Abstract
There are conflicting data regarding what motions increase ACL injury risk. More specifically, the mechanical role of valgus collapse positions during ACL injury remains controversial. Our objective was to evaluate ACL elongation in a model that mimics knee movements thought to occur during ACL injury. Eight healthy male subjects were imaged using MR and biplanar fluoroscopy to measure the in vivo elongation of the ACL and its functional bundles during three static knee positions: full extension, 30° of flexion, and a position intended to mimic a valgus collapse position described in the literature. For this study, the valgus collapse position consisted of 30° of knee flexion, internal rotation of the hip, and 10° of external tibial rotation. ACL length decreased significantly from full extension (30.2 ± 2.6 mm) to 30° of flexion (27.1 ± 2.2 mm). ACL length further decreased in the valgus collapse position (25.6 ± 2.4 mm). Both functional bundles of the ACL followed similar trends with regards to decreases in length in each of the three positions. Since strain would follow patterns of ACL length, landing on an extended knee may be a more relevant risk factor for ACL injuries than the valgus collapse position in males. Future studies should evaluate the effects of dynamic motion patterns on in vivo ACL strains.
Keywords: Anterior cruciate ligament (ACL), Magnetic resonance imaging (MRI), Fluoroscopy, Injury mechanisms, Valgus collapse, Dynamic valgus
INTRODUCTION
With an estimated annual incidence between 200,000 and 400,000 in the United States, anterior cruciate ligament (ACL) rupture is a common problem in young, active populations.22,26 For these patients, the repercussions of ACL injury likely involve temporary or permanent disability, absence from work or sports, high costs from operative or rehabilitation treatment, and early onset osteoarthritis (OA).26 Although surgical reconstruction is a common treatment option, its ability to restore normal joint function and mitigate the long-term development of osteoarthritis is unclear.4,19,20,39,50 For example, some studies have reported radiographic evidence of early-onset osteoarthritis in more than 50% of patients 10–20 years after ACL reconstruction.39,49 As a result of these outcomes, there has been a great interest in studying ACL injury mechanisms11,48,57 in order to design training programs aimed at prevention.25,29,47
Prevention programs have been aimed at non-contact ACL injuries, which comprise approximately 70% of all ACL injuries.24,57 Although some studies have reported significant decreases in the incidence of ACL injuries with training programs,29,41 other studies have been less successful.6,47,54 Furthermore, despite these efforts, ACL injury rates remain high.3 The ability of current prevention programs to reduce the incidence of ACL injury is likely hindered by controversies regarding the mechanisms of injury.48,53,60 Specifically, an understanding of what motions elevate in vivo ACL loading is needed to improve the efficacy of prevention programs.
Previous studies have shown that most ACL injuries occur during change of direction or cutting maneuvers, such as landing from a jump or pivoting on a planted foot.16,46 ACL ruptures also frequently occur with the knee in 30° of flexion or less.13,26,40 Although there is consensus on the association of lower flexion angles with ACL injury, there are conflicting opinions on how these injuries are influenced by valgus collapse positions described from videographic analyses of in-game ACL tears.10,31,36,46 In general, these positions have been described as excessive medial collapse of the knee, resulting in a valgus knee orientation as measured by the angle between long axes of the tibia and femur in the coronal plane.30,52 However, since the precise time of injury and ACL loading are not known, it is difficult to determine from videographic analyses what specific motions elevate ACL strains and initiate ACL injury.
A number of studies have used cadaver models to quantify the effects of valgus and other knee motions on ACL function.5,7,42,58 While these studies provide valuable data on the biomechanical function of the ACL, it is unclear how well these models reproduce the complex loading experienced under in vivo conditions. Furthermore, several techniques, including direct measurement from implanted strain gauges or a combination of 3D modeling and biplanar fluoroscopy, have been used to measure ACL function during various in vivo activities.8,23,33,37 However, there is limited in vivo data available on ACL function in positions thought to be high risk for injury. This information could play a vital role in the improvement of prevention programs, which is crucial to lowering the incidence of ACL injuries.
To address this lack of data in the literature, the objective of this study was to evaluate in vivo ACL elongation in a static model that mimics a valgus collapse knee position31,36,46 thought to occur at the time of injury. In addition, positions of full extension and low flexion were also considered. Elongation was measured since it is proportional to strain, a critical parameter for predicting ACL failure and injury risk.56 We hypothesized that ACL elongation would be elevated in the valgus collapse knee position since these positions are believed to be high risk for ACL injury.31,36,46
METHODS
Eight healthy male subjects (mean age, 30 ± 7 years; range, 23–41 years) were evaluated using an IRB approved protocol. All volunteers had no previous history of lower extremity injury or surgery prior to completing the test protocol.
One knee of each subject was imaged using a 3T MR scanner (Trio Tim, Siemens Medical Solutions USA, Malvern, PA) at the Center for Advanced Magnetic Resonance Development at Duke University. Coronal, sagittal, and axial images were acquired from the subjects while lying in a supine position using a double-echo steady-state sequence (DESS) and an eight-channel receive-only knee coil with a field of view of 15 × 15 cm2, a matrix of 512 × 512 pixels2, and slice thickness of 1 mm (flip angle: 25°; repetition time: 17 ms; echo time: 6 ms). From the three views of the MRI scans, outlines of the femur and tibia were segmented using solid-modeling software (Rhinoceros 4.0, Robert McNeel and Associates, Seattle, WA), as described in previous studies.2,56 Additionally, the attachment site of the ACL was outlined in the three planes of view. Knowing the voxel size, these outlines were then used to create 3D models of the distal femur and proximal tibia, as well as the footprints of the ACL on each. Orthogonal image sets were used to confirm the shape and position of the ACL. The ACL footprint was further divided into anteromedial (AM) and posterolateral (PL) bundles,28 as described previously in the literature (Fig. 1).33,38 A previous validation study has shown that this methodology can locate the center of the ACL footprint to within 0.3 mm.2 Based on a previous parametric study,38 we expect this relatively small difference to have minimal effect on our results.
