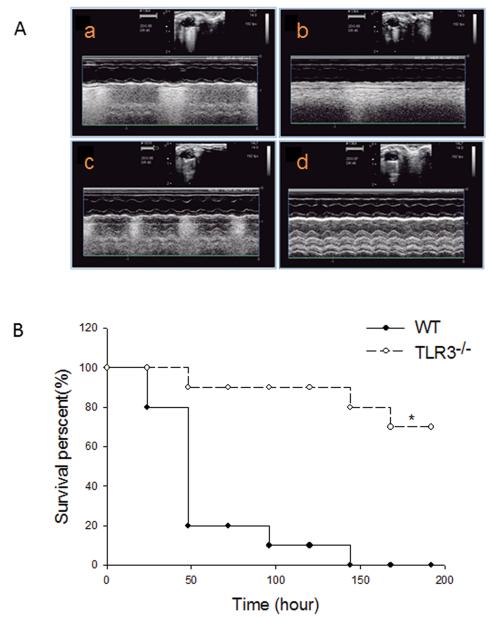
CLP significantly induced cardiac dysfunction as evidenced by decreased ejection fraction (%EF) by 25.7% and fractional shortening (%FS) by 29.8%, respectively, compared with baseline (Table 1). In contrast, TLR3−/− mice maintained the levels of %EF and %FS at baseline levels following CLP. Figure 1A shows representative images of M-model images of parasternal short-axis view at the papillary muscle level at base line and after CLP. CLP WT mice exhibited marked ventricular dilatation and poor left ventricular (LV) wall motion compared with base line. There was no significant change in LV dilatation and LV wall motion in TLR3−/− CLP mice.
Table 1.
TLR3 deficiency attenuated cardiac dysfunction in CLP-induced sepsis
| Group | Heart rate | %EF | %FS | LVESD(mm) | LVEDD(mm) | SV(mmHg*μl) | CO(μl) |
|---|---|---|---|---|---|---|---|
| WT pre- CLP |
440.9 ± 21.72 | 61.2 ± 5.49 | 32.4 ± 3.99 | 2.2 ± 0.34 | 5.5 ± 0.33 | 33816.9 ± 3279.01 | 15170.2 ± 1609.34 |
| WT CLP | 368.1 ± 40.52* | 45.5 ± 7.32* | 22.8 ± 8.15* | 0.6 ± 0.13* | 1.3 ± 0.51* | 6696.6 ± 3787.75* | 2483.9 ± 1241.00* |
| TLR3−/− pre-CLP |
454.2 ± 19.34 | 61.8 ± 6.93 | 33.0 ± 5.25 | 2.1 ± 0.39 | 5.6 ± 0.61 | 34270.8 ± 5730.02 | 17249.0 ± 3648.22 |
| TLR3−/− CLP |
392.8 ± 6.55 # | 62.6 ± 6.08# | 33.2 ± 4.58# | 1.5 ± 0.58# | 4.4 ± 0.65# | 28924.5 ± 6411.69# | 12682.1 ± 3525.39# |
TLR3 deficiency attenuated cardiac dysfunction in CLP-induced sepsis. TLR3−/− mice (n=12) and WT mice (n=12) were subjected to CLP. Cardiac function was examined by echocardiography before and 6 hrs after CLP. EF: Ejection fraction; FS: Fractional Shortening; LVESD: left ventricle end systolic diameter; LVEDD: left ventricle end diastolic diameter; SV: Stroke volume; CO: cardiac output.
p<0.01, compared with the WT pre-CLP
p<0.01, compared with the WT CLP
Figure 1. TLR3 deficiency attenuated cardiac dysfunction and increased survival outcome following CLP-induced sepsis in mice.
TLR3−/− and age-matched WT mice (n=12/group) were subjected to CLP and cardiac function was measured before and 6 hrs after CLP. (A) Representative images generated by echocardiography. a: WT mice base line (before CLP); b: WT mice after CLP; c: TLR3−/− mice base line (before CLP); d: TLR3−/− mice after CLP. (B) TLR3 deficiency increases survival outcome in CLP-induced septic mice. TLR3−/− and age-matched WT mice (10/group) were subjected to CLP and the survival was carefully monitored daily. * P<0.05 compared with indicated groups.
We also evaluated the effect of TLR3 deficiency on survival outcome. TLR3−/− (n=10) and age-matched WT (n=10) mice were subjected to CLP. Survival rate was carefully monitored for 192 hrs, i.e. 8 days. Figure 1B shows that TLR3−/− mice were more resistant to CLP-induced mortality than WT mice. In WT mice, time to 50% mortality was 48 hrs and to 100% mortality was 145 hrs after CLP. In striking contrast, 70% of TLR3−/− mice went on to survive indefinitely, i.e. >192 hrs (8 days).
