Abstract
Diquat and paraquat are non-specific defoliants that induce toxicity in many organs including the lung, liver, kidney and brain. This toxicity is thought to be due to the generation of reactive oxygen species (ROS). An important pathway leading to ROS production by these compounds is redox cycling. In the present studies, diquat and paraquat redox cycling was characterized using human recombinant NADPH-cytochrome P450 reductase, rat liver microsomes, and Chinese Hamster Ovary (CHO) cells constructed to overexpress cytochrome P450 reductase (CHO-OR) and wild type control cells (CHO-WT). In redox cycling assays with recombinant cytochrome P450 reductase and microsomes, diquat was 10-40 times more effective in generating ROS when compared to paraquat (KM = 1.0 and 44.2 μM, respectively for H2O2 generation by diquat and paraquat using recombinant enzyme, and 15.1 and 178.5 μM, respectively for microsomes). In contrast, at saturating concentrations, these compounds showed similar redox cycling activity (Vmax ≈ 6.0 nmoles H2O2/min/mg protein) for recombinant enzyme and microsomes. Diquat and paraquat also redox cycle in CHO cells. Significantly more activity was evident in CHO-OR cells than CHO-WT cells. Diquat redox cycling in CHO cells was associated with marked increases in protein carbonyl formation, a marker of protein oxidation, as well as cellular oxygen consumption, measured using oxygen microsensors; greater activity was detected in CHO-OR cells than CHO-WT cells. These data demonstrate that ROS formation during diquat redox cycling can generate oxidative stress. Enhanced oxygen utilization during redox cycling may reduce intracellular oxygen available for metabolic reactions and contribute to toxicity.
Keywords: redox cycling, herbicides, paraquat, diquat, reactive oxygen species, oxidative stress
Introduction
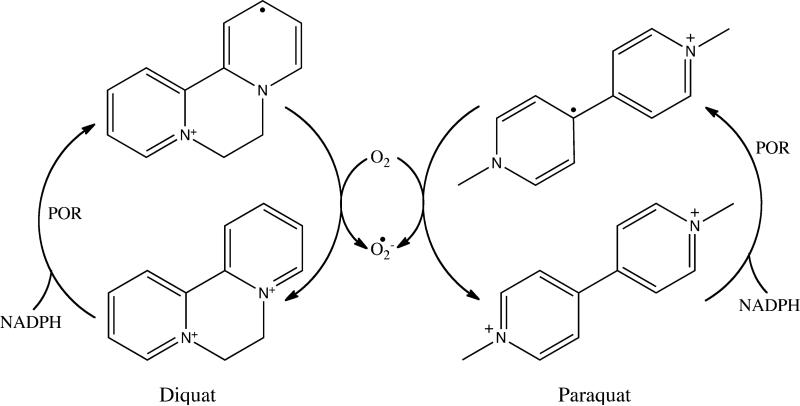
Diquat (1,1’-ethylene-2,2’-bipyridylium) and paraquat (1,1′-dimethyl-4,4′-bipyridylium) are widely used agricultural chemicals and environmental contaminants, whose toxicities result in damage to numerous tissues including the lung, liver, kidney and brain [1-7]. Redox cycling and consequent ROS generation are thought to be key cytotoxic mechanisms induced by these bipyridyl herbicides. During redox cycling, diquat and paraquat undergo an enzymatic one electron reduction, forming radical cations (Fig. 1). Under aerobic conditions, these radicals react rapidly with molecular oxygen, forming superoxide anion and regenerating the parent compounds. Dismutation of superoxide anion generates H2O2 and, in the presence of redox active transition metals, highly toxic hydroxyl radicals. These reactive oxygen species (ROS) can cause oxidative stress leading to damage to lipids, proteins and nucleic acids [8]. Under hypoxic conditions, the bipyridyl radicals are stabilized creating reactive electrophiles [9]. The diquat and paraquat radicals are also capable of reacting with a variety of nucleophiles in cells, directly causing damage to cellular components, a process that contributes to toxicity.
Fig. 1. Diquat and paraquat redox cycling and ROS generation.
The one-electron reduction of diquat and paraquat by cytochrome P450 reductase (POR) generates a bipyridyl cation radical. In the presence of molecular oxygen, reoxidation of the radical forms superoxide anion and regenerates the parent compound.
Enzyme-mediated redox cycling consumes considerable quantities of molecular oxygen [9-11]. Depending on the localized concentrations of redox cycling chemicals and enzymes mediating the process, this can diminish the availability of oxygen required for cellular metabolism, creating hypoxic conditions. This can exacerbate oxidative stress and promote tissue injury [12]. In the present studies, we compared diquat and paraquat redox cycling using mouse and human recombinant NADPH-cytochrome P450 reductase and rat liver microsomes. In each of these preparations, diquat and paraquat redox cycling generated ROS in an oxygen-dependent process. A marked increase in oxygen consumption was also observed in Chinese Hamster Ovary (CHO) cells overexpressing cytochrome P450 reductase during bipyridyl herbicide redox cycling. Since redox cycling has the potential to reduce localized oxygen tension in target cells, in addition to forming ROS, our data suggest that there may be additional mechanisms mediating the toxicity of the bipyridyl defoliants that include formation of bipyridyl radicals [9, 13-19], as well as hypoxia.
Methods
Chemicals and reagents
Human recombinant cytochrome P450 reductase expressed in microsomal fractions of insect cells (Supersomes; Cat. No. 456244; Lot No. 02708) was obtained from BD Gentest (Woburn, MA). Microsomal fractions from β-naphthoflavone-induced rat livers were from Xenotech (Lenexa, KS). Amplex Red (10-acetyl-3, 7-dihydroxyphenoxazine) was from Invitrogen (Eugene, OR) and diquat from ChemService, Inc. (West Chester, PA). Paraquat, NADPH, and all other chemicals were from Sigma-Aldrich (St. Louis, MO).
