Abstract
BACKGROUND & AIMS
The type of immune response during development of acute pancreatitis (AP) determines disease severity. Pancreatic epithelial cells express the interleukin (IL)-22 receptor A1 (IL-22RA1). The aryl hydrocarbon receptor (AhR) is a ligand-dependent transcription factor that regulates expression of IL-22. We investigated sources and role of IL-22 in the pancreas, along with the effects of AhR activation on IL-22 expression and AP progression in mice.
METHODS
We analyzed the effects of recombinant IL-22, a monoclonal antibody against IL-22, and agonists and antagonists of AhR in mice with AP (induced with caerulein or a choline-deficient diet supplemented with DL-ethionine) and control mice. We also analyzed transgenic mice with AhR deficiency (AhRd and AhR−/− mice).
RESULTS
CD4+ T cells were the main source of IL-22 in pancreatic tissues from healthy mice. During development of AP, numbers of IL-22+ CD4+ T cells were reduced, whereas IL-22RA1 was up-regulated. Consistent with high levels of IL-22RA1 expression, pancreatic acinar cells responded to IL-22 signaling via signal transducers and activators of transcription 3; administration of IL-22 reduced AP and associated lung injury in mice. AhR was required for production of IL-22 and protected mice from AP. Mice that did not respond to AhR activation developed AP, but administration of IL-22 reduced AP; blockade of IL-22 reversed the ability of activated AhR to protect against AP.
CONCLUSIONS
AhR activation protects mice from AP by inducing expression of IL-22. AhR therefore mediates interactions between pancreatic leukocytes and epithelial cells and might be developed as a therapeutic target.
Keywords: Immune Regulation, Inflammatory Response, T-Cell Signaling, Gene Expression
Acute pancreatitis (AP) is associated with significant morbidity and can account for up to 30% mortality in those who follow a severe course.1 AP can develop as a result of an injury to pancreatic acini, leakage, and inappropriate activation of pancreatic enzymes, leading to significant pancreatic necrosis and the feared complication of multiple organ failure.2 Aside from supportive therapy, currently, no active treatment exists for treating AP. The unmet need for therapies against AP and the paucity of immune response understanding in AP led us to explore the role of interleukin (IL)-22 and its regulation by the aryl hydrocarbon receptor (AhR) in AP.
IL-22 is a member of the IL-10 cytokine family and plays a critical role in modulation of tissue response during inflammation. IL-22 is an important cytokine allowing for cross talk between leukocytes and epithelial cells because IL-22 production and receptor expression are restricted to leukocytes and epithelial cells, respectively.3 In addition to the well-studied T-helper (Th) 17 cells, various other leukocyte subsets such as γδ T cells, Th22 cells, NK cells, monocytes, dendritic cells, and lymphoid tissue-inducer (LTi) cells have been shown to be important sources of IL-22.4 IL-22 signals via the IL-22 receptor that consists of IL-22 receptor A1 (IL-22RA1) and IL-10R2 chains.5 IL-22 receptor activation leads to signal transducers and activators of transcription (STAT) 3-mediated proliferative and antiapoptotic pathway signaling, as well as antimicrobial induction that helps prevent damage and aid tissue repair.6 IL-10R2 is expressed ubiquitously in various organs; however, IL-22RA1 has a more restricted expression with highest level of messenger RNA (mRNA) reported in the pancreas followed by the intestines and the skin.7 Despite these findings and detailed studies, for example outlining importance of IL-22 in gut immunity,8 regulation of IL-22 and activation of IL-22 receptor in the pancreas remain poorly understood both under homeostatic and inflammatory states.
In elegant studies by several groups, AhR has been established as a critical ligand-dependent transcription factor for IL-22 production.9 AhR ligands include environmental toxins and endogenous natural ligands, which have become subject of great research interest.10 In this study, we investigate IL-22RA1 and IL-22 induction during AP and demonstrate ex vivo and in vivo pancreatic IL-22RA1 activation and downstream signaling. Consistent with our result of IL-22’s protective role in ongoing severe AP and pancreatic acinar cell apoptosis, AhR antagonism with either chemical blockade or use of AhR nonresponsive transgenic mice leads to decrease in pancreatic IL-22 production and worsening of pancreatitis. In addition, we show that biliverdin, an endogenous AhR ligand, increases pancreatic IL-22 and protects against AP. Furthermore, we confirm that AhR activation increases IL-22 production in the pancreas and mediates protective role of IL-22 in AP using 2 independent approaches by reconstituting AhR nonresponsive mice with IL-22 and neutralizing IL-22 in mice where AhR has been activated.
Materials and Methods
Mice
Mice including Balb/c, C57B6/J, and AhR deficient (AhRd) were purchased from Jackson Laboratories (Bar Harbor, ME) and housed under pathogen-free conditions. All animal experiments were approved by Stanford University institutional animal care and use committees.
AP Models
For caerulein-induced pancreatitis, age- and sex-matched mice were fasted for 12–16 hours. Mice then received 7 hourly intraperitoneal injections of saline (control) or 50 µg/kg caerulein in saline and followed up to 12 hours or indicated times. For the choline-deficient diet supplemented with DL-ethionine (CDE) model of pancreatitis, young female mice (16–20 g) were fasted then fed a choline-deficient diet (Harlan Teklad, Madison, WI) supplemented with 0.5% DL-ethionine (Sigma-Aldrich, St Louis, MO) or normal chow (control group).11,12
Mice Treatments
Mice were given phosphate-buffered saline or IL-22 (200 ng/mouse, Miltenyi Biotech) intraperitoneally at 24 hours after onset of CDE-induced AP. For experiments involving anti-IL-22 monoclonal antibodies (mAb), mice were treated with 1 µg of either anti-IL-22 or isotype control mAb (R&D Systems, Minneapolis, MN) at indicated times. CH-223191 (100 µg/mouse, Sigma-Aldrich) was dissolved in dimethyl sulfoxide and biliverdin hydrochloride (35 mg/kg, Frontier Scientific, West Logan, UT) in 20 mmol/L NaOH adjusted to pH of 7.0. CH-223191, biliverdin hydrochloride, or vehicle control were used to treat normal mice or mice undergoing AP at indicated times.
Histology and Immunofluorescence
Mice were killed by CO2 inhalation. Pancreata and other tissues were rapidly removed. Pancreas and lung pieces were immediately fixed in 10% formalin and embedded in paraffin. Fixed tissues were sectioned and stained with H&E (performed by Histo-Tec Laboratory). The severity of pancreatitis and lung injury were scored blindly as described previously.11,12 Other pancreas pieces were frozen in Tissue-Tek OCT compound and sectioned for fluorescence staining. Immunofluorescence staining for phosphorylation of STAT3 (Cell Signaling Technologies, Danvers, MA) and 4′,6-diamidino-2-phenylindole (DAPI) (nuclei) was performed according to manufacturer’s guidelines. Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling assay was performed according to the manufacturer’s instructions (ApopTag Red In Situ Apoptosis Detection Kit; Millipore, Billerica, MA).