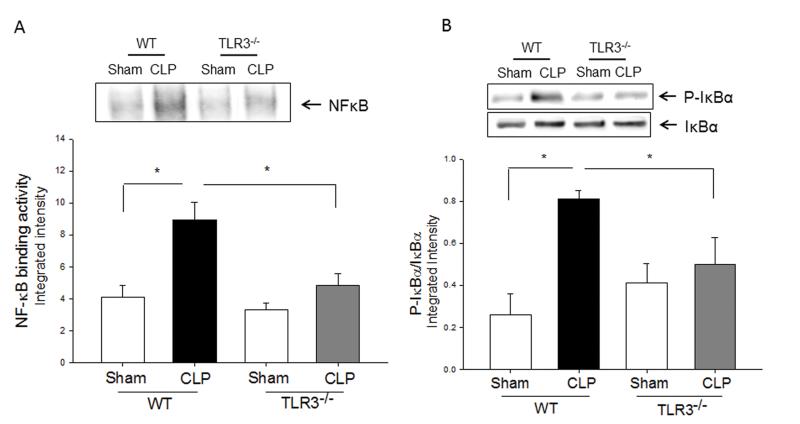
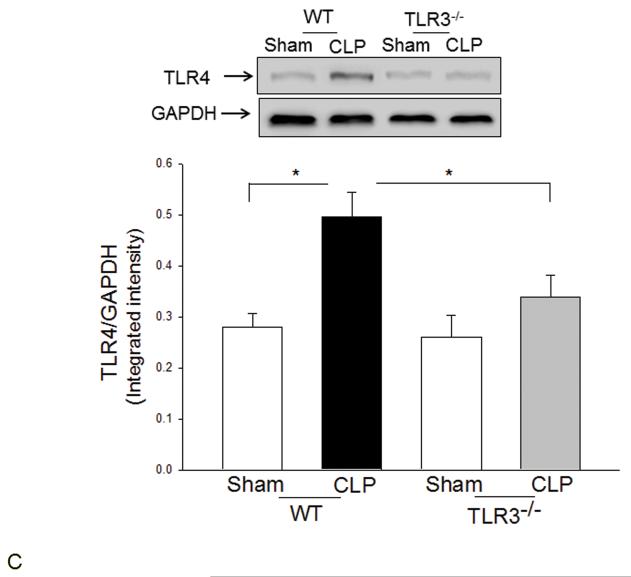
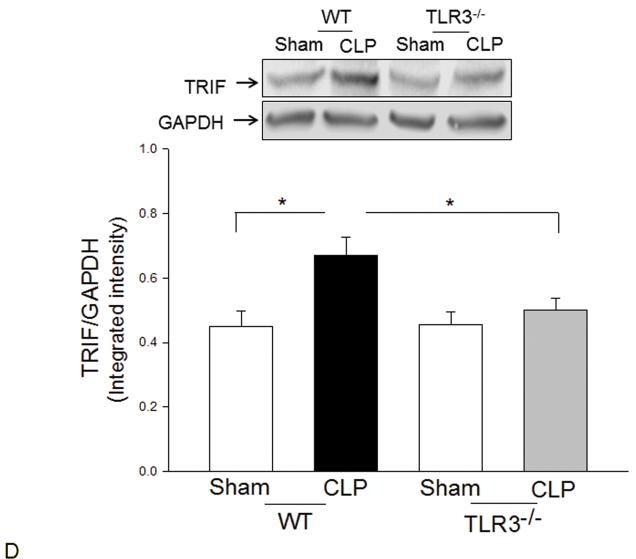
TLR3 deficiency prevented sepsis-induced activation of TLR4 mediated NF-κB and TRIF/IRF signaling pathways
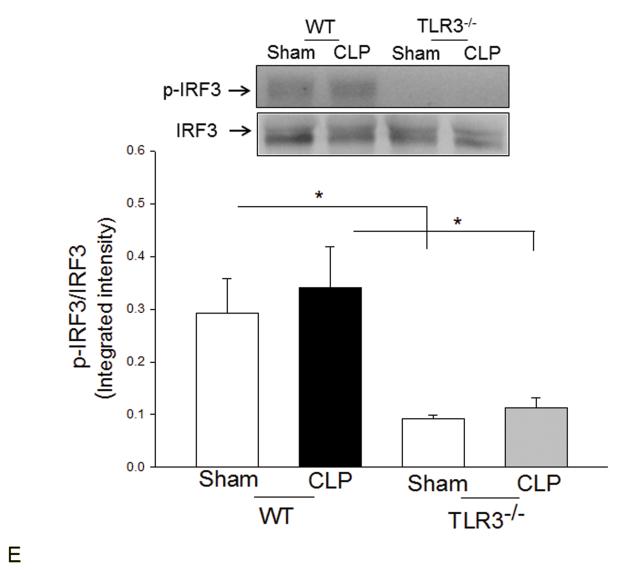
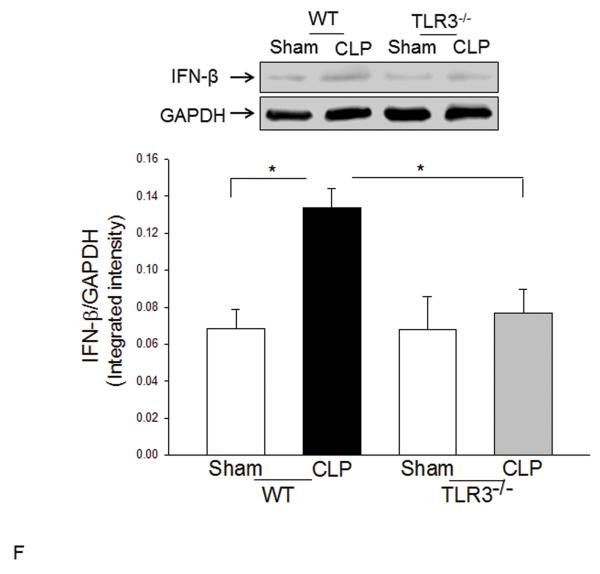
TLR4-mediated signaling activates both MyD88-dependent NF-κB and TRIF/IRF-dependent IFN pathways(3). Figure 2 shows that CLP significantly increased myocardial NF-κB binding activity (A) and the levels of phosphorylated IκBα/IκBα (B) in the myocardium in WT mice but not in TLR3−/− mice. CLP-increased levels of TLR4 (C), and TRIF (D), and IFN-β (E) in the myocardium were significantly prevented by TLR3 deficiency. CLP did not significantly induce IRF3 phosphorylation (F); however, the levels of phosphorylated IRF3 were significantly lower in both TLR3−/− sham and CLP mice compared with respective WT groups.
Figure 2. TLR3 deficiency prevented sepsis-induced activation of TLR4-mediated NF-κB activation and TRIF/IRF-dependent IFN signaling pathways.
TLR3−/− and WT mice were subjected to CLP (5-6/group). Sham operation served as sham control (5-6/group). Hearts were harvested six hrs after CLP and the nuclear and cytoplasmic proteins were prepared. (A) NFκB binding activity was determined by EMSA with nuclear proteins. The levels of p-IκBα/IκBα (B), TLR4 (C), TRIF (D), p-IRF3/IRF3 (E), and IFNβ (F) were examined by Western blot with specific antibodies, respectively. * p<0.05 compared with indicated groups.