Cells and treatments
CHO cells overexpressing mouse cytochrome P450 reductase (CHO-OR cells) and control cells expressing empty vector (CHO-WT cells) were generous gifts of Dr. Jun Yan Hong, University of Medicine and Dentistry of New Jersey (Piscataway, NJ). Cells were maintained in Ham's F12K medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin (all from Gibco BRL, Grand Island, NY) and 500 g/ml hygromycin B (Invitrogen, Carlsbad, CA). Cells were incubated at 37 C in 5% CO2 in a humidified incubator. CHO-OR cells contain approximately 30-fold more cytochrome P450 reductase activity than CHO-WT cells as measured by cytochrome c reductase activity (2.9 ± 0.6 vs. 87.1 ± 5.9 units/mg protein, respectively, n = 3 ± SE) [20]. To prepare lysates, cells were resuspended in phosphate-buffered saline (106 cells/ml) and disrupted on ice using a sonicator (Artek Systems Inc., Farmingdale, NY). Protein concentrations were quantified using the DC protein assay kit (Bio-Rad, Hercules, CA) with bovine serum albumin as the standard.
Analysis of ROS production
H2O2 production was assayed using the Amplex red/horseradish peroxidase method as previously described [10, 21]. Reactions were run at 37°C and contained 50 μl PBS and 50 μl potassium phosphate buffer (50 mM, pH 7.8) supplemented with 0-0.3 mM NADPH, 0-1 mM diquat or paraquat, 100 μM Amplex Red, 1 unit/ml horseradish peroxidase, and 5 μg/ml cytochrome P450 reductase (790 U/mg), 10 μg/ml rat liver microsomes or 50 μg/ml cell lysate protein. In some experiments, enzyme assays were supplemented with 5 μM diphenyleneiodonium (DPI) to inhibit flavoenzymes. The fluorescent product, resorufin (excitation 540 nm/emission 595 nm), was recorded using a SpectraMax M5 fluorescent microplate reader (Molecular Devices, Sunnyvale, CA).
The generation of superoxide anion by cell lysates was quantified by the reduction of acetylated cytochrome c, as measured by increases in absorbance at 550 nm. Standard reaction mixes contained 100 μM NADPH, 100 μM diquat or paraquat, 625 μM acetylated cytochrome c and 100 μg/ml cell lysate protein in 600 μl PBS. Enzyme assays were supplemented with 5 μM DPI to inhibit flavoenzymes, in some experiments. Reactions were run at room temperature in a Lambda 20 UV/Vis spectrophotometer (Perkin-Elmer Life Sciences, Shelton, CT). In some assays, superoxide anion production by recombinant enzyme and microsomes was assayed using dihydroethidium as previously described [10] and expressed in arbitrary fluorescence units (AFU). Reactions were run at 37°C in reaction mixes containing 100 μM NADPH, 0-30 μM diquat or 0-300 μM paraquat, 80 μM dihydroethidium, and 5 μg/ml cytochrome P450 reductase or 10 μg/ml rat liver microsomes. In some experiments, enzyme assays were supplemented with 5 μM DPI to inhibit flavoenzymes. The fluorescent product (excitation 510 nm/emission 595 nm) was recorded using the fluorescent microplate reader.
Hydroxyl radical production in enzyme assays was analyzed by the production of 2-hydroxyterephthalate (2-OH-TPT) [22]. Reaction mixes contained 100 μM NADPH, 5 μg/ml cytochrome P450 reductase, 10 μg/ml rat liver microsomes or 50 μg/ml cell lysate protein, 100 μM FeCl3, 110 μM EDTA, 1 mM terephthalate and appropriate concentrations of diquat or paraquat in 100 μl. Fluorescence was measured using the fluorescent microplate reader with emission and excitation set at 315 nm and 425 nm, respectively.
Diquat and paraquat bipyridyl radical formation
Diquat and paraquat radical formation was quantified spectrophotometrically in standard reaction mixes containing 100 μM diquat or paraquat, cytochrome P450 reductase (20 U/ml) and NADPH (100 μM) supplemented with 20 mM glucose, 12.5 U/ml glucose oxidase and 50 U/ml catalase to eliminate oxygen in the assays [23]. Depletion of oxygen by the two-enzyme system was confirmed in an Oxygraph fitted with a Clark-type oxygen electrode (Yellow Springs Instruments, Yellow Springs, OH).
Cellular oxidative stress
Oxidative stress was measured in CHO cells as protein carbonyl formation using an OxyBlot™ Protein Oxidation Detection Kit (S7150, Chemicon, Billerica, MA). Briefly, cells (5 × 105 in 10 cm culture dishes) were treated with 10 μM diquat or vehicle control. After 1 hr, cell lysates were prepared, protein carbonyl groups derivatized with 2, 4-dinitrophenylhydrazine and then analyzed by western blotting [24]. Previous studies have shown that cell culture medium contains redox active phenolic compounds [25]. To eliminate interference by these compounds cells were exposed to diquat in PBS.
Assays for cellular oxygen utilization
Depletion of oxygen in a closed system by recombinant NADPH-cytochrome P450 reductase was quantified in an Oxygraph with a Clark-type oxygen electrode (Yellow Springs Instruments, Yellow Springs, OH). Reaction mixes contained increasing concentrations of bipyridyl herbicide (0.1-30 μM), NADPH (120 μM), glucose-6-phosphate (4 mM), glucose-6-phosphate dehydrogenase (4 U/ml) and 50 μg/ml of recombinant human cytochrome P450 reductase.
Oxygen flux into CHO cells was quantified using Whelan oxygen microsensors as previously described [26, 27]. In this analysis, the microsensors are used in a self-referencing format which minimizes noise and random drift, processes that often limit the use of standard oxygen electrodes. Unique noise characteristics were evident in each of the tracings and this was due to the fact that each microsensor was hand manufactured.