Biochemical Analysis and Myeloperoxidase Activity Assay
Blood was collected by intracardiac puncture, and serum was isolated for subsequent lipase level determination by diagnostic laboratory at Stanford University. Lung tissues were collected and myeloperoxidase assay performed according to manufacturer’s guidelines (Biovision, Mountain View, CA).
Western Blotting and Enzyme-Linked Immunosorbent Assay
Mouse pancreata were isolated and frozen immediately in liquid nitrogen. Total tissue and primary pancreatic acinar cells isolated as described below were homogenized in RIPA buffer containing protein inhibitors and analyzed by Western blot as described previously.12,13 IL-22RA1 and cleaved caspase 3 antibodies were purchased from Abcam and Cell Signaling Technologies, respectively. Supernatant from pancreas homogenate was analyzed using enzyme-linked immunosorbent assay (ELISA) kit for mouse IL-22 (Biolegend, San Diego, CA).
Quantitative Polymerase Chain Reaction
Pancreas tissue was lysed with Trizol reagent (Invitrogen, Carlsbad, CA) for total RNA preparation according to manufacturer’s instructions. Briefly, complementary DNA was generated using GoScript reverse transcription system (Promega, Madison, WI). Quantitative polymerase chain reaction was performed with an ABI-7900 Sequence Detection System (Applied Biosystems, Grand island, NY) using designed specific TaqMan probes and primers as follows: Regenerating gene (Reg)IIIβ (forward, 5′-TGGAAGACAGACAAGATGCTG-3′; reverse, 5′-TAAGAGCATCAGGCAGGAGA-3′; Probe, 5′-CCTCCAACAGCCTGCTCCGTC-3′); RegIIIγ (forward, 5′-TCCTGTCCTCCATGATCAAA-3′; reverse, 5′-TGGGTT CATAGCCCAGTGT-3′; Probe, 5′-CGGGTCATGGAGCCCAATCC-3′); β-defensin2 (forward, 5′-CACTCCAGCTGTTGGAAGTTT-3′; reverse, 5′-GGGTTCTTCTCTGGGAAACA-3′; Probe, 5′-CCTCCTTCTGCCAGGCGTCC-3′); serum amyloid A (SAA) 3 (forward, 5′-GCATCTTGATCCTGGGAGTT-3′; reverse, 5′-AGACCCTTGACCAGCTTCTTT-3′; Probe, 5′-ACAGCCAAAGATGGGTCCAGTTCA-3′); IL-22RA1 (forward, 5′-ACATCACCAAGCCACCTGTA-3′; reverse, 5′-GGTCCAAGACAGGGATCAGT-3′; Probe, 5′-TCCCTGAACGTCCAACGTGTCC-3′). Samples were normalized to glyceraldehyde-3-phosphate dehydrogenase (GADPH) and displayed as fold induction over vehicle-treated controls unless otherwise stated.
Isolation of Pancreatic Acinar Cells and Leukocytes
Mice were killed and pancreas removed carefully by trimming fat and mesentery. Dispersed pancreatic acinar cells were isolated using collagenase digestion method described previously.14 Isolated primary pancreatic acinar cells were in vitro treated with either recombinant IL-22 (rIL-22) or vehicle control for up to 4 hours. Pancreatic leukocytes were prepared with minor modification of method developed by Hawkins et al.15 Briefly, 3 or 4 pancreata were pooled together and minced with scissors then washed twice with buffer A (Hank’s balanced salt solution +10% fetal calf serum). The tissue was resuspended in buffer A containing 2 mg/mL collagenase type IV (Sigma-Aldrich) and incubated in a shaker at 37°C for 15 minutes. The suspension was then vortexed at low speed for 20 seconds and centrifuged, and the cell pellet was resuspended in red blood cell lysing buffer (Sigma-Aldrich) for 5 minutes. The cells were spun down, washed 3 times with Hank’s balanced salt solution + 2% bovine calf serum, and used for surface and intracellular staining.
Flow Cytometry
For surface staining, cells were stained with the following Fluorochrome conjugated antibodies (Biolegend): AF700 or FITC CD45.2, PE/Cy7-CD3, PE/Cy7-CD19, APC/Cy7-CD4, FITC-CD90, Percp/Cy5.5-CD11b, APC-Sca-1, PB-CD11c, APC/Cy7-NK1.1, and APC-NKp46. For intracellular cytokine staining, immediately after isolation, cells were cultured in RPMI complete medium and stimulated with phorbol myristate acetate (50 ng/mL), ionomycin (1 µg/mL), and brefeldin A (10 µg/mL, eBioscience) for 4 hours. The cells were washed and stained with surface markers. The cells were then fixed and permeabilized using eBioscience kit and following manufacturer’s guidelines. PE-IL-22 and isotype control PE-immunoglobulin G1 (eBioscience, San Diego, CA) were used for intracellular staining. Dead cells were excluded from analysis using violet viability stain (Invitrogen). Flow cytometry data collection was performed on Fortessa LSRII (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Tree Star, Street Ashland, OR).
Statistical Analysis
Unpaired Student t test was used to determine statistical significance, and P value of less than .05 was considered significant. One-way analysis of variance plus Tukey post hoc test were used to determine the difference among multiple groups, and P value less than .05 was considered statistically significant. Values are expressed as mean ± standard error of mean (Prism 4; GraphPad Software, San Jose, CA). Unless indicated, results are from at least 3 independent experiments.
