Abstract
There are currently approximately 33.9 million individuals with Alzheimer's disease (AD) worldwide, and prevalence is expected to triple over the next 40 years. The goal of this review was to summarize the evidence regarding seven potentially modifiable AD risk factors: diabetes, mid-life hypertension, mid-life obesity, smoking, depression, low educational attainment and physical inactivity. In addition, we projected the impact of risk factor reduction on AD prevalence by calculating population attributable risks (PARs, the percent of cases attributable to a given factor) and the number of AD cases that could potentially be prevented by 10% and 25% risk factor reductions worldwide and in the US. Together, these factors contributed to up to half of AD cases globally (17.2 million) and in the US (2.9 million). A 10%–25% reduction in all seven risk factors could potentially prevent as many as 1.1–3.0 million cases worldwide and 184,000–492,000 cases in the US.
BACKGROUND
Alzheimer's disease (AD) is the most common cause of dementia, accounting for 60–80% of cases, although there is growing awareness that AD is often mixed with other dementia etiologies. There are currently approximately 33.9 million individuals with AD worldwide and 5.3 million in the US, and it is anticipated that prevalence will triple over the next 40 years due to demographic changes and longer life expectancies.1, 2 Currently available medications for dementia and AD have relatively small effect sizes and do not clearly alter disease progression,3 and several promising new agents have recently failed in Phase III clinical trials.4, 5 Given the current lack of disease-modifying treatments, as well as increasing awareness that symptoms develop over many years or even decades, there has been growing interest in identifying effective strategies for prevention. Delaying symptom onset by as little as one year could potentially lower AD prevalence by more than 9 million cases over the next 40 years.1
Observational studies have identified a wide range of potentially modifiable risk factors for AD and dementia, including cardiovascular risk factors (e.g., hypertension, diabetes, obesity), psychosocial factors (e.g., depression) and health behaviors (e.g., low level of physical or mental activity, smoking).6 However, few randomized, controlled trials (RCTs) have examined the impact of risk factor modification on AD incidence and even fewer have investigated several factors at once.
The goal of this review was to provide an updated summary of the evidence related to several potentially modifiable risk factors and AD risk and to project the impact of risk factor reduction on AD prevalence by calculating population attributable risks (PARs), which take into account the prevalence of a given risk factor as well as the strength of its association with the outcome of interest. PAR estimates are important because they can help identify the intervention strategies that are likely to result in the greatest impact on disease prevalence.7
METHODS
Search strategy and selection criteria
The National Institutes of Health (NIH) in the US recently commissioned an independent state-of-the-science (SOS) report that included a comprehensive systematic review of the evidence related to risk factors for AD and cognitive decline.6 Although the report highlighted many limitations of the available evidence, several potentially modifiable factors were identified as being associated with increased risk of cognitive decline and/or AD. The factors with the most consistent evidence included diabetes mellitus, current smoking, depression, cognitive inactivity, physical inactivity, and poor diet (high saturated fat/low vegetable intake). Therefore, we initially focused our review on these six factors. We subsequently chose to include hypertension and obesity based on findings from more recent meta-analyses.8, 9 In addition, we excluded diet due to heterogeneity in the types of dietary factors studied and lack of data on prevalence. Thus, our final list of potentially modifiable risk factors included diabetes, hypertension, obesity, current smoking, depression, cognitive inactivity and physical inactivity.
For each of these risk factors, we searched the Cochrane Database of Systematic Reviews (all years) and PUBMED (2005–2011) to identify English-language systematic reviews and meta-analyses for associations with AD or dementia. Searches were performed separately for each risk factor of interest, and references in articles identified also were searched.
Relative risk of AD
Relative risk (RR) estimates were based on the best available adjusted estimates from Cochrane reviews when available or on the most recent and comprehensive meta-analysis when Cochrane reviews had not been performed. If no Cochrane reviews or meta-analyses were identified, we performed a meta-analysis based on studies included in the most recent and comprehensive systematic review(s). RR estimates for AD were used when available; otherwise, RR estimates for dementia were used.
Prevalence of AD and risk factors
Current prevalence of AD worldwide was estimated as approximately 33.9 million through linear extrapolation of estimates from 2006.1 Prevalence of AD in the US was estimated as 5.3 million based on estimates from the Alzheimer's Association.2
For each risk factor, current global and US prevalence estimates were determined by searching PUBMED, Google and the US Census website. When risk factor data suggested that risk was restricted to a specific age range (e.g., mid-life only), risk factor prevalence estimates also were restricted to that age group (i.e., by calculating the percentage of the population that had the risk factor and was in the age group of interest).
Population attributable risk (PAR), number of attributable cases and number of cases prevented
Population attributable risk (PAR) refers to the proportion of cases of a disease in a population that can be `attributed' to a given risk factor, assuming that there is a causal relationship.10, 11 It takes into account the strength of the association between the risk factor and the outcome as well as the prevalence of the risk factor. We calculated PAR for each individual risk factor using the Levin formula:10
where PRF refers to the population prevalence of the risk factor and RR refers to the relative risk. As others have described,11, 12 this formula was originally developed for use with unadjusted RR estimates in the setting of a single risk factor and single outcome, whereas we were interested in calculating PAR estimates for multiple inter-related risk factors. Several alternative formulas are available for calculating PARs adjusted for confounders and effect modifiers and in the setting of multiple collinear risk factors.11, 13 However, we were unable to use these alternative formulas because they require analysis of raw data from a single study. Therefore, we applied adjusted RR estimates from meta-analyses to Levin's original formula. Because adjusted RR estimates from meta-analyses are calculated using adjusted RR estimates from individual studies, each of which may have adjusted for different factors, it is difficult to determine precisely what is “adjusted” for in these estimates. None-the-less, we acknowledge that use of adjusted RR estimates with Levin's formula is a limitation.14 However, a recent study found that PAR estimates are biased toward the null when adjusted RR estimates used in Levin's formula are smaller than crude RR estimates,12 which is the case in most studies of AD. Therefore, assuming a causal relationship between the risk factors examined and AD, our PAR estimates are likely to be underestimates rather than overestimates.
We also calculated a combined PAR, or the effect of simultaneous reduction of all of the risk factors examined, using the formula:
This formula is superior to simply adding PAR estimates together because it ensures that the total combined PAR does not exceed 100%. However, it assumes that risk factors are independent and that an additive relationship exists between them, which is unlikely to be the case with the risk factors under consideration. Therefore, these combined PAR estimates should be considered as maximums. Other methods for calculating combined PAR estimates—such as sequential or average sequential PAR—can only be used when raw data are available.13
Finally, we estimated the total number of AD cases currently attributable to risk factors by multiplying the PAR estimates by the current prevalence of AD. We also determined the number of cases that could potentially have been prevented if risk factor prevalence were 10% or 25% lower than their current levels by using the formulas above and reducing current prevalence estimates by 0.90 and 0.75, respectively, and subtracting the revised number of attributable cases from the original number. We also calculated “confidence ranges” for our estimates of PAR, number of cases attributable and number of cases potentially prevented using the 95% confidence intervals from the RR estimates.