FIGURE 1.
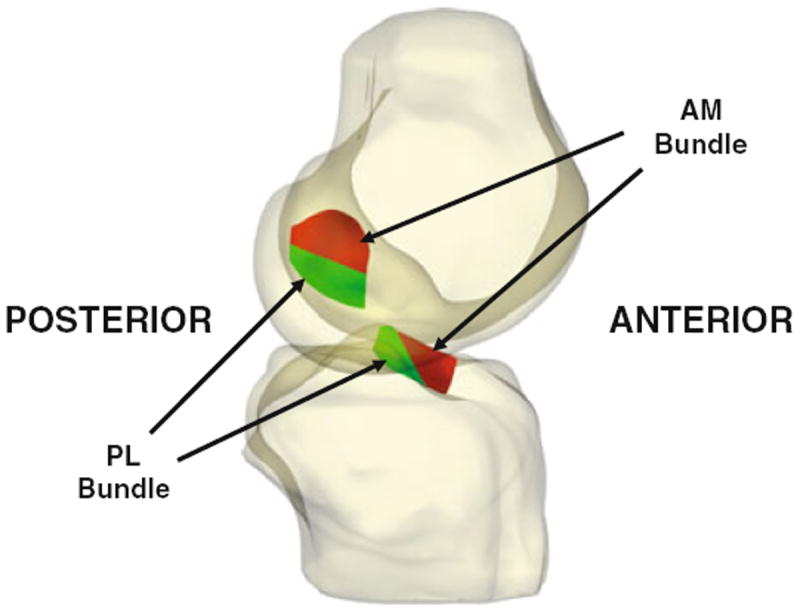
Three-dimensional knee models were created from high resolution MR images. These models included the attachment sites of the ACL on the femur and tibia. These attachment sites were divided into anteromedial (AM) and posterolateral (PL) bundles, as described previously.33,38
Following MRI, each subject’s knee was imaged while standing on a level platform from orthogonal directions using fluoroscopes (BV Pulsera, Philips, The Netherlands).33 Each fluoroscopic image had a resolution of 1024 × 1024 pixels2. The protocol consisted of the following single-legged static knee positions (Fig. 2): full extension, 30° of flexion, and 30° of flexion with 10° of external rotation of the tibia and maximal internal rotation at the hip to simulate a valgus collapse position.36,46,52,60 For each pose, subjects were guided on how to position their knees by one investigator using a goniometer.
FIGURE 2.
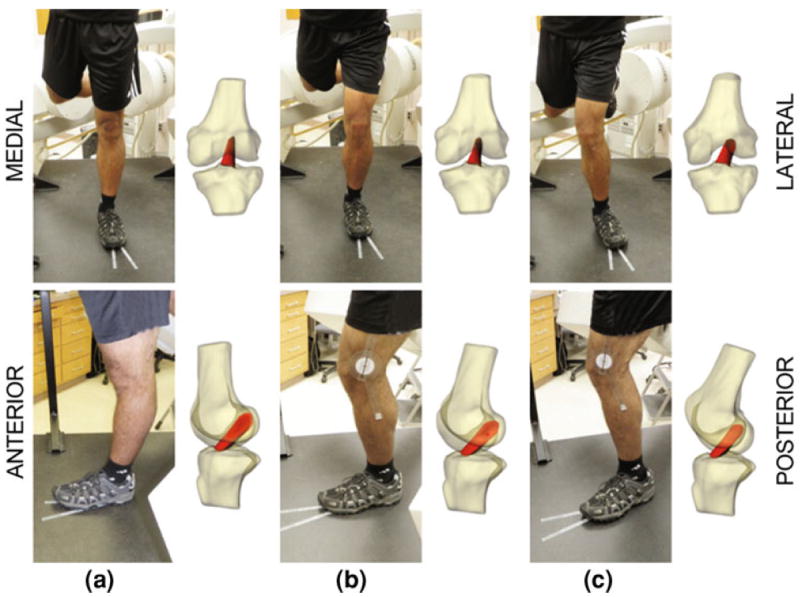
Each subject was imaged using biplanar fluoroscopy. Using 3D MR-based models of the knee joint, we then reproduced the motion of each subject’s knee during testing as demonstrated in coronal (top) and sagittal views (bottom). These models were used to measure ACL length and joint kinematics in each of the three positions: (a) full extension, (b) 30° flexion, (c) a pose mimicking a valgus collapse position, with 30° flexion, 10° external rotation of tibia, and internal rotation of the hip.