Results
Characterization of redox cycling
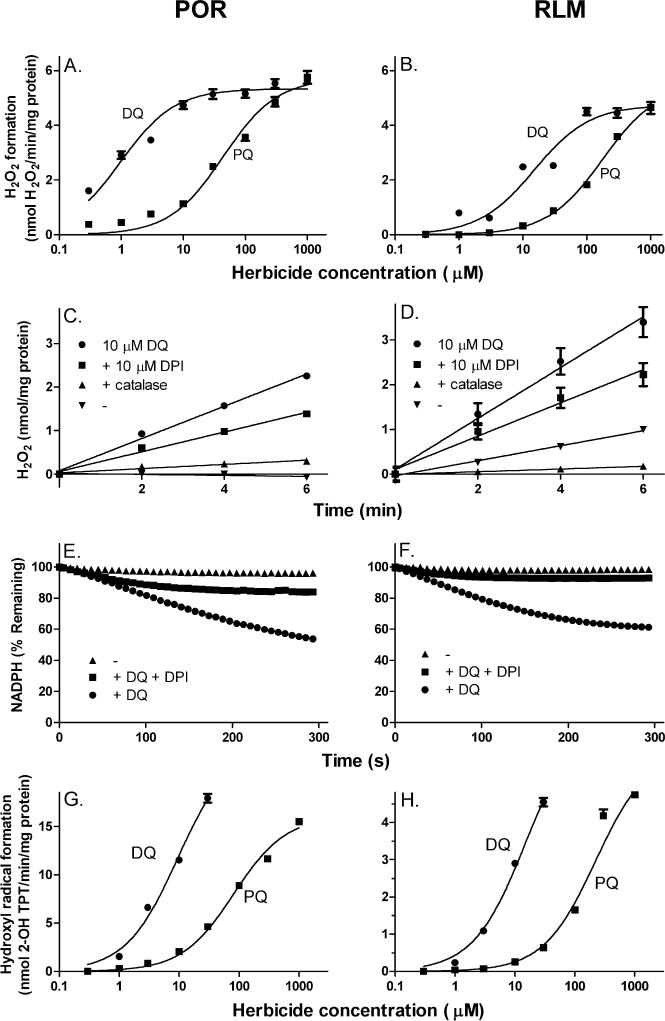
In initial studies we characterized diquat and paraquat redox cycling using recombinant human cytochrome P450 reductase and rat liver microsomes by quantifying H2O2 production in enzyme assays. The rate of H2O2 generation by both herbicides was linear with respect to time and concentration (Fig. 2, panels A-D). At low concentrations (0.3-30 μM), diquat was approximately 40- and 12-times more efficient than paraquat as a generator of H2O2 with the recombinant enzyme and microsomes, respectively. The apparent KM values for diquat and paraquat were 1.0 and 44.2 μM, respectively, for the recombinant enzyme, and 15.1 and 178.5 μM, respectively, for microsomes. At saturating concentrations, however, the herbicides showed similar redox cycling activity (Vmax's ≈ 6.0 nmoles H2O2/min/mg protein) for both enzyme preparations. Redox cycling for diquat and paraquat required NADPH; NADPH was readily metabolized in redox cycling assays using recombinant cytochrome P450 reductase and liver microsomes (Fig. 2, panels E and F, and not shown).
Fig. 2. Redox cycling of diquat by cytochrome P450 reductase.
Generation of hydrogen peroxide and hydroxyl radicals in enzyme reaction mixes containing recombinant cytochrome P450 reductase (5 μg/ml) or rat liver microsomes (10 μg/ml) were assayed using the Amplex Red/horseradish peroxidase and terephthalate assays, respectively. Reactions contained 100 μM NADPH. Data were collected in triplicate and presented as the mean ± SEM. Panels A and B, Effects of increasing concentration of diquat (DQ) and paraquat (PQ) on H2O2. Panels C and D, Effects of catalase and DPI on diquat-stimulated H2O2 production. Reactions were run in the absence and presence of 10 μM diquat. Some reactions were supplemented with 10 μM DPI or 100 units/ml catalase. Panels E and F, NADPH utilization during redox cycling. Reactions were run in the absence and presence of 10 μM diquat or diquat plus DPI and contained 100 μM NADPH. NADPH utilization was measured as decreases in absorbance at 340 nm. Assay mixtures contained either 100 μg/ml recombinant cytochrome P450 reductase or 200 μg/ml rat liver microsomes. Panels G and H, Effects of increasing concentrations of diquat or paraquat on hydroxyl radical formation.
In the presence of iron, redox cycling by the herbicides also generated hydroxyl radicals (Fig. 2, panels G and H). As observed with H2O2 formation, diquat was more efficient in generating hydroxyl radicals than paraquat. Whereas the apparent KM's for diquat and paraquat were 9.4 and 81.9 μM, respectively, and the Vmax's, 23.4 and 16.1 nmol 2-OH-TPT/min/mg protein, respectively, for the recombinant enzyme, for liver microsomes the apparent KM's for diquat and paraquat were 14.9 and 221.5 μM, respectively, and Vmax's, 6.9 and 5.9 nmol 2-OH-TPT/min/protein. The accumulation of H2O2 during redox cycling was blocked by catalase, and inhibited by DPI (Fig. 2, panels C and D and not shown). NADPH utilization in enzyme assays was also inhibited by DPI (Fig. 2, panels E and F). These inhibitory effects of DPI are consistent with the fact that cytochrome P450 reductase and other enzymes known to mediate redox cycling are flavoproteins [28].
It is generally thought that H2O2 is formed from superoxide anion generated via the one electron reduction of oxygen by bipyridyl cation radicals. Both diquat and paraquat were found to generate superoxide anion during redox cycling with recombinant cytochrome P450 reductase and microsomes. This reaction was inhibited by DPI and superoxide dismutase (Fig. 3 and not shown). Consistent with the measurements of H2O2 and hydroxyl radical formation, diquat was more effective than paraquat in generating superoxide anion (KM's were 49.0 and 407.1 μM, for diquat and paraquat, respectively, for recombinant cytochrome P450 reductase, and 11.1 and 43.3 μM, for microsomes. The calculated Vmax's for diquat and paraquat were 398.1 and 246.6 AFU/min/mU cytochrome P450 reductase, respectively, for the recombinant enzyme, and 678.5 and 656.4 AFU/min/ng total protein, for microsomes).
Fig. 3. Diquat-stimulated superoxide production by NADPH-cytochrome P450 reductase.
Generation of superoxide anion in enzyme reaction mixes containing recombinant cytochrome P450 reductase (5 μg/ml) or rat liver microsomes (10 μg/ml) were assayed using dihydroethidium. Reactions contained 100 μM NADPH. Data were collected in triplicate and presented as the mean ± SEM. Panels A and B, Effects of DPI on diquat-stimulated superoxide anion production. Reactions were run in the absence and presence of 10 μM diquat, or diquat plus 10 μM DPI. Panels C and D, Effects of increasing concentrations of diquat or paraquat on superoxide anion generation.