FINDINGS
Diabetes mellitus
Relative Risk for AD
Diabetes has been associated with an increased risk of AD and dementia in several studies. A recent meta-analysis by Lu et al.15 identified eight prospective, population-based studies that have examined the association between diabetes mellitus and risk of AD, vascular dementia (VaD) and all-cause dementia. For AD, two studies found a statistically significant increase in AD risk in subjects with diabetes while five studies found a non-significant increase resulting in a combined RR estimate of 1.39 (95% confidence interval [CI]: 1.17, 1.66). When all-cause dementia was considered, the combined RR was 1.47 (1.25, 1.73).
Another recent meta-analysis by Profenno et al.9 identified nine prospective studies that examined the association between diabetes and dementia, six of which overlapped with the Lu meta-analysis. Four of these nine studies found a significant association between diabetes and all-cause dementia with a pooled RR estimate of 1.54 (1.33, 1.79). AD was not examined as a specific outcome in this meta-analysis.
A Cochrane review updated in 2005 identified five RCTs that examined the effects of treatment of type 2 diabetes on cognitive outcomes.16 Two provided limited evidence of a beneficial treatment effect while three did not include objective measures of cognitive function. None of the RCTs examined the impact of treatment on AD incidence. Therefore, we based our PAR calculations on the Lu et al. meta-analysis RR summary estimate from observational studies of 1.39 for risk of AD.
Prevalence
The global prevalence of diabetes mellitus in the year 2010 was 6.4% (285 million adults), and this was projected to increase to 7.7% (439 million adults) by 2030.17, 18 Diabetes prevalence was highest in North America (10.2%) and lowest in Africa (3.8%). In the US, the age-adjusted prevalence of diabetes in adults age 18 years or older in 2009 was 8.7%.19
Population Attributable Risk and Number of Cases Prevented
Approximately 2% (825,000) of AD cases worldwide are currently attributable to diabetes (Table 1), including 3% (nearly 175,000) in the US (Table 2). If diabetes prevalence were 10% lower than current levels, we estimate more than 80,000 AD cases worldwide and nearly 17,000 cases in the US could potentially be prevented; a 25% lower diabetes prevalence could potentially prevent more than 200,000 cases worldwide and 40,000 cases in the US.
Table 1.
Estimated Percent and Number of Alzheimer's Disease Cases Attributable to Potentially Modifiable Risk Factors Globally
| RISK FACTOR | POPULATION PREVALENCE | RELATIVE RISK (95% CI) | PAR% (Confidence Range) | NO. CASES ATTRIBUTABLE, Millions (Confidence Range) |
|---|---|---|---|---|
| Low education | 40.0% | 1.59 (1.35, 1.86) | 19.1% (12.3%, 25.6%) | 6.5 (4.2, 8.7) |
| Smoking | 27.4% | 1.59 (1.15, 2.20) | 13.9% (3.9%, 24.7%) | 4.7 (1.3, 8.4) |
| Physical inactivity | 17.7% | 1.82 (1.19, 2.78) | 12.7% (3.3%, 24.0%) | 4.3 (1.1, 8.1) |
| Depression | 13.2% | 1.90 (1.55, 2.33) | 10.6% (6.8%, 14.9%) | 3.6 (2.3, 5.1) |
| Mid-life hypertension | 8.9% | 1.61 (1.16, 2.24) | 5.1% (1.4%, 9.9%) | 1.7 (0.5, 3.4) |
| Diabetes | 6.4% | 1.39 (1.17, 1.66) | 2.4% (1.1%, 4.1%) | 0.8 (0.4, 1.4) |
| Mid-life obesity | 3.4% | 1.60 (1.34, 1.92) | 2.0% (1.1%, 3.0%) | 0.7 (0.4, 1.0) |
| Combined (maximum) | 50.7% | 17,187,028 |
PAR, population attributable risk.
Table 2.
Estimated Percent and Number of Alzheimer's Disease Cases Attributable to Potentially Modifiable Risk Factors in the US
| RISK FACTOR | POPULATION PREVALENCE | RELATIVE RISK (95% CI) | PAR% (Confidence Range) | NO. CASES ATTRIBUTABLE Thousands (Confidence Range) |
|---|---|---|---|---|
| Physical inactivity | 32.5% | 1.82 (1.19, 2.78) | 21.0% (5.8%, 36.6%) | 1115 (308, 1942) |
| Depression | 19.2% | 1.90 (1.55, 2.33) | 14.7% (9.6%, 20.3%) | 781 (506, 1078) |
| Smoking | 20.6% | 1.59 (1.15, 2.20) | 10.8% (3.0%, 19.8%) | 574 (159, 1050) |
| Mid-life hypertension | 14.3% | 1.61 (1.16, 2.24) | 8.0% (2.2%, 15.1%) | 425 (119, 798) |
| Mid-life obesity | 13.1% | 1.60 (1.34, 1.92) | 7.3% (4.3%, 10.8%) | 386 (226, 570) |
| Low education | 13.3% | 1.59 (1.35, 1.86) | 7.3% (4.4%, 10.3%) | 386 (236, 544) |
| Diabetes | 8.7% | 1.39 (1.17, 1.66) | 3.3% (1.5%, 5.4%) | 174 (77, 288) |
| Combined (maximum) | 54.1% | 2,866,951 |
PAR, population attributable risk.
Hypertension
Relative Risk of AD
Several articles have recently performed systematic reviews of the evidence that hypertension is associated with an increased risk of AD or dementia20–22 and that treatment of hypertension is associated with reduced risk of AD or dementia.8, 23 An initial systematic review published in 2005 found that the association between blood pressure and risk of dementia is complex and appears to differ based on age.22 Hypertension in mid-life was consistently associated with increased risk of AD and dementia in late-life, with four of five studies finding a significant association in fully adjusted models. One study found that the association was restricted to those with untreated mid-life hypertension. In contrast, hypertension in late-life was not consistently associated with risk of AD or dementia, with eight of 13 studies finding no significant association. Instead, hypotension in late-life was consistently associated with increased risk of AD and dementia, particularly in individuals who took antihypertensive medications. These early findings were confirmed in two more recent systematic reviews.21, 24 However, none of these studies included meta-analyses to quantify the magnitude of the association between hypertension—particularly mid-life hypertension—and risk of AD or dementia.
