Abstract
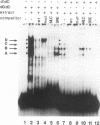
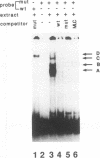
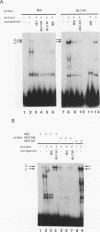
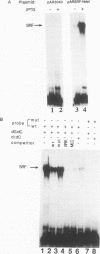
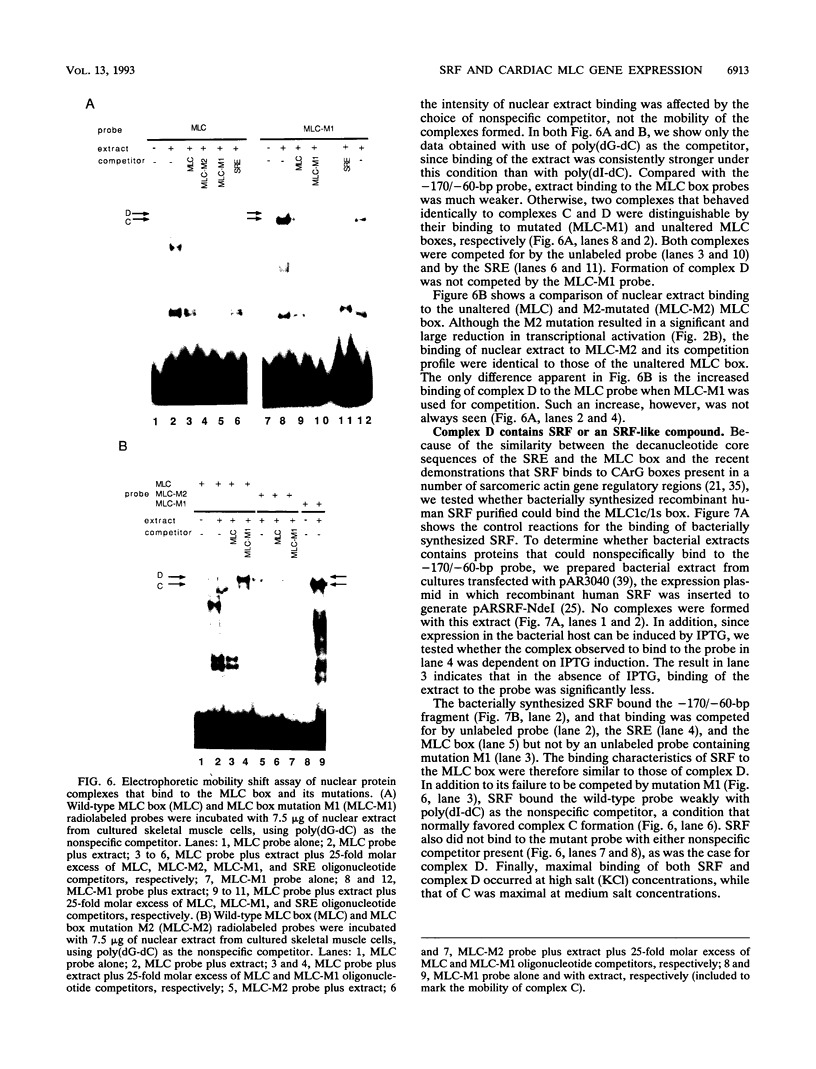
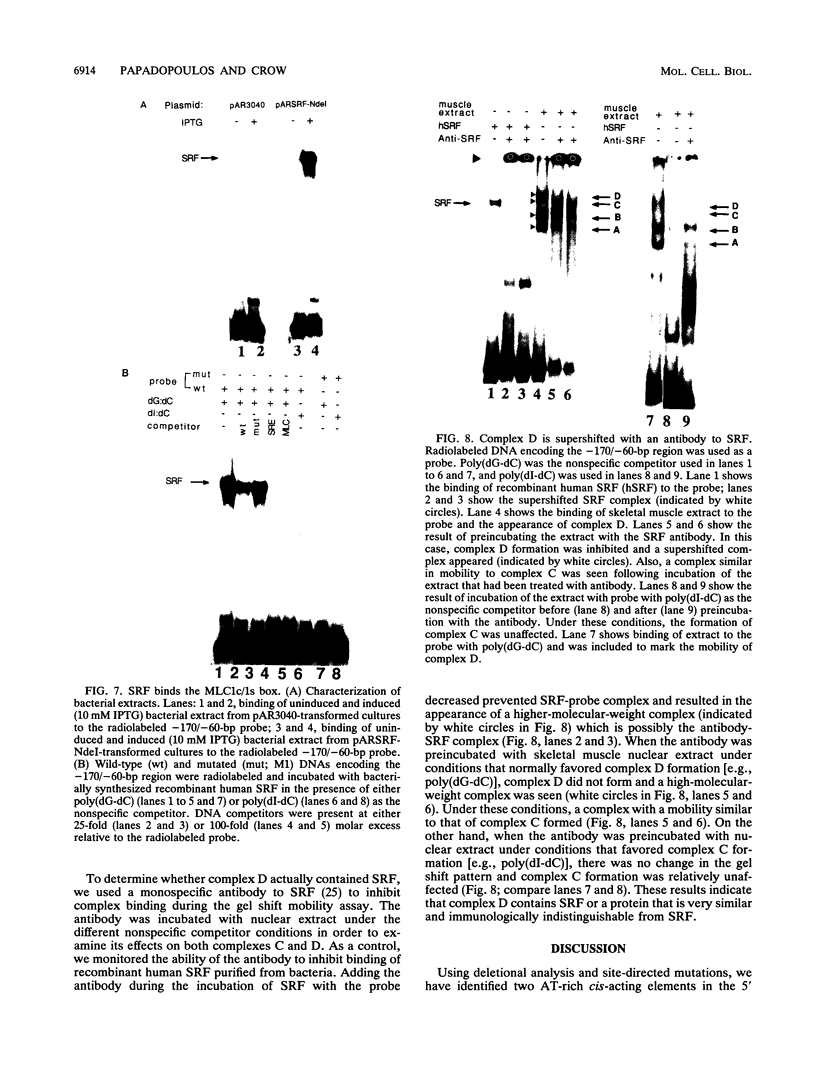
Transcriptional control of the cardiac/slow skeletal alkali myosin light-chain (MLC1c/1s) gene is mediated, in part, by two highly conserved AT-rich cis-acting elements present in the immediate 5' flanking region. These elements cooperate to form an enhancer that can impart tissue specificity to heterologous promoters that are themselves not tissue specific in their pattern of expression. In the chicken, one of these elements matches the binding site for myocyte-specific enhancer-binding factor 2, while the other is a cis-acting element present in the transcriptional control regions of all striated alkali MLC genes (except MLC3f) and is referred to as the MLC box. The central decanucleotide core region of the MLC box closely resembles the CArG (CC[A/T]6GG) box of the serum response element, and the binding of muscle nuclear protein complexes to this element can be competed for with a synthetic serum response element. On the basis of their competition profiles and requirements for nonspecific competitor, two nuclear protein complexes, which compete for binding to the CArG-like region of the MLC box, have been identified. One of the complexes binds to a mutation of the CArG-like region that inactivates transcription of a linked reporter gene, while binding of the other complex is inhibited by this mutation. This latter complex reacts with an antibody to serum response factor (SRF) and exhibits the same binding characteristics as purified SRF. These results demonstrate that transcriptional control of the chicken MLC1c/1s gene resides in an upstream enhancer that is composed of two separate AT-rich elements, both of which are required to drive expression of a linked reporter gene. The binding of a nuclear protein complex containing SRF to one of these elements, the MLC box, is required for gene activation and apparently inhibited by other nuclear factors whose binding overlaps that of the SRF complex.