The effect of TLR3 deficiency on CLP-induced inflammatory cytokine production
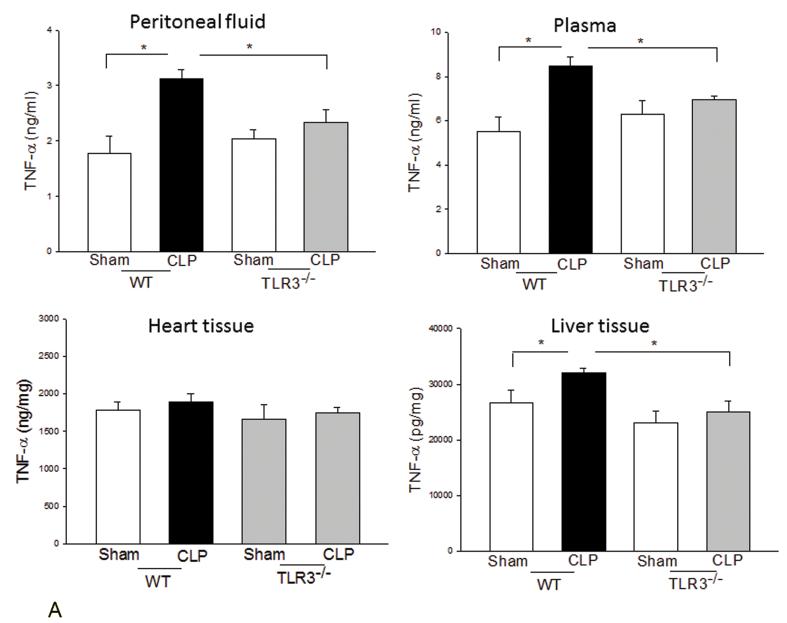
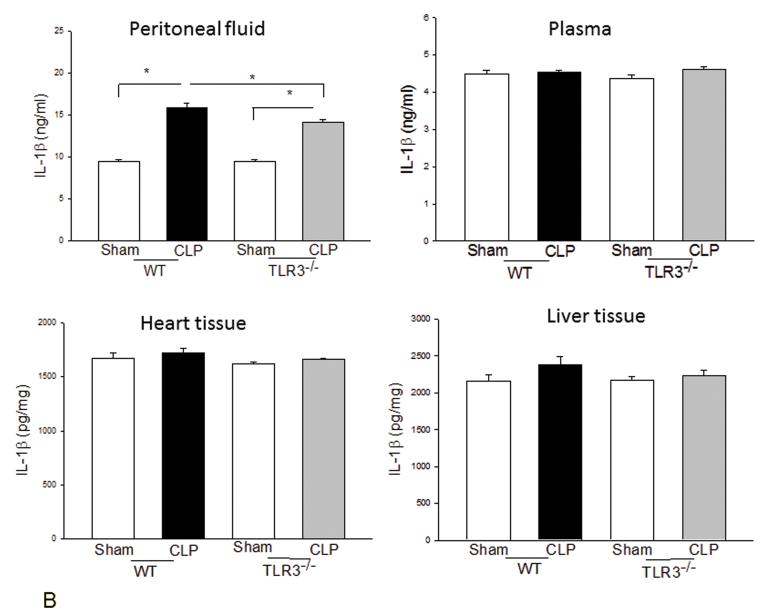
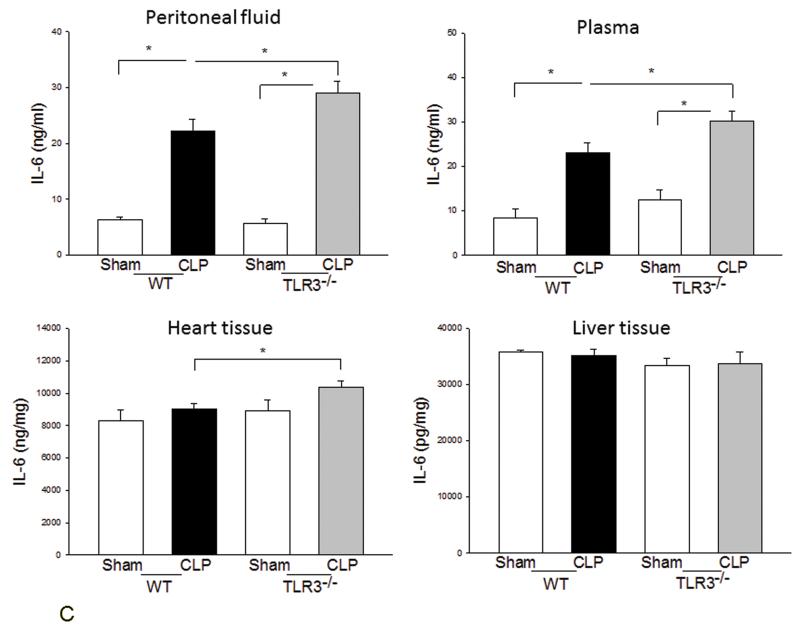
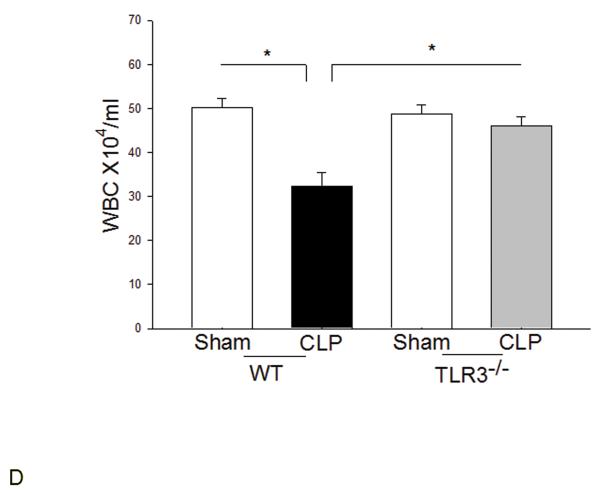
We examined the systemic inflammatory responses of WT and TLR3−/− mice to CLP-induced sepsis. Figure 3A shows that CLP significantly increased levels of TNFα in the peritoneal fluid, plasma, and liver tissue in WT mice but did not in TLR3−/− mice. TLR3 deficiency also significantly attenuated CLP-increased IL-1β levels in the peritoneal fluid (Fig. 3B). Interestingly, CLP significantly increased IL-6 levels in peritoneal fluid and plasma in both WT and TLR3−/− mice (Fig. 3C). The levels of IL-6 in TLR3−/− CLP mice were higher than that in WT CLP mice. The levels of myocardial IL-6 were also increased in TLR3−/− mice, but not in WT mice after CLP. In addition, CLP caused a decrease in the number of white blood cells (WBCs) in WT, but not in TLR3−/−, mice (Fig. 3D).