A Cochrane systematic review and meta-analysis examined the effects of hypertension treatment on dementia risk, identifying four RCTs that included a total of 15,936 individuals with hypertension and studied dementia incidence as a secondary outcome.8 A pooled meta-analysis of these studies found no significant difference in dementia incidence in the treatment versus placebo groups (odds ratio [OR], 0.89; 95% CI: 0.74, 1.07, p=0.21). However, cognitive decline as measured by the Mini-Mental State Examination (MMSE) was significantly lower in the treatment versus placebo groups (weighted mean difference [WMD], 0.42 points; 95% CI: 0.30, 0.53). Another meta-analysis of hypertension treatment trials found a comparable effect size for dementia incidence that was of borderline statistical significance (pooled OR, 0.87; 95% CI: 0.76, 1.00; p=0.045).25 A third meta-analysis found differential effects based on the type of hypertension treatment,26 although conflicting findings were observed in another study that included observational studies and RCTs.27
Given the available evidence from epidemiologic studies and RCTs, we conclude that mid-life, but not late-life, hypertension is associated with an increased risk of AD and dementia. We therefore pooled results from studies of mid-life hypertension that have been included in systematic reviews28–32 to calculate a weighted OR of 1.61 (95% CI: 1.16, 2.24) (Appendix 1), which was used to calculate PAR estimates for mid-life hypertension.
Prevalence
Because the association between hypertension and AD was restricted to mid-life, prevalence for PAR estimates was calculated for mid-life hypertension only (i.e., by calculating the joint probability of being both middle-aged and hypertensive in the population). To estimate prevalence of mid-life hypertension, we combined data on age- and gender-specific hypertension prevalence estimates globally33 and in the US34 with corresponding population estimates obtained through the U.S. Census Bureau International Data Base population calculator to obtain estimates of 8.9% worldwide and 14.3% in the US (Appendices 2a and 2b).
Population Attributable Risk and Number of Cases Prevented
Worldwide, approximately 5% (1.7 million) AD cases are potentially attributable to mid-life hypertension (Table 1). If the prevalence of mid-life hypertension were 10% lower than current levels, we estimate that there would be >160,000 fewer AD cases; a 25% lower prevalence of mid-life hypertension would be associated with more than 400,000 fewer AD cases. In the US, approximately 8% (>425,000) AD cases are potentially attributable to mid-life hypertension (Table 2). A 10% reduction in prevalence of mid-life hypertension could potentially lower AD prevalence by nearly 40,000 cases; a 25% reduction could lower prevalence by nearly 100,000 cases.
Obesity
Relative Risk of AD
A recent systematic review identified 10 prospective studies that examined the association between various measures of body weight and dementia, of which 7 were suitable for inclusion in a meta-analysis.35 Three of four studies found that body mass index (BMI) (as a continuous measure) was associated with an increased risk of all-cause dementia; two of five studies found that obesity (BMI ≥ 30) was associated with increased risk of all-cause dementia; and two of five studies found that obesity was associated with an increased risk of AD. Pooled results indicated that the association between obesity and AD was statistically significant (OR, 1.80; 95% CI: 1.00, 3.29),35 which was confirmed in a more recent meta-analysis that included six studies on obesity and AD (RR, 1.59; 95% CI: 1.02, 2.48).9
Similar to hypertension, there is evidence that the association between weight and AD may change with age.36 A recent study that was not included in either of the meta-analyses above found that obesity in mid-life was associated with a significantly increased risk of dementia (HR, 1.39; 95% CI: 1.03, 1.87); however, in late-life, obesity was associated with reduced dementia risk (HR, 0.63; 95% CI: 0.44, 0.91) while being underweight was associated with increased risk (HR, 1.62; 95% CI: 1.02, 2.64).37 Some studies have found that low BMI in late life is associated with an increased risk of AD and dementia,38, 39 and that BMI declines up to ten years prior to development of symptoms,39, 40 although other studies have found the opposite.41
Based on the available evidence, we conclude that there is evidence of an association between mid-life obesity and increased risk of dementia. We therefore calculated a pooled RR estimate of 1.60 (95% CI: 1.34, 1.92) based on studies included in prior systematic reviews (Appendix 3).
Prevalence
We determined the prevalence of mid-life obesity by combining age- and gender-specific obesity prevalence rates globally42 and in the US43 with corresponding population estimates from the US Census International Data Base (Appendices 4a and 4b). We estimated that 3.4% of adults worldwide were both obese and middle-aged in 2005.42 Obesity rates were consistently higher in women than men, but they varied substantially by country, with the lowest rates observed in India, Asia and Sub-Saharan African men and the highest rates observed in established industrialized economies such as the US and USSR. In the US, prevalence of mid-life obesity was estimated as 13.1%.
Population Attributable Risk and Number of Cases Prevented
Approximately 2% (677,000) AD cases worldwide are potentially attributable to mid-life obesity. In the US, the PAR is higher—7.3% (386,000 cases)—due to the higher prevalence of mid-life obesity. A 10% reduction in mid-life obesity prevalence could potentially prevent more than 66,000 AD cases worldwide and 36,000 cases in the US; a 25% reduction could potentially lower AD prevalence by more than 166,000 cases worldwide and 91,000 cases in the US.
Depression
Relative Risk of AD
An early meta-analysis of 13 studies found that a history of depression was associated with approximately a two-fold increase in risk of dementia, with pooled relative risk estimates of 2.01 (1.16, 3.50) for 7 case-control studies and 1.87 (1.09, 3.20) for 6 prospective studies.44 A more recent systematic review and meta-analysis identified 20 studies of 102,172 individuals from 8 countries and found very similar results for AD with pooled odds ratio estimates of 2.03 (1.73, 2.38) for 9 case-control studies and 1.90 (1.55, 2.33) for 11 cohort studies.45
Several RCTs have found that treatment of depression in older adults results in improved cognitive function,46–49 although some studies have found no improvement,50 and cognitive function typically remains below normal levels. In addition, some types of anti-depressant therapies—particularly those with anti-cholinergic properties—may impair or worsen cognitive function.51 To our knowledge, no studies have been published to determine whether treatment of late-life depression can lower or delay dementia incidence. Therefore, PAR calculations were based on the more recent estimate from longitudinal studies of 1.90 as our estimate of relative risk.45
Prevalence
Estimates of depression prevalence vary widely depending on the study population and definition of depression52, 53 and are more widely available for 12-month prevalence than lifetime prevalence. A recent study found that the 12-month prevalence of major depressive disorder worldwide is 5.5% for developed countries and 5.9% for developing countries, with estimates ranging from 2.2% (Japan) to 10.4% (Brazil); however, lifetime prevalence of depression was not reported.54 In the US, the prevalence of 12-month major depressive disorder is 8.3% while the prevalence of lifetime major depressive disorder is 19.2%.55 Because lifetime depression prevalence estimates were available for the US but not globally, we estimated lifetime prevalence worldwide by assuming that the US/global ratio would be similar for 12-month and lifetime estimates: that is, 12-month estimates were 8.3% in the US and 5.7% (median value) globally (ratio, 1.46:1); given a lifetime prevalence of 19.2% in the US, we estimated that the lifetime prevalence of depression globally was approximately 13.2%.