Full text
PDF


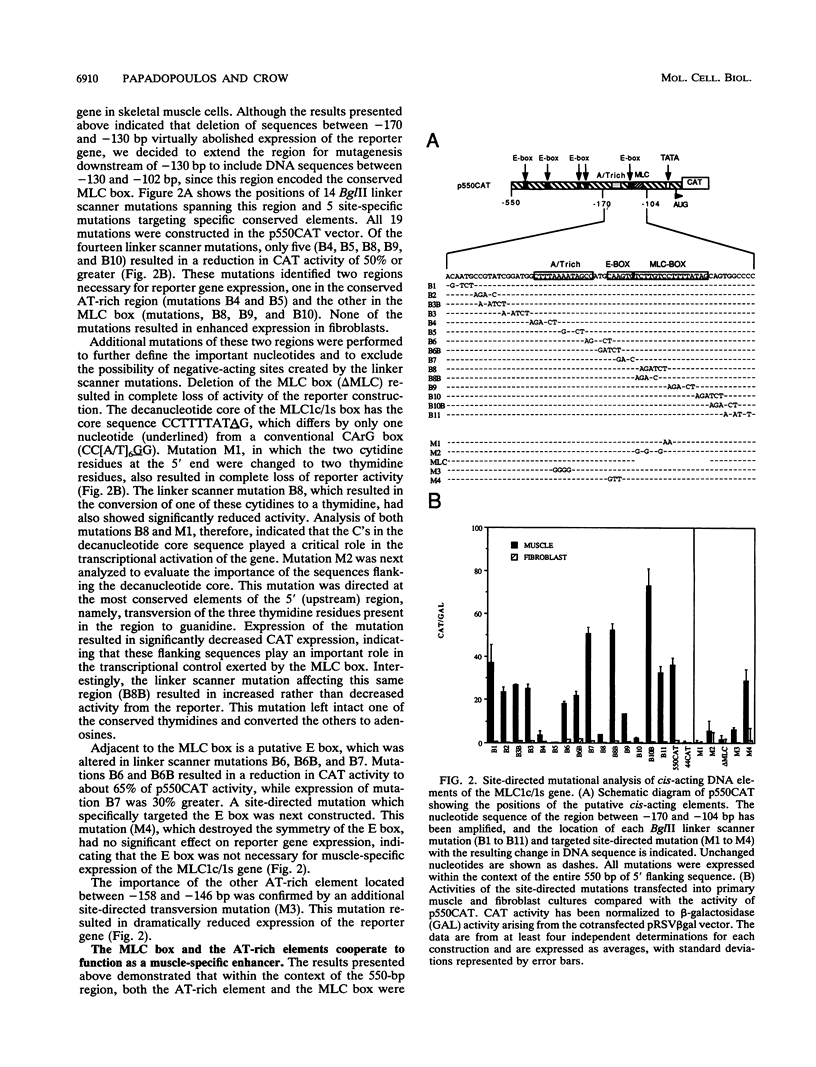




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton P. J., Buckingham M. E. The myosin alkali light chain proteins and their genes. Biochem J. 1985 Oct 15;231(2):249–261. doi: 10.1042/bj2310249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton P. J., Robert B., Cohen A., Garner I., Sassoon D., Weydert A., Buckingham M. E. Structure and sequence of the myosin alkali light chain gene expressed in adult cardiac atria and fetal striated muscle. J Biol Chem. 1988 Sep 5;263(25):12669–12676. [PubMed] [Google Scholar]
- Bergsma D. J., Grichnik J. M., Gossett L. M., Schwartz R. J. Delimitation and characterization of cis-acting DNA sequences required for the regulated expression and transcriptional control of the chicken skeletal alpha-actin gene. Mol Cell Biol. 1986 Jul;6(7):2462–2475. doi: 10.1128/mcb.6.7.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. M., Prywes R., Roeder R. G., Kedes L. The sarcomeric actin CArG-binding factor is indistinguishable from the c-fos serum response factor. Mol Cell Biol. 1989 Feb;9(2):515–522. doi: 10.1128/mcb.9.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow K. L., Schwartz R. J. A combination of closely associated positive and negative cis-acting promoter elements regulates transcription of the skeletal alpha-actin gene. Mol Cell Biol. 1990 Feb;10(2):528–538. doi: 10.1128/mcb.10.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Barton P. J., Robert B., Garner I., Alonso S., Buckingham M. E. Promoter analysis of myosin alkali light chain genes expressed in mouse striated muscle. Nucleic Acids Res. 1988 Nov 11;16(21):10037–10052. doi: 10.1093/nar/16.21.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. T., Olson P. S., Stockdale F. E. Myosin light-chain expression during avian muscle development. J Cell Biol. 1983 Mar;96(3):736–744. doi: 10.1083/jcb.96.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserjesi P., Lilly B., Bryson L., Wang Y., Sassoon D. A., Olson E. N. MHox: a mesodermally restricted homeodomain protein that binds an essential site in the muscle creatine kinase enhancer. Development. 1992 Aug;115(4):1087–1101. doi: 10.1242/dev.115.4.1087. [DOI] [PubMed] [Google Scholar]