Results
IL-22 Induces Phosphorylation of STAT3 and RegIII Genes in the Pancreas
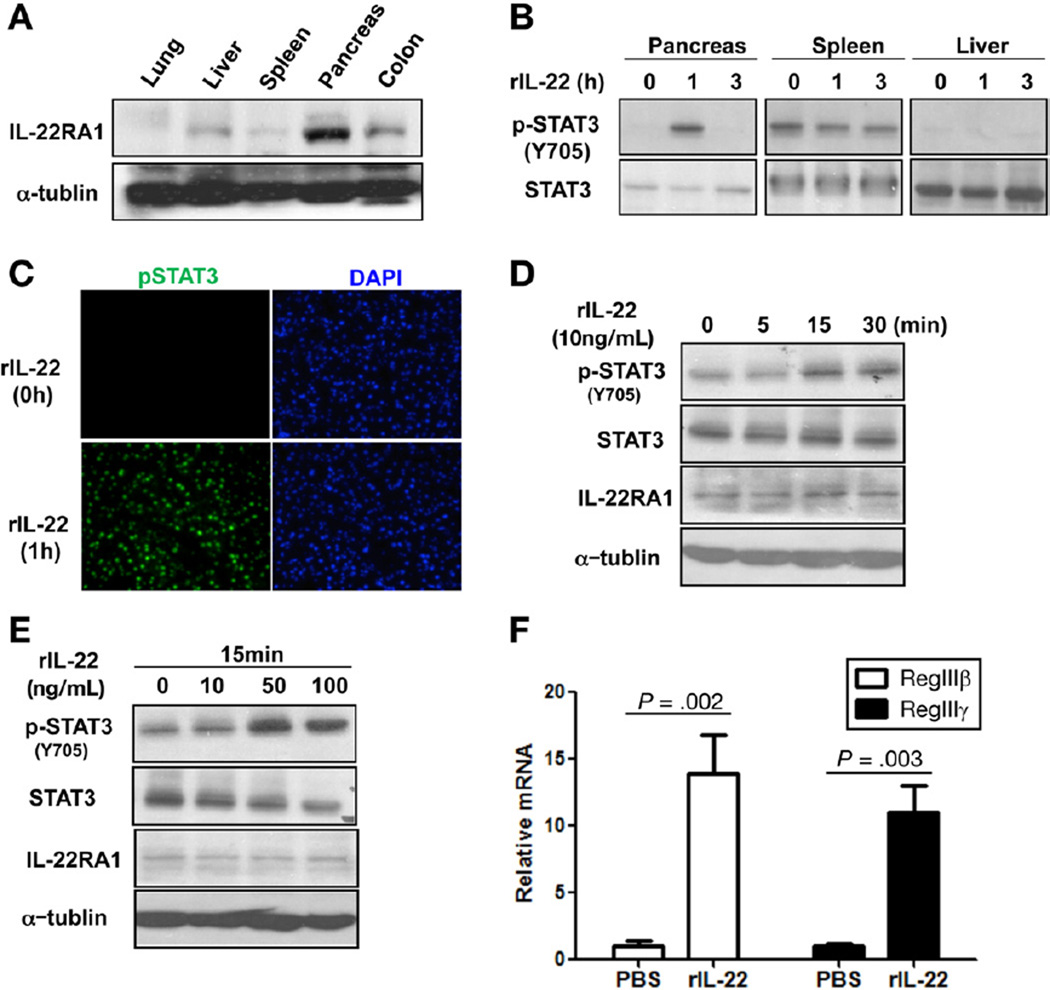
Pancreatic acinar cells express IL-22RA1 mRNA and have been shown to be a target for IL-22 action in vitro.16 IL-22RA1 exhibits a restricted expression pattern, with highest level of mRNA expression reported in the pancreas and detectable expression in multiple other tissues, particularly the colon and liver.7 Therefore, we first determined the expression of IL-22RA1 in different tissues at a protein level. Compared with the colon and liver, the pancreas has the highest level of IL-22RA1 expression (Figure 1A). In contrast to other tissues, in vivo activation of IL-22 receptor in the pancreas has not been well defined. Based on the high expression of IL-22RA1, we hypothesized that the pancreas would respond strongly to IL-22 administration. To test this hypothesis, we administered a relatively low dose of rIL-22 (200 ng/mouse) systemically and assessed IL-22 downstream signaling pathway. Consistent with our hypothesis, significant STAT3 activation was observed in the pancreas at 1 hour following exogenous IL-22 administration as compared with other tissues (Figure 1B). Activation of STAT3 in pancreas was also confirmed by immunofluorescence staining (Figure 1C). To further confirm acinar cell response, we isolated pancreatic acinar cells and treated them with different doses of rIL-22 and assessed IL-22 receptor activation. As shown, pSTAT3 was induced by rIL-22 in pancreatic acinar cell in a time- and dose-dependent manner (Figure 1D and E).
Figure 1.
IL-22 induces phosphorylation of STAT3 and RegIII genes in the pancreas. (A) Tissue lysates prepared from lung, liver, spleen, pancreas, and colon were examined for the expression of IL-22RA1 by Western blot. (B) Balb/c mice were treated with rIL-22, and, after 1 and 3 hours, tissue extracts from pancreas, liver, and spleen were prepared, and phosphorylation of STAT3 (p-STAT3) and total STAT3 were examined by Western blot. (C) Pancreas sections from rIL-22 treated mice (0, 1h) were costained with phospho-STAT3 (pSTAT3; green) and 4′,6-diamidino-2-phenylindole (DAPI) (nuclei; blue) and assessed by confocal microscopy. (D) Primary pancreatic acinar cells were treated with 10 ng/mL rIL-22 for indicated times and then lysed for immunoblotting with pSTAT3 and total STAT3. (E) Primary pancreatic acinar cells were treated with different doses of rIL-22 for 15 minutes and then assayed for pSTAT3 and total STAT3 by Western blotting. (F) Mice were treated with phosphate-buffered saline (PBS) or rIL-22. After 24 hours, pancreas RNA was isolated and complementary DNA prepared for quantitative polymerase chain reaction analysis. Results are shown as fold change in RegIIIβ and RegIIIγ messenger RNA expression relative to the control group.
IL-22 has been shown to induce multiple downstream targets in various tissues, such as RegIII genes (also known as pancreatitis-associated protein [PAP]), SAA, and β-defensins. PAPs are mainly expressed by pancreatic acinar cells and are up-regulated during AP.17 Emerging evidence supports that PAP proteins play regulatory roles during the inflammatory process in pancreatitis.18,19 Such studies showed the protective role of RegIII/PAPs in AP using PAP knockout mice and small interfering RNA knockdown of PAP1 and PAP3 (RegIIIγ). IL-22 was previously shown to up-regulate PAP1 in cultured pancreatic acinar cells.16 Therefore, to determine whether IL-22 in vivo can induce these gene expressions, we treated mice with rIL-22 and harvested the pancreas 24 hours later for quantitative polymerase chain reaction analysis. As shown, rIL-22 stimulated expression of RegIIIβ (PAP1) and RegIIIγ (PAP3) genes involved in tissue regeneration (Figure 1F); however, there was no significant induction in β-defensin2, SAA3, and IL-22RA1 (Supplementary Figure 1).