Population Attributable Risk and Number of Cases Prevented
More than 10% (nearly 3.6 million) AD cases worldwide and almost 15% (>780,000) in the US may be attributable to depression. A 10% reduction in depression prevalence could potentially result in more than 325,000 fewer AD cases worldwide and 67,000 fewer cases in the US; a 25% reduction in depression prevalence could potentially result in more than 826,000 fewer AD cases worldwide and 172,000 cases in the US.
Physical inactivity
Relative Risk of AD
A recent systematic review and meta-analysis identified 16 prospective studies on the association between physical activity and dementia that included 163,797 non-demented older adults at baseline and 3,219 cases of dementia at follow-up.56 The combined RR in the highest versus lowest physical activity groups was 0.72 (95% CI: 0.60, 0.80) for all-cause dementia and 0.55 (95% CI: 0.36, 0.84) for AD. Reversing these values to reflect risks associated with inactivity yields 1.39 (95% CI: 1.16, 1.67) for all-cause dementia and 1.82 (95% CI: 1.19, 2.78) for AD. Another systematic review that included a wider range of cognitive outcomes reached similar conclusions, finding that physical inactivity was associated with an increased risk of cognitive impairment in 20 of 24 longitudinal studies identified, but did not provide pooled RR estimates.57
These findings from observational studies are supported by RCTs which have found that healthy, sedentary elders who begin exercise programs experience significant improvements in cognitive function, particularly mental processing speed.58 To our knowledge, there are no published RCTs to determine whether an exercise intervention can lower or delay AD incidence, although several trials are planned or ongoing. Therefore, our PAR estimates used a RR of 1.82 for AD.56
Prevalence
A recent study that included 51 countries worldwide found that 17.7% of the pooled sample were inactive, including 15.2% of men and 19.8% of women.59 In most countries, prevalence of inactivity was higher in women, in the elderly and in those living in urban environments. In the US, 32.5% of adults age 18 years or older were considered inactive in 2009 while 32.5% had some leisure-time physical activity and 34.9% were regularly active.19 As in other countries, prevalence of inactivity increased with age. Because there is evidence that physical activity throughout the life-course is associated with better cognitive function,60 PAR estimates were based on the prevalence of inactivity in the total population.
Population Attributable Risk and Number of Cases Prevented
Worldwide, approximately 13% (nearly 4.3 million) AD cases may be attributable to physical inactivity, including 21% (>1.1 million) in the US. A 10% reduction in the prevalence of physical inactivity could potentially prevent more than 380,000 AD cases globally and nearly 90,000 cases in the US, while a 25% reduction in physical inactivity prevalence could potentially prevent nearly 1 million AD cases globally and 230,000 in the US.
Smoking
Relative Risk of AD
Although several early case-control studies found that smoking was associated with a reduced risk of AD,61 more recent longitudinal studies have found that the risks of AD and dementia are increased with smoking.62–64 A meta-analysis of 19 prospective studies found that current smoking was associated with a significantly increased risk of dementia (RR, 1.27; 95% CI: 1.02, 1.60) and AD (RR, 1.79; 95% CI: 1.43, 2.23).62 However, a more recent meta-analysis that included 23 longitudinal studies found slightly lower risk estimates of 1.16 (95% CI: 0.90, 1.50) for all-cause dementia and 1.59 (95% CI: 1.15, 2.20) for AD.64 A third meta-analysis was published more recently but only included 17 longitudinal studies and focused on the effects of tobacco industry affiliations: in longitudinal studies without tobacco industry-affiliated authors, the RR for AD among smokers was 1.45 (95% CI: 1.16, 1.80).63 Our PAR calculations used a RR of 1.59 for AD since this was based on the most comprehensive meta-analysis.64 Former smoking was not associated with AD risk in most studies.62
Prevalence
The worldwide prevalence of smoking in individuals aged 15 years or older in 1995 was 29%, with the highest prevalence observed in Europe and Asia (34%) and the lowest in sub-Saharan Africa (18%).65 Smoking prevalence was more than four times higher in men (47%) than women (11%). More recent surveys also have found a wide range of smoking prevalence globally (3.9% to 36%) with a median of 27.4%.66 In the US, 20.6% of adults age 18 years or older were current cigarette smokers in 2009.67
Population Attributable Risk and Number of Cases Prevented
We estimate that nearly 14% (4.7 million) AD cases worldwide and 11% (575,000) in the US are attributable to smoking. A 10% reduction in smoking prevalence could potentially lower AD prevalence by more than 400,000 cases globally and 50,000 cases in the US; a 25% reduction in smoking prevalence could potentially prevent more than 1 million cases worldwide and 130,000 cases in the US.
Cognitive inactivity
Relative Risk of AD
We identified two systematic reviews/meta-analyses related to cognitive inactivity and risk of AD or dementia. The first study examined risk of dementia associated with a wide range of markers of `brain reserve,' which refers generally to the capacity of the brain to withstand the effects of pathology by recruiting alternative neurological processes or pathways.68 A total of 22 longitudinal studies that included 21,456 individuals and 1,733 cases of dementia were identified. The risk of dementia was significantly lower for those with higher education (OR, 0.53; 95% CI: 0.45, 0.62), occupational attainment (OR, 0.56; 95% CI: 0.49, 0.65), intelligence/IQ (OR, 0.58; 95% CI: 0.44, 0.77) and mentally stimulating leisure activities (OR, 0.50; 95% CI: 0.42, 0.61). When all of these brain reserve markers were combined, the pooled OR was 0.54 (95% CI: 0.49, 0.59). This can also be expressed as its inverse: the odds of dementia were significantly increased in those with low brain reserve (OR, 1.85; 95% CI: 1.69, 2.04).