- Cserjesi P., Olson E. N. Myogenin induces the myocyte-specific enhancer binding factor MEF-2 independently of other muscle-specific gene products. Mol Cell Biol. 1991 Oct;11(10):4854–4862. doi: 10.1128/mcb.11.10.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor W. L., Darras B., Seharaseyon J., Falkenthal S., Francke U., Vanin E. F. Human ventricular/slow twitch myosin alkali light chain gene characterization, sequence, and chromosomal location. J Biol Chem. 1989 Feb 5;264(4):2143–2149. [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S., Benfield P. A., Hobbs A. W. Distribution and properties of myosin isozymes in developing avian and mammalian skeletal muscle fibers. J Cell Biol. 1982 Feb;92(2):471–484. doi: 10.1083/jcb.92.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Siegfried Z., Ziff E. B. Mutation of the c-fos gene dyad symmetry element inhibits serum inducibility of transcription in vivo and the nuclear regulatory factor binding in vitro. Mol Cell Biol. 1987 Mar;7(3):1217–1225. doi: 10.1128/mcb.7.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualberto A., LePage D., Pons G., Mader S. L., Park K., Atchison M. L., Walsh K. Functional antagonism between YY1 and the serum response factor. Mol Cell Biol. 1992 Sep;12(9):4209–4214. doi: 10.1128/mcb.12.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Herrera R. E., Shaw P. E., Nordheim A. Occupation of the c-fos serum response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature. 1989 Jul 6;340(6228):68–70. doi: 10.1038/340068a0. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Chow K. L., Fang P., Schwartz R. J. Activation of skeletal alpha-actin gene transcription: the cooperative formation of serum response factor-binding complexes over positive cis-acting promoter serum response elements displaces a negative-acting nuclear factor enriched in replicating myoblasts and nonmyogenic cells. Mol Cell Biol. 1991 Oct;11(10):5090–5100. doi: 10.1128/mcb.11.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. C., Schwartz R. J. Differential detection of multiple DNA-binding complexes using dissimilar polyanion competitors. Nucleic Acids Res. 1992 Jan 11;20(1):140–140. doi: 10.1093/nar/20.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. C., Shi Y., Schwartz R. J. Displacement of BrdUrd-induced YY1 by serum response factor activates skeletal alpha-actin transcription in embryonic myoblasts. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9814–9818. doi: 10.1073/pnas.89.20.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung S., Miyamoto N. G. Point mutational analysis of the human c-fos serum response factor binding site. Nucleic Acids Res. 1989 Feb 11;17(3):1177–1195. doi: 10.1093/nar/17.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manak J. R., de Bisschop N., Kris R. M., Prywes R. Casein kinase II enhances the DNA binding activity of serum response factor. Genes Dev. 1990 Jun;4(6):955–967. doi: 10.1101/gad.4.6.955. [DOI] [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
