ABSTRACT
The field of solid organ transplantation has historically concentrated research efforts on basic science and translational studies. However, there has been increasing interest in health services and outcomes research. The aim was to build an effective and sustainable, inter- and transdisciplinary health services and outcomes research team (NUTORC), that leveraged institutional strengths in social science, engineering, and management disciplines, coupled with an international recognized transplant program. In 2008, leading methodological experts across the university were identified and intramural funding was obtained for the NUTORC initiative. Inter- and transdisciplinary collaborative teams were created across departments and schools within the university. Within 3 years, NUTORC became fiscally sustainable, yielding more than tenfold return of the initial investment. Academic productivity included funding for 39 grants, publication of 60 manuscripts, and 166 national presentations. Sustainable educational opportunities for students were created. Inter- and transdisciplinary health services and outcomes research in transplant can be innovative and sustainable.
KEYWORDS: Transdisciplinary research teams, Health Services and Outcomes Research, Educational opportunities, Academic productivity, Sustainable research efforts
INTRODUCTION
The first successful organ transplant occurred in 1954, as a patient in kidney failure received a kidney from an identical twin [1]. This event marked the beginning of clinical transplantation as we know it today. In 1983, the discovery of cyclosporine, an effective anti-rejection medication, catapulted transplants from investigative and experimental, to standard of care and therapeutic status. By the early 1990s, this success resulted in organ shortages that raised a number of social issues, including but not limited to the ethics of rationing, and the notion of utility. This equipoise led to a focus on outcomes, first targeted at 1-year graft and patient survival rates, because these are important milestones that predict longer term outcomes. Therefore, the main focus of transplant research in the past several decades has been on transplantation immunology and clinical trials aimed at informing optimal anti-rejection treatments, with 1-year outcomes endpoints [2, 3]. From a regulatory perspective, these same endpoints have become the bright-line tests for transplant program and center performance [4]. The evolution of these endpoints as central outcomes for both scientific and regulatory purposes stems from the fact that the National Organ Transplant Act (NOTA) of 1984 mandates the extensive collection of data on every transplant patient in the United States (US), resulting in the ability to generate and publicly report program-specific, risk-adjusted graft and patient outcomes that have been used to inform transplant policy since 1987, and to assess transplant program performance. These endpoints have allowed for regulations to certify and de-certify programs [4, 5]. However, as the field of transplantation has evolved, 1-year graft and patient survival rates are no longer sufficient as quality measures. Other factors, including patient-reported outcomes of transplant recipients, the adequacy of the informed consent process, the economic implications of clinical decisions, and systems and process vulnerabilities in the delivery of care (patient safety), have taken on an increasing role in determining true outcomes [6]. Whereas these types of parameters have been studied and integrated into the decision-making process in other healthcare disciplines, especially those that involve the management of chronic diseases, little attention has been paid to these types of outcomes in transplantation [7]. For example, quality of life following transplantation is increasingly relevant as survival rates improve, akin to the notion of “survivorship” following treatment for cancer [8]. Yet, no transplant-specific validated tool for the assessment of quality of life (QOL) exists [9]. By its very nature and complexity, the field of transplantation lends itself well to the investigation of many of these topics, particularly patient QOL, patient safety, ethical issues, informed consent, risk assessment and economic modeling. Healthcare in general, and transplantation in particular, lag behind many other industries in these areas. For example, the airline and nuclear power industries have championed process improvement toward improved safety. Retail sales and marketing make much more extensive use of much less thorough data [10]. The financial industry applies advanced decision analysis methodologies towards decisions with far less significant consequences than healthcare choices. Primary and secondary school districts make much better use of advanced approaches to individualized education.
Given that federal agencies, such as the Agency for Healthcare Research and Quality (AHRQ) and the Patient Centered Outcomes Research Institute (PCORI), established as part of the Affordable Care Act, have a central mission to support outcomes and health services research, we believe that transplantation research is well positioned to attract these funds for several reasons. As a highly complex field, transplantation crosses the boundaries of medicine and surgery, includes both focused episodic and longitudinal care, relies on multidisciplinary care teams and complex healthcare systems for delivery of care, maintains the potential for significant consequences upon errors, and represents a clearly defined area with high continuity of care. Transplant is therefore an ideal field to study preliminary applications of methodologies in a specific small number of patients, prior to disseminating successful interventions to other disciplines.
Recognizing this opportunity for innovative collaborative research [11], we sought to build an outcomes and health services research initiative in transplantation [12]. The following is an account of this experience to date.
NUTORC conceptual framework
The thesis behind the NUTORC model is that innovative and transformative research necessitates collaboration between investigative disciplines that, at first glance, are seemingly disparate, and in all likelihood, know very little about each other. Yet, if brought together at the intersection of their respective disciplines (the intersection may not be automatically intuitive), and assuming that this intersection is relevant to the respective disciplines, there is a potential for true innovation that advances the field that assumes the center of gravity. In the case of NUTORC, it was clear that the center of gravity was represented by transplantation. Therefore, the first step in assembling NUTORC was to identify potential disciplines in the health services and outcomes arena that might be relevant to transplantation. In order to define these areas, it was important to have an understanding of the following: (1) What are some of the important unanswered questions that relate to transplantation outcomes? (2) What health services and outcomes disciplines might be useful in helping format and eventually answer those questions? (3) What local expertise currently exists in these disciplines? With regards to the first question, we took a step back and looked at trends in the transplantation literature, as well as in non-transplant healthcare literature in order to better inform the formulation of a list of questions. This was done for each of several organs (kidney, liver, etc.) as these questions might be different for each. It was important that the list of questions included not only the transplant, but the condition that led to the transplant, and life after transplantation. With regards to the second question, given that the transplant surgeons who were formulating these questions did not have significant expertise in health services and outcomes research, they sought input from several people, including investigators and clinicians with expertise in other healthcare disciplines that had been at the forefront of this type of research. Finally, with regards to the third question, given that our university does not have a school of public health, it was necessary for the transplant surgeons to educate themselves about the various pockets of expertise within the university both in the school of medicine, as well as in the health sciences, undergraduate and graduate programs at the various schools within the university.
A second piece of the conceptual framework deals directly with the issue of incentives. The first question related to incentives is: how do you get someone, who is an expert in his/her field, presumably successful (i.e. busy) to give you the time to present your case for collaboration? This is a particular problem for investigators in health services and outcomes research, given that most investigators rely entirely on grant support. Therefore, in putting together NUTORC, there was an immediate recognition that (1) a compelling case needed to be made to “sell the plan”, and (2) the plan needed to align incentives between the Comprehensive Transplant Center (CTC) and the various schools/departments/divisions/centers where collaborations were important. This led to the second question: what is the best construct that is likely to yield a successful outcome? The fact that we were “selling” a collaboration with transplant surgeons worked to our advantage. The idea of life and death decisions driven by organ shortages where outcomes measures might inform policy was scintillating. We found that speaking to health services and outcomes investigators about transplantation became a lot of fun. Our “product” was intriguing and sexy, and it made it relatively easy to get their attention. But that was not sufficient. Once we got their attention, we had to keep them interested. This too was relatively easy, because we could align our incentives quite easily. We laid out what we were hoping to get out of the collaboration. None of our transplant surgeons/clinicians were dependent on grant support. We merely wanted to advance the field of transplantation and gain visibility for our program by exploring areas relevant to our field that our colleagues would find interesting. In contrast, we were willing to provide content and expertise so that investigators in these various disciplines could develop research areas that coincided with their interests. We would add transplantation to the research portfolios of these investigative teams. We would be happy being co-authors on manuscripts, and co-investigators on grants with the notion that if some of these areas of investigation became extremely successful and fertile, we would then take more of a leadership role. This strategy resonated well with our collaborators. Finally, we were willing to partially support the initiation phase of these collaborations by paying for a percent effort of new faculty, recruited purposely to develop NUTORC. We were not out to reproduce anything that already existed within these disciplines. We merely wanted newly recruited faculty, embedded in their respective home (e.g. departments/centers), to be dedicated to transplantation research.