IL-22RA1 Expression in the Pancreas Is Up-regulated in AP
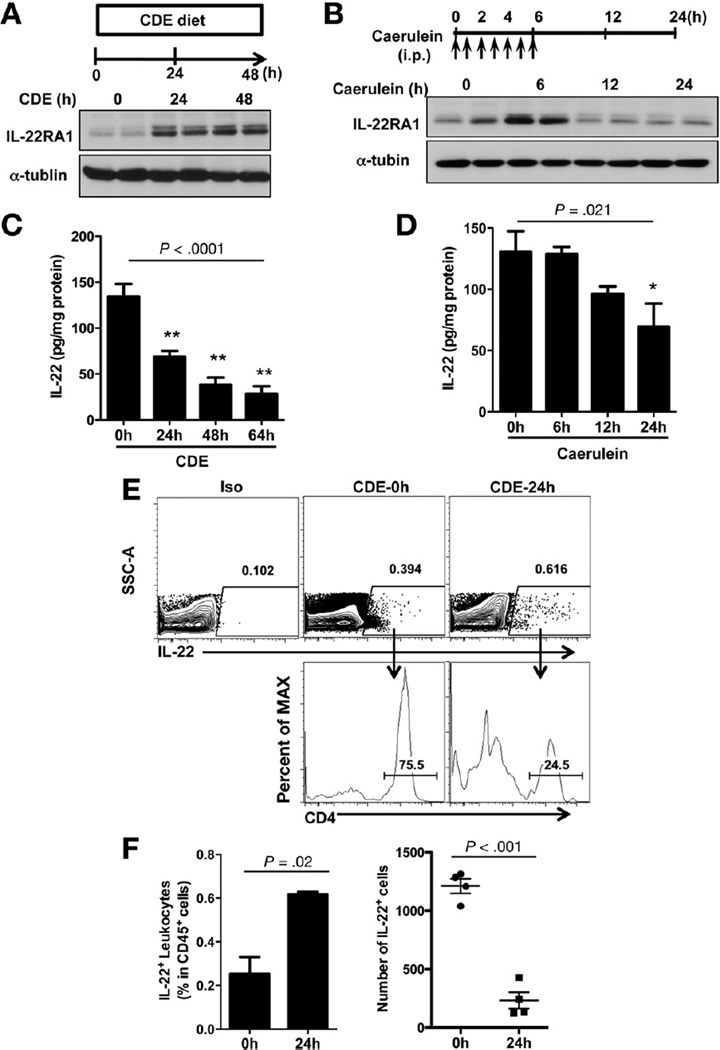
Because IL-22 receptor is highly expressed in the pancreas (Figure 1A), we next examined IL-22RA1 expression during pancreatic inflammation using 2 widely accepted independent mouse models of AP: (1) caerulein hyperstimulation, which causes mild to moderate AP,12 and (2) choline-deficient diet supplemented with DL-ethionine (CDE) feeding, which causes severe hemorrhagic AP associated with significant mortality.11 IL-22RA1 expression increased significantly in both models (Figure 2A and B). Sustained IL-22RA1 expression was noted during the induction and progression of AP but was reversible during the recovery phase in the caerulein model (Figure 2B). Given the high induction of IL-22RA1 in the AP models, these findings suggest that IL-22RA1 expression is stress inducible, and its activation leads to effects that could modulate pancreatic injury.
Figure 2.
Pancreatic IL-22RA1 is up-regulated whereas IL-22 is down-regulated during acute pancreatitis. (A and B) Total tissue homogenates were obtained from pancreata of CDE-fed and caerulein-treated mice at indicated times and blotted with IL-22RA1 and α-tublin antibodies. Representatives from 2 age- and sex-matched mice are shown for each time point. (C and D) Total pancreatic homogenates were obtained from CDE-fed and caerulein-treated mice at indicated times and IL-22 levels determined by ELISA. (E) Pancreas leukocytes were isolated for IL-22 intracellular staining from Balb/c mice prior to and 24 hours after CDE feeding (3 mice pooled per test/stain). Flow cytometry plots are those derived from sequential gating on total leukocytes (CD45.2+) and live cells as shown in Supplementary Figure 2. Iso indicates isotype control antibody staining. (F) Solid bars represent quantification of IL-22+ cells as a percent of total pancreatic leukocytes (CD45+ cells) from mice fed with CDE diet at times 0 and 24 hours. In the second panel, dots show absolute number of IL-22+ cells from 3 pooled pancreata (3 mice). Data shown are that of mean ± standard error of mean from 4 independent experiments.
Pancreatic IL-22 Is Reduced During AP
To determine the availability of IL-22 during disease progression, we evaluated the expression of IL-22 over time using both AP models. In contrast to IL-22RA1 expression, IL-22 levels decreased significantly over time (Figure 2C and D). Considerable decrease in IL-22 levels was associated with the more severe disease, as shown with the CDE feeding over time (Figure 2C). To further assess pancreatic IL-22 expression during AP and determine the source for the IL-22, we isolated pancreas leukocytes and performed intracellular cytokine staining and then analyzed by flow cytometry. Because pancreas IL-22 expression by ELISA declined significantly by 24 hours in the CDE model (Figure 2C), we compared leukocyte IL-22 production at time 0 and 24 hours. Following dead cell exclusion and gating on total (CD45+) leukocytes, we found that the percent of IL-22+ leukocytes increased at 24 hours (Figure 2E and F). However, because the total number of leukocytes per harvested pancreas decreases over time (Supplementary Figure 2A), the absolute number of IL-22+ leukocytes was much lower at 24 hours (Figure 2F, second panel). These results are consistent with the above ELISA findings (Figure 2C) suggesting depletion of IL-22 during AP progression. Under homeostatic conditions, most of the pancreatic IL-22 is produced by CD4+ T cells and during AP IL-22+ CD4+ T cells are markedly decreased (Figure 2E). Interestingly, using previously described phenotyping strategy,20 the relative increase in percent of IL-22+ leukocyte during AP is in part accounted for by innate immune cells including CD4− LTi cells and NKp46+ innate lymphoid cells (ILCs) (Supplementary Figure 2). In particular NKp46+ ILCs account for the IL-22+ cells with high fluorescence intensity.