Figure 3. The effect of TLR3 deficiency on systemic inflammatory responses following CLP.
TLR3−/− and WT mice were subjected to CLP (4-6/group). Sham operation served as sham control (4-6/group). Peritoneal fluid, plasma, and heart and liver tissues were harvested six hrs after CLP. Inflammatory cytokines TNFα (A), IL-1β (B), and IL-6 (C) were measured using commercially available ELISA kits, respectively. (D) White blood cells (WBC) in the plasma were accounted. * p<0.05 compared with indicated groups.
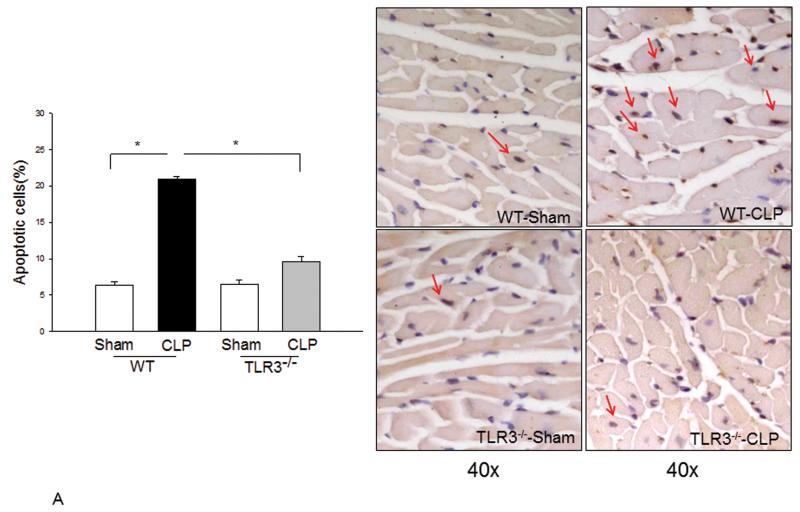
TLR3 deficiency prevented CLP-increased myocardial apoptosis and Fas/FasL-mediated apoptotic signaling
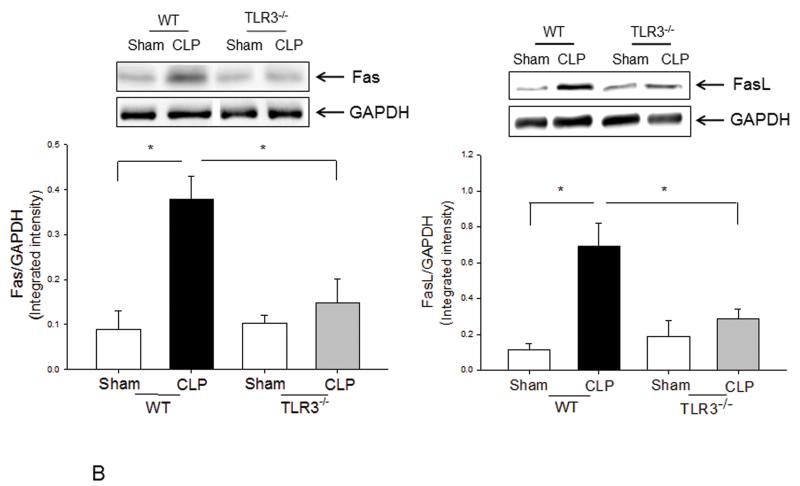
We examined the role of TLR3 in cardiac myocyte apoptosis following CLP-sepsis. Figure 4A shows that cardiac myocyte apoptosis was significantly increased in WT-CLP mice compared with WT sham control (20.9 ± 0.29 vs 6.4 ± 0.48). CLP-induced cardiac myocyte apoptosis was 34.9% (p<0.05) lower in TLR3−/− mice when compared with WT CLP mice. Figure 4B show that CLP increased the levels of Fas by 315.9% and FasL by 540.7% in WT mice compared with sham control. However, the CLP-increased levels of Fas and FasL were prevented in TLR3−/− mice. There was no significant difference in the levels of Fas and FasL between TLR3−/− CLP and TLR3−/− sham control mice.
Figure 4. TLR3 deficiency prevented CLP-apoptosis and Fas/FasL-mediated apoptotic signaling in the myocardium.
TLR3−/− and WT mice were subjected to CLP (6/group). Sham operation served as sham control (6/group). Hearts were harvested for tissue section and cellular protein preparations. (A) Cardiac myocyte apoptosis was examined by TUNEL assay. Red arrows indicate cardiac myocyte apoptosis. Magnification is 40X. Quantitated data are shown on the left. (B) The levels of Fas and FasL were examined by Western blot with specific antibodies. * p<0.05 compared with indicated groups.