To create the in vivo joint model (Fig. 2), the orthogonal images were imported into the solid-modeling software in order to recreate the biplanar fluoroscopic system used during testing.1,12 Next, the 3D MR knee model was imported into the virtual fluoroscopic environment. Using custom-written edge detection software as a modeling aid to highlight the bone contours on the fluoroscopic images,1,12 the bones were moved individually in six degrees of freedom until their projections matched the bony outlines in the two orthogonal planes when viewed from the x-ray sources. Previous validation studies have shown that this approach can reproduce joint motion to within 0.1 mm and 0.3°.12,15
From these 3D models, knee joint kinematics and the length of the ACL and its functional bundles were measured. First, a coordinate system was drawn on each knee model.15 The long axis of the tibia was determined by fitting a cylinder to the tibial shaft. Next, a mediolateral axis was drawn perpendicular to the long axis of the tibia and tangent to the posterior extremes of the tibial plateau. Finally, the anteroposterior axis was drawn orthogonal to the long and mediolateral axes of the tibia. On the femur, the long axis was determined by fitting a cylinder to the femoral shaft. The femoral coordinate system consisted of this proximodistal axis and an axis through the transepicondylar line. The kinematic measures examined by this study included flexion, internal/external rotation, and varus/valgus angle.27 The transepicondylar line was used as a flexion/extension rotational axis. The internal/external rotation of the tibia was measured as the angle between the mediolateral axis of the tibia and the transepicondylar line projected on to the tibial plateau. Varus/valgus angle was measured as the change in angle between the long axis of tibia and transepicondylar line of the femur (Fig. 3). However, varus/valgus calculated this way is different from valgus measurements made by various videographic studies.11,46 Therefore, we used the coronal plane angle to approximate these measurements of valgus when viewed from a broad perspective outside the knee. Coronal plane angle was defined as the angle between the long axis of the femur and the long axis of the tibia projected on the tibial coronal plane (Fig. 3). ACL and bundle lengths were calculated as the distance between the area centroids of the femoral and tibial ACL attachment sites.1,56
FIGURE 3.
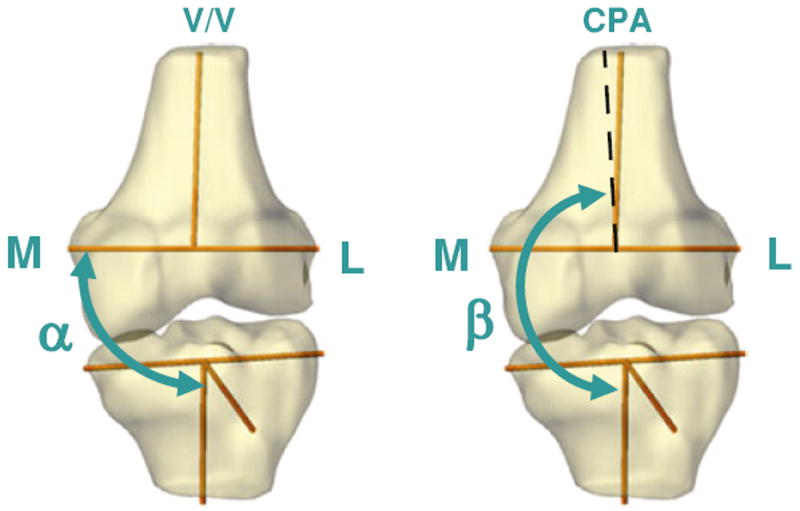
Varus/valgus angle (V/V, left) was defined as the angle between the tibial long axis and femoral transepicondylar line (α – 90°). Coronal plane angle (CPA, right) was defined as the angle between the long axis of the tibia and the long axis of the femur projected onto the tibial coronal plane (β – 180°).
Repeated measures ANOVA and Student–Newman–Keuls post hoc tests were used to detect statistically significant differences in flexion angle, as well as the lengths of the ACL and its functional bundles at each of the three knee positions. In addition, a two-way repeated measures ANOVA was used to detect differences between the coronal plane and varus/valgus angles in each knee position. Differences were considered statistically significant where p < 0.05.
RESULTS
The average flexion angles measured at the three different positions were −5.4 ± 7.3° for full extension, 30.0 ± 6.1° for 30° of flexion, and 29.3 ± 6.7° for the valgus collapse position (Fig. 4). At full extension, flexion was significantly different (p < 0.001) when compared with 30° of flexion and the valgus collapse position. No significant difference in flexion was detected between 30° of flexion and the valgus collapse position (p = 0.81). In the transverse plane, the tibia was externally rotated by 3.3 ± 4.0° at full extension, and was internally rotated by 4.2 ± 4.0° at 30° of flexion (Fig. 5). In the valgus collapse position, the tibia was externally rotated by 12.1 ± 4.0°, which was close to the 10° of desired external rotation we designed into the protocol. With regard to the orientation of the knee in the coronal plane, knee position and measurement type (varus/valgus angle vs. coronal plane angle) had statistically significant interactions (p < 0.001). No statistically significant differences were detected between the varus/valgus angle and coronal plane angle measurements with the knee in full extension or 30° of flexion (p > 0.76; Fig. 6). However, coronal plane angle was significantly different (p < 0.001) from the varus/valgus angle in the valgus collapse position. In particular, the varus/valgus angle was −1.0 ± 1.2°, while the coronal plane angle was 5.3 ± 5.5°.
FIGURE 4.