The second study identified 19 studies (13 cohort, 6 case-control) that examined the association between low education and risk of AD or dementia.69 For AD, the combined RR was 1.80 (95% CI: 1.43, 2.27); however, the estimate from cohort studies (RR, 1.59; 95% CI: 1.35, 1.86) was markedly lower than the estimate from case-control studies (RR, 2.40; 95% CI: 1.32, 4.38). For dementia, the combined RR for low versus high education also was 1.59 (95% CI: 1.26, 2.01).
These observational findings are supported by results from RCTs, which have found that cognitive interventions in healthy, older adults are associated with domain-specific improvements in cognitive function.70, 71 A recent systematic review identified 10 RCTs that were associated with a mean effect size (Cohen's d) for improvement in cognitive function of 0.16 (95% CI: 0.138, 0.186).72 Similarly, a Cochrane review identified 36 RCTs that included a total of 2,229 participants and found significant improvements in immediate and delayed recall when compared with a no-contact control.73 However, to date, no published RCT has examined the impact of a mental activity intervention on AD incidence. Because prevalence estimates are available for low education but not low brain reserve, our PAR estimates were calculated using the estimate of 1.59 that was based on cohort studies of the association between low education and risk of AD.69
Prevalence
Data from 146 countries indicate that, in 2010, 14.8% of individuals worldwide had not received any formal schooling and an additional 25.2% had only attended primary school for a total of 40.0% with low educational attainment.74 In the US, 13.3% of individuals age 25 years or older had completed less than 12 years of high school in 2009.75
Population Attributable Risk and Number of Cases Prevented
Worldwide, approximately 19% (6.5 million) of AD cases are potentially attributable to low education, including 7% (>385,000) cases in the US. A 10% reduction in the prevalence of low educational attainment could potentially lower AD prevalence by more than 500,000 cases globally and 36,000 cases in the US; a 25% reduction could potentially lower AD prevalence by nearly 1.4 million cases globally and 91,000 cases in the US.
Combined
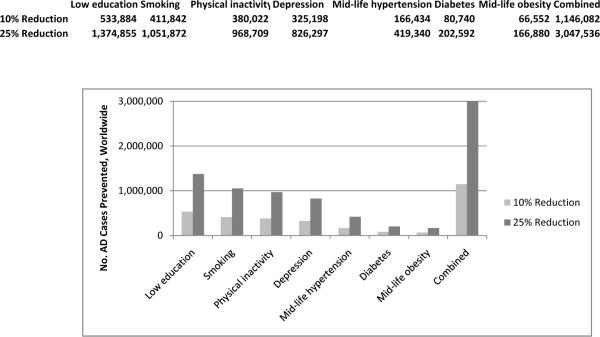
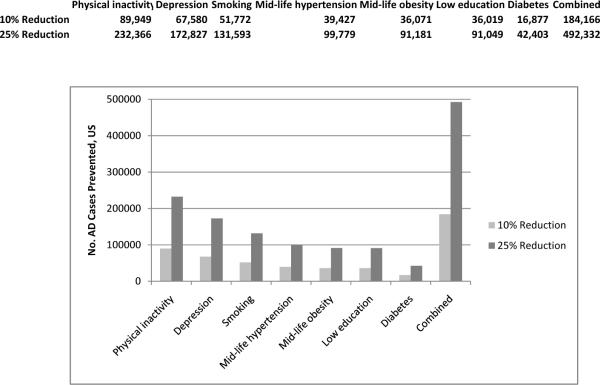
Together, we estimate that these seven potentially modifiable risk factors contribute to up to half of AD cases globally (17.2 million, Figure 1) and in the US (2.9 million) (Figure 2). If the prevalence of all seven risk factors were 10% lower, we estimate that there would be as many as 1.1 million fewer AD cases globally and 184,000 fewer cases in the US; if risk factor prevalence were 25% lower, AD prevalence could potentially be reduced by up to 3.0 million cases globally and 492,000 in the US.
Figure 1.
The number of Alzheimer's disease (AD) cases that could potentially be prevented through risk factor reductions of 10% or 25% worldwide (Figure 1a) and in the US (Figure 1b) was estimated by multiplying current prevalence estimates by 0.90 and 0.75, respectively, and subtracting the revised number of attributable cases from the original number. These estimates assume that a causal relationship exists between the risk factor and AD and that the relative risk estimate is a good approximation of the impact of risk factor reduction. Therefore, the actual number of cases prevented could be higher or lower depending on the extent to which these assumptions are valid. In addition, the combined estimate assumes that the individual risk factors are independent and have an additive relationship. Because several of the risk factors examined are inter-related, the combined PAR estimates should be considered as maximums.
Figure 2.
INTERPRETATION
Our findings suggest that up to half of AD cases may be attributable to modifiable risk factors. Furthermore, we expect that these findings would be similar for all-cause dementia. Our review focused on AD because most of the meta-analyses identified focused on AD. However, AD contributes to most cases of dementia, and risk factors for AD and all-cause dementia are generally similar. Therefore, it is likely that attributable risk estimates for all-cause dementia would be similar to the estimates presented here for AD.
Low education contributed to the largest proportion of AD cases worldwide. Mechanistically, it is believed that education and mental stimulation throughout life may lower risk of AD and dementia by helping to build a “cognitive reserve” that enables individuals to continue functioning at a `normal' level despite experiencing neurodegenerative changes.76 This theory is supported by neuropathological studies which show that many older adults with normal cognitive function meet neuropathological criteria for AD at autopsy.68 Similarly, AD biomarkers appear to be less predictive of development of AD in those with high cognitive reserve.77 When combined with mostly positive RCT results for cognitive training,72, 73 these findings suggest that interventions to enhance educational opportunities throughout the lifecourse could potentially prevent millions of AD cases from becoming symptomatic, thereby substantially reducing future AD prevalence.
Smoking contributed to the second-largest number of AD cases globally as well as a substantial proportion in the US. The most likely mechanism underlying the association between smoking and AD is vascular disease.78 Smoking contributes to a variety of subclinical and clinical vascular disorders including atherosclerosis and cerebrovascular disease79 which, in turn, could lead to increased risk of AD.80, 81 However, tobacco smoke also contains hundreds of chemicals that are known to be neurotoxins and could contribute to AD risk through oxidative stress or inflammatory processes.79
Physical inactivity contributed to the largest proportion of AD cases in the US and the third-largest proportion globally. There are several potential mechanisms by which physical inactivity could contribute to risk of AD and dementia.82 First, physical inactivity is associated with increased risk of several cardiovascular risk factors—such as diabetes, hypertension and obesity83, 84—that in turn are associated with increased risk of dementia.9, 24 Second, physical activity appears to have a direct beneficial effect on brain structure and function in both animals and humans.{Cotman, 2007 #222; Voss, 2010 #673} As with mental activity, the benefits of physical activity may accrue over the lifecourse.85 Therefore, public health campaigns targeted at increasing levels of physical activity on a societal level could have a profound impact on future AD prevalence.