Sepsis-induced neutrophil and macrophage infiltration into the myocardium was prevented by TLR3 deficiency
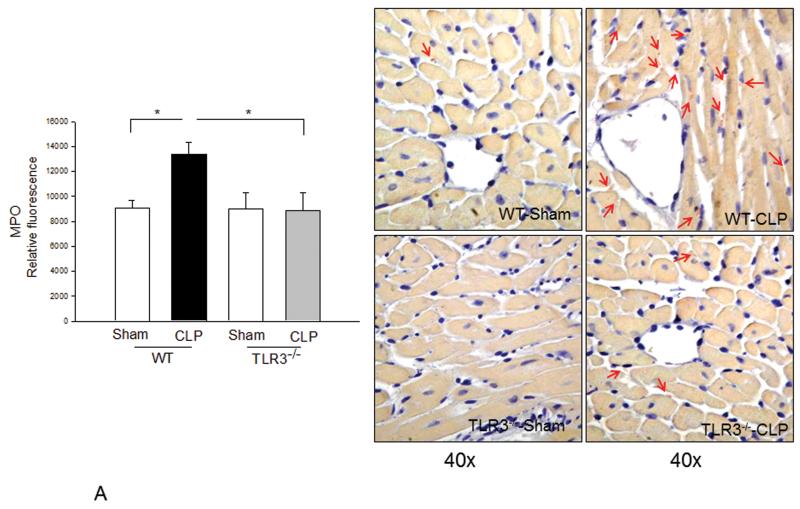
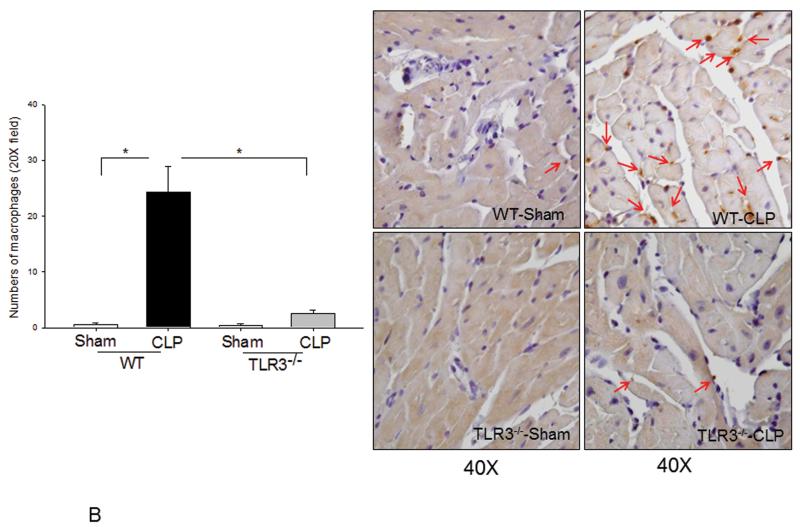
Neutrophil and macrophage infiltration into the myocardium contribute to cardiac dysfunction during the development of sepsis/septic shock(20,21). Figure 5A shows that CLP increased MPO activity by 52.6% (p<0.05) in WT mice compared with sham control, indicating increased neutrophil infiltration into the myocardium. There were more neutrophils staining in WT CLP heart tissues compared with WT sham. In contrast, MPO activity was not significantly increased and there was less neutrophil staining in TLR3−/− mice following CLP. Figure 5B shows that CLP significantly increased the number of macrophages in the myocardium of WT mice compared with WT sham control. However, the numbers of macrophages in the myocardium of TLR3−/− mice were not significantly increased following CLP.
Figure 5. Neutrophil and macrophage myocardial infiltration was attenuated in TLR3 deficiency.
TLR3−/− and WT mice were subjected to CLP (6/group). Sham operation served as sham control (6/group). Hearts were harvested six hrs after CLP and sectioned. (A) MPO activity was measured in cellular protein preparations by a kit (left). Neutrophil accumulation (pink color) was marked by red arrows (n=3/group). (B) Macrophages in the myocardium were examined by immunohistochemistry (right) with a specific antibody (n=3/group). The dark brown color indicates macrophage infiltration marked by red arrows. Quantitated data are shown on the left. * p<0.05 compared with indicated groups.
TLR3 deficiency prevented sepsis-induced expression of adhesion molecules in the myocardium
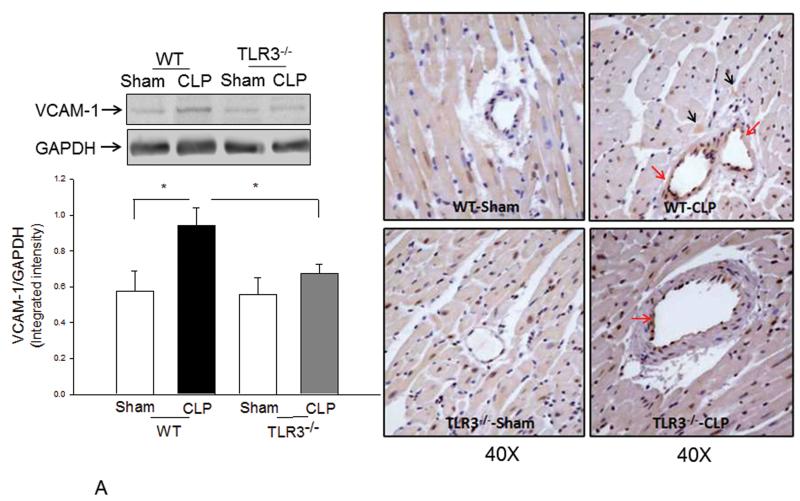
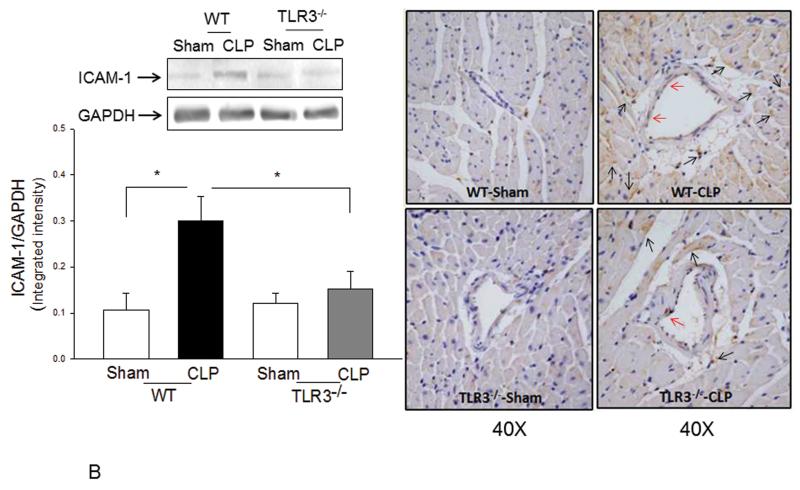
Increased expression of adhesion molecules, such as VCAM-1 and ICAM-1 mediate neutrophil and macrophage infiltration into the myocardium during sepsis/septic shock(22,21). Figure 6A and B show that CLP significantly increased both VCAM-1 (↑63.8%) and ICAM-1 (↑182.7%) expression in the myocardium of WT mice compared with sham control. However, TLR3 deficiency prevented sepsis-induced increases in VCAM-1 and ICAM-1 expression. Specifically, the levels of VCAM-1 and ICAM-1 in septic TLR3−/− mice were not significantly different from TLR3−/− sham control. Immunohistochemistry staining demonstrated that increased expression of VCAM-1 is predominantly located in endothelium while ICAM-1 is expressed by both in endothelial cells and cardiac myocytes. There was less staining of VCAM-1 and ICAM-1 in myocardial tissue sections of septic TLR3−/− mice compared with that of septic WT mice.