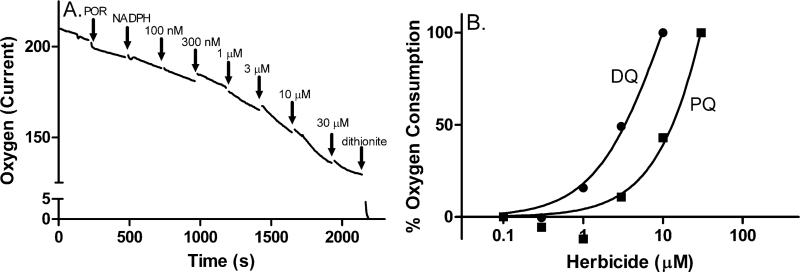
Since redox cycling consumes molecular oxygen, we next measured changes in oxygen levels in enzyme assays. With both recombinant cytochrome P450 reductase and microsomes, diquat and paraquat were found to stimulate oxygen utilization during redox cycling (Fig. 4, panel A and not shown). This reaction was dependent on NADPH and inhibited by DPI. As observed with ROS formation, diquat was significantly more active than paraquat in utilizing oxygen during redox cycling (Fig. 4, panel B and not shown).
Fig. 4. Redox cycling by diquat and paraquat increases oxygen consumption by microsomal enzyme preparations.
The rate of oxygen depletion as a function of increasing concentrations of herbicides was assessed with a Clark-type electrode in a closed system containing NADPH (120 μM), glucose-6-phosphate (4 mM), glucose-6-phosphate dehydrogenase (4 U/ml) and 50 μg/ml of recombinant human cytochrome P450 reductase (Panel A). Current was measured in pAmps.
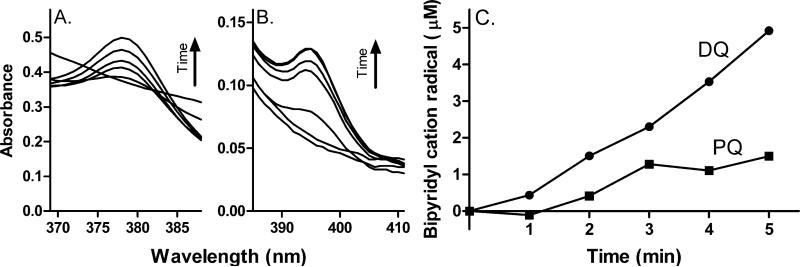
As indicated above, diquat and paraquat cation radicals are unstable due to their reactivity with oxygen. However, they can accumulate in reaction mixes under anoxic conditions. Using a two-enzyme system to induce anoxia in our reaction mixes [23], we found that recombinant cytochrome P450 reductase readily generated diquat and paraquat cation radicals (Fig. 5).
Fig. 5. Cytochrome P450 reductase catalyzes diquat and paraquat radical formation under anoxic conditions.
Diquat and paraquat radical formation was quantified spectrophotometrically in reaction mixes containing 100 μM diquat or paraquat, cytochrome P450 reductase (20 U/ml) and NADPH (100 μM) supplemented with 20 mM glucose, 12.5 U/ml glucose oxidase and 50 U/ml catalase to induce anoxia. Panel A, Diquat radical formation, Panel B, Paraquat radical formation. Panel C, Time-dependent formation of diquat and paraquat radicals. The extinction coefficients for diquat and paraquat used in our calculations were ε379 = 2.52 × 104 and ε400 = 4.6 × 104, respectively.
Diquat redox cycling by CHO cells and oxidative stress
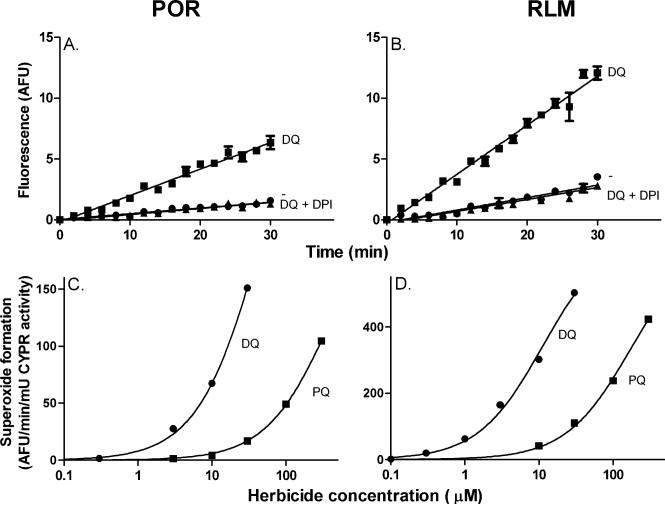
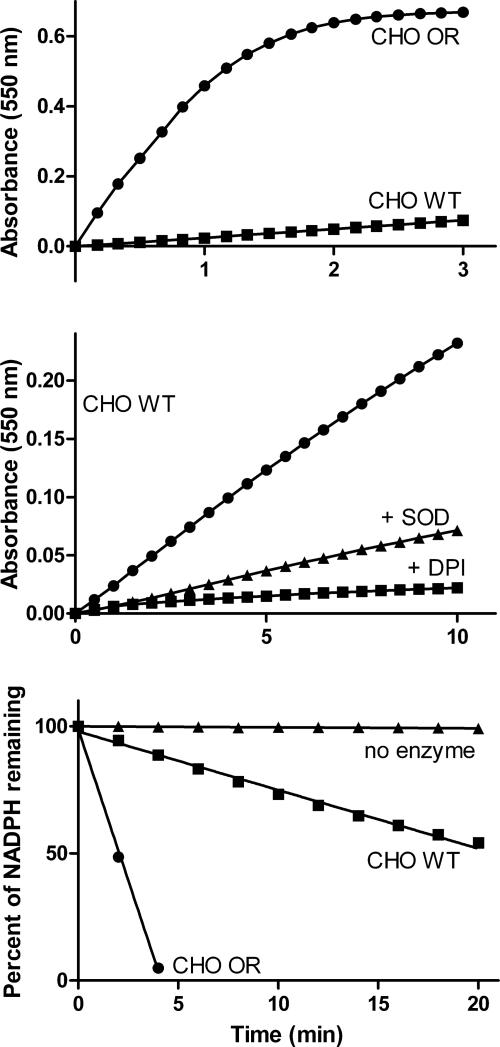
We next characterized redox cycling in CHO cells constructed to overexpress mouse cytochrome P450 reductase (CHO-OR cells). Diquat was used in these experiments since it was a more effective redox cycling agent when compared to paraquat. Lysates from both CHO-WT and CHO-OR cells catalyzed diquat redox cycling; significantly more activity was evident in CHO-OR cells, relative to CHO-WT cells, as measured by the generation of superoxide anion, hydrogen peroxide, and hydroxyl radicals, and NADPH metabolism (Figs. 6 and 7). ROS production in cell lysates was inhibited by DPI (Fig. 6 and not shown). The accumulation of superoxide anion and H2O2 in lysates during redox cycling was inhibited by SOD and catalase, respectively (Fig. 6, panel B and not shown), while hydroxyl radicals were scavenged by DMSO, a hydroxyl radical trap (not shown). In intact cells, diquat-induced redox cycling was associated with cellular oxidative stress, as measured by protein oxidation (e.g., the formation of protein carbonyl groups) [29]. Basal levels of protein oxidation were evident in both CHO-WT and CHO-OR cells with protein bands ranging from 24-200 kDa (Fig. 8, panel A). Diquat increased oxidation of at least six proteins with molecular weights of 24, 26, 45, 51, 63 and 112 kDa (Fig. 8, arrows). Greater protein oxidation was evident in CHO-OR cells, as compared to CHO-WT cells.
