To the Editor: Bacteria of the genus Bordetella are gram-negative, rod-shaped organisms that cause respiratory tract diseases in humans and animals. In 1995, Bordetella hinzii was isolated from poultry and 2 patients in the United States and France (1). This pathogen colonizes the respiratory tract of poultry and is closely related to B. avium, which is a commensal species in poultry. However, information on the etiologic role, hosts, and transmission routes of B. hinzii is incomplete because infections in human who did not have any close contact with poultry have been reported, mainly in immunocompromised patients (1–5). We obtained a single isolate of B. hinzii from blood agar culture during screening for bacterial zoonotic diseases in blood samples of rodents in Southeast Asia during the Ceropath project (www.ceropath.org).
During 2008–2010, we collected rodents along the Mekong River areas of 3 countries in Southeast Asia (Cambodia, Laos, and Thailand). Rodents were trapped in urban areas and in rural areas, which consisted of forests, upland and dry agricultural areas (orchards, cassava fields), unirrigated and irrigated agricultural areas (rice fields), and domestic areas (isolated farms and villages). Each animal was identified at the species level by using morphologic or molecular methods. Two hundred six blood samples were cultured on Columbia agar containing 5% sheep blood and incubated at 37°C for 3–7 days. A single atypical isolate was observed after 2 days of culture. This isolate was identified by using matrix-assisted laser desorption ionization time-of-flight mass spectrometry as described by Seng et al. (6). However, this isolate was identified only at the genus level as a Bordetella sp. (score 1.7).
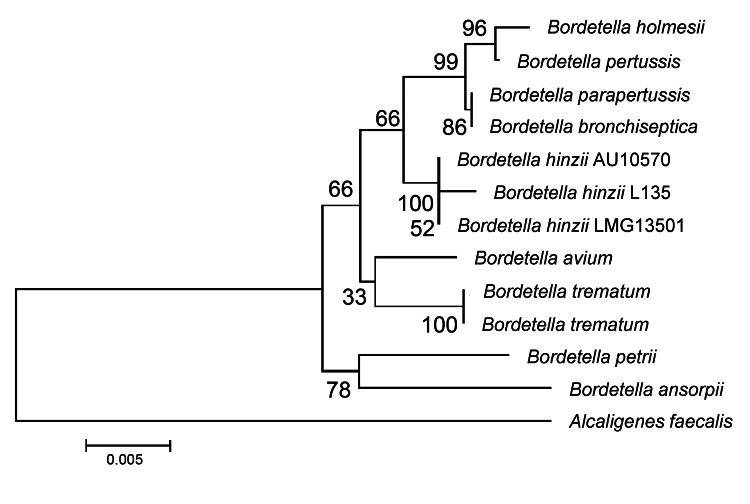
To identify the Bordetella species, DNA from the isolate was extracted by using the QIAamp DNA Kit (QIAGEN, Hilden, Germany). Partial PCR amplification and sequencing of 16S rRNA gene was performed as described (7). Sequence analysis showed that the isolate was closely related to B. hinzii LMG 13501 (99.0% homology), which was isolated from the blood of a patient who died of septicemia in 2000 (2). The 16S rRNA sequence of our isolate (B. hinzii L135) has been deposited in GenBank under accession no. JX188059. A phylogenetic analysis of the new sequence and sequences of other bacteria in the genus Bordetella is shown in the Figure.
Figure.
Maximum-parsimony phylogenetic tree of 16S rRNA gene of Bordetella hinzii isolate from this study (L135) and validated Bordetella species. Numbers along branches indicate bootstrap values. Scale bar indicates nucleotide substitutions per site.
B. hinzii is a causative agent of respiratory tract illnesses in birds and has been described as an emerging and opportunistic pathogen in immunocompromised patients; and in patients with AIDS, cystic fibrosis, and fatal septicemia (1–5). However, the source of transmission is not clear. Although B. hinzii is commensal in birds, several cases were reported in persons who did not have any close contact with birds (2–5), suggesting alternative sources of contamination. Thus, transmission routes and reservoirs of B. hinzii infection are ambiguous. B. hinzii infection has also been reported in rabbits and laboratory mice in Hungary and Japan (8–10). Rodents were suspected to be potential reservoirs but, to the best of our knowledge, this emerging pathogen has not been reported in wild rodents.