The final piece of the conceptual framework was to create workgroups or teams for each collaborative initiative that would include at least one health services and outcomes investigator (as the leader), and at least one transplant clinician (as a co-leader). These two (or more) individuals would spend a considerable amount of time educating each other about their respective fields, in an effort to find common ground at the intersection of the two fields, often exploring several directions that led nowhere. Their task was to formulate four to five questions that would be relevant to both fields. Once these questions were formulated, they were vetted by other members of both teams, often including more senior members, for validation purposes. The next step was to perform a comprehensive literature review, in order to identify the most updated state of the science relevant to each of the questions formulated, and in order to identify the opportunities. The final step was to identify “methodologists” that might provide state-of-the art approaches to each particular question being addressed.
NUTORC—IMPLEMENTING THE CONCEPTUAL FRAMEWORK
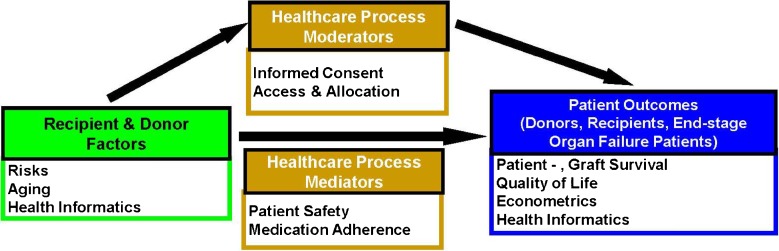
The abdominal solid organ transplant program ranks in the top five of approximately 250 transplant programs in the US [13]. The transplant clinicians are well known nationally and internationally and play important roles in the major trade organizations and professional societies including the United Network for Organ Sharing/Organ Procurement and Transplant Network, (UNOS/OPTN) the American Society for Transplantation (AST), and the American Society for Transplant Surgeons (ASTS). As national leaders in transplantation, the transplant clinicians are acutely aware of gaps in knowledge, as well as in how correcting these gaps might result in changes in national policies and regulations that govern transplantation (Fig. 1).
Fig. 1.
The conceptual model demonstrates the need for risk stratification (recipient and donor factors) to measure outcomes (patient- , graft survival, quality of life, econometrics); moderators and mediators affect the outcomes (informed consent, medication adherence, access to transplant and allocation of organs, patient safety)
Therefore, the initial 3-year goal was to build, on the shoulders of a large and successful clinical transplant program, a sustainable collaborative that was inter- and transdisciplinary, that would reach the pinnacle of academic excellence for transplant-related health services and outcomes research. To achieve this goal, we created what we termed the MATRIX construct—a Modular Approach to Transplantation Research by Inter-disciplinary eXperts. Rather than reproducing expertise already existent within the various collaborative entities, the MATRIX construct allowed for the implementation of the conceptual model by creating a number of “modules” each built around a team of leading experts from different disciplines focused on a specific topic and problem that was deemed relevant to the field of transplantation. We have used the terms inter- and transdisciplinary to mean two different things. Inter-disciplinary refers to the individuality of each discipline, and transdisciplinary refers to the commonality between collaborating disciplines. Thus, each module is designed to include inter-disciplinary teams that apply transdisciplinary approaches to resolve a commonly identified problem. It is important to note that the “disciplines” in question are often seemingly disparate. For instance, what does transplantation surgery have in common with industrial engineering, or for that matter, epidemiologists or psychometricians? Yet, when these seemingly disparate collaborators seek relevance in their collaborations, the “eureka” moment does not take long, as evidenced by our experience. In this construct, the transplant clinicians pose pertinent and relevant clinical/healthcare questions. It should be noted however that in order for the non-transplant collaborators to understand these questions and their implications requires a significant amount of education on the part of the transplant expert. For instance, what is a Model for End-stage Liver Disease (MELD) score? And for that matter, what is a serum creatinine? A bilirubin? An INR? Once the clinical questions/problems are defined, it is the turn of the health services and outcomes research expert to frame the specific question/problem in the context and boundaries of their respective field of expertise. Are there validated instruments that can be used to frame these problems/questions? If so, is the investigator familiar enough with these instruments to apply them to the specific transplant issue at hand, or does this require building and validating instruments to do so? And does this require further help/input from methodologists that need to be brought into the fold, in order to ensure the use of state-of-the art methodologies? This now becomes the flip side of the coin. What does a transplant expert really know about validated QOL instruments, or about the psychometric properties of these instruments? What about the statistical or informatics needs of the analyses? Again, the MATRIX construct requires continued back-and-forth education between the various investigators in order that all participants in the research module feel comfortable at the intersection of their respective areas of expertise. Each module is considered to be an incubator for innovative research in transplant outcomes. We have utilized the MATRIX construct to also create training opportunities for postdoctoral students in our T-32 postdoctoral training grants, as we believe that this framework provides excellent opportunities for novel research approaches.
In 2008, the university and the hospital provided the CTC with a commitment to support the NUTORC initiative, based on the MATRIX construct, allowing us to secure 3 years of seed funding, with a clear understanding that it was expected that the initiative would be able to sustain itself financially beyond the 3-year start-up without any need for additional institutional support. Shortly thereafter, the CTC was awarded a NIH/NIDDK (National Institute for Health/National Institute for Digestive Diseases and Kidney Diseases) funded T-32 postdoctoral fellowship award (Transplant Surgery Scientist Program [5T32DK077662-05]), providing a vehicle for postdoctoral trainees to work at the intersection of disciplines within a NUTORC team and effectively serve as a bridge among MATRIX mentors.
In an effort to develop a multi-layered modular mentorship structure, the CTC Director identified two nationally recognized senior health services and outcomes research investigators/leaders, one in patient-reported outcomes and the other in patient safety, who were intrigued by, and interested in building NUTORC. Together, they identified four more faculty members with methodological expertise and interest in working at the intersection of transplant and of their respective fields of knowledge. Furthermore, as there was a clinical need for an additional transplant surgeon, the CTC Director recruited a transplant surgeon (D.L.) with expertise in health services and outcomes research to lead and grow the nascent NUTORC initiative. Seed funds were used to fund these recruitments. In complying with the guiding principles of NUTORC, each recruited faculty was embedded into a mentorship network of senior researchers, to whom they would regularly report. In addition, postdoctoral students funded by the postdoctoral T-32 were assigned to individual collaboratives. Thus, the first four transdisciplinary teams were created, each co-led by a transplant expert and an outcomes research expert: (1) QOL, (2) patient safety, (3) informed consent, and (4) risk and econometrics.
Two new research faculty were specifically recruited to lead the quality of life and the informed consent team, respectively. For example, a clinical health psychologist (Z.B.) with extensive training in psychometrics and the development and application of patient-reported outcomes measure, and a junior investigator in the Department of Medical Social Sciences - MSS (the Chair of MSS was one of the two senior leaders who had joined forces with the CTC to create NUTORC), was recruited jointly by the CTC and MSS into NUTORC to focus on transplant-related QOL initiatives. It is important to reiterate that the NUTORC model emphasizes that investigators reside, both physically and intellectually amongst their peers in their home centers and departments, providing a fertile environment of continued academic growth, within their native disciplines, and therefore that NUTORC initiatives be embedded in these home centers and departments. At the same time, these faculty members are provided structured opportunities to work closely with a transplant clinician co-leader. Research faculty were recruited into NUTORC for 3-year terms with the expectation of obtaining independent extramural funding by the end of that period. The rationale and importance behind joint recruitments goes beyond financial implications. The main driver of the model is to make sure that principles behind the MATRIX construct are not only theoretical, but that it is palpable in every aspect of the otherwise virtual collaborative arrangement, providing a construct that does not require a physical structure. We believe that these subtle elements are essential in building a culture of inter- and transdisciplinary collaboration that is indifferent to the academic silos of departments/divisions and instead culturally align incentives between collaborating entities and individuals.