Fig. 6. Superoxide anion production during diquat redox cycling in CHO cells.
Production of superoxide anion by lysates from CHO cells was measured spectrophotometrically by the reduction of acetylated cytochrome c. Reaction mixes contained 100 μM diquat, 100 μM NADPH, 625 μg/ml acetylated cytochrome c, and cell lysate protein (100 μg/ml) from either CHO-WT or CHO-OR cells. Upper panel, Superoxide anion generating activity in lysates from CHO-WT and CHO-OR cells. Center panel, Effects of superoxide dismutase (SOD) and DPI on superoxide anion production in lysates from CHO-WT cells. Lysates contained either 10,000 units/ml SOD or 10 μM DPI. Lower panel. NADPH utilization in lysates from CHO-WT and CHO-OR cells were assayed by decreases in absorbance at 340 nm. Data are presented as percentage decreases in NADPH in reaction mixes. Data shown are representative of two experiments.
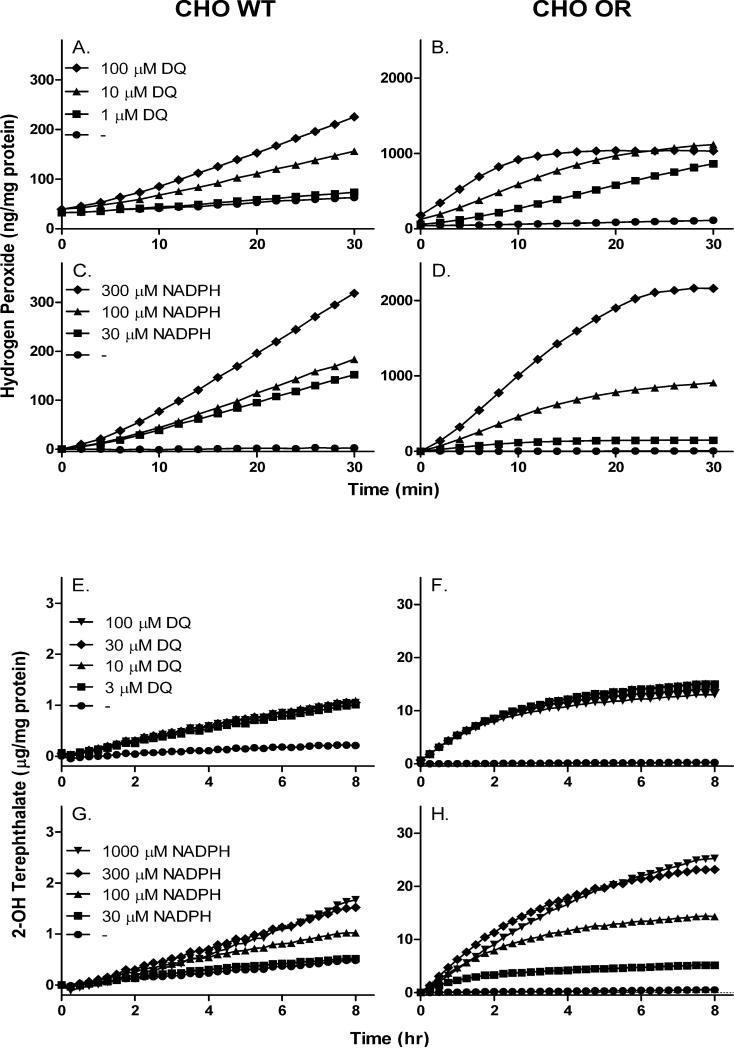
Fig. 7. Generation of H2O2 and hydroxyl radicals during diquat redox cycling by CHO cells.
Upper panels, H2O2 production was assayed using the Amplex Red/horseradish peroxidase method. Redox cycling in lysates from CHO-WT (panel A) and CHO-OR cells (panel B) was assayed with increasing concentrations of diquat (DQ) in the presence of 100 μM NADPH. Redox cycling in lysates from CHO-WT (panel C) and CHO-OR cells (panel D) was assayed with increasing concentrations of NADPH in the presence of 10 μM diquat. Lower panels, Hydroxyl radicals were assayed using the terephthalate assay. Hydroxyl radical production in lysates from CHO-WT (panel E) and CHO-OR cells (panel F) was assayed with increasing concentrations of diquat in the presence of 100 μM NADPH. Hydroxyl radicals in lysates from CHO-WT (panel G) and CHO-OR cells (panel H) was assayed with increasing concentrations of NADPH in the presence of 10 μM diquat. All assays contained 5 μg/ml of cell lysate protein and were performed in triplicate and presented as the mean ± SEM. In all panels, note the change in axis scale between CHO-WT and CHO-OR cells.