We detected in B. hinzii in a Rattus tanezumi rat that was trapped in upland agricultural area in Laos. R. tanezumi rats are the most common rodent in southeastern Asia and can be found in various habitats, including forests, agricultural areas, and houses. In Southeast Asia, human populations in several countries (Cambodia, Laos, and Thailand) live in close contact with rodents (including R. tanezumi) or share the environment with them. These rodents are known to be a reservoir and possible source of bacterial zoonoses such as leptospirosis, plague, scrub typhus, and murine typhus.
Our findings suggest that B. hinzii isolated from wild rodents may serve as reservoir for this bacterial species that could be transmitted to human or pets. B. hinzii should be added to the list of emerging bacterial zoonotic agents in wild rodents that could be pathogenic for humans. Further studies are warranted to evaluate the prevalence of this bacterium in rodents in other countries and to demonstrate that rodents may be a source of transmission of this bacterium to humans, especially immunocompromised patients.
Acknowledgments
We thank Annick Bernard and Linda Hadjadj for technical assistance.
This study was supported by the French National Research Agency; CERoPath (Community Ecology of Rodents and their Pathogens in Southeast Asia Project ANR 07 BDIV 012); Infectiopôle Sud; Center for Excellence on Agricultural Biotechnology; the Science and Technology Postgraduate Education and Research Development Office; the Office of Higher Education Commission, Ministry of Education (AG-BIO/PERDO-CHE); and the Center of Advanced Studies for Agriculture and Food, Institute for Advanced Studies, Kasetsart University.
Footnotes
Suggested citation for this article: Jiyipong T, Morand S, Jittapalapong S, Raoult D, Rolain J-M. Bordetella hinzii in rodents, Southeast Asia [letter]. Emerg Infect Dis [Internet]. 2013 Mar [date cited]. http://dx.doi.org/10.3201/eid1903.120987
References
- 1.Vandamme P, Hommez J, Vancanneyt M, Monsieurs M, Hoste B, Cookson B, et al. Bordetella hinzii sp. nov., isolated from poultry and humans. Int J Syst Bacteriol. 1995;45:37–45. 10.1099/00207713-45-1-37 [DOI] [PubMed] [Google Scholar]
- 2.Kattar MM, Chavez JF, Limaye AP, Rassoulian-Barrett SL, Yarfitz SL, Carlson LC, et al. Application of 16S rRNA gene sequencing to identify Bordetella hinzii as the causative agent of fatal septicemia. J Clin Microbiol. 2000;38:789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvand M, Feldhues R, Mieth M, Kraus T, Vandamme P. Chronic cholangitis caused by Bordetella hinzii in a liver transplant recipient. J Clin Microbiol. 2004;42:2335–7. 10.1128/JCM.42.5.2335-2337.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fry NK, Duncan J, Edwards MT, Tilley RE, Chitnavis D, Harman R, et al. A UK clinical isolate of Bordetella hinzii from a patient with myelodysplastic syndrome. J Med Microbiol. 2007;56:1700–3. 10.1099/jmm.0.47482-0 [DOI] [PubMed] [Google Scholar]
- 5.Funke G, Hess T, von Graevenitz A, Vandamme P. Characteristics of Bordetella hinzii strains isolated from a cystic fibrosis patient over a 3-year period. J Clin Microbiol. 1996;34:966–9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seng P, Drancourt M, Gouriet F, La Scola B, Fournier PE, Rolain JM, et al. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis. 2009;49:543–51. 10.1086/600885 [DOI] [PubMed] [Google Scholar]
- 7.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Register KB, Sacco RE, Nordholm GE. Comparison of ribotyping and restriction enzyme analysis for inter- and intraspecies discrimination of Bordetella avium and Bordetella hinzii. J Clin Microbiol. 2003;41:1512–9. 10.1128/JCM.41.4.1512-1519.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashimoto N, Morita H, Yasuda M, Ishida T, Kameda S, Takakura A, et al. Prevalence of Bordetella hinzii in mice in experimental facilities in Japan. Res Vet Sci. 2012. [DOI] [PubMed]
- 10.Hayashimoto N, Yasuda M, Goto K, Takakura A, Itoh T. Study of a Bordetella hinzii isolate from a laboratory mouse. Comp Med. 2008;58:440–6 . [PMC free article] [PubMed] [Google Scholar]