Figure 6. Sepsis-increased expression of myocardial VCAM-1 and ICAM-1 was attenuated by TLR3 deficiency.
TLR3−/− and WT mice were subjected to CLP (6/group). Sham operation served as sham control (6/group). Hearts were harvested six hrs after CLP and sectioned. Expression of (A) VCAM-1 and (B) ICAM-1 was examined by Western blot (left) and immunohistochemistry (right). The dark brown color indicates expression of VCAM-1 and ICAM-1 marked by red and black arrows, respectively. * p<0.05 compared with indicated groups.
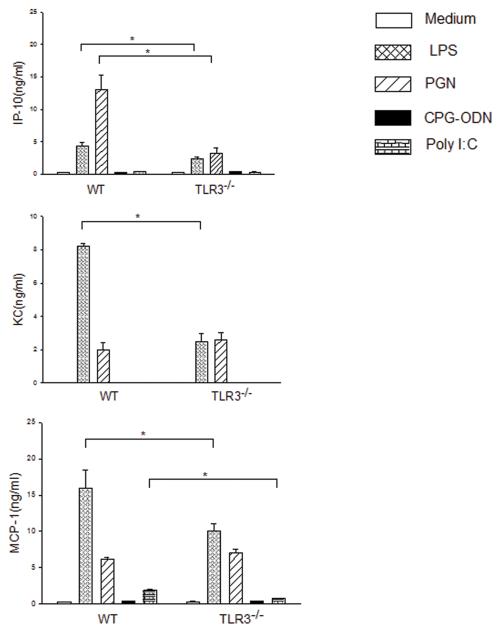
Attenuation of chemokine secretion by TLR3−/− macrophages following stimulation with TLR ligands
We examined the effect of TLR3 deficiency on the response to TLR ligand stimulation by macrophages. Figure 7 shows that both LPS and Poly I:C stimulation significantly increased the levels of MCP-1 and KC in WT macrophages but not in TLR3−/− macrophages. Both LPS and PGN significantly increased the levels of IP-10 in WT mice, however, the increased IP-10 by LPS and PGN was attenuated in TLR3−/− macrophages. CpG-ODN, a ligand for TLR9, did not stimulate the secretion of chemokines in WT and TLR3−/− macrophages.
Figure 7. Attenuated chemokine secretion by TLR3−/− macrophages following TLR ligand stimulation.
Peritoneal macrophages were isolated from TLR3−/− and WT mice and stimulated with LPS, PGN, CpG-ODN and Poly I:C, respectively. Secretion of chemokines into the supernatants was measured using commercial kits. There were four replicates in each group (4/group). * p<0.05 compared with indicated groups.
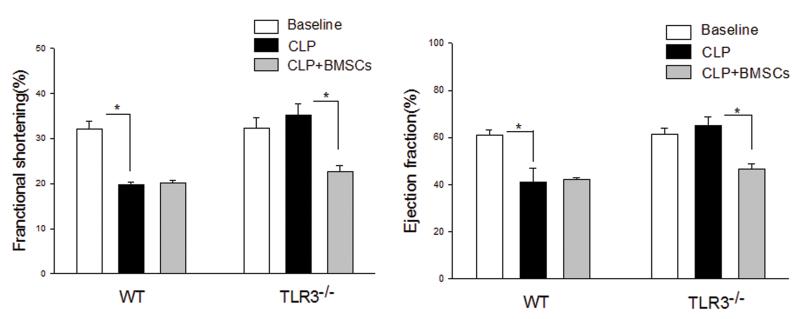
Adoptive transfer of wild type bone marrow stromal cells abolished the cardioprotective effect of TLR3 deficiency in sepsis
To examine the role of peripheral cells in TLR3 deficiency-induced protection against sepsis-induced cardiac dysfunction, we isolated bone marrow stromal cells (BMSCs) from WT mice and transplanted them into TLR3−/− mice immediately prior to induction of CLP. Cardiac function was assessed 6 hrs after CLP. As shown in Figure 8, adoptive transfer of WT BMSCs into WT mice did not affect the CLP-induced decrease in cardiac function. However, TLR3−/− mice that received WT-BMSCs showed a loss of cardioprotection 6 hrs after sepsis. Both %EF (61.8%) and %FS (33.0%) values in TLR3−/− mice that received BMSCs were similar to that of WT CLP mice (46.1%, and 22.3%) and significantly lower than TLR3−/− mice (66.6%, 36.2%) that did not receive BMSCs.
Figure 8. Adoptive transfer of WT bone marrow stromal cells abolished the cardioprotective effect of TLR3 deficiency.
Bone marrow stromal cells were isolated from WT mice and transplanted to TLR3−/− mice and WT mice, immediately before the mice were subjected to CLP (5/group). Cardiac function was measured by echocardiography before and 6 hrs after CLP. * p<0.05 compared with indicated groups.
Discussion
The major finding in this study was that the TLR3 plays a key role in the pathophysiology of sepsis-induced cardiac dysfunction. Cavassani et al have reported that TLR3−/− mice were resistant to CLP-induced lethality(12). We also observed that TLR3 deficiency significantly improved survival outcome in CLP-sepsis/septic shock. Our current study provides insights into the mechanisms by which TLR3 mediates septic morbidity and mortality.
Clinical and experimental studies have shown that myocardial dysfunction is an early and fatal complication of septic shock(1). The current study demonstrated that sepsis-induced myocardial dysfunction was dramatically attenuated in the absence of TLR3. In addition, TLR3 deficiency reduced myocardial apoptosis in response to sepsis. We have previously shown that cardiac myocyte apoptosis contributes to cardiac dysfunction during the development of sepsis/septic shock(8). The mechanisms by which TLR3 deficiency attenuated CLP-induced cardiac myocyte apoptosis is by inhibition of Fas/FasL-mediated apoptotic signaling. It is unclear how TLR3 deficiency attenuates sepsis-induced Fas/FasL-mediated apoptotic signaling. However, activation of TLR3 is known to stimulate the extrinsic and intrinsic apoptotic pathways by up-regulating TNF-related apoptosis-inducing ligand (TRAIL) and its receptors and by down-regulating the anti-apoptotic protein Bcl2(23).