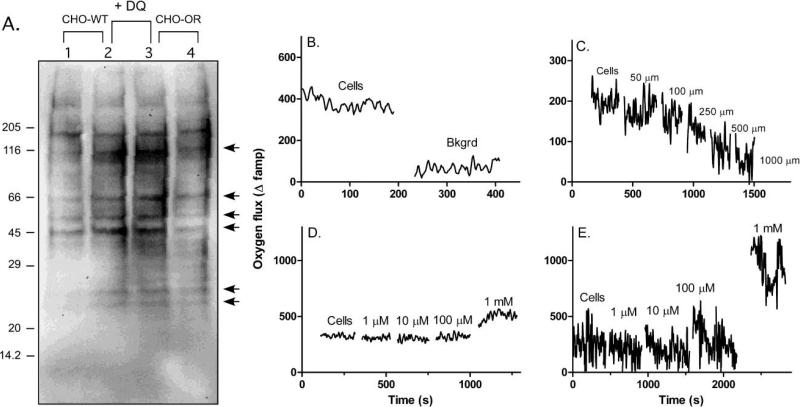
Fig. 8. Effects of diquat on protein oxidation and oxygen flux in CHO cells.
Panel A, Basal (Lanes 1 and 4) and diquat (DQ)-stimulated (Lanes 2 and 3) protein carbonylation was assayed in CHO-WT (Lanes 1-2) and CHO-OR cells (Lanes 3-4). Cells were treated with 10 μM diquat, after 1 h, cells lysates were prepared, derivatized with 2,4-dinitrophenylhydrazine and then analyzed by western blotting using an antibody to 2,4-dinitrophenylhydrazine. Arrows indicate an increase in carbonylated proteins following diquat treatment. Coomassie staining of the gel confirmed equal loading of cell lysate protein (not shown). Panels B-D, Oxygen flux into CHO cells was measured using self-referencing microsensors. Panel B, Oxygen flux by CHO-WT cells was measured near the cell surface and at a background (Bkgrd) distance 1 mm from the cell surface. Note the loss of signal at the background. Panel C, Oxygen flux was measured at increasing distances from cells. Panel D, Effects of diquat on oxygen flux by CHO-WT cells. An increase in oxygen flux into the cells was measured with 1 mM diquat. Panel E, Effects of diquat on oxygen flux by CHO-OR cells.
Diquat stimulates oxygen flux into CHO cells
In further studies we determined if diquat redox cycling occurred at sufficient rates in CHO cells to stimulate oxygen utilization. For these experiments, we used self-referencing microsensors to quantify oxygen flux [30]. Low basal rates of oxygen consumption were noted in both CHO-WT and CHO-OR cells (Fig. 8, panels B and C). The addition of 1 mM diquat to the cells caused a marked increase in cellular oxygen consumption (Fig. 8, panels D and E). Lower concentrations of diquat (1-100 μM) had little or no effect on oxygen flux. The response of CHO-OR cells to diquat was significantly greater than the response of CHO-WT cells, a finding consistent with increased diquat redox cycling in these cells.
Discussion
The cytotoxic activity of bipyridyl herbicides is thought to be due to their ability to redox cycle; a process by which these compounds undergo a one electron reduction to form stable free radicals which are readily oxidized back to the parent cations in a process that generates ROS [9, 31]. This activity is related to the coplanarity of the pyridine rings in the bipyridyl molecules and the relative positions of the nitrogen atoms which allow optimal electron resonance to stabilize the reduced compounds. Earlier studies demonstrated that redox cycling, measured as diquat- or paraquat-induced NADPH oxidase activity, was mediated by microsomal enzymes with NADPH as the source of reducing equivalents [9]. Cytochrome P450 reductase purified from rat liver was identified as a key enzyme in microsomes mediating this process [32, 33], a finding confirmed in the present studies with diquat and paraquat using human recombinant enzyme. Our studies also demonstrated that redox cycling by recombinant cytochrome P450 reductase and rat liver microsomes was suppressed by DPI, an inhibitor of flavoproteins [20, 34]. These findings are consistent with the fact that cytochrome P450 reductase contains both FAD and FMN [28]. Of interest was our observation that diquat was more active than paraquat in generating hydrogen peroxide and hydroxyl radicals during redox cycling, a finding likely due to the more positive redox potential of diquat (-350 mV) when compared to paraquat (-446 mV) [6, 35]. For hydrogen peroxide and hydroxyl radical production, the apparent KM's for diquat and paraquat redox cycling were lower with the recombinant enzyme, relative to the microsomes. This is probably a result of the reduced concentrations of cytochrome P450 reductase in the microsomal preparations.
It is generally thought that redox cycling generates ROS via the univalent reduction of oxygen resulting in the formation of superoxide anion [8]. In this reaction, one molecule of oxygen oxidizes two molecules of NADPH generating two molecules of superoxide anion [9]. We found that under aerobic conditions, recombinant cytochrome P450 reductase- and liver microsome-mediated redox cycling by diquat and paraquat generated superoxide anion and consequently, readily consumed oxygen. These data are consistent with studies in rat liver microsomes, showing that oxygen consumption was correlated with increased NADPH oxidase activity during bipyridyl herbicide redox cycling [9]. It should be noted that under anaerobic conditions, the recombinant enzyme can still support the reduction of diquat and paraquat, as shown by the appearance of the radical cations in our enzyme assays. Thus, oxygen is not required for the reduction of diquat or paraquat. As expected, enzyme catalyzed diquat radical formation was much more rapid than paraquat radical formation, confirming that the one electron reduction of the herbicides is the rate limiting step.
Since the diquat and paraquat radicals are reactive intermediates, we speculate that under hypoxic conditions, the bipyridyl radicals can directly initiate toxicity by binding to cellular macromolecules. The resulting balance between direct- and ROS-mediated toxicities may depend on localized oxygen tension and/or the extent of redox cycling in the tissues. In this regard, our studies show that diquat increases oxygen flux into CHO-OR cells, a response due to decreased intracellular oxygen tension during redox cycling. Therefore, redox cycling by diquat can create localized hypoxic conditions in the cells, a process that stabilizes bipyridyl radicals. Thus, in addition to generating cytotoxic ROS, the toxicity of the bipyridyl herbicides may be mediated, at least in part, by localized depletion of molecular oxygen and the production of bipyridyl cation radicals.