The MATRIX construct provides transdisciplinary modules for inter-disciplinary collaboration. One of the advantages of the construct is that a number of these modules can also be assembled rapidly in response to a funding opportunity, which may cut across expertise in more than one module. Thus, NUTORC assembled all four newly created modules in response to the NIH/NIDDK U01 competitive renewal of the Adult-to-Adult Living Liver Transplant Cohort Study (A2ALL). Over a period of three months, NUTORC brought together team leaders and other collaborators weekly to create and submit a grant proposal with four aims, each aligned with one of the NUTORC modules (QOL, patient safety, informed consent, risk and econometrics). The intense collaborative inter- and transdisciplinary experience required to prepare this large grant submission led to the rapid and effective education of all participants about liver transplantation in general, and live donor liver transplantation in particular, and about new methodological approaches and their limitations. During this time, NUTORC created the foundation for its current organizational structure. After submission of the U01 grant proposal (5U01DK062467-10), which was funded, the weekly grant preparation meetings were transitioned into weekly NUTORC meetings. In those meetings, junior as well as more senior faculty members that include health services and outcomes investigators, clinicians, and students assemble to discuss research topics that each team is exploring, as well as to brainstorm on new ideas. While individual teams regularly meet to advance their work, this weekly NUTORC-wide meeting provides a forum for inter-team feedback, as well as a chance to identify new projects that leverage multiple teams.
NUTORC—THE FIRST 4 YEARS
Teams
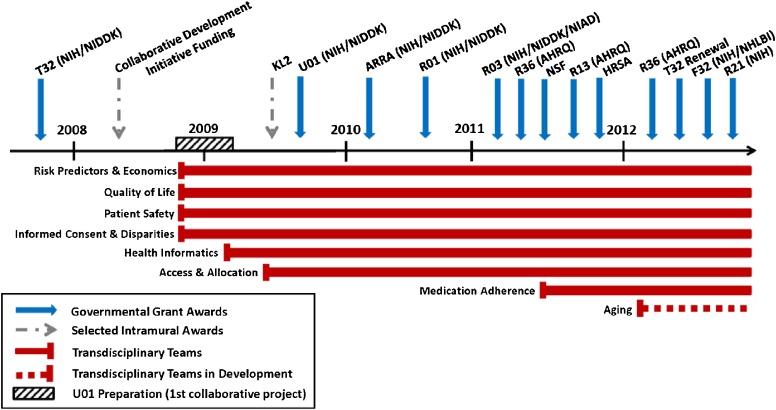
Since its creation in November 2008, NUTORC has expanded from four to eight teams. The fifth team was created to focus on Health Informatics. It had become obvious that it was difficult to extract data from electronic health records for analysis as many systems were used for the care of patients. This team focuses on resolving the informatics hurdles with the goal to provide the most granular data for all research teams. We then had the opportunity to create a collaboration with the department of Industrial Engineering in the school of Engineering, to apply novel modeling methodologies to transplant Access and Allocation. The two most recently added teams focus on Medication Adherence, a significant challenge in organ transplantation, as well as Aging, a topic of interest to the National Institute for Aging (NIA), given that the elderly constitute the fastest growing age segment in transplantation. See timeline in Fig. 2. Productivity by team is outlined in Table 1.
Fig. 2.
NUTORC Development Timeline provides an overview of major milestones and accomplishments from 2008 to present, noting that the creation of the first transdisciplinary teams was drawn together through the U01 grant submission; federal funding and the creation of individual transdisciplinary teams are highlighted
Table 1.
Total NUTORC productivity by team
| NUTORC teams | Total awarded | NIH grants | Manuscripts | Abstracts |
|---|---|---|---|---|
| Quality of life | $1,802,707 | 3 | 19 | 25 |
| Patient safety | $3,824,697 | 2 | 5 | 34 |
| Informed consent and disparities | $1,452,855 | 4 | 18 | 28 |
| Risk predictors and economics | $240,324 | 3 | 10 | 52 |
| Health informatics | $853,000 | N/A | N/A | 4 |
| Access and allocation | $354,172 | 2 | N/A | 21 |
| Health literacy and medication adherence | $3,000 | N/A | N/A | 2 |
| Aging | N/A | N/A | N/A | N/A |
| Cross teams | $1,971,205 | 2 | N/A | N/A |
Grant funding
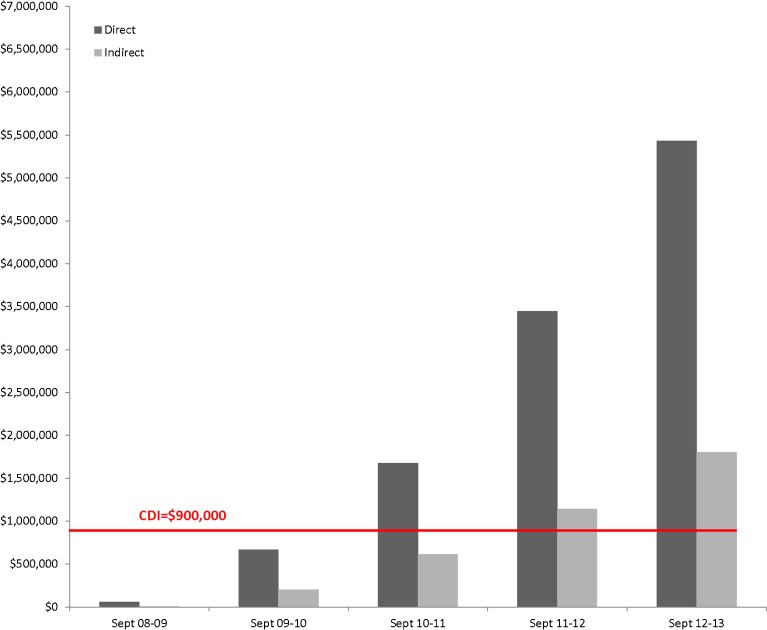
As it was essential to support the NUTORC operations in a sustainable manner within 3 years, emphasis was placed on submission of intra- and extramural grants. To date, NUTORC has submitted 81 grant proposals, 39 of which have been funded for a total of $11,619,500. Seventeen grants from federal agencies were received from the National Institute of Health (NIH), the National Science Foundation (NSF), the Agency for Healthcare Research and Quality (AHRQ), and the Health Resources and Services Administration (HRSA) (total federal funding $9,562,800). Additional funding was obtained from industry and intramural sources ($2,056,700). Thus, the initial financial investment of $900,000 over 3 years to build and support NUTORC, yielded a more than tenfold return within 3 years. Given that the institution recovers the indirect costs of grant awards, in Fig. 3 we demonstrate the cumulative indirect revenue compared to the initial investment of $900,000. The figure shows that the investment is recovered by the institution in form of indirect costs within less than 3 years.
Fig. 3.
The black indicates the initial intramural investment. The bars demonstrate the cumulative amount (direct: black; indirect: gray). The initial investment of the hospital and university was recovered in indirect funds within less than 3 years
Publications
To date NUTORC teams have presented 114 posters and 52 oral presentations at national and international conferences, and published 60 peer-reviewed manuscripts [8, 9, 14–66].
Diffusion among disciplines
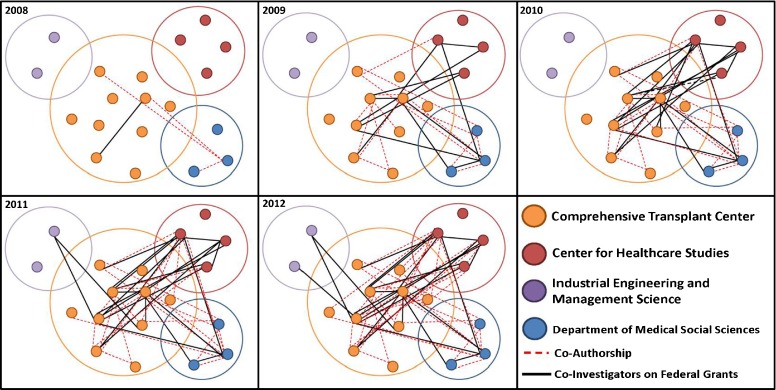
To demonstrate how much of this work was indeed inter- and transdisciplinary, we manually created a network map using PowerPoint. Each dot represents an individual investigator, most of them team leaders and senior investigators. Investigators are color coded based on their primary affiliation: for example all transplant clinicians are orange, while the industrial engineers are purple. Co-investigators of federal grants (NIH, AHRQ, NSF, HRSA) are connected with a black line, while co-authors of peer-reviewed publications are connected with a red dotted line. Figure 4 displays the significant increase in interconnectivity between transplant clinicians and researchers with specific methodological expertise from 2008 to present.