We have previously reported that CLP significantly increased the levels of TLR4 and NF-κB binding activity in the myocardium(15). In the present study, we observed that CLP sepsis markedly increased TLR4-mediated MyD88-dependent NF-κB activation and TRIF-dependent IRF3-mediated IFN-β signaling pathways. Importantly, TLR3 deficiency prevented CLP sepsis activation of both TLR4-mediated MyD88-dependent NF-κB activation and TRIF-dependent IFN-β signaling pathways. Indeed, the levels of inflammatory cytokines, such as TNFα, IL-1β and myocardial IFN-β, were markedly lower in TLR3−/− septic mice than in WT septic mice. At present we do not understand why the levels of IL-6 were significantly higher in TLR3−/− mice than in WT mice after CLP. It could be possible that increased IL-6 levels in TLR3−/− mice may serve as an anti-inflammatory effect.
Neutrophil and macrophage infiltration plays a critical role in mediating cardiac dysfunction during sepsis/septic shock(20,21). Infiltrated neutrophils release inflammatory cytokines, including TNF-α and IL-1β which are the important suppressors of cardiac function(20). Activated macrophages also release chemokines such as MCP-1 and KC, which attract neutrophils into the myocardium(24). In addition, macrophages release macrophage inhibitory factor (MIF) which contributes to cardiac dysfunction and correlates with sepsis severity(25). In the present study, we observed that CLP sepsis resulted in significantly increased numbers of neutrophils and macrophages in the myocardium of WT mice. However, neutrophil and macrophage infiltration into the myocardium was markedly attenuated in septic TLR3−/− mice. It is well known that increased expression of adhesion molecules, such as ICAM-1 and VCAM-1 plays a critical role in recruitment of neutrophils and macrophages into the myocardium during sepsis/septic shock(20,21,24) and are associated with myocardial dysfunction induced by LPS(26). We observed that CLP-sepsis significantly increased expression of ICAM-1 and VCAM-1 in the myocardium of WT mice. In contrast, TLR3−/− deficiency blunted CLP-increased expression of adhesion molecules in the myocardium. When taken together, these data indicate that TLR3 plays a crucial role in sepsis-induced adhesion molecule expression, and neutrophil and macrophage infiltration into the myocardium during sepsis/septic shock.
To more critically evaluate this conclusion, we isolated peritoneal macrophages from WT and TLR3−/− mice and examined chemokine secretion following stimulation with TLR ligands. We observed that the TLR3 ligand, Poly I:C markedly increased secretion of MCP-1 and KC in WT macrophages but not in TLR3−/− macrophages, suggesting that TLR3 mediates activation of chemokines following ligand stimulation. Interestingly, LPS, a TLR4 ligand and PGN, a TLR2 ligand, significantly increased secretion of MCP-1 and IP-10 in WT macrophages. However, increased secretion of MCP-1 and IP-10 by LPS and PGN was also significantly attenuated in TLR3−/− macrophages. This may be due to cross talk within the TLR signaling network. Cavassani et al reported that the levels of peritoneal and tissue cytokine (TNFα) and chemokines (CCL5, CCL3, CXCL10, and MIP-2) were significantly increased in WT mice following CLP, but markedly decreased in TLR3−/− mice(12). We have observed a similar phenomenon in TNFα and IL-1β levels in WT and TLR3−/− mice after CLP. In addition, Cavassani et al have shown that TLR3−/− macrophages responded normally to the other TLR ligands but did not respond to RNA from necrotic neutrophils. Their observation suggests that RNA released from necrotic neutrophils could be an endogenous ligand that triggers activation of TLR3-mediated signaling.
As part of this study, we examined the role of immunocytes in the TLR3-mediated response to sepsis. Bone marrow stromal cells (BMSC) were isolated from WT mice and transferred into TLR3−/− mice immediately before induction of CLP. We observed that the cardioprotective effect of TLR3 deficiency was completely abrogated in presence of WT BMSCs. This suggests that TLR3 positive BMSCs contribute to cardiac dysfunction in CLP-sepsis/septic shock. Similarly, Binck et al reported that TLR4−/− mice showed protection against LPS-induced cardiac dysfunction(27). However, TLR4−/− mice, after receiving bone marrow-derived cells from WT mice, lost the ability to protect against LPS-induced cardiac dysfunction(27). These data confirm the importance of TLR3 positive bone marrow cells in this response. However, the role and importance of TLR3 expression by cardiac myocytes during sepsis/septic shock remains to be determined.
In summary, our data clearly demonstrate a role of TLR3 in the pathophysiology of cardiac dysfunction in sepsis. These data also indicate that TLR3 could be a promising therapeutic target for the preservation of cardiac function during sepsis/septic shock. At present, Poly I:C is considered as a TLR3 agonist(11). Endogenous ligands such as dsRNA released from apoptotic and necrotic cells can be recognized by TLR3(12,13,14). It is possible that endogenous ligands released from damaged cells during the development of sepsis, could stimulate TLR3, resulting in the pathophysiologic mechanisms of sepsis/septic shock. Further studies are needed to determine the endogenous ligands that trigger activation of TLR3-mediated signaling and examine how TLR3-mediated signaling causes cardiac dysfunction.
Acknowledgments
This work was supported, in part, by NIH HL071837 to C.L., NIH GM083016 to C.L. and D.L.W., NIH GM53552 to D.L.W., NIH GM093878 to RLK., HL-091405 and HL-092459 to KS., and a Merit Review Grant from the Department of Veterans Affairs to KS.