Exogenous IL-22 Ameliorates Established AP
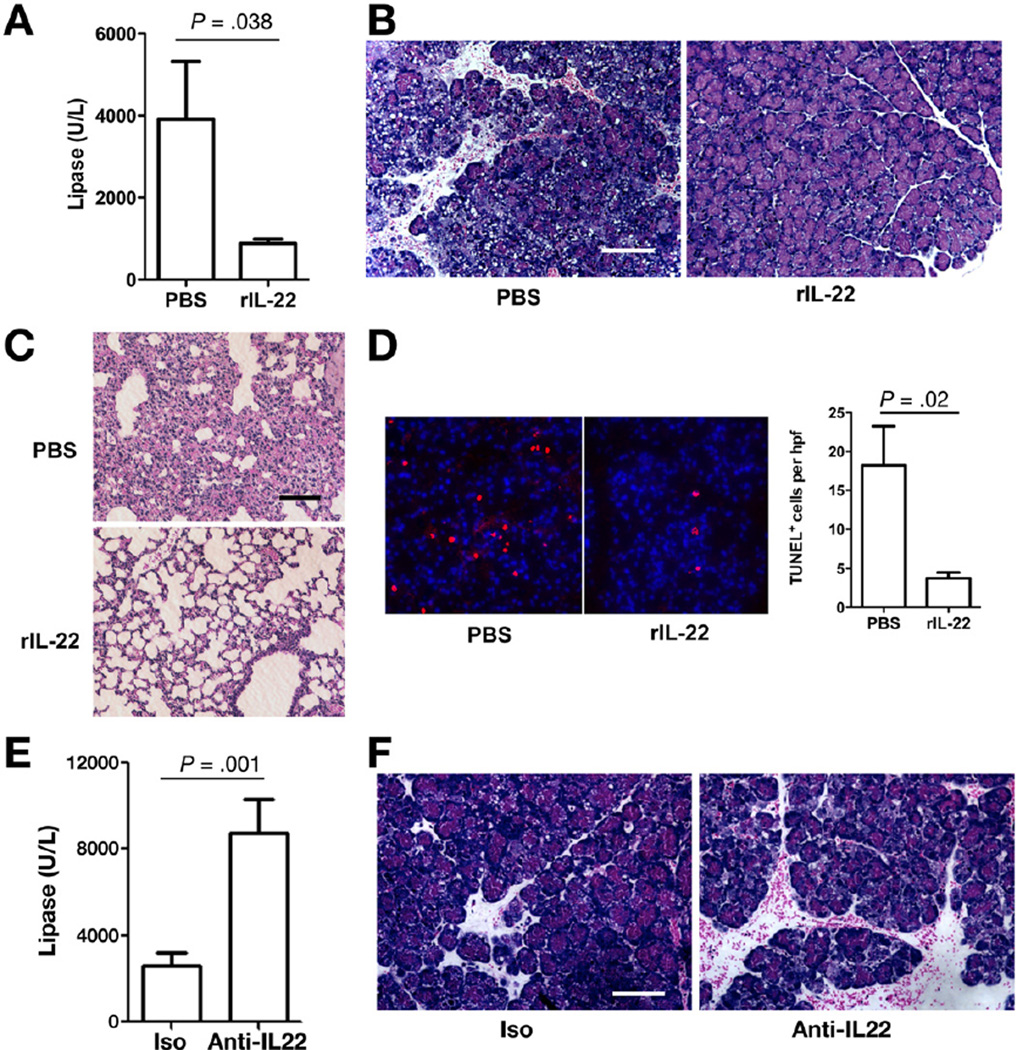
IL-22 expression significantly decreased in the pancreas 12 hours and 24 hours following AP induction in mild to moderate and severe models of AP, respectively (Figure 2). We explored whether supplementation of IL-22 could contribute to attenuation of ongoing and established AP. We used CDE-induced AP model, where we previously showed a notable pancreatic injury by day 1.11 Mice were given rIL-22 or control 24 hours after initiation of the CDE feeding then serum, pancreas, and lung were harvested at 60 hours. Serum lipase levels were significantly lower in the group that received rIL-22 (Figure 3A). Histologic examination and blinded scoring of the pancreata showed a less severe pancreatitis in mice treated with rIL-22 (Figure 3B and Supplementary Figure 3B). Furthermore, morphologic evidence of lung injury and lung myeloperoxidase levels are significantly reduced by rIL-22 treatment (Figure 3C and Supplementary Figure 3C). Blinded lung histology scores were significantly lower in the rIL-22 treatment group (2.8 ± 0.37 for control vs 1.6 ± 0.24 for rIL-22 treated mice; P = .028). In addition, rIL-22 treatment group had lower mortality compared with the controls (Supplementary Figure 3A). Effective pancreas IL-22 reconstitution following systemic rIL-22 administration was confirmed by ELISA (Supplementary Figure 3D). To determine further the role of rIL-22 on cell death during AP in vivo, we used terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling assay, and rIL-22 treatment significantly reduced apoptosis (Figure 3D). Similarly, treatment of isolated primary pancreatic acinar cells with rIL-22 in vitro delayed spontaneous apoptosis as assessed by level of cleaved Caspase 3 (Supplementary Figure 4). To define further the role of IL-22 in AP, we treated mice with either isotype control or anti-IL-22 neutralizing mAb. Consistent with the protective role of rIL-22, mice treated with anti-IL-22 mAb had higher mortality and increased serum lipase levels (Supplementary Figure 3E and Figure 3E). In addition, blinded histologic scoring confirmed increased pancreatic injury with blockade of IL-22 (Figure 3F and Supplementary Figure 3F).
Figure 3.
Exogenous IL-22 ameliorates established acute pancreatitis. (A) Balb/c mice were intraperitoneally injected with rIL-22 (single dose, 200 ng/mouse) or vehicle at 24 hours after feeding CDE diet and killed at 60 hours. Bar graph shows results from serum lipase measurements. (B and C) Representative H&E staining of pancreas and lung tissue sections are shown. Scale bar, 100 µm. (D) Pancreatic tissue from phosphate-buffered saline (PBS)- and rIL-22-treated AP mice (at 60 hours) were processed for antibody staining of apoptotic cells (red, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling [TUNEL] assay) and nuclei (blue, 4’,6-diamidino-2-phenylindole [DAPI]). Bar graph represents the mean ± standard error of mean of TUNEL-positive cells per multiple high-powered fields of 3 independent experiments. (E) Bar graph represents results of serum lipase measurements. (F) Representative H&E-stained pancreatic sections of isotype control (Iso) and anti-IL-22 mAb-treated CDE mice are shown. Scale bar, 100 µm. All data are presented as mean ± standard error of mean of at least 3 independent experiments (n > 5 mice per group and per experiment).