Fig. 4.
The NUTORC Network map demonstrates individual investigators with small circles and the color of their primary affiliation. Small circles are connected with dotted lines for co-authorships, and black lines connect co-investigators on funded federal grants. Since the inception of NUTORC in late 2008, the connectivity has grown considerably demonstrating the growing transdisciplinary productivity in form of federal grants and manuscripts
CURRENT ADMINISTRATIVE NUTORC STRUCTURE
To sustain the continuous growth of NUTORC a core administrative support structure was created, consisting of the NUTORC director, a NUTORC manager, and two NUTORC research assistants. This core team manages the following areas: (1) general operations (e.g., meeting), (2) regulatory oversight, (3) outreach, (4) student programs, (5) data management, (6) unfunded research activities with high likelihood for federal funding, and (7) grant submission support. Critical tasks within each area are listed in Table 2. This administrative structure has allowed for continuous growth and adaptation as new collaborations are formed and funding opportunities become available, often on short notice. The administrative team also provides key support for collaborators to address organizational questions.
Table 2.
Critical administrative NUTORC tasks
| NUTORC Area | Tasks |
|---|---|
| General operations | Scheduling and organization of meetings |
| Monitoring of NUTORC performance and productivity | |
| Quarterly progress reports | |
| Establishing management procedures and protocols | |
| Cataloguing NUTORC output | |
| Quarterly budget review | |
| Regulatory oversight | Oversight for Institutional Review Board (IRB) compliance |
| IRB submission and revisions | |
| Management of physical and electronic data access for collaborators | |
| Outreach | Maintenance of NUTORC website |
| Creation of brand identity | |
| Weekly dissemination of team related publications | |
| Outreach to student programs | |
| Promotional and marketing materials | |
| Student program | Organization and management of summer internship program (high school, undergraduate, graduate, and postdoctoral students) |
| Organization of student training and educational workshops | |
| Student oversight and mentorship | |
| Data management | Creation of NUTORC databases |
| Data acquisition/collection | |
| Data management, coding, cataloguing | |
| Basic data analysis | |
| Research dissemination | Creation and maintenance of standardized templates for NUTORC presentations, posters, and materials |
| Abstract and manuscript development (literature searches, reference management, creation of graphs and figures) | |
| Editing NUTORC research output | |
| Grant support | Grant preparation and revisions (obtaining and organizing supporting documentation) (e.g. biosketches, letters of support, etc.), drafting supplemental grant sections (e.g. budget justification, data sharing plan, etc.) |
| Routine dissemination of current grant opportunities |
NUTORC TEAMS
To date, NUTORC has created eight teams, each co-chaired by a transplant clinician and an expert in outcomes research. In addition, each team is joined by clinicians (nurses, physician assistants, nurse practitioners, pharmacists, and physicians), pre- and postdoctoral students, as well as various methodologists. In frequent team meetings and the weekly NUTORC meetings the transplant clinicians educate their collaborators about transplantation while the research faculty educate the clinicians and the health services and outcomes researchers about their cutting-edge methodologies and their respective strengths and limitations. This process can be lengthy as transplantation is a complex field with many caveats that need to be properly and repeatedly communicated. Furthermore, during these meetings, transplant clinicians communicate the gaps in knowledge and pertinent clinical questions. Health services and outcomes researchers help to frame the questions, and content experts provide the state-of-the art tools to answer them. Hence, within and across the teams experimental designs are formulated and advanced methodologies are applied for the purpose of grant proposals and data collection for manuscripts. Teams often meet weekly in addition to the weekly NUTORC meetings, to move their respective projects forward, as well as prepare manuscripts and grant applications.
Presently, 40 transplant clinicians, outcomes researchers and methodological experts are actively involved, and have either received transdisciplinary federal funding, and/or have successfully published transdisciplinary peer-reviewed manuscripts. The breakdown of productivity is provided in Table 1.
Quality of life
[8, 22–40] QOL is a multi-dimensional concept that refers to an individual’s usual or expected physical, emotional, and social well-being. The importance of QOL was particularly recognized in transplantation as an important aspect of living donor liver transplantation. As mentioned above the QOL team is co-led by a clinical psychologist/patient-reported outcomes researcher (Z.B.) and a transplant clinician (E.A.). A senior and highly funded expert (D.C.) with an international reputation provides ongoing methodological mentorship in addition to the clinical mentorship from the transplant division chief (M.A.). He was specifically recruited for the role of team leader for QOL and early on successfully obtained an intramural KL-2 award (1KL2RR025740-01) for his project focused on increasing his own expertise in transplant outcomes. Also, as one of the contributors to the competitive A2ALL collaborative grant renewal, he was invited by the nine center steering committee to co-chair the central QOL effort of this 5-year study. This effort provides him with considerable funding as well as national exposure. NUTORC also received an American Recovery and Reinvestment Act (ARRA) award to study long-term QOL in living liver donors (3U01DK062467-08S1). He and other members of the QOL team provided mentorship to two T-32 postdoctoral fellows and students, who had a focus on QOL research in kidney and liver transplantation.
Patient safety
[8, 41, 67–69] Patient Safety is focused on the study of the systems and processes within which we provide transplantation care. Despite the occurrence of high profile safety events in transplantation, such as errors in ABO compatibility [70] and transmission of communicable disease [71] here has been limited research examining the range of patient safety vulnerabilities in transplantation [72]. This relative lack of empirical patient safety data stands in stark contrast to other high-risk industries such as air transport and nuclear power [8, 68, 73, 74]. Hence this team, which includes many transplant clinicians, focuses on systems and process vulnerabilities. A patient safety expert (D.W), added transplantation to her portfolio. She is provided mentorship by senior patient safety expert (J.H.) and together with a transplant surgeon (D.L.) co-leads this group. They jointly received an R-01 award by the NIH/NIDDK as Co-Principal Investigators, for their work: “A2ALL-Patient Safety System Improvements in Living Donor Liver Transplantation.”(R01DK090129)
Informed consent and disparities
[41–58] Transplantation is rife with ethical dilemmas, including challenges with obtaining informed consent for donors and recipients, and the preponderance of ethnic/racial disparities in access to and outcomes of transplantation.[45, 46, 48, 49] Together with a transplant surgeon (J.C.) a nationally recognized methodological expert (E.G.), with training in medical anthropology and bioethics, who was hired from an outside institution in 2008 leads this team. She (E.G.) is mentored by a senior clinical mentor (M.A.) and a senior health services and outcomes research mentor (J.H.). In close collaboration with transplant clinicians, she successfully obtained an R03, HRSA and R21 grant with a R01 under review to develop Internet-based interventions to optimize informed consent and reduce ethnic disparities transplantation.
Risk predictors and econometrics
[8, 9, 59–66]Risk models and decision analytics can be leveraged to better inform clinician, patient, and payer decision-making, facilitating personalized medicine in transplantation. This group is co-led by a transplant clinician (A.S.) and a decision analyst (G.H.) from the department of Industrial Engineering. As a full professor and a nationally recognized decision analyst, he demonstrated interest in extending his portfolio to transplantation and co-leads the risk and econometrics group. Together with transplant clinicians he provided mentoring for two postdoctoral fellows to create intricate decision models for transplant recipients from donor after cardiac death organ donors. This group also provides mentorship to a predoctoral engineering student, who received an R-36 (AHRQ) award for his thesis project in transplantation stochastic game modeling in liver transplantation. One of our new postdoctoral fellows received an F-32 through the NIH/National Heart, Lung and Blood Institute to study cardiovascular risk assessment in transplantation and receives senior mentorship from a senior health services and outcomes researcher as well as transplant clinicians.