Depression contributed to the second-largest proportion of AD cases in the US and was the fourth-largest contributor globally. Although there remains controversy regarding whether depression reflects a true etiologic risk factor for AD or is a prodromal symptom, several recent studies with long (10–20 year) follow-up periods have begun to shift the weight of evidence toward the risk factor hypothesis in at least some cases.86, 87 Vascular disease has been hypothesized as one of the potential mechanisms by which depression could increase risk of dementia and cognitive impairment88, 89 since there is evidence of a reciprocal relationship between depression and vascular disease, and vascular disease contributes to the clinical manifestation of AD and dementia.80, 81 Depression also is associated with alterations in stress-related hormones, lower levels of neuronal growth factors and reduced hippocampal volume.88
Mid-life obesity, mid-life hypertension and diabetes also contributed to a substantial proportion of cases worldwide and in the US. These conditions are inter-related, and it is generally hypothesized that they contribute to AD largely through a vascular mechanism.80, 81 However, adipose tissue produces several substances that are important in metabolism (adipokines) and inflammation (cytokines) and are correlated with insulin resistance and hyperinsulemia. It has been hypothesized that peripheral hyperinsulemia could potentially inhibit brain insulin production, which could in turn result in impaired amyloid clearance in the brain.90 Diabetes could also impact cognition through its affects on blood glucose levels, insulin resistance, inflammation or alterations in beta-amyloid metabolism.91–93
Strengths and limitations
The primary strength of our study is that estimates were based on the best available prevalence and relative risk estimates from recent systematic reviews and meta-analyses. However, there are several limitations. First, PAR estimates assume that there is a causal relationship between the risk factor and the outcome and that the magnitude of the RR estimate is a good approximation of the impact of risk factor removal on disease incidence. However, AD is a multifactorial disease, and it is not known whether removal of a single risk factor will actually lower AD incidence. Many of the risk factors we examined are inter-related. For example, hypertension, diabetes and obesity often co-occur94 and can be affected by physical activity.83, 84, 95 In addition, most of the risk factors we examined are associated with greater cardiovascular disease, which has been implicated as a contributing factor in the clinical manifestation of AD and dementia.80, 81 Therefore, risk reduction strategies that target multiple risk factors may be required to lower AD risk.
Second, our global PAR estimates may not apply to most individual countries or communities. PAR estimates are based on risk factor prevalence and relative risk. Since there was not much variation in the relative risk estimates (RR range: 1.39 – 1.90), differences in PAR estimates were largely driven by differences in risk factor prevalence. Therefore, the most important AD risk factors for a given country or community are likely be the ones that are most prevalent.
Third, there are other potentially modifiable risk factors that were not included in our estimates. In particular, there is growing evidence that dietary patterns such as the Mediterranean diet are associated with lower AD risk.96, 97 We did not include diet due to the heterogeneity of dietary factors that have been studied, the relatively small number of studies on each individual dietary factor and lack of prevalence data, but we acknowledge that diet may be another important modifiable AD risk factor.
Finally, we acknowledge that these are estimates and that they may change as additional data become available. They are provided to guide policy- and decision-makers regarding the AD prevention strategies that are likely to have the greatest impact on AD prevalence given current risk factor profiles.
Ultimately, RCTs are critically needed to directly assess the impact of single and multiple risk factor reduction strategies on AD incidence and prevalence. Several ongoing RCTs—including the Multi-domain Intervention in the Prevention of Age-related Cognitive Decline (MAPT) in France, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) and the Lifestyle Interventions and Independence for Elders (LIFE) in the US—will provide important insights into the impact of risk factor modification on cognitive impairment and decline; additional RCTs should include AD incidence as the primary outcome.
Summary
Up to half of AD and dementia cases worldwide may be attributable to potentially modifiable risk factors. Globally, lack of education and smoking were the greatest contributors to AD risk, suggesting that the most effective strategies for lowering AD prevalence may be public education campaigns and smoking cessation initiatives. Physical inactivity contributed to the largest proportion of AD cases in the US as well as a substantial proportion of cases globally. Since physical inactivity is associated with most of the other AD risk factors identified—including depression, mid-life obesity, mid-life hypertension and diabetes—public health initiatives to increase physical activity levels throughout life could potentially have a dramatic impact on dementia prevalence over time. In addition, societal-level interventions—such as community planning initiatives to emphasize open spaces, walking and natural physical activities—may be particularly effective at the population level. Depression, mid-life hypertension, mid-life obesity and diabetes also contributed to a substantial proportion of AD cases highlighting the importance of identification and management of these conditions. Randomized, controlled trials of multimodal risk factor reduction strategies to prevent AD are critically needed, and public health campaigns targeted at AD risk factor modification should be considered.
Supplementary Material
Acknowledgments
Role of funding source. None.
Funding. Dr. Barnes is supported by in part by the Alzheimer's Association, NARSAD and the University of California, San Francisco, School of Medicine. Dr. Yaffe is supported in part by the National Institute on Aging (K24 AG031155).
Footnotes
Conflict of Interest Statement. We have no conflicts of interest.
Contributions. Dr. Barnes conceived of the project and drafted the manuscript. Dr. Yaffe provided guidance on methodology and interpretation of findings and critical feedback on manuscript drafts.
REFERENCES
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–91. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed January 10, 2011];2010 Alzheimer's Disease Facts & Figures. 2010 at http://www.alz.org/documents_custom/report_alzfactsfigures2010.pdf.