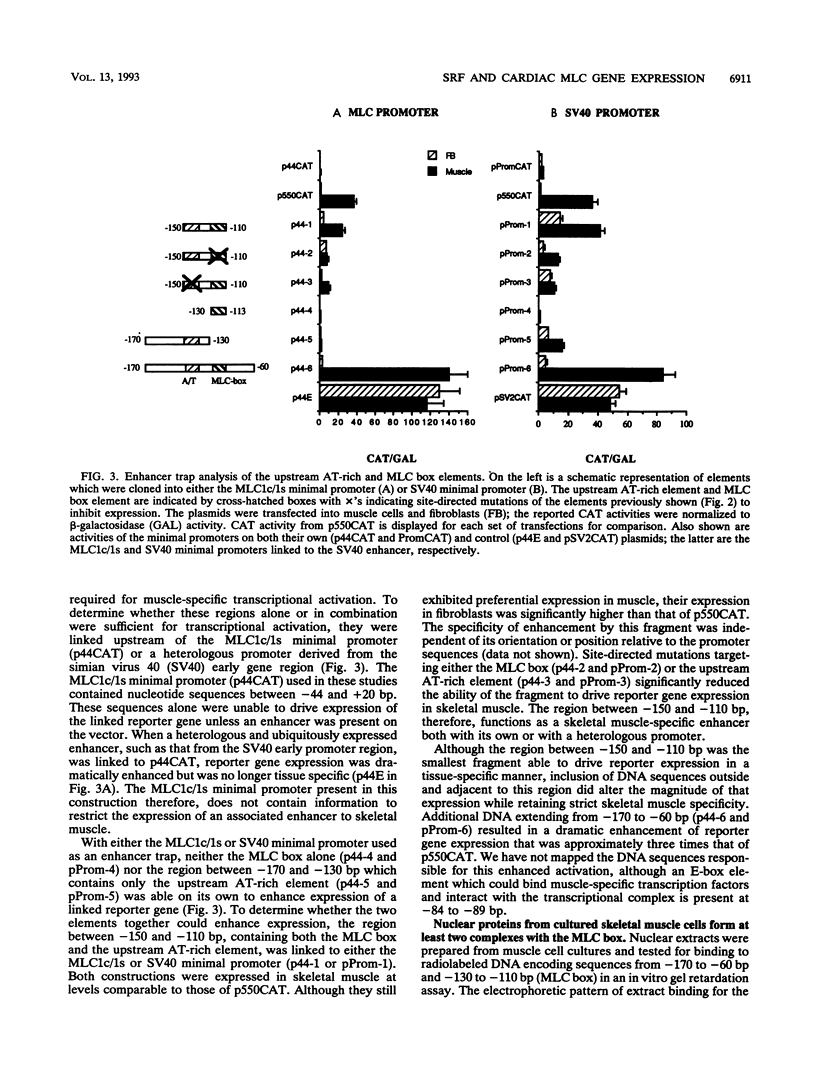
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Norman C., Runswick M., Pollock R., Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988 Dec 23;55(6):989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- Prywes R., Roeder R. G. Purification of the c-fos enhancer-binding protein. Mol Cell Biol. 1987 Oct;7(10):3482–3489. doi: 10.1128/mcb.7.10.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera V. M., Sheng M., Greenberg M. E. The inner core of the serum response element mediates both the rapid induction and subsequent repression of c-fos transcription following serum stimulation. Genes Dev. 1990 Feb;4(2):255–268. doi: 10.1101/gad.4.2.255. [DOI] [PubMed] [Google Scholar]
- Rubinstein N. A., Kelly A. M. Myogenic and neurogenic contributions to the development of fast and slow twitch muscles in rat. Dev Biol. 1978 Feb;62(2):473–485. doi: 10.1016/0012-1606(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Ryan W. A., Jr, Franza B. R., Jr, Gilman M. Z. Two distinct cellular phosphoproteins bind to the c-fos serum response element. EMBO J. 1989 Jun;8(6):1785–1792. doi: 10.1002/j.1460-2075.1989.tb03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli V., Webster K. A., Kedes L. Muscle-specific expression of the cardiac alpha-actin gene requires MyoD1, CArG-box binding factor, and Sp1. Genes Dev. 1990 Oct;4(10):1811–1822. doi: 10.1101/gad.4.10.1811. [DOI] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Stoflet E. S., Schmidt L. J., Elder P. K., Korf G. M., Foster D. N., Strauch A. R., Getz M. J. Activation of a muscle-specific actin gene promoter in serum-stimulated fibroblasts. Mol Biol Cell. 1992 Oct;3(10):1073–1083. doi: 10.1091/mbc.3.10.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Taylor M., Treisman R., Garrett N., Mohun T. Muscle-specific (CArG) and serum-responsive (SRE) promoter elements are functionally interchangeable in Xenopus embryos and mouse fibroblasts. Development. 1989 May;106(1):67–78. doi: 10.1242/dev.106.1.67. [DOI] [PubMed] [Google Scholar]
- Treisman R. Identification and purification of a polypeptide that binds to the c-fos serum response element. EMBO J. 1987 Sep;6(9):2711–2717. doi: 10.1002/j.1460-2075.1987.tb02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Treisman R. The serum response element. Trends Biochem Sci. 1992 Oct;17(10):423–426. doi: 10.1016/0968-0004(92)90013-y. [DOI] [PubMed] [Google Scholar]