Health informatics
[75–77] This team concentrates its efforts on the creation of a comprehensive transplant datamart within the medical school’s Enterprise Data Warehouse, integrating multi-source data and making non-discreet text data more usable by applying indexing strategies and natural language processing algorithms. The team is led by the administrative director of the transplant center (L.P.) and a transplant nephrologist (B.H.), both of whom have a background in medical informatics. Their team includes programmers and database architects with experience utilizing data from electronic medical records.
Access and allocation
[78–81] Geographic inequity in organ allocation is an unsolved conundrum, which can be addressed with advanced modeling methodologies. A full professor in Industrial Engineering (S.M.) had interest in extending his research portfolio to transplantation. In fact, one of the predoctoral students wanted to focus her thesis on transplant and organ allocation. We integrated her into our clinical operations for 3 months to teach her about transplantation while she is physically situated at the School of Engineering and primarily mentored by her engineering professors. She successfully received an AHRQ R-36 award for her thesis project “Addressing Geographic Inequities in Kidney Transplantation” (1R36HS021078-01). Furthermore this team obtained collaborative funding by the National Science Foundation to study “Geographic disparity in organ allocation” (CMMI-1131568).
Health literacy and medication adherence
[82, 83] Health literacy is the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions. Adherence is the extent to which a patient follows a healthcare provider’s recommendations. Limited health literacy and non-adherence have profound effects on clinical outcomes and quality of care in a variety of medical conditions, but have not been rigorously examined in transplantation. The mission of this nascent team is to apply the existing expertise within the Health Literacy and Learning Program to advance the study of limited health literacy and medication adherence in liver and kidney transplantation. This group is led by a senior investigator in medication adherences (M.W.) with extensive funding and an interest in transplantation. One of our present postdoctoral fellows focuses her 2-year T-32 postdoctoral fellowship on medication adherence and transplantation.
Aging
Earlier this year, the NIA has demonstrated its interest in aging and transplantation and brought together experts in transplants and geriatrics to discuss the gaps in knowledge [84]. It was very evident that this age segment is growing rapidly and that better understanding of the risk and barriers to transplantation need to better delineated and understood. Hence this latest team focusing on the elderly in transplantation is being created bringing together geriatricians, transplant clinicians and methodological experts.
NUTORC EDUCATIONAL AND MENTORSHIP PROGRAM
The structure of NUTORC was purposefully designed to create opportunities for collaboration for junior faculty as well as pre- and postdoctoral students. Each team leader is closely mentored by one senior clinical and one senior research mentor. Additional peer mentorship is achieved through regular NUTORC and team meetings. Each investigator also assumes a mentorship role for students at different levels. Important educational opportunities have been created for postdoctoral students through the T-32 postdoctoral Transplant Surgery Scientist Training Program, which supports one health services and outcomes research position each year for physician-scientists committed to an academic career in transplantation. The three T-32 postdoctoral fellows have catalyzed transdisciplinary collaboration, serving as conduits among busy senior investigators and coalescing broad input into project design. T-32 students obtain a master’s in health services and outcomes research and are integrated into one or two of the teams. The mentorship model provides each trainee with a primary clinical and primary methodological mentor for day-to-day mentorship, and quarterly senior level mentorship in addition to the weekly NUTORC meetings. This has created a highly interactive environment for each student. This year successful funding was obtained for an individual F-32 NRSA grant: “Derivation and Validation of a Cardiac Risk Index in Liver Transplantation,” (1F32HL116151-01) adding an additional trainee to the collaborative.
Through the AHRQ R-36 mechanism two predoctoral students are currently supported to complete their doctoral theses in Industrial Engineering with a focus in transplantation: “Addressing Geographic Inequities in Kidney Transplantation,” (1R36HS021078-01) and “Stochastic models in transplantation” (1R36HS021609). The Industrial Engineering students focus their efforts on both the theoretical advancement of engineering methodology as well as the practical application of these methods to address pertinent questions in transplantation.
Lastly, in an effort to recruit students at all levels (high school, college, and graduate school) we created a coveted summer internship, which integrates students into NUTORC teams. Weekly transplant teaching sessions are provided by clinical and research faculty, and basic health services and outcomes research skills are imparted by the NUTORC staff. Each summer intern is given a concise project supervised by a primary mentor, leading to abstract submission to the national transplant meeting (American Transplant Congress) and a poster presentation for the intramural university student fair. Since 2008, this summer program has grown from 2 summer interns to 15 in 2012.
BARRIERS AND CHALLENGES OF INTER- AND TRANSDISCIPLINARY RESEARCH IN TRANSPLANTATION
The institutional support to develop the transdisciplinary collaborative NUTORC was essential. Although the initial investment was returned more than tenfold within the first 3 years, the initial stipend of $900,000 was substantial. Without significant financial investment, NUTORC was unlikely to become successful and self-sustaining this quickly and at this scale. Furthermore, as is the nature of transdisciplinary and inter-disciplinary work, investigators have different disciplinary backgrounds, cultures, and vocabularies. If not resolved, this can pose a barrier to innovative research. In NUTORC this barrier was preemptively removed through frequent and ongoing in-person collaborative team meetings creating ongoing discussions and intellectual exchange, as well as dedication from the senior leaders to openly and decisively working through cultural barriers. The frequent meetings leading to mutual education of all participants were a crucial ingredient for the success. Most notably, tight deadlines with considerable involvement from all the teams often effectively and swiftly removed cultural barriers. Other barriers were different incentives and goals of collaborators. For example, some collaborators are more incentivized by academic productivity in the form of high impact publications than obtaining federal funding. Clear identification of common and mutually beneficial objectives for each collaboration was helpful. Moreover, the serious investment of all parties in the success of the collaboration is essential. To this end, sharing of fiscal responsibility for newly recruited or retained faculty was novel, but essential.
Historically, for every cent of federal funding spent on health services and outcomes research one dollar is spent on basic science and transplantation research [85]. However, despite the less prominent historical roots compared to basic science, health services and outcomes research has increasingly been utilized and recognized for its scientific rigor and value in advancing medicine [86]. This greater acclaim is noteworthy as the traditional federal funding agencies for transplantation (e.g., NIDDK: National Institute of Diabetes and Digestive and Kidney Diseases) have begun to support such projects (e.g., A2ALL Patient Safety R01). Championing the field of rigorous health services and outcomes research in transplantation through transplant societies (e.g., AST, ASTS) and engaging the NIH in discussions, will be essential to provide R funding opportunities for research faculty and clinical investigators. Facilitating collaborations between agencies (e.g., NIA and NIDDK), might provide more opportunities and productivity for this developing field of research.
CONCLUSION
The field of transplantation is well-suited to implement inter- and transdisciplinary research to address the many unanswered clinically relevant questions. Therefore, we created an inter- and transdisciplinary research group called NUTORC that has grown to eight content teams each co-led by a transplant clinician and an outcomes research expert, and reinforced with methodological support as needed. The institution made a substantive initial investment of $900,000 to enable the creation of NUTORC, which has now been leveraged to become highly productive academically and financially. NUTORC has succeeded in connecting inter-disciplinary collaborators across the university through transdisciplinary research (Network Fig. 4). To date, NUTORC has secured $11,619,500 in federal and extramural grants and was fiscally self-sustaining within 3 years after inception. Furthermore, NUTORC faculty have published 60 manuscripts, and given 166 national and international presentations and posters. Table 3 demonstrates that transdisciplinary collaboration in transplant as exemplified with NUTORC, is feasible, sustainable, and highly productive.
Table 3.