- 3.O'Brien JT, Burns A. Clinical practice with anti-dementia drugs: a revised (second) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2010 doi: 10.1177/0269881110387547. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Green RC, Schneider LS, Amato DA, et al. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302:2557–64. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304:1903–11. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daviglus ML, Bell CC, Berrettini W, et al. National Institutes of Health State-of-the-Science Conference statement: preventing alzheimer disease and cognitive decline. Annals of internal medicine. 2010;153:176–81. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- 7.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 8.McGuinness B, Todd S, Passmore P, Bullock R. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane database of systematic reviews (Online) 2009:CD004034. doi: 10.1002/14651858.CD004034.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biological psychiatry. 2010;67:505–12. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Levin ML. The occurrence of lung cancer in man. Acta Unio Internationalis Contra Cancrum. 1953;9:531–41. [PubMed] [Google Scholar]
- 11.Benichou J. A review of adjusted estimators of attributable risk. Statistical methods in medical research. 2001;10:195–216. doi: 10.1177/096228020101000303. [DOI] [PubMed] [Google Scholar]
- 12.Darrow LA, Steenland NK. Confounding and bias in the attributable fraction. Epidemiology (Cambridge, Mass. 2011;22:53–8. doi: 10.1097/EDE.0b013e3181fce49b. [DOI] [PubMed] [Google Scholar]
- 13.Ruckinger S, von Kries R, Toschke AM. An illustration of and programs estimating attributable fractions in large scale surveys considering multiple risk factors. BMC Med Res Methodol. 2009;9:7. doi: 10.1186/1471-2288-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gefeller O. Comparison of adjusted attributable risk estimators. Statistics in medicine. 1992;11:2083–91. doi: 10.1002/sim.4780111606. [DOI] [PubMed] [Google Scholar]
- 15.Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS ONE. 2009;4:e4144. doi: 10.1371/journal.pone.0004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimley EV, Areosa SA. Effect of the treatment of Type II diabetes mellitus on the development of cognitive impairment and dementia. Cochrane database of systematic reviews (Online) 2003:CD003804. doi: 10.1002/14651858.CD003804. [DOI] [PubMed] [Google Scholar]
- 17.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. National Center for Health Statistics . Vital and Health Statistics: Summary Health Statistics for U.S. Adults: National Health Interview Survey, 2009. Hyattsville, MD: 2010. [Google Scholar]
- 20.Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and dementia - a comprehensive review. Ther Adv Neurol Disord. 2009;2:241–60. doi: 10.1177/1756285609103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purnell C, Gao S, Callahan CM, Hendrie HC. Cardiovascular risk factors and incident Alzheimer disease: a systematic review of the literature. Alzheimer disease and associated disorders. 2009;23:1–10. doi: 10.1097/WAD.0b013e318187541c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet neurology. 2005;4:487–99. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 23.Ligthart SA, Moll van Charante EP, Van Gool WA, Richard E. Treatment of cardiovascular risk factors to prevent cognitive decline and dementia: a systematic review. Vasc Health Risk Manag. 2010;6:775–85. doi: 10.2147/vhrm.s7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk for dementia: a double edged sword. Ageing Res Rev. 2009;8:61–70. doi: 10.1016/j.arr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet neurology. 2008;7:683–9. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 26.Wang JG, Staessen JA, Birkenhager WH. Antihypertensive treatment and prevention of stroke and dementia. Seminars in Cerebrovascular Diseases and Stroke. 2003;2003:155–64. [Google Scholar]
- 27.Shah K, Qureshi SU, Johnson M, Parikh N, Schulz PE, Kunik ME. Does use of antihypertensive drugs affect the incidence or progression of dementia? A systematic review. Am J Geriatr Pharmacother. 2009;7:250–61. doi: 10.1016/j.amjopharm.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ (Clinical research ed. 2001;322:1447–51. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiology of aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 30.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–81. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 31.Wu C, Zhou D, Wen C, Zhang L, Como P, Qiao Y. Relationship between blood pressure and Alzheimer's disease in Linxian County, China. Life sciences. 2003;72:1125–33. doi: 10.1016/s0024-3205(02)02367-6. [DOI] [PubMed] [Google Scholar]
- 32.Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G. Association between dementia and midlife risk factors: the Radiation Effects Research Foundation Adult Health Study. J Am Geriatr Soc. 2003;51:410–4. doi: 10.1046/j.1532-5415.2003.51117.x. [DOI] [PubMed] [Google Scholar]
- 33.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 34.National Center for Health Statistics . Health: Unites States, 2010: With Special Feature on Death and Dying. Hyattsville, MD: 2011. [PubMed] [Google Scholar]
- 35.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev. 2008;9:204–18. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Archives of neurology. 2007;64:392–8. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Archives of neurology. 2009;66:336–42. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes DE, Covinsky KE, Whitmer RA, Kuller LH, Lopez OL, Yaffe K. Predicting risk of dementia in older adults: The late-life dementia risk index. Neurology. 2009;73:173–9. doi: 10.1212/WNL.0b013e3181a81636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dahl AK, Lopponen M, Isoaho R, Berg S, Kivela SL. Overweight and obesity in old age are not associated with greater dementia risk. J Am Geriatr Soc. 2008;56:2261–6. doi: 10.1111/j.1532-5415.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- 40.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69:739–46. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 41.Beydoun MA, Lhotsky A, Wang Y, et al. Association of adiposity status and changes in early to mid-adulthood with incidence of Alzheimer's disease. American journal of epidemiology. 2008;168:1179–89. doi: 10.1093/aje/kwn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–7. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 43.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 44.Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry. 2001;35:776–81. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- 45.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Archives of general psychiatry. 2006;63:530–8. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. The American journal of psychiatry. 2000;157:1949–54. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 47.Doraiswamy PM, Krishnan KR, Oxman T, et al. Does antidepressant therapy improve cognition in elderly depressed patients? The journals of gerontology. 2003;58:M1137–44. doi: 10.1093/gerona/58.12.m1137. [DOI] [PubMed] [Google Scholar]
- 48.Nebes RD, Pollock BG, Houck PR, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 49.Gallassi R, Di Sarro R, Morreale A, Amore M. Memory impairment in patients with late-onset major depression: the effect of antidepressant therapy. Journal of affective disorders. 2006;91:243–50. doi: 10.1016/j.jad.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 50.Portella MJ, Marcos T, Rami L, Navarro V, Gasto C, Salamero M. Residual cognitive impairment in late-life depression after a 12-month period follow-up. International journal of geriatric psychiatry. 2003;18:571–6. doi: 10.1002/gps.895. [DOI] [PubMed] [Google Scholar]
- 51.Knegtering H, Eijck M, Huijsman A. Effects of antidepressants on cognitive functioning of elderly patients. A review. Drugs Aging. 1994;5:192–9. doi: 10.2165/00002512-199405030-00005. [DOI] [PubMed] [Google Scholar]