Diquat stimulated redox cycling and increased oxygen flux in CHO-OR cells was associated with a marked increase in protein carbonylation, a major form of protein oxidation and an important biomarker of oxidative stress. Protein oxidation can confer irreversible loss of function and contribute to toxicity [36]. A number of carbonylated proteins ranging in molecular weight from 24-112 kDa were identified in CHO-OR cells treated with diquat. Additional studies are needed to identify these proteins and to assess their role in mediating the actions of diquat. Most clinical studies on paraquat and diquat poisoning report micromolar to millimolar serum concentrations, depending on time after exposure and methods used to measure the bipyridyl herbicides [37-39]. Thus, the concentrations of diquat and paraquat used in our biochemical and cell studies are within the ranges that can be observed within a clinical setting and are thus relevant.
Consistent with previous studies [9], we found that diquat is more efficient than paraquat in generating ROS during redox cycling in microsomes, as well as in recombinant cytochrome P450 reductase. A question arises as to whether this is responsible for the increased toxicity of diquat, relative to paraquat in different tissues. It is likely that the toxicity of herbicides depends on their relative uptake into target cells and the cellular content of cytochrome P450 reductase or other enzymes that have the capacity to mediate redox cycling. It is well established that the toxicity of diquat and paraquat are distinct [6, 40, 41]. Thus, whereas diquat damages the liver and kidneys [40], paraquat causes lung injury and fibrosis which may be due to selective uptake into tissues [41].
It should be noted, however, that redox cycling is not always correlated with cytotoxicity. For example, in recent studies nitrofurantoin and mitomycin c were shown to redox cycle in lysates of CHO cells, which was significantly greater in CHO-OR cells, when compared to CHO-WT cells. However, no differences in cytotoxicity were observed between the cells [20, 34]. Similar results were observed in nine different tumor cell lines that varied in cytochrome P450 reductase content [20, 34]. Stabilization of the mitomycin c radical by hypoxia in CHO-WT cells and in the tumor cell lines also had no effect on cytotoxicity [34]. These data suggest that redox cycling and intracellular ROS generation do not necessarily mediate cytotoxicity. Thus, the precise role of redox cycling and cation radical formation in mediating diquat and paraquat toxicity in different tissues remains to be determined.
In summary, our studies demonstrate that both human and mouse recombinant cytochrome P450 reductase and liver microsomes mediate redox cycling by diquat and paraquat, generating ROS in an NADPH- and oxygen-dependent process. In intact CHO cells, this increases oxygen utilization, inducing oxidative stress, and possibly reducing intracellular oxygen available for metabolic processes. Increased redox cycling of diquat, when compared paraquat, may lead to selective tissue toxicity; however, this depends on the localized tissue concentrations of the herbicides, their capacity to redox cycle, as well as antioxidant defense mechanisms. One can also not exclude the possibility of alternative actions of the bipyridyl herbicides in mediating toxicity. For example, in keratinocytes, paraquat stimulated redox cycling can induce antioxidant expression, including Cu,Zn-superoxide dismutase, catalase, hemeoxygenase-1 and several glutathione-S-transferases (GSTP1, GSTA3 and GSTA4), in an adaptive response that has the potential to mitigate diquat- and paraquat-induced oxidative stress. Further studies are required to determine if similar changes in antioxidant expression occur in CHO cells and if they are mediated by the redox cycling process.
Acknowledgements
This work was supported in part by National Institutes of Health grants CA093798, ES005022, and AR055073. This work was also funded in part by the National Institutes of Health CounterACT Program through the National Institute of Arthritis and Musculoskeletal and Skin Diseases (award #U54AR055073). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government.
List of Abbreviations
- CHO-WT
Chinese hamster ovary cells
- CHO-OR
Chinese hamster ovary cells overexpressing cytochrome P450 reductase
- DPI
diphenyleneiodonium
- H2O2
hydrogen peroxide
- 2-OH-TPT
2-hydroxyterephthalate
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinis-Oliveira RJ, Duarte JA, Sanchez-Navarro A, Remiao F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38:13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 3.Franco R, Li S, Rodriguez-Rocha H, Burns M, Panayiotidis MI. Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson's disease. Chem Biol Interact. 2010;188:289–300. doi: 10.1016/j.cbi.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones GM, Vale JA. Mechanisms of toxicity, clinical features, and management of diquat poisoning: a review. J Toxicol Clin Toxicol. 2000;38:123–128. doi: 10.1081/clt-100100926. [DOI] [PubMed] [Google Scholar]
- 5.Onyeama HP, Oehme FW. A literature review of paraquat toxicity. Vet Hum Toxicol. 1984;26:494–502. [PubMed] [Google Scholar]
- 6.Pasi A. The toxicology of paraquat, diquat and morfamquat. H. Huber; Bern: 1978. [Google Scholar]
- 7.Van Vleet TR, Schnellmann RG. Toxic nephropathy: environmental chemicals. Semin Nephrol. 2003;23:500–508. doi: 10.1016/s0270-9295(03)00094-9. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Clarendon Press; Oxford University Press; Oxford New York: 2007. [Google Scholar]
- 9.Gage JC. The action of paraquat and diquat on the respiration of liver cell fractions. Biochem J. 1968;109:757–761. doi: 10.1042/bj1090757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray JP, Heck DE, Mishin V, Smith PJS, Hong JY, Thiruchelvam M, Cory-Slechta DA, Laskin DL, Laskin JD. Paraquat increases cyanide-insensitive respiration in murine lung epithelial cells by activating an NAD(P)H:paraquat oxidoreductase. Identification of the enzyme as thioredoxin reductase. J Biol Chem. 2007;282:7939–7949. doi: 10.1074/jbc.M611817200. [DOI] [PubMed] [Google Scholar]