NUTORC overview
| Significance |
| Addresses important problems and critical barriers in the field of transplantation, identified by transplant leaders |
| Expands scientific knowledge across seven research areas (patient safety, HRQOL, access and allocation, risk and econometrics, informed consent and disparities, health literacy and medication adherence, infectious diseases) |
| Improves technical capabilities through the advances achieved in the health informatics team |
| Achieves recognition of through publication of peer-reviewed manuscripts, national presentations and receipt of federal and other extramural funding |
| NUTORC has demonstrated that at the intersection of transdisciplinary collaboration, new areas of innovative research in health services and outcomes research can be created, that go well beyond secondary data analysis |
| Funded projects are in progress to improve systems and processes surrounding transplantation, expand applied methodologies for allocation and access to transplantation, provide deeper understanding about informed consent, provide culturally sensitive education, services, and care, identify the appropriate treatments for liver and kidney transplantation, study and improve medication adherence, reduce infectious disease and create data systems for analysis that are far richer than the existing national data sources |
| Focuses on existing expertise within the university and collaborative efforts across disciplines and schools |
| Innovation |
| Creates transdisciplinary teams to address pertinent questions with cutting-edge methodologies |
| Introduces state-of-the art health services and outcomes research into the field of transplantation, beyond secondary data analysis |
| Shifts practice paradigm in transplantation from basic science to health services and outcomes research in transplantation |
| Creates and maintains exclusively transdisciplinary teams bringing together seemingly disparate sciences |
| Introduces novel specific topics to transplant-specific funding institutions (e.g., patient safety to NIH/NIDDK) |
| Diffuses transdisciplinary expertise successfully |
| Shared financial investment by collaborating partners, consolidating shared interest |
| Approach |
| Identifies national leaders in research with expertise pertinent to health services and outcomes research across the university |
| Creates pipeline for nascent scientists with interest in transplantation through NIH/NIDDK funded T-32 postdoctoral fellowship program |
| Secures buy-in from the university and hospital and secures seed funding |
| Significant investment up front with 3-year timeline to become self-sustaining |
| Incentive and expectation from clinical transplant leadership for all clinicians to be involved in research |
| No financial penalty if research funding is obtained and clinical productivity has to be reduced |
| Joint hiring or financial support of research faculty on 3-year timeline |
| Investigators |
| Fosters collaboration between junior, mid-career and senior researchers and clinical investigators |
| All key investigators (co-chairs) have successfully published in peer-reviewed manuscripts and have received grant funding |
| Each team is led by a transplant clinician and an expert previously foreign to the topic on transplantation |
| Senior mentorship by highly federal funded clinical and research investigators |
| Environment |
| Leading university with abundance of national leaders in diverse research areas |
| Support for collaborative and transdisciplinary work by the university, the schools and the division |
| Commitment and full support of transplant leadership (Director CTC) |
| Senior mentorship accessible to and integrated into NUTORC |
| Excellent track-record of successful mentoring |
| Large transplant center with all transplant clinicians immersed in clinical and national policy activities |
| MATRIX: multi-layered mentorship involving senior leadership, mid-career, junior level research and clinical faculty as well as students |
Acknowledgments
This work was funded by Northwestern Medicine’s Collaborative Development Initiative (CDI), and the Northwestern Transplant Outcomes Research Collaborative (NUTORC). The authors would like to thank all Northwestern University Transplant Outcomes Research Collaborative (NUTORC) transdisciplinary team members and collaborators for their opinions and suggestions for this work.
Footnotes
Implications
Practice: To build a robust, effective and sustainable institutional, inter- and transdisciplinary health services and outcomes research team in transplantation, strengths in various disciplines should be leveraged with the clinical strength of the multi-organ transplant program.
Policy: To build productive and sustainable transdisciplinary research collaborations in health services and outcomes research in transplantation, resources should be aimed toward investigators that bridge expertise from different departments and schools.
Research: At the intersection of seemingly disparate disciplines, strong and innovative outcomes and health services research can be achieved in organ transplantation
References
- 1.Tilney NL. Transplant: from myth to reality. New Haven: Yale University Press; 2003. [Google Scholar]
- 2.Chinen J, Buckley RH. Transplantation immunology: solid organ and bone marrow. The Journal of Allergy and Clinical Immunology. 2010;125(2 Suppl 2):S324–S335. doi: 10.1016/j.jaci.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical Trials In Organ Transplantation (CTOT). www.ctotstudies.org.
- 4.42 FR. Parts 405, 482, 488, and 498 Medicare Program Hospital Conditions of Participation: Requirements for Approval and Re-Approval of Transplant Centers To Perform Organ Transplants; Final Rule. In: Department of Health and Human Services Centers for Medicare & Medicaid Services, ed2007. [PubMed]
- 5.Services CfMaM. (2006) Final Rule, CMS-3064-F: Conditions for Coverage (CfCs) & Conditions of Participations (CoPs). Federal Register, 30981–31054. [PubMed]
- 6.Weinstein MC, Skinner JA. Comparative effectiveness and health care spending—implications for reform. The New England Journal of Medicine. 2010;362(5):460–465. doi: 10.1056/NEJMsb0911104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeve BB, Burke LB, Chiang YP, et al. Enhancing measurement in health outcomes research supported by Agencies within the US Department of Health and Human Services. Quality of life Research : An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation. 2007;16(Suppl 1):175–186. doi: 10.1007/s11136-007-9190-8. [DOI] [PubMed] [Google Scholar]
- 8.Butt Z, Parikh ND, Skaro AI, Ladner D, Cella D. Quality of life, risk assessment, and safety research in liver transplantation: new frontiers in health services and outcomes research. Current Opinion in Organ Transplantation. 2012;17(3):241–247. doi: 10.1097/MOT.0b013e32835365c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jay CL, Butt Z, Ladner DP, Skaro AI, Abecassis MM. A review of quality of life instruments used in liver transplantation. Journal of Hepatology. 2009;51(5):949–959. doi: 10.1016/j.jhep.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duhigg C. (2012) How companies learn your secrets. The New York Times.
- 11.Magill-Evans J, Hodge M, Darrah J. Establishing a transdisciplinary research team in academia. Journal of Allied Health. 2002;31(4):222–226. [PubMed] [Google Scholar]
- 12.Ladner D, Gordon E, Ross O, et al. (2011) NUTORC—the implementing of a transdisciplinary scientific collaboration to advance transplantation outcomes research. Paper presented at: International Science of Team Science (SciTS) Conference; Chicago, IL.
- 13.Northwestern University Department of Surgery—Feinberg School of Medicine. Northwestern’s Transplant Program. Organ Transplantation Overviewhttp://www.surgery.northwestern.edu/divisions/transplant/overview/index.html.