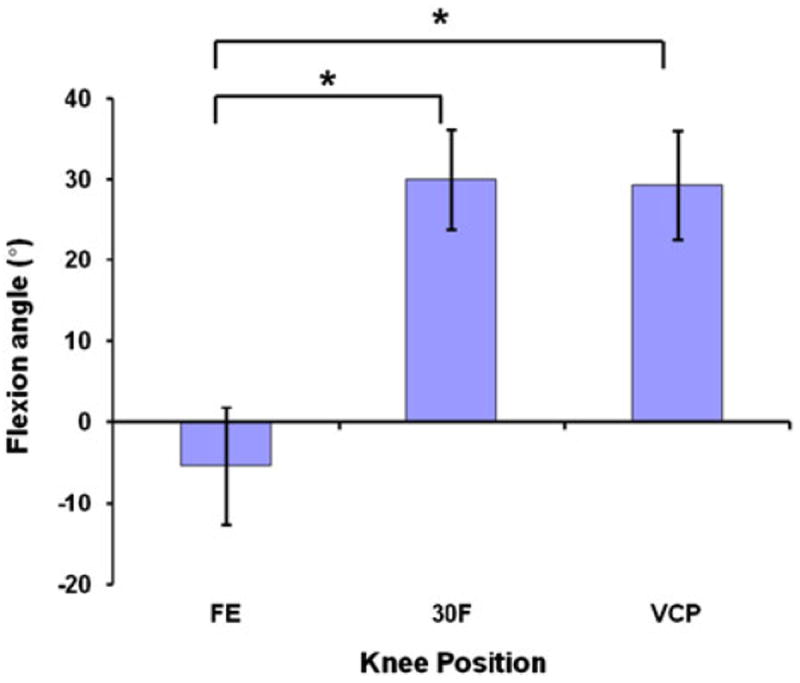
Flexion angle at each knee position (FE = full extension, 30F = 30° of flexion, VCP = valgus collapse position). Positive values indicate flexion and negative values indicate hyperextension (*p < 0.05).
FIGURE 5.
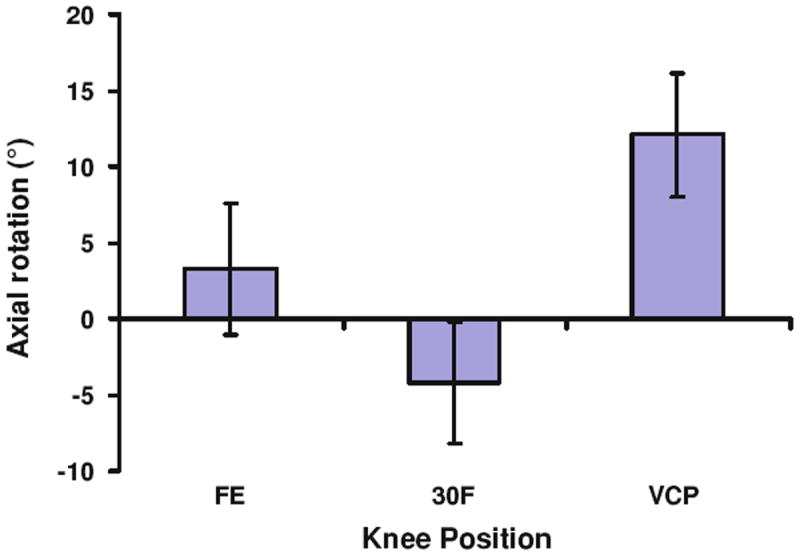
Axial rotation of the tibia at each knee position (FE = full extension, 30F = 30° of flexion, VCP = valgus collapse position). Positive values denote external rotation of the tibia, while negative values indicate internal rotation of the tibia.
FIGURE 6.
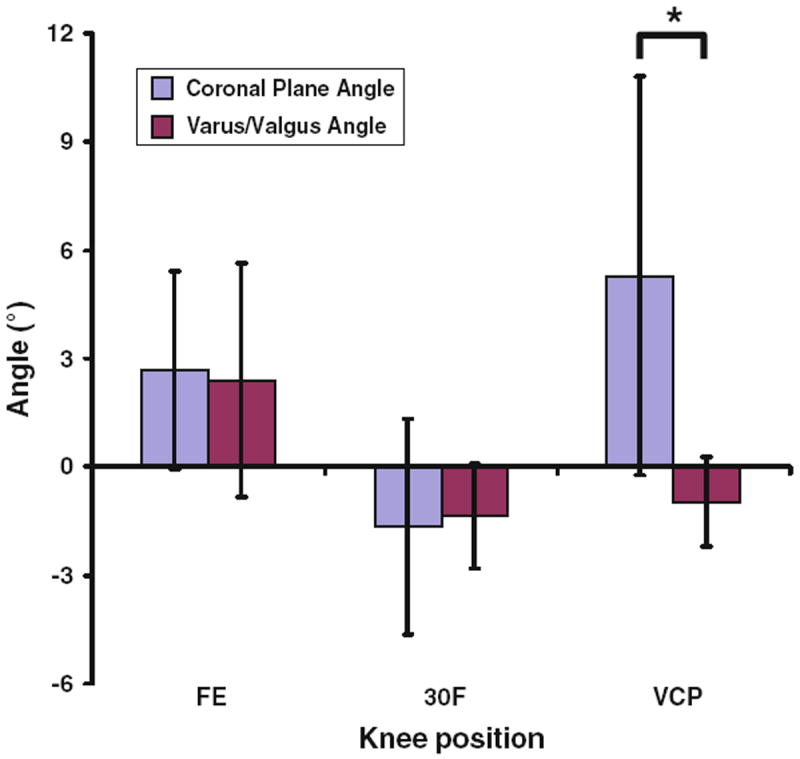
Coronal plane angle and varus-valgus angle at each knee position (FE = full extension, 30F = 30° of flexion, VCP = valgus collapse position). Positive values denote valgus and negative values indicate varus (*p < 0.05).