- Tuil D., Clergue N., Montarras D., Pinset C., Kahn A., Phan-Dinh-Tuy F. CC Ar GG boxes, cis-acting elements with a dual specificity. Muscle-specific transcriptional activation and serum responsiveness. J Mol Biol. 1990 Jun 20;213(4):677–686. doi: 10.1016/S0022-2836(05)80255-4. [DOI] [PubMed] [Google Scholar]
- Uetsuki T., Nabeshima Y., Fujisawa-Sehara A., Nabeshima Y. Regulation of the chicken embryonic myosin light-chain (L23) gene: existence of a common regulatory element shared by myosin alkali light-chain genes. Mol Cell Biol. 1990 Jun;10(6):2562–2569. doi: 10.1128/mcb.10.6.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner B. J., Hayes T. E., Hoban C. J., Cochran B. H. The SIF binding element confers sis/PDGF inducibility onto the c-fos promoter. EMBO J. 1990 Dec;9(13):4477–4484. doi: 10.1002/j.1460-2075.1990.tb07898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K. Cross-binding of factors to functionally different promoter elements in c-fos and skeletal actin genes. Mol Cell Biol. 1989 May;9(5):2191–2201. doi: 10.1128/mcb.9.5.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K., Schimmel P. DNA-binding site for two skeletal actin promoter factors is important for expression in muscle cells. Mol Cell Biol. 1988 Apr;8(4):1800–1802. doi: 10.1128/mcb.8.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K., Schimmel P. Two nuclear factors compete for the skeletal muscle actin promoter. J Biol Chem. 1987 Jul 15;262(20):9429–9432. [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Yu Y. T., Breitbart R. E., Smoot L. B., Lee Y., Mahdavi V., Nadal-Ginard B. Human myocyte-specific enhancer factor 2 comprises a group of tissue-restricted MADS box transcription factors. Genes Dev. 1992 Sep;6(9):1783–1798. doi: 10.1101/gad.6.9.1783. [DOI] [PubMed] [Google Scholar]
- Zhou C., Abaigar L., Jong A. Y. A protocol for using T7 DNA polymerase in oligonucleotide site-directed mutagenesis. Biotechniques. 1990 May;8(5):503–503. [PubMed] [Google Scholar]
- Zhu Y. Y., Schwartz R. J., Crow M. T. Phorbol esters selectively downregulate contractile protein gene expression in terminally differentiated myotubes through transcriptional repression and message destabilization. J Cell Biol. 1991 Nov;115(3):745–754. doi: 10.1083/jcb.115.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]