- 14.Baker T, Jay C, Ladner D, et al. Laparoscopy-assisted and open living donor right hepatectomy: a comparative study of outcomes. Surgery. 2009;146(4):817–825. doi: 10.1016/j.surg.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Garzoni C, Ison MG. (2011) Uniform definitions for donor-derived infectious disease transmissions in solid organ transplantation. Transplantation. Oct 12. [DOI] [PubMed]
- 16.Gilmour SM, Sorensen LG, Anand R, Yin W, Alonso EM. School outcomes in children registered in the studies for pediatric liver transplant (SPLIT) consortium. Liver Transplantation. 2010;16(9):1041–1048. doi: 10.1002/lt.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khosla N, Gordon E, Nishi L, Ghossein C. (2010) Impact of a chronic kidney disease clinic on preemptive kidney transplantation and transplant wait times. Progress in Transplantation. 20(3). [DOI] [PubMed]
- 18.Miloh T, Anand R, Yin W, Vos M, Kerkar N, Alonso E. (2011) Pediatric liver transplantation for primary sclerosing cholangitis (PSC). Liver Transplant Apr 19. [DOI] [PMC free article] [PubMed]
- 19.Sundaram SS, Alonso EM, Anand R. Outcomes after liver transplantation in young infants. Journal of Pediatric Gastroenterology and Nutrition. 2008;47(4):486–492. doi: 10.1097/MPG.0b013e318175d7d2. [DOI] [PubMed] [Google Scholar]
- 20.Sundaram SS, Alonso EM, Narkewicz MR, Zhang S, Squires RH. (2011) Characterization and outcomes of young infants with acute liver failure. Journal Pediatrics May 27. [DOI] [PMC free article] [PubMed]
- 21.Trotter JF, Gillespie BW, Terrault NA, et al. Laboratory test results after living liver donation in the adult-to-adult living donor liver transplantation cohort study. Liver Transplantation. 2011;17(4):409–417. doi: 10.1002/lt.22246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alonso EM. Growth and developmental considerations in pediatric liver transplantation. Liver Transplantation. 2008;14(5):585–591. doi: 10.1002/lt.21488. [DOI] [PubMed] [Google Scholar]
- 23.Alonso EM. Quality of life for pediatric liver recipients. Liver Transplantation. 2009;15(Suppl 2):S57–S62. doi: 10.1002/lt.21904. [DOI] [PubMed] [Google Scholar]
- 24.Alonso EM, Limbers CA, Neighbors K, et al. Cross-sectional analysis of health-related quality of life in pediatric liver transplant recipients. The Journal of Pediatrics. 2010;156(2):270–276. doi: 10.1016/j.jpeds.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso EM, Neighbors K, Barton FB, et al. Health-related quality of life and family function following pediatric liver transplantation. Liver Transplantation. 2008;14(4):460–468. doi: 10.1002/lt.21352. [DOI] [PubMed] [Google Scholar]
- 26.Alonso EM, Sorensen LG. Cognitive development following pediatric solid organ transplantation. Current Opinion in Organ Transplantation. 2009;14(5):522–525. doi: 10.1097/MOT.0b013e3283307a62. [DOI] [PubMed] [Google Scholar]
- 27.Bucuvalas JC, Alonso E, Magee JC, Talwalkar J, Hanto D, Doo E. Improving long-term outcomes after liver transplantation in children. American Journal of Transplantation. 2008;8(12):2506–2513. doi: 10.1111/j.1600-6143.2008.02432.x. [DOI] [PubMed] [Google Scholar]
- 28.Butt Z, Gordon E, Penrod D, Sherman LA. Which patients with ESRD are willing to pay for a kidney? American Journal of Transplantation. 2010;10(11):2560. doi: 10.1111/j.1600-6143.2010.03243.x. [DOI] [PubMed] [Google Scholar]
- 29.Butt Z, Ladner D, Parikh N. Quality of life in actual living liver donors versus potential living liver donors. Liver Transplantation. 2010;16(5):691. doi: 10.1002/lt.22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butt Z, Parikh ND, Beaumont JL, et al. (2012) Development and validation of a symptom index for advanced hepatobiliary and pancreatic cancers: The NCCN-FACT Hepatobiliary-Pancreatic Symptom Index (NFHSI). Cancer. [DOI] [PMC free article] [PubMed]
- 31.Butt Z, Yount SE, Caicedo JC, Abecassis MM, Cella D. Quality of life assessment in renal transplant: review and future directions. Clinical Transplantation. 2008;22(3):292–303. doi: 10.1111/j.1399-0012.2007.00784.x. [DOI] [PubMed] [Google Scholar]
- 32.Limbers CA, Neighbors K, Martz K, et al. (2010) Health-related quality of life in pediatric liver transplant recipients compared with other chronic disease groups. Pediatrics Transplant Dec 29. [DOI] [PMC free article] [PubMed]
- 33.Mohammad S, Alonso EM. Approach to optimizing growth, rehabilitation, and neurodevelopmental outcomes in children after solid-organ transplantation. Pediatric Clinics of North America. 2010;57(2):539–557. doi: 10.1016/j.pcl.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Mohammad S, Hormaza L, Neighbors K, et al. (2012) Health status in young adults two decades after pediatric liver transplantation. American Journal of Transplant May 8. [DOI] [PMC free article] [PubMed]
- 35.Parikh N, Butt Z. The relative contribution of hepatic encephalopathy to burden in patients with cirrhosis. The American Journal of Gastroenterology. 2012;107(3):486. doi: 10.1038/ajg.2011.488. [DOI] [PubMed] [Google Scholar]
- 36.Parikh ND, Ladner D, Abecassis M, Butt Z. Quality of life for donors after living donor liver transplantation: a review of the literature. Liver Transplantation. 2010;16(12):1352–1358. doi: 10.1002/lt.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorensen LG, Neighbors K, Martz K, Zelko F, Bucuvalas JC, Alonso EM. Cognitive and academic outcomes after pediatric liver transplantation: functional outcomes group (FOG) results. American Journal of Transplantation. 2011;11(2):303–311. doi: 10.1111/j.1600-6143.2010.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varni JW, Limbers CA, Neighbors K, et al. The PedsQL Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Quality of Life Research : An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation. 2011;20(1):45–55. doi: 10.1007/s11136-010-9730-5. [DOI] [PubMed] [Google Scholar]
- 39.Varni JW, Limbers CA, Sorensen LG, et al. (2010). PedsQL Cognitive Functioning Scale in pediatric liver transplant recipients: feasibility, reliability, and validity. Quality of Life Research : An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation. Dec 24. [DOI] [PMC free article] [PubMed]
- 40.Weissberg-Benchell J, Zielinski TE, Rodgers S, et al. Pediatric health-related quality of life: Feasibility, reliability and validity of the PedsQL transplant module. American Journal of Transplantation. 2010;10(7):1677–1685. doi: 10.1111/j.1600-6143.2010.03149.x. [DOI] [PubMed] [Google Scholar]
- 41.Ison MG, Holl JL, Ladner D. (2012) Preventable errors in organ transplantation: an emerging patient safety issue? Am J Transplant. Jun 15. [DOI] [PMC free article] [PubMed]
- 42.Buoy AG, Yoo S, Alam M, et al. Distribution of skin type and skin cancer in organ transplant recipients. Archives of Dermatology. 2010;146(3):344–346. doi: 10.1001/archdermatol.2009.397. [DOI] [PubMed] [Google Scholar]
- 43.Fischer MJ, Ahya SN, Gordon EJ. Interventions to reduce late referrals to nephrologists. American Journal of Nephrology. 2011;33(1):60–69. doi: 10.1159/000322704. [DOI] [PubMed] [Google Scholar]
- 44.Gordon E, Bergeron A, McNatt G, Friedewald J, Abecassis M, Wolf M. Are informed consent forms for organ transplantation and donation too difficult to read? Clinical Transplantation. 2012;26(2):275–283. doi: 10.1111/j.1399-0012.2011.01480.x. [DOI] [PubMed] [Google Scholar]
- 45.Gordon EJ, Beauvais N, Theodoropoulos N, et al. The challenge of informed consent for increased risk living donation and transplantation. American Journal of Transplantation. 2011;11(12):2569–2574. doi: 10.1111/j.1600-6143.2011.03814.x. [DOI] [PubMed] [Google Scholar]
- 46.Gordon EJ, Frader J, Goldberg AM, Penrod D, McNatt G, Franklin J. In response to: Testa et al. ‘elective surgical patients as living organ donors: a clinical and ethical innovation’. American Journal of Transplantation. 2010;10(3):704–705. doi: 10.1111/j.1600-6143.2009.02946.x. [DOI] [PubMed] [Google Scholar]
- 47.Gordon EJ, Caicedo JC. Ethnic advantages in kidney transplant outcomes: the hispanic paradox at work? Nephrology, Dialysis, Transplantation. 2009;24(4):1103–1109. doi: 10.1093/ndt/gfn691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon EJ, Caicedo JC, Ladner DP, Reddy E, Abecassis MM. Transplant center provision of education and culturally and linguistically competent care: a national study. American Journal of Transplantation. 2010;10(12):2701–2707. doi: 10.1111/j.1600-6143.2010.03304.x. [DOI] [PubMed] [Google Scholar]