Knee position had a statistically significant effect on the length of the ACL (Fig. 7a; p < 0.001), the length of the AM bundle (Fig. 7b; p < 0.001), and the length of the PL bundle (Fig. 7c; p < 0.001). ACL length at full extension was 30.2 ± 2.6 mm and decreased to 27.1 ± 2.2 mm at 30° of flexion (p < 0.01). ACL length further decreased to 25.6 ± 2.4 mm in the valgus collapse position (p < 0.01). Similarly, the AM and PL bundles each experienced significant decreases in length as the knee was flexed from full extension to 30° of flexion (p < 0.05). Both bundles also had minimal length in the valgus collapse position (p < 0.01).
FIGURE 7.
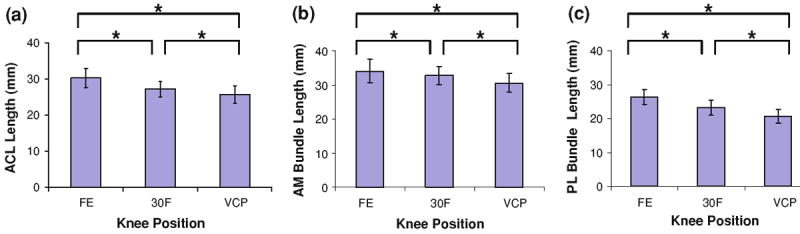
(a) ACL length at each knee position. (b) Anteromedial (AM) bundle of ACL at each knee position. (c) Posterolateral (PL) bundle of ACL at each knee position (FE = full extension, 30F = 30° of flexion, VCP = valgus collapse position, *p < 0.05).
DISCUSSION
Non-contact ACL injuries frequently occur during cutting or jumping maneuvers. Although the contributions of low flexion angles to ACL rupture are generally agreed upon,13,26,40 the role of valgus collapse remains controversial.10,23,31,53 Understanding what specific motions elevate ACL loading is critical to improving programs aimed at injury prevention. Because there are limited data on in vivo ACL loading during knee positions proposed to increase injury risk, the objective of this study was to evaluate ACL deformations in a static model that mimics low flexion and valgus positions thought to occur at the time of ACL rupture. Our results suggest that flexion has a greater influence on ACL elongation than valgus knee positioning in male subjects. These findings might suggest that landing on an extended knee is a position of higher risk for ACL injury than landing in a valgus collapse position. However, more studies are needed to evaluate what motions increase in vivo ACL loading under dynamic loading conditions.
In the current study, three different knee positions were simulated: full extension, 30° of flexion, and a valgus collapse position. Each position was associated with significant changes in the length of the ACL and its function bundles (Fig. 7). In particular, the length of the ACL and both the AM and PL bundles decreased from full extension to 30° of flexion. This trend was in agreement with several previous in vivo studies investigating changes in ACL and functional bundle length with flexion during similar activities.1,33,38 Interestingly, the length of the ACL and both functional bundles decreased further in the valgus collapse position at 30° of flexion. This finding was consistent with that of Fleming et al., who performed an in vivo study of the strains experienced by the ACL during the application of several joint torque and compressive load combinations.23 They reported that valgus alone did not directly increase ACL strain. Together, these results suggest that flexion has a greater influence on ACL elongation than valgus knee positioning under these in vivo loading conditions.
Landing on an extended knee has been hypothesized to be a risk factor for ACL injury due to increased loading on the ligament from muscular forces.17,60 Specifically, with increasing extension, the proximal attachment of the patellar tendon is more anteriorly oriented.14,45 This could allow the quadriceps to apply anterior shear forces to the tibia, thus loading the ACL.9,32 In support of this mechanism, a number of in vivo studies have shown that ACL elongation is maximal near full extension under a variety of loading conditions.9,21,33,37,56,59 With increasing flexion, the anterior orientation of the patellar tendon decreases,14,45 which may protect the ACL from large anterior shear forces exerted by the quadriceps even in the presence of valgus.
External rotation of the tibia has been proposed to be a part of valgus collapse,36,60 and was therefore included in our protocol. An average external rotation of 12° was achieved in the current study, which was close to 10° of external rotation reported by Olsen et al.46 External tibial torque, when isolated, has previously been shown to have no significant effect on ACL strain.5,7,23 Furthermore, an in vitro study by Markolf et al. reported that with an increase in the flexion angle, the combination of external tibial torque along with valgus moment decreased ACL force when compared to valgus alone.42 This may also help to explain why the ACL length decreased in the valgus collapse position when compared to 30° of flexion.
Previous studies have indicated a significant change in valgus angles during ACL injury using measurements of the long axes of the femur and tibia projected onto a frontal plane as observed from the perspective of a camera.11,36,46 In this study, rotations in the coronal plane were measured in two fashions (Fig. 3): as the change in angle between the transepicondylar line and long axis of the tibia (varus/valgus), and as the angle of the femoral and tibial long axes projected onto the coronal plane of the tibia (coronal plane angle). The purpose of the coronal plane angle measurement was to establish a method similar to those used in such videographic analyses. Our data indicate that the subjects in this study were successful in achieving a valgus knee position, as measured by the coronal plane angle. However, this relatively large change in coronal plane angle corresponded to very little change in the varus/valgus angle measured in this study. Thus, these results reinforce the notion that valgus approximated from video footage of an ACL injury may differ from mathematical descriptions of valgus used in laboratory studies.27,51,55 Regardless, our results indicate that the valgus collapse position did not elongate the ACL, while extension caused significant increases in ACL length.