AhR Inactivation Decreases Pancreatic IL-22 and Worsens AP
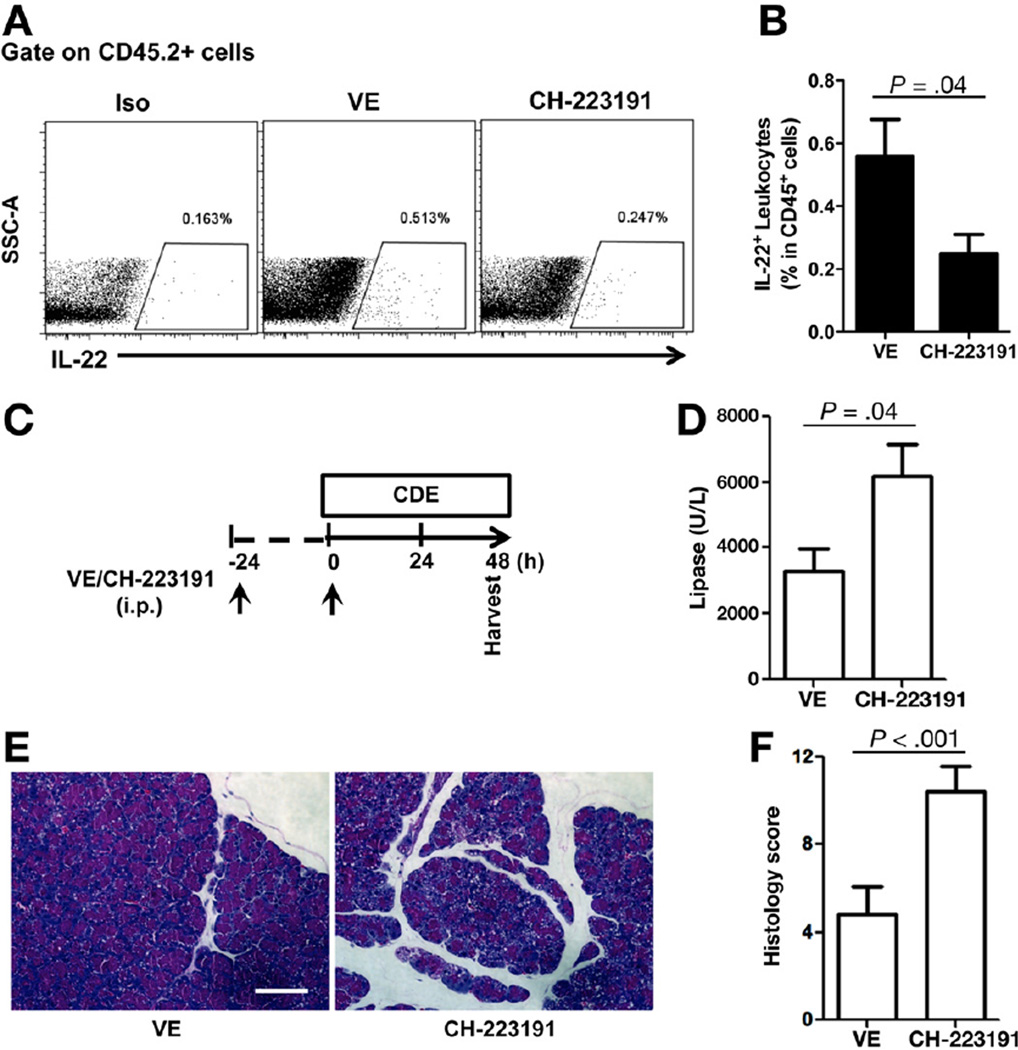
Recent reports show that the transcription factor AhR is required for most leukocyte IL-22 production. Therefore, we investigated whether inhibition of AhR signaling would reduce IL-22 expression in the pancreas and accelerate AP. CH-223191 is a novel chemical compound that has been used widely to inhibit AhR-dependent ligand activation and signaling.21 Balb/c mice were treated with either vehicle or CH-223191 (100 µg/mouse) for 2 consecutive days followed by isolation of pancreatic leukocytes at day 3. The frequency of IL-22 expressing leukocytes in the pancreas were markedly decreased in mice treated with the AhR antagonist relative to mice treated with vehicle control by flow cytometry (Figure 4A and B) and ELISA (Supplementary Figure 5). Furthermore, to determine whether inhibiting AhR signaling would result in enhanced pancreatitis, we pretreated Balb/c mice with CH-223191 and induced CDE-mediated AP and analyzed at day 2 (Figure 4C) because of the increased mortality relative to control-treated mice by day 3. Consistent with AhR requirement for IL-22 production and protective role of IL-22 in AP, mice pretreated with AhR antagonist CH-223191 developed more severe pancreatitis as compared with mice treated with vehicle control (Figure 4D–F).
Figure 4.
AhR antagonist CH-223191 decreases pancreatic IL-22 and worsens acute pancreatitis. (A) Balb/c mice were treated with vehicle control (VE) or AhR antagonist CH-223191 on day 1 and 2 (once per day); pancreata were then harvested at day 3. Pancreatic leukocytes were isolated for IL-22+ cell identification. Iso, intracellular staining with isotype control antibody. (B) Bar graph represents IL-22+ cells as percent of total leukocytes from vehicle or CH-223191-treated mice. Data shown are mean ± standard error of mean from 3 or more independent experiments. (C) Balb/c mice were injected with vehicle control (VE) or AhR antagonist CH-223191 daily (arrows) prior to initiation of CDE feeding. (D) After 2 days of CDE feeding, sera were collected for lipase measurement and presented as a bar graph. (E and F) Pancreata were also collected for H&E staining and histologic scores. Scale bar, 100 µm. Data are presented as mean ± standard error of mean (n ≥ 5 mice per group).
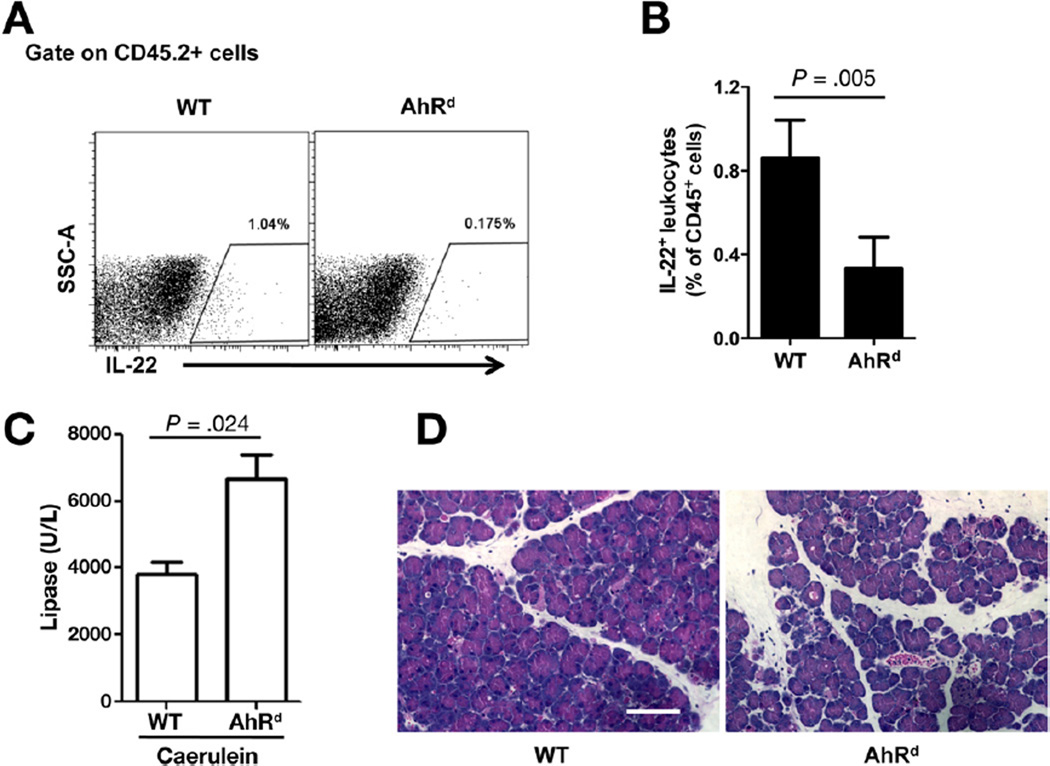
To further delineate and confirm the protective role of AhR in AP, we induced AP in the previously well-described AhR non-responsive (AhRd) mice22 and their wild-type counterparts. The d allele codes for AhR protein with reduced affinity for known ligands as a result of mutations in its ligand-binding site. Consistent with AhR antagonist use above, pancreatic leukocytes from AhRd mice had reduced level of IL-22 as compared with those from their wild-type counterparts by flow cytometry (Figure 5A and B) and ELISA (Supplementary Figure 5). Reduced level of pancreatic IL-22 was also present in the pancreas of AhR mutant (AhR−/−) (Supplementary Figure 6). We next investigated whether AhRd mice with decreased expression of IL-22 would be more sensitive to AP induction. To explore this possibility, we used caerulein to induce AP because the AhRd mice are on C57BL/6 background. As expected, caerulein hyperstimulation in AhRd mice resulted in a more severe pancreatitis (Figure 5C and D).
Figure 5.