- 49.Gordon EJ, Conti D. The quality of health insurance service delivery for kidney transplant recipients: a patient perspective. American Journal of Transplantation. 2010;10(10):2208–2214. doi: 10.1111/j.1600-6143.2010.03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon EJ, Daud A, Caicedo JC, et al. Informed consent and decision-making about adult-to-adult living donor liver transplantation: a systematic review of empirical research. Transplantation. 2011;92(12):1285–1296. doi: 10.1097/TP.0b013e31823817d5. [DOI] [PubMed] [Google Scholar]
- 51.Gordon EJ, Ladner DP, Caicedo JC, Franklin J. Disparities in kidney transplant outcomes: a review. Seminars in Nephrology. 2010;30(1):81–89. doi: 10.1016/j.semnephrol.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gordon EJ, Lash JP. A timely change in CKD delivery: promoting patient education. American Journal of Kidney Diseases. 2011;57(3):375–377. doi: 10.1053/j.ajkd.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gordon EJ, Prohaska TR, Gallant MP, et al. Prevalence and determinants of physical activity and fluid intake in kidney transplant recipients. Clinical Transplant. 2010;24(3):E69–E81. doi: 10.1111/j.1399-0012.2009.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gordon EJ, Prohaska TR, Gallant MP, et al. Longitudinal analysis of physical activity, fluid intake, and graft function among kidney transplant recipients. Transplant International. 2009;22(10):990–998. doi: 10.1111/j.1432-2277.2009.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gordon E, Wolf M. Health literacy among kidney transplant recipients. Progress in Transplantation. 2009;19(1):25–34. doi: 10.1177/152692480901900104. [DOI] [PubMed] [Google Scholar]
- 56.Lora CM, Gordon EJ, Sharp LK, Fischer MJ, Gerber BS, Lash JP. Progression of CKD in Hispanics: potential roles of health literacy, acculturation, and social support. American Journal of Kidney Diseases. 2011;58(2):282–290. doi: 10.1053/j.ajkd.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robinson JK, Alam M, Ashourian N, et al. Skin cancer prevention education for kidney transplant recipients: a systematic evaluation of Internet sites. Progress in Transplantation. 2010;20(4):344–349. doi: 10.1177/152692481002000407. [DOI] [PubMed] [Google Scholar]
- 58.Robinson JK, Turrisi R, Mallett KA, et al. Efficacy of an educational intervention with kidney transplant recipients to promote skin self-examination for squamous cell carcinoma detection. Archives of Dermatology. 2011;147(6):689–695. doi: 10.1001/archdermatol.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jay CL, Lyuksemburg V, Ladner DP, et al. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta-analysis. Annals of Surgery. 2011;253(2):259–264. doi: 10.1097/SLA.0b013e318204e658. [DOI] [PubMed] [Google Scholar]
- 60.Jay CL, Lyuksemburg V, Kang R, et al. The increased costs of donation after cardiac death liver transplantation: caveat emptor. Annals of Surgery. 2010;251(4):743–748. doi: 10.1097/SLA.0b013e3181d3d3da. [DOI] [PubMed] [Google Scholar]
- 61.Jay C, Skaro A, Ladner D, et al. (2012). The comparative effectiveness of donation after cardiac death versus donation after brain death liver transplantation: recognizing who can benefit. Liver Transplant [DOI] [PMC free article] [PubMed]
- 62.Jay C, Ladner D, Wang E, et al. A comprehensive risk assessment of mortality following donation after cardiac death liver transplant—an analysis of the national registry. Journal of Hepatology. 2011;55(4):808–813. doi: 10.1016/j.jhep.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raval Z, Harinstein ME, Skaro AI, et al. Cardiovascular risk assessment of the liver transplant candidate. Journal of the American College of Cardiology. 2011;58(3):223–231. doi: 10.1016/j.jacc.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 64.Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140(2):497–507. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skaro AI, Jay CL, Baker TB, et al. The impact of ischemic cholangiopathy in liver transplantation using donors after cardiac death: the untold story. Surgery. 2009;146(4):543–552. doi: 10.1016/j.surg.2009.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skaro AI, Jay CL, Ladner D, Abecassis MM. Trends in donation after cardiac death and donation after brain death—reading between the lines. American Journal of Transplantation. 2010;10(11):2390–2391. doi: 10.1111/j.1600-6143.2010.03299.x. [DOI] [PubMed] [Google Scholar]
- 67.Pondrom S. The AJT, report: news and issues that affect organ and tissue transplantation. American Journal of Transplantation. 2011;11(7):1345–1346. doi: 10.1111/j.1600-6143.2011.03658.x. [DOI] [PubMed] [Google Scholar]
- 68.Ladner DP, Ison M. Safety issues in organ transplantation—AST Podcast. http://www.a-s-t.org/podcasts/safety-issues-organ-transplantation.
- 69.Ladner DP, Skaro AI, Abecassis MM. (2012). Are all readmissions the same? Liver Transplant Jul 28. [DOI] [PubMed]
- 70.Friedman AL, Lee KC, Lee GD. Errors in ABO labeling of deceased donor kidneys: case reports and approach to ensuring patient safety. American Journal of Transplantation. 2007;7(2):480–483. doi: 10.1111/j.1600-6143.2006.01630.x. [DOI] [PubMed] [Google Scholar]
- 71.Ison MG, Nalesnik MA. An update on donor-derived disease transmission in organ transplantation. American Journal of Transplantation. 2011;11(6):1123–1130. doi: 10.1111/j.1600-6143.2011.03493.x. [DOI] [PubMed] [Google Scholar]
- 72.Ross O, McNatt G, Ladner D, et al. (2009). Adult living donor liver transplantation safety: a systematic literature review. American Transplant Congress.
- 73.Commission Electrotechnique Internationale. (2006). Analysis techniques for system reliability: procedure for failure mode and effects analysis (FMEA). 2nd ed. Geneva, Switzerland.
- 74.Pondrom S. The AJT, report: news and issues that affect organ and tissue transplantation. American Journal of Transplantation. 2010;10(5):1109–1110. doi: 10.1111/j.1600-6143.2009.03122.x. [DOI] [PubMed] [Google Scholar]
- 75.Preczewski L. (2011). UNOS May Have Underestimated the Data Collection Burden It Places on Transplant Centers in Its Required Justification to the White House Office of Management and Budget. Paper presented at: American Transplant Congress; Philadelphia, PA.
- 76.Preczewski L, McNatt G. (2007). Physician practice relationship with the transplant center. Paper presented at: UNOS Transplant Management Forum; Washington, DC.
- 77.Preczewski L, McNatt G. (2011). Building a hospital–physician practice relationship. Paper presented at: American Association of Surgery Administrators; Chicago, IL.
- 78.Davis A, Ladner D, Friedewald J, Daskin M, Mehrotra S, Lyuksemburg V. (2010). Dialysis centers are placed closer to healthy than the sick. Paper presented at: American Transplant Congress; San Diego, CA.
- 79.Davis A, Ladner D, Friedewald J, Mehrotra S, Daskin M, Skaro A. (2011). Demonstrating geographic equity in kidney organ allocation satisfying the final rule at last. Paper presented at: American Society of Nephrology; Philadelphia, PA.
- 80.Davis A, Ladner D, Gonzales A, Rigney C, Friedewald J. (2010). Use of the kidney donor risk index to assess organ utilization in a single OPO. Paper presented at: American Transplant Congress; San Diego, CA.
- 81.Davis A, Ladner D, Mehrotra S, Skaro A, Friedewald J. (2012). Ensuring the gift of life is not wasted: a sharing perspective on hard to place kidney allocation. Paper presented at: American Transplant Congress; Boston, MA.
- 82.Serper M, Patzer RE, Przytula K, et al. (2012). Medication non-adherence, treatment understanding, and health literacy in liver transplant recipients. Paper presented at: American Association for the Study of Liver Diseases; Boston, MA.
- 83.Serper M, Patzer RE, Przytula K, et al. (2012). Medication non-adherence, treatment understanding, and health literacy in kidney transplant recipients. Paper presented at: American Society of Nephrology; San Diego, CA.
- 84.ASP Workshop on Solid Organ Transplantation in Older Adults. (2012) http://www.im.org/AcademicAffairs/Aging/IGP/ExpandingResearchEfforts/Pages/SolidOrganTransplantation.aspx.
- 85.Pronovost PJ, Colantuoni E. Measuring preventable harm: helping science keep pace with policy. JAMA : The Journal of the American Medical Association. 2009;301(12):1273–1275. doi: 10.1001/jama.2009.388. [DOI] [PubMed] [Google Scholar]
- 86.Moses H, 3rd, Martin JB. Biomedical research and health advances. The New England Journal of Medicine. 2011;364(6):567–571. doi: 10.1056/NEJMsb1007634. [DOI] [PubMed] [Google Scholar]