It should be noted that the parameters for our valgus collapse positioning protocol were based on previous videographic analyses.31,36,46 However, in one study, Krosshaug et al. detailed the difficulties associated with visually interpreting bone position and segment orientation in the video analysis of ACL injuries.35 This is an important point because it implies that the measurements made may have over- or underestimated the actual joint angles experienced. Additionally, videographic analyses are currently unable to detect the behavior of ligaments during various activities, including identifying the exact moment of ACL injury. Therefore, it is unclear if valgus collapse positions would necessarily induce high ACL loads or if the ligament had already ruptured and the knee was simply collapsing into this position due to the loss of supporting structures. Recently, an in vivo study of dynamic jumping mechanics found that peak ACL strain occurs prior to ground impact with the knee in maximum extension.56 This maximum extension was followed by an increase in flexion and a decrease in ACL strain. Besides reinforcing the inverse relationship between flexion and ACL length demonstrated by this study, these previous results might suggest that injury transpires near ground impact34,56 prior to collapsing into flexion and valgus. Regardless, additional studies are needed to further clarify the precise mechanisms of ACL injury.
There are several limitations to this study. First, the study positions were captured statically due to the small field of view and low capture speed of our fluoroscopic system. Due to the complex loading conditions applied, it is difficult to predict ACL strains during dynamic activities such as jumping or cutting. Thus, additional studies using dynamic measurement techniques with higher sampling rates18,43,44,56 and larger fields of view might investigate the effects of valgus collapse positions on ACL strains during such high demand tasks. Another limitation of this study is the inclusion of only male subjects. Many studies have reported that females are more predisposed to higher coronal plane valgus angles compared to males.30,40,52 Since recent studies have suggested that males and females may have different ACL injury mechanisms,48 a similar study performed using females could help to determine the potential relationship between sex and the biomechanical role of valgus collapse. As mentioned above, we generalized valgus collapse as 30° of flexion with 10° of external rotation of the tibia and internal rotation at the hip, which may not be inclusive of all types of valgus collapse injuries. Future studies could vary these parameters to check the effects of a broader range of knee poses on ACL loading. Finally, we approximated the length of the ACL and its bundles as straight lines connecting the femoral and tibial attachment sites. However, this assumption would not provide accurate measurements of length if the ACL is slack. Despite this limitation, this approximation would not affect our conclusion that the valgus collapse position does not elongate the ACL under these loading conditions.
In conclusion, we used MR and fluoroscopic imaging techniques to measure in vivo ACL length in several positions that mimic high risk motions reported in the literature. Our results demonstrate that, under weightbearing conditions, ACL length decreased when moving from full extension to 30° of flexion. Notably, the valgus collapse knee position simulated in this study did not elongate the ACL. Future studies should include dynamic data acquisitions, which may more closely replicate the conditions of ACL injury. Detailed data describing what knee motions increase ACL loading is imperative to the continued improvement of ACL injury prevention programs.
Acknowledgments
This work was supported by the National Institutes of Health (Grant no. R03AR055659) and a grant from the National Football League Charities.
References
- 1.Abebe ES, Kim JP, Utturkar GM, et al. The effect of femoral tunnel placement on ACL graft orientation and length during in vivo knee flexion. J Biomech. 2011;44(10):1914–1920. doi: 10.1016/j.jbiomech.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abebe ES, Moorman CT, 3rd, Dziedzic TS, et al. Femoral tunnel placement during anterior cruciate ligament reconstruction: an in vivo imaging analysis comparing transtibial and 2-incision tibial tunnel-independent techniques. Am J Sports Med. 2009;37(10):1904–1911. doi: 10.1177/0363546509340768. [DOI] [PubMed] [Google Scholar]
- 3.Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524–530. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- 4.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 5.Arms SW, Pope MH, Johnson RJ, Fischer RA, Arvidsson I, Eriksson E. The biomechanics of anterior cruciate ligament rehabilitation and reconstruction. Am J Sports Med. 1984;12(1):8–18. doi: 10.1177/036354658401200102. [DOI] [PubMed] [Google Scholar]
- 6.Barber-Westin SD, Noyes FR, Smith ST, Campbell TM. Reducing the risk of noncontact anterior cruciate ligament injuries in the female athlete. Phys Sports-med. 2009;37(3):49–61. doi: 10.3810/psm.2009.10.1729. [DOI] [PubMed] [Google Scholar]
- 7.Berns GS, Hull ML, Patterson HA. Strain in the anteromedial bundle of the anterior cruciate ligament under combination loading. J Orthop Res. 1992;10(2):167–176. doi: 10.1002/jor.1100100203. [DOI] [PubMed] [Google Scholar]
- 8.Beynnon BD, Fleming BC, Johnson RJ, Nichols CE, Renstrom PA, Pope MH. Anterior cruciate ligament strain behavior during rehabilitation exercises in vivo. Am J Sports Med. 1995;23(1):24–34. doi: 10.1177/036354659502300105. [DOI] [PubMed] [Google Scholar]