Defective AhR signaling accelerates acute pancreatitis. (A) Pancreatic leukocytes were isolated from C57BL/6 wild-type (WT) and AhRd. Isolated cells were gated on live cells, leukocytes (CD45.2+), and then analyzed for frequency of IL-22+ cells. (B) Bar graph represents IL-22+ cells as a percent of total leukocytes. (C and D) WT and AhRd mice were injected with caerulein to induce AP. Sera and pancreata were collected for lipase measurement and H&E staining, respectively. Data are presented as mean ± standard error of mean (n = 5 mice per group).
AhR Mediates Protective Function of IL-22 in AP
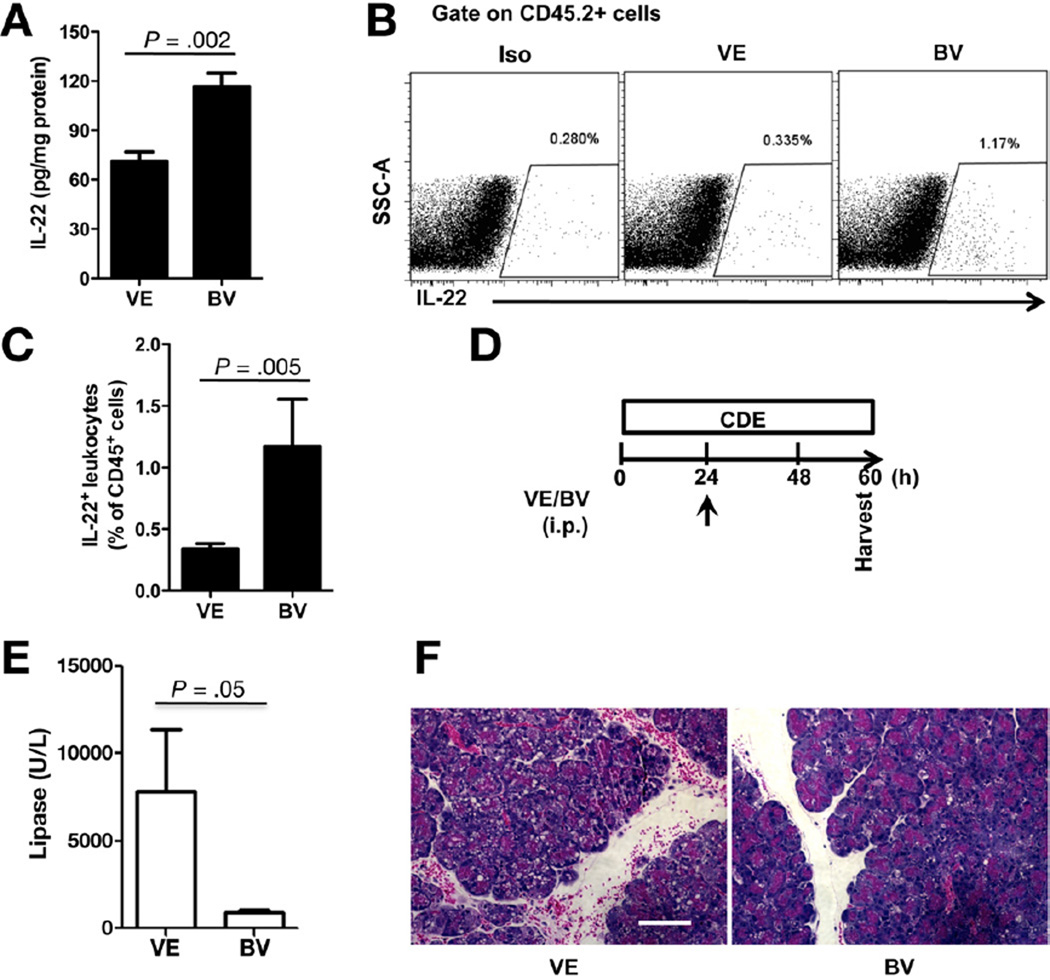
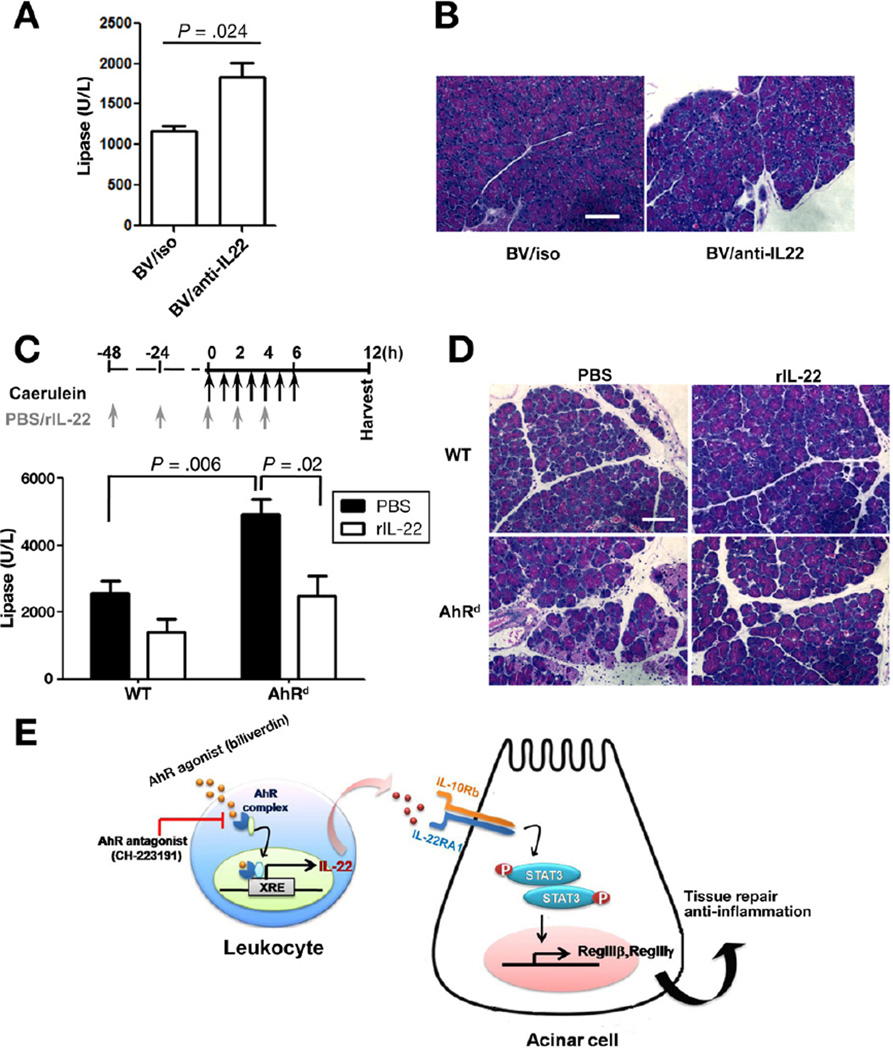
Although environmental toxins remain the main characterized ligands, in recent years endogenous AhR ligands are being more recognized and appreciated.23 Based on the AhR blockade studies above, we hypothesized that biliverdin, an endogenous AhR ligand, would alter pancreatic IL-22 levels and protect against AP. In agreement with this, biliverdin administration led to significant increase in pancreatic IL-22 by ELISA (Figure 6A) and flow cytometry (Figure 6B and C). Moreover, mice treated with a single dose of biliverdin (Figure 6D) were protected against AP as shown by the serum lipase (Figure 6E) and histologic assessment (Figure 6F). To confirm the relationship between AhR activation and IL-22-mediated protection, we set IL-22 blockade experiments in mice pretreated with biliverdin and undergoing CDEmediated AP. Anti-IL-22 in part reversed the protective effect of biliverdin as shown by the elevated serum lipase (Figure 7A) and pancreatic injury (Figure 7B).