- 11.Mason RP. Redox cycling of radical anion metabolites of toxic chemicals and drugs and the Marcus theory of electron transfer. Environ Health Perspect. 1990;87:237–243. doi: 10.1289/ehp.9087237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward PA. Oxidative stress: acute and progressive lung injury. Ann N Y Acad Sci. 2010;1203:53–59. doi: 10.1111/j.1749-6632.2010.05552.x. [DOI] [PubMed] [Google Scholar]
- 13.Adam A, Smith LL, Cohen GM. An evaluation of the redox cycling potencies of paraquat and nitrofurantoin in microsomal and lung slice systems. Biochem Pharmacol. 1990;40:1533–1539. doi: 10.1016/0006-2952(90)90451-p. [DOI] [PubMed] [Google Scholar]
- 14.Adam A, Smith LL, Cohen GM. An assessment of the role of redox cycling in mediating the toxicity of paraquat and nitrofurantoin. Environ Health Perspect. 1990;85:113–117. doi: 10.1289/ehp.85-1568326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan HM, Fridovich I. Regulation of the synthesis of superoxide dismutase in Escherichia coli. Induction by methyl viologen. J Biol Chem. 1977;252:7667–7672. [PubMed] [Google Scholar]
- 16.Hassan HM, Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem. 1978;253:8143–8148. [PubMed] [Google Scholar]
- 17.Rossouw DJ, Engelbrecht FM. The effect of paraquat on the respiration of lung cell fractions. S Afr Med J. 1978;54:1101–1104. [PubMed] [Google Scholar]
- 18.Rossouw DJ, Engelbrecht FM. The effect of oxygen and paraquat on the 14C-glucose oxidation of rabbit alveolar macrophages and lung slices. S Afr Med J. 1979;55:558–560. [PubMed] [Google Scholar]
- 19.Rossouw DJ, Engelbrecht FM. The effect of paraquat on the aerobic metabolism of rabbit alveolar macrophages and lung fibroblasts. S Afr Med J. 1979;55:20–23. [PubMed] [Google Scholar]
- 20.Wang Y, Gray JP, Mishin V, Heck DE, Laskin DL, Laskin JD. Role of cytochrome P450 reductase in nitrofurantoin-induced redox cycling and cytotoxicity. Free Radic Biol Med. 2008;44:1169–1179. doi: 10.1016/j.freeradbiomed.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 22.Mishin VM, Thomas PE. Characterization of hydroxyl radical formation by microsomal enzymes using a water-soluble trap, terephthalate. Biochem Pharmacol. 2004;68:747–752. doi: 10.1016/j.bcp.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Baumann RP, Penketh PG, Seow HA, Shyam K, Sartorelli AC. Generation of oxygen deficiency in cell culture using a two-enzyme system to evaluate agents targeting hypoxic tumor cells. Radiat Res. 2008;170:651–660. doi: 10.1667/RR1431.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR. Protein oxidation in the brain in Alzheimer's disease. Neuroscience. 2001;103:373–383. doi: 10.1016/s0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 25.Long LH, Hoi A, Halliwell B. Instability of, and generation of hydrogen peroxide by, phenolic compounds in cell culture media. Arch Biochem Biophys. 2010;501:162–169. doi: 10.1016/j.abb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Smith PJS, Sanger RS, Messerli MA. Principles development and applications of self-referencing electrochemical microelectrodes to the determination of fluxes at cell membrane. In: Michael AC, editor. Methods and New Frontiers in Neuroscience. CRC Press; 2007. pp. 373–405. [PubMed] [Google Scholar]
- 27.Messerli MA, Robinson KR, Smith PJS. Electrochemical sensor applications to the study of molecular physiology and analyte flux in plants. In: Volkov AG, editor. Plant Electrophysiology - Theory and Methods. Springer; 2006. [Google Scholar]
- 28.Djordjevic S, Roberts DL, Wang M, Shea T, Camitta MG, Masters BS, Kim JJ. Crystallization and preliminary x-ray studies of NADPH-cytochrome P450 reductase. Proc Natl Acad Sci U S A. 1995;92:3214–3218. doi: 10.1073/pnas.92.8.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarzer C, Illek B, Suh JH, Remington SJ, Fischer H, Machen TE. Organelle redox of CF and CFTR-corrected airway epithelia. Free Radic Biol Med. 2007;43:300–316. doi: 10.1016/j.freeradbiomed.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Land SC, Porterfield DM, Sanger RH, Smith PJ. The self-referencing oxygen-selective microelectrode: detection of transmembrane oxygen flux from single cells. J Exp Biol. 1999;202:211–218. doi: 10.1242/jeb.202.2.211. [DOI] [PubMed] [Google Scholar]
- 31.Mees GC. Experiments on the herbicidal action of 1,1′-ethylene-2,2′-dipyridylium dibromide. Ann Appl Biol. 1960;48:601–612. [Google Scholar]
- 32.Han JF, Wang SL, He XY, Liu CY, Hong JY. Effect of genetic variation on human cytochrome P450 reductase-mediated paraquat cytotoxicity. Toxicol Sci. 2006;91:42–48. doi: 10.1093/toxsci/kfj139. [DOI] [PubMed] [Google Scholar]
- 33.Saito M, Thomas CE, Aust SD. Paraquat and ferritin-dependent lipid peroxidation. J Free Radic Biol Med. 1985;1:179–185. doi: 10.1016/0748-5514(85)90116-3. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Gray JP, Mishin V, Heck DE, Laskin DL, Laskin JD. Distinct roles of cytochrome P450 reductase in mitomycin C redox cycling and cytotoxicity. Mol Cancer Ther. 2010;9:1852–1863. doi: 10.1158/1535-7163.MCT-09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewinsohn E, Gressel J. Benzyl Viologen-Mediated Counteraction of Diquat and Paraquat Phytotoxicities. Plant Physiol. 1984;76:125–130. doi: 10.1104/pp.76.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 37.Fell AF, Jarvie DR, Stewart MJ. Analysis for paraquat by second- and fourth-derivative spectroscopy. Clin Chem. 1981;27:286–292. [PubMed] [Google Scholar]
- 38.Hantson P, Wallemacq P, Mahieu P. A case of fatal diquat poisoning: toxicokinetic data and autopsy findings. J Toxicol Clin Toxicol. 2000;38:149–152. doi: 10.1081/clt-100100930. [DOI] [PubMed] [Google Scholar]
- 39.Kim JH, Gil HW, Yang JO, Lee EY, Hong SY. Effect of glutathione administration on serum levels of reactive oxygen metabolites in patients with paraquat intoxication: a pilot study. Korean J Intern Med. 2010;25:282–287. doi: 10.3904/kjim.2010.25.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litchfield MH, Daniel JW, Longshaw S. The tissue distribution of the bipyridylium herbicides diquat and paraquat in rats and mice. Toxicology. 1973;1:155–165. doi: 10.1016/0300-483x(73)90029-2. [DOI] [PubMed] [Google Scholar]
- 41.Rose MS, Smith LL. Tissue uptake of paraquat and diquat. Gen Pharmacol. 1977;8:173–176. doi: 10.1016/0306-3623(77)90045-3. [DOI] [PubMed] [Google Scholar]