- 9.Beynnon BD, Johnson RJ, Fleming BC, Stankewich CJ, Renstrom PA, Nichols CE. The strain behavior of the anterior cruciate ligament during squatting and active flexion-extension. A comparison of an open and a closed kinetic chain exercise. Am J Sports Med. 1997;25(6):823–829. doi: 10.1177/036354659702500616. [DOI] [PubMed] [Google Scholar]
- 10.Boden BP, Dean GS, Feagin JA, Jr, Garrett WE., Jr Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 11.Boden BP, Torg JS, Knowles SB, Hewett TE. Video analysis of anterior cruciate ligament injury: abnormalities in hip and ankle kinematics. Am J Sports Med. 2009;37(2):252–259. doi: 10.1177/0363546508328107. [DOI] [PubMed] [Google Scholar]
- 12.Caputo AM, Lee JY, Spritzer CE, et al. In vivo kinematics of the tibiotalar joint after lateral ankle instability. Am J Sports Med. 2009;37(11):2241–2248. doi: 10.1177/0363546509337578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cochrane JL, Lloyd DG, Buttfield A, Seward H, McGivern J. Characteristics of anterior cruciate ligament injuries in Australian football. J Sci Med Sport. 2007;10(2):96–104. doi: 10.1016/j.jsams.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 14.DeFrate LE, Nha KW, Papannagari R, Moses JM, Gill TJ, Li G. The biomechanical function of the patellar tendon during in-vivo weight-bearing flexion. J Biomech. 2007;40(8):1716–1722. doi: 10.1016/j.jbiomech.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFrate LE, Papannagari R, Gill TJ, Moses JM, Pathare NP, Li G. The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. Am J Sports Med. 2006;34(8):1240–1246. doi: 10.1177/0363546506287299. [DOI] [PubMed] [Google Scholar]
- 16.Delfico AJ, Garrett WE., Jr Mechanisms of injury of the anterior cruciate ligament in soccer players. Clin Sports Med. 1998;17(4):779–785. vii. doi: 10.1016/s0278-5919(05)70118-6. [DOI] [PubMed] [Google Scholar]
- 17.DeMorat G, Weinhold P, Blackburn T, Chudik S, Garrett W. Aggressive quadriceps loading can induce noncontact anterior cruciate ligament injury. Am J Sports Med. 2004;32(2):477–483. doi: 10.1177/0363546503258928. [DOI] [PubMed] [Google Scholar]
- 18.Deneweth JM, Bey MJ, McLean SG, Lock TR, Kolowich PA, Tashman S. Tibiofemoral joint kinematics of the anterior cruciate ligament-reconstructed knee during a single-legged hop landing. Am J Sports Med. 2010;38(9):1820–1828. doi: 10.1177/0363546510365531. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez JW, Akbarshahi M, Crossley KM, Shelburne KB, Pandy MG. Model predictions of increased knee joint loading in regions of thinner articular cartilage after patellar tendon adhesion. J Orthop Res. 2011;29(8):1168–1177. doi: 10.1002/jor.21345. [DOI] [PubMed] [Google Scholar]
- 20.Fithian DC, Paxton EW, Stone ML, et al. Prospective trial of a treatment algorithm for the management of the anterior cruciate ligament-injured knee. Am J Sports Med. 2005;33(3):335–346. doi: 10.1177/0363546504269590. [DOI] [PubMed] [Google Scholar]
- 21.Fleming BC, Beynnon BD, Renstrom PA, Peura GD, Nichols CE, Johnson RJ. The strain behavior of the anterior cruciate ligament during bicycling. An in vivo study. Am J Sports Med. 1998;26(1):109–118. doi: 10.1177/03635465980260010301. [DOI] [PubMed] [Google Scholar]
- 22.Fleming BC, Oksendahl HL, Mehan WA, et al. Delayed gadolinium-enhanced MR imaging of cartilage (dGEMRIC) following ACL injury. Osteoarthr Cartil. 2010;18(5):662–667. doi: 10.1016/j.joca.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming BC, Renstrom PA, Beynnon BD, et al. The effect of weightbearing and external loading on anterior cruciate ligament strain. J Biomech. 2001;34(2):163–170. doi: 10.1016/s0021-9290(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 24.Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622–627. doi: 10.1016/j.jsams.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Gilchrist J, Mandelbaum BR, Melancon H, et al. A randomized controlled trial to prevent noncontact anterior cruciate ligament injury in female collegiate soccer players. Am J Sports Med. 2008;36(8):1476–1483. doi: 10.1177/0363546508318188. [DOI] [PubMed] [Google Scholar]
- 26.Griffin LY, Albohm MJ, Arendt EA, et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34(9):1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 27.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105(2):136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 28.Harner CD, Baek GH, Vogrin TM, Carlin GJ, Kashiwaguchi S, Woo SL. Quantitative analysis of human cruciate ligament insertions. Arthroscopy. 1999;15(7):741–749. doi: 10.1016/s0749-8063(99)70006-x. [DOI] [PubMed] [Google Scholar]
- 29.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27(6):699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- 30.Hewett TE, Myer GD, Ford KR. Decrease in neuromuscular control about the knee with maturation in female athletes. J Bone Joint Surg Am. 2004;86-A(8):1601–1608. doi: 10.2106/00004623-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 32.Hirokawa S, Solomonow M, Lu Y, Lou ZP, D’Ambrosia R. Anterior-posterior and rotational displacement of the tibia elicited by quadriceps contraction. Am J Sports Med. 1992;20(3):299–306. doi: 10.1177/036354659202000311. [DOI] [PubMed] [Google Scholar]
- 33.Jordan SS, DeFrate LE, Nha KW, Papannagari R, Gill TJ, Li G. The in vivo kinematics of the anteromedial and posterolateral bundles of the anterior cruciate ligament during weightbearing knee flexion. Am J Sports Med. 2007;35(4):547–554. doi: 10.1177/0363546506295941. [DOI] [PubMed] [Google Scholar]
- 34.Koga H, Nakamae A, Shima Y, et al. Mechanisms for noncontact anterior cruciate ligament injuries: knee joint kinematics in 10 injury situations from female team handball and basketball. Am J Sports Med. 2010;38(11):2218–2225. doi: 10.1177/0363546510373570. [DOI] [PubMed] [Google Scholar]