- 52.Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307–11. doi: 10.1192/bjp.174.4.307. [DOI] [PubMed] [Google Scholar]
- 53.Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr Scand. 2006;113:372–87. doi: 10.1111/j.1600-0447.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 54.Kessler RC, Birnbaum HG, Shahly V, et al. Age differences in the prevalence and comorbidity of DSM-IV major depressive episodes: results from the WHO World Mental Health Survey Initiative. Depress Anxiety. 2010;27:351–64. doi: 10.1002/da.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kessler RC, Birnbaum H, Bromet E, Hwang I, Sampson N, Shahly V. Age differences in major depression: results from the National Comorbidity Survey Replication (NCS-R) Psychol Med. 2010;40:225–37. doi: 10.1017/S0033291709990213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 57.Rolland Y, Abellan van Kan G, Vellas B. Physical activity and Alzheimer's disease: from prevention to therapeutic perspectives. J Am Med Dir Assoc. 2008;9:390–405. doi: 10.1016/j.jamda.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane database of systematic reviews (Online) 2008:CD005381. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- 59.Guthold R, Ono T, Strong KL, Chatterji S, Morabia A. Worldwide variability in physical inactivity a 51-country survey. Am J Prev Med. 2008;34:486–94. doi: 10.1016/j.amepre.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Middleton LE, Barnes DE, Lui LY, Yaffe K. Physical activity over the life course and its association with cognitive performance and impairment in old age. J Am Geriatr Soc. 2010;58:1322–6. doi: 10.1111/j.1532-5415.2010.02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Almeida OP, Hulse GK, Lawrence D, Flicker L. Smoking as a risk factor for Alzheimer's disease: contrasting evidence from a systematic review of case-control and cohort studies. Addiction. 2002;97:15–28. doi: 10.1046/j.1360-0443.2002.00016.x. [DOI] [PubMed] [Google Scholar]
- 62.Anstey KJ, von Sanden C, Salim A, O'Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. American journal of epidemiology. 2007;166:367–78. doi: 10.1093/aje/kwm116. [DOI] [PubMed] [Google Scholar]
- 63.Cataldo JK, Prochaska JJ, Glantz SA. Cigarette smoking is a risk factor for Alzheimer's Disease: an analysis controlling for tobacco industry affiliation. J Alzheimers Dis. 2010;19:465–80. doi: 10.3233/JAD-2010-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:36. doi: 10.1186/1471-2318-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jha P, Ranson MK, Nguyen SN, Yach D. Estimates of global and regional smoking prevalence in 1995, by age and sex. Am J Public Health. 2002;92:1002–6. doi: 10.2105/ajph.92.6.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Storr CL, Cheng H, Alonso J, et al. Smoking estimates from around the world: data from the first 17 participating countries in the World Mental Health Survey Consortium. Tobacco control. 2010;19:65–74. doi: 10.1136/tc.2009.032474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vital signs: current cigarette smoking among adults aged >or=18 years --- United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1135–40. [PubMed] [Google Scholar]
- 68.Valenzuela MJ. Brain reserve and the prevention of dementia. Current opinion in psychiatry. 2008;21:296–302. doi: 10.1097/YCO.0b013e3282f97b1f. [DOI] [PubMed] [Google Scholar]
- 69.Caamano-Isorna F, Corral M, Montes-Martinez A, Takkouche B. Education and dementia: a meta-analytic study. Neuroepidemiology. 2006;26:226–32. doi: 10.1159/000093378. [DOI] [PubMed] [Google Scholar]
- 70.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. Jama. 2002;288:2271–81. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. Jama. 2006;296:2805–14. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papp KV, Walsh SJ, Snyder PJ. Immediate and delayed effects of cognitive interventions in healthy elderly: a review of current literature and future directions. Alzheimers Dement. 2009;5:50–60. doi: 10.1016/j.jalz.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 73.Martin M, Clare L, Altgassen AM, Cameron MH, Zehnder F. Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane database of systematic reviews (Online) 2011;1:CD006220. doi: 10.1002/14651858.CD006220.pub2. [DOI] [PubMed] [Google Scholar]
- 74.A New Data Set of Eductional Attainment in the World, 1950–2010: NBER Working Paper No. 15902. [Accessed January 19, 2011];The National Bureau of Economic Research (NBER) 2010 at http://www.nber.org/papers/w15902.
- 75.International Data Base (IDB) [Accessed January 19, 2011];2010 at http://www.census.gov/ipc/www/idb/region.php.
- 76.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–60. [PubMed] [Google Scholar]
- 77.Yaffe K, Weston A, Graff-Radford NR, et al. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011;305:261–6. doi: 10.1001/jama.2010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barnes DE, Haight TJ, Mehta KM, Carlson MC, Kuller LH, Tager IB. Secondhand smoke, vascular disease, and dementia incidence: findings from the cardiovascular health cognition study. American journal of epidemiology. 2010;171:292–302. doi: 10.1093/aje/kwp376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychology review. 2007;17:259–73. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- 80.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997;277:813–7. [PubMed] [Google Scholar]
- 81.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. The New England journal of medicine. 2003;348:1215–22. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 82.Barnes DE, Whitmer RA, Yaffe K. Physical activity and dementia: The need for prevention trials. Exercise and sport sciences reviews. 2007;35:24–9. doi: 10.1097/JES.0b013e31802d6bc2. [DOI] [PubMed] [Google Scholar]
- 83. [Accessed January 13, 2011];High Blood Pressure. 2010 at http://www.cdc.gov/bloodpressure/index.htm.
- 84. [Accessed January 13, 2011];Overweight and Obesity. 2011 at http://www.cdc.gov/obesity/index.html.
- 85.Middleton LE, Yaffe K. Targets for the prevention of dementia. J Alzheimers Dis. 2010;20:915–24. doi: 10.3233/JAD-2010-091657. [DOI] [PubMed] [Google Scholar]
- 86.Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75:27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA. Change in depressive symptoms during the prodromal phase of Alzheimer disease. Archives of general psychiatry. 2008;65:439–45. doi: 10.1001/archpsyc.65.4.439. [DOI] [PubMed] [Google Scholar]
- 88.Alexopoulos GS. Vascular disease, depression, and dementia. J Am Geriatr Soc. 2003;51:1178–80. doi: 10.1046/j.1532-5415.2003.51373.x. [DOI] [PubMed] [Google Scholar]
- 89.Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Archives of general psychiatry. 2006;63:273–9. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 90.Luchsinger JA, Gustafson DR. Adiposity and Alzheimer's disease. Curr Opin Clin Nutr Metab Care. 2009;12:15–21. doi: 10.1097/MCO.0b013e32831c8c71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet neurology. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 92.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–63. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 93.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. Jama. 2004;292:2237–42. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 94.Yaffe K. Metabolic syndrome and cognitive disorders: is the sum greater than its parts? Alzheimer disease and associated disorders. 2007;21:167–71. doi: 10.1097/WAD.0b013e318065bfd6. [DOI] [PubMed] [Google Scholar]
- 95.U.S. Department of Health and Human Services. National Institutes of Health. National Heart Lung and Blood Institute. National High Blood Pressure Education Program The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure; 2003. [DOI] [PubMed] [Google Scholar]
- 96.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Archives of neurology. 2006;63:1709–17. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189–96. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.