Footnotes
The authors have not disclosed any potential conflicts of interest
Conflict of Interest
There was no conflict of interest for the authors in the present study.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krishnagopalan S, Kumar A, Parrillo JE, Kumar A. Myocardial dysfunction in the patient with sepsis. Curr Opin Crit Care. 2002;8:376–388. doi: 10.1097/00075198-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis Syndromes: Understanding the Role of Innate and Acquired Immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 3.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Takeda K. Toll-Like Receptor Signalling. Nature Reviews. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 5.Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2007;4:444–454. doi: 10.1038/ncpcardio0938. [DOI] [PubMed] [Google Scholar]
- 6.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medshitov R, Lee RT, Kelly RA. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–280. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DL, Ha T, Li C, Kalbfleisch JH, Schweitzer J, Vogt W, Browder IW. Modulation of tissue toll-like receptor 2 and 4 during the early phases of polymicrobial sepsis correlates with mortality. Crit Care Med. 2003;31:1808–1818. doi: 10.1097/01.CCM.0000069343.27691.F3. [DOI] [PubMed] [Google Scholar]
- 8.Ha T, Lu C, Liu L, Hua F, Hu Y, Kelley J, Singh K, Kao RL, Kalbfleisch J, Williams DL, Gao X, Li C. TLR2 ligands attenuate cardiac dysfunction in polymicrobial sepsis via a phosphoinositide-3-kinase dependent mechanism. Am J Physiol Heart Circ Physiol. 2010;298:H984–H991. doi: 10.1152/ajpheart.01109.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browder W, Ha T, Li C, Kalbfleisch JH, Ferguson DAJr, Williams DL. Early Activation of Pulmonary Nuclear Factor kB and Nuclear Factor Interleukin-6 in Polymicrobial Sepsis. Journal of Trauma: Injury, Infection, and Critical Care. 1999;46:590–596. doi: 10.1097/00005373-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Williams DL, Ha T, Li C, Kalbfleisch JH, Laffan JJ, Ferguson DA. Inhibiting early activation of tissue nuclear factor-kB and nuclear factor interleukin 6 with (1-->3)-b-D-glucan increases long-term survival in polymicrobial sepsis. Surgery. 1999;126:54–65. doi: 10.1067/msy.1999.99058. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto M, Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv Drug Deliv Rev. 2008;60:805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, Hogaboam CM, Kunkel SL. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 14.Sloane JA, Blitz D, Margolin Z, Vartanian T. A clear and present danger: endogenous ligands of Toll-like receptors. Neuromolecular Medicine. 2010;12:149–163. doi: 10.1007/s12017-009-8094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha T, Hua F, Grant D, Xia Y, Ma J, Gao X, Kelley J, Williams DL, Kablfleisch J, Browder IW, Li C. Glucan phosphate attenuates cardiac dysfunction and inhibits cardiac MIF expression and apoptosis in septic mice. Am J Physiol Heart Circ Physiol. 2006;291:H1910–H1918. doi: 10.1152/ajpheart.01264.2005. [DOI] [PubMed] [Google Scholar]
- 16.Williams DL, Li C, Ha T, Ozment-Skelton T, Kalbfleisch JH, Preiszner J, Brooks L, Breuel K, Schweitzer JB. Modulation of the phosphoinositide 3-Kinase pathway alters innate resistance to polymicrobial sepsis. J Immunol. 2004;172:449–456. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- 17.Ha T, Hu Y, Liu L, Lu C, McMullen JR, Shioi T, Isumo S, Kelley J, Kao RL, Williams DL, Gao X, Li C. TLR2 ligands induce cardioprotection against ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Cardiovascular Research. 2010;87:694–703. doi: 10.1093/cvr/cvq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Z, Hu Y, Wu W, Ha T, Kelley J, Deng C, Chen Q, Li C, Li J, Li Y. The TIR/BB-loop mimetic AS-1 protects the myocardium from ischaemia/reperfusion injury. Cardiovascular Research. 2009;84:442–451. doi: 10.1093/cvr/cvp234. [DOI] [PubMed] [Google Scholar]
- 19.Williams DL, Ha T, Li C, Kalbfleisch JH, Ferguson DA., Jr Early activation of hepatic NFkB and NF-IL6 in polymicrobial sepsis correlates with bacteremia, cytokine expression and mortality. Ann Surg. 1999;230:95–104. doi: 10.1097/00000658-199907000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alves-Filho JC, de Freitas A, Spiller F, Souto FO, Cunha CQ. The role of neutrophils in severe sepsis. Shock. 2008;30:3–9. doi: 10.1097/SHK.0b013e3181818466. [DOI] [PubMed] [Google Scholar]
- 21.Cavaillon J-M, Adib-Conquy M. Monocytes/macrophages and sepsis. Crit Care Med. 2005;33:S506–S509. doi: 10.1097/01.ccm.0000185502.21012.37. [DOI] [PubMed] [Google Scholar]
- 22.Davani EY, Boyd JH, Dorscheid DR, Wang Y, Meredith A, Chau E, Singhera GK, Walley KR. Cardiac ICAM-1 mediates leukocyte-dependent decreased ventricular contractility in endotoxemic mice. Cardiovascular Research. 2006;72:134–142. doi: 10.1016/j.cardiores.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Sun R, Zhang Y, Lv Q, Liu B, Jin M, Zhang W, He Q, Deng M, Liu S, Li G, Li Y, Zhou G, Xie P, Xie X, Duan Z, Hu J. Toll-like receptor 3 (TLR3) induces apoptosis via death receptors and mitochondria by up-regulating the transactivating p63 isoform {alpha} (tap63{alpha}) J Biol Chem. 2011;286:15918–15928. doi: 10.1074/jbc.M110.178798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raeburn CD, Calkins CM, Zimmerman MA, Song Y, Ao L, Banerjee A, Harken AH, Meng X. ICAM-1 and VCAM-1 mediate endotoxemic myocardial dysfunction independent of neutrophil accumulation. Am J Physiol Regulatory Integrative Comp Physiol. 2002;283:R477–R486. doi: 10.1152/ajpregu.00034.2002. [DOI] [PubMed] [Google Scholar]
- 25.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nature Reviews Immunology. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 27.Binck BW, Tsen MF, Islas M, White DJ, Schultz RA, Willis MS, et al. Bone marrow-derived cells contribute to contractile dysfunction in endotoxic shock. Am J Physiol Heart Circ Physiol. 2005;288:577–583. doi: 10.1152/ajpheart.00745.2004. [DOI] [PubMed] [Google Scholar]