Figure 6.
AhR activation with biliverdin increases pancreatic IL-22 and protects against acute pancreatitis. (A) Balb/c mice were treated with vehicle (VE) or AhR agonist biliverdin (BV) on day 1. Pancreata were harvested at day 3 and IL-22 determined by ELISA (A) and by flow cytometry (B). (C) Bar graph represents IL-22+ cells as percent of total leukocytes from vehicle or BV-treated mice. Data shown are mean ± standard error of mean from 3 or more independent experiments. (D) Balb/c mice were injected with VE or BV 24 hours after CDE feeding and killed at 60 hours. (E) Serum lipase measurement is presented as a bar graph. (F) A representative H&E staining of pancreata is shown. Scale bar, 100 µm. Data are represented as mean ± standard error of mean (n = 5 mice per group).
Figure 7.
AhR mediates protective function of IL-22 in acute pancreatitis. (A) Balb/c mice were treated with biliverdin (BV) at time 0 and treated with isotype control (Iso) or anti-IL-22 antibody at 12 and 36 hours after CDE feeding. Serum lipase results are shown as a bar graph. (B) A representative of pancreata H&E staining is shown. Scale bar, 100 µm. (C) C57BL/6 wild-type (WT) and AhRd mice underwent caerulein-induced AP. Phosphate-buffered saline (PBS) or rIL-22 was administrated to WT or AhRd mice, respectively, at the indicated times (arrows). Serum lipase measurement results are shown as a bar graph. (D) Representative H&E staining of the pancreata are shown. Scale bar, 100 µm. Data are presented as mean ± standard error of mean from 3 independent experiments with minimum of 5 mice per group. (E) Schematic representation summarizing findings of AhR activity and IL-22 cross talk in maintaining tissue repair and homeostasis in the pancreas.
Finally, to verify further the importance of AhR activation in protecting against AP via IL-22, we tested whether IL-22 supplementation can rescue AhRd mice susceptibility to AP. Exogenous IL-22 protected AhRd mice from AP as shown by the decrease in serum lipase levels (Figure 7C) and pancreatic injury (Figure 7D). Taken together, these results show the significance of AhR activation in IL-22 induction that allows for a cross talk between immune cells and pancreatic acinar cells to confer protection in AP as depicted by the schematic diagram (Figure 7E).
Discussion
AP remains a challenging clinical problem, particularly in patients with severe disease. Relative to progress in understanding pancreatic acinar cell physiology and pathophysiology, there is lack of understanding in immune cell response and cross talk during AP.24 IL-22 receptor is expressed by epithelial cells and not leukocytes, whereas IL-22 is produced by leukocyte subsets offering a unique opportunity to study cross talk between leukocytes and nonimmune cells in the pancreas. As a result, we examined the expression of IL-22 receptor and IL-22 in the pancreas prior to and following AP induction, whether IL-22 treats established and severe AP, and evaluated the significance of AhR activation in modulating IL-22 and pancreatic injury during AP. To our knowledge, a source for pancreas IL-22 and a role for AhR have not been previously described in AP.
Regulation and IL-22 signaling has been subject of intensive research, particularly in the intestine where it was shown to promote antimicrobial defense and tissue regeneration, maintain lymphoid follicles, and protect against colonic infection and inflammation.9 In agreement with previous studies showing high IL-22 receptor mRNA expression and IL-22 mediated up-regulation of PAP1, the pancreas even relative to the colon expresses high level of IL-22RA1. Moreover, the pancreas responds readily to IL-22 in a time- and dose-dependent manner as a strong activator of STAT3 without altering IL-22 receptor expression both at a protein and gene level under homeostatic conditions. However, IL-22 receptor expression is up-regulated during ongoing and severe AP but returns to baseline as the pancreas recovers, supporting the notion that IL-22 receptor activation is essential for protecting against tissue damage and allowing tissue regeneration. In contrast, IL-22 decreases with pancreatic injury and is associated with depletion of IL-22–producing CD4+ T cells. This led us to examine whether exogenous IL-22 would treat and its blockade enhance ongoing AP. Recently, Feng et al25 showed that IL-22 pretreatment attenuated the severity of caerulein-induced AP. Our study differs in that we used IL-22 treatment in mice with established disease using a severe model of AP. Taken together, IL-22 is likely to offer benefit as a prophylactic and therapeutic agent in mild and severe AP.
Initial studies found that T-cell subsets, such as Th17 and IL-17–producing cytotoxic T cells, are primary sources for IL-22 in mice.3 Subsequent studies identified other leukocyte subsets such as γδ T cells, NK T cells, and ILCs (including NK cells and LTi cells)26 as important sources of IL-22. Because IL-22–producing leukocyte subsets in the pancreas have not been described, we isolated pancreas leukocytes from pre- and post-AP induction and find that CD4+ T cells are the primary source for IL-22 under homeostatic conditions. However, following induction of AP, there is a decrease in total number of leukocytes and IL-22–producing CD4+ T cells, but there is a notable small increase in IL-22+ innate immune cells such as CD4− LTi cells and NKp46+ ILCs. It is not clear at this time whether this is a compensatory mechanism for the great depletion of IL-22–producing CD4+ T cells associated with progression of AP. Whether the depletion is related to apoptosis or changes in CD4+ T-cell recruitment is a future area of investigation. Demols et al using T cell-deficient mice and CD4+ T cell-depleting antibodies showed that CD4+ T cells play an important role in the pathogenesis of AP.27 Because the majority of the CD4+ T cells in the pancreas are non-IL-22 producers, depletion of total CD4+ T cells may have greater effect on dampening effector or pathogenic T cells rather than regulatory function of T-cell subsets. This may be supported by the fact that systemic low-dose (relative to previous studies)28 rIL-22 administration as shown in the current study significantly reconstitutes the pancreas and treats AP effectively.
Based on the protective role of IL-22 in the pancreas, we were interested in studying the regulation of IL-22. Because AhR has been shown to be a critical ligand-dependent transcription factor for IL-22 production in T cells and other leukocyte subsets, we modulated AhR signaling using chemical agents and transgenic mice. Pancreatic IL-22 is significantly decreased in AhR nonresponsive mice, AhR−