- 35.Krosshaug T, Bahr R. A model-based image-matching technique for three-dimensional reconstruction of human motion from uncalibrated video sequences. J Biomech. 2005;38(4):919–929. doi: 10.1016/j.jbiomech.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 36.Krosshaug T, Nakamae A, Boden BP, et al. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med. 2007;35(3):359–367. doi: 10.1177/0363546506293899. [DOI] [PubMed] [Google Scholar]
- 37.Li G, Defrate LE, Rubash HE, Gill TJ. In vivo kinematics of the ACL during weight-bearing knee flexion. J Orthop Res. 2005;23(2):340–344. doi: 10.1016/j.orthres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Li G, DeFrate LE, Sun H, Gill TJ. In vivo elongation of the anterior cruciate ligament and posterior cruciate ligament during knee flexion. Am J Sports Med. 2004;32(6):1415–1420. doi: 10.1177/0363546503262175. [DOI] [PubMed] [Google Scholar]
- 39.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 40.Malinzak RA, Colby SM, Kirkendall DT, Yu B, Garrett WE. A comparison of knee joint motion patterns between men and women in selected athletic tasks. Clin Biomech (Bristol, Avon) 2001;16(5):438–445. doi: 10.1016/s0268-0033(01)00019-5. [DOI] [PubMed] [Google Scholar]
- 41.Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33(7):1003–1010. doi: 10.1177/0363546504272261. [DOI] [PubMed] [Google Scholar]
- 42.Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GA, Slauterbeck JL. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13(6):930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- 43.Miranda DL, Schwartz JB, Loomis AC, Brainerd EL, Fleming BC, Crisco JJ. Static and dynamic error of a biplanar videoradiography system using marker-based and markerless tracking techniques. J Biomech Eng. 2011;133(12):121002. doi: 10.1115/1.4005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Myers CA, Torry MR, Peterson DS, et al. Measurements of tibiofemoral kinematics during soft and stiff drop landings using biplane fluoroscopy. Am J Sports Med. 2011;39(8):1714–1722. doi: 10.1177/0363546511404922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nunley RM, Wright D, Renner JB, Yu B, Garrett WE. Gender comparison of patellar tendon tibial shaft angle with weight bearing. Res Sports Med. 2003;11:173–185. [Google Scholar]
- 46.Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- 47.Pfeiffer RP, Shea KG, Roberts D, Grandstrand S, Bond L. Lack of effect of a knee ligament injury prevention program on the incidence of noncontact anterior cruciate ligament injury. J Bone Joint Surg Am. 2006;88(8):1769–1774. doi: 10.2106/JBJS.E.00616. [DOI] [PubMed] [Google Scholar]
- 48.Quatman CE, Hewett TE. The anterior cruciate ligament injury controversy: is “valgus collapse” a sex-specific mechanism? Br J Sports Med. 2009;43(5):328–335. doi: 10.1136/bjsm.2009.059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salmon LJ, Russell VJ, Refshauge K, et al. Long-term outcome of endoscopic anterior cruciate ligament reconstruction with patellar tendon autograft: minimum 13-year review. Am J Sports Med. 2006;34(5):721–732. doi: 10.1177/0363546505282626. [DOI] [PubMed] [Google Scholar]
- 50.Scanlan SF, Blazek K, Chaudhari AM, Safran MR, Andriacchi TP. Graft orientation influences the knee flexion moment during walking in patients with anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(11):2173–2178. doi: 10.1177/0363546509339574. [DOI] [PubMed] [Google Scholar]
- 51.Scanlan SF, Chaudhari AM, Dyrby CO, Andriacchi TP. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. J Biomech. 2010;43(9):1817–1822. doi: 10.1016/j.jbiomech.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmitz RJ, Shultz SJ, Nguyen AD. Dynamic valgus alignment and functional strength in males and females during maturation. J Athl Train. 2009;44(1):26–32. doi: 10.4085/1062-6050-44.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shin CS, Chaudhari AM, Andriacchi TP. The effect of isolated valgus moments on ACL strain during single-leg landing: a simulation study. J Biomech. 2009;42(3):280–285. doi: 10.1016/j.jbiomech.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soderman K, Werner S, Pietila T, Engstrom B, Alfredson H. Balance board training: prevention of traumatic injuries of the lower extremities in female soccer players? A prospective randomized intervention study. Knee Surg Sports Traumatol Arthrosc. 2000;8(6):356–363. doi: 10.1007/s001670000147. [DOI] [PubMed] [Google Scholar]
- 55.Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):975–983. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- 56.Taylor KA, Terry ME, Utturkar GM, et al. Measurement of in vivo anterior cruciate ligament strain during dynamic jump landing. J Biomech. 2011;44(3):365–371. doi: 10.1016/j.jbiomech.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinhold PS, Stewart JD, Liu HY, Lin CF, Garrett WE, Yu B. The influence of gender-specific loading patterns of the stop-jump task on anterior cruciate ligament strain. Injury. 2007;38(8):973–978. doi: 10.1016/j.injury.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 58.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech (Bristol, Avon) 2006;21(9):977–983. doi: 10.1016/j.clinbiomech.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Wu JL, Hosseini A, Kozanek M, Gadikota HR, Gill TJT, Li G. Kinematics of the anterior cruciate ligament during gait. Am J Sports Med. 2010;38(7):1475–1482. doi: 10.1177/0363546510364240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu B, Garrett WE. Mechanisms of non-contact ACL injuries. Br J Sports Med. 2007;41(Suppl 1):i47–i51. doi: 10.1136/bjsm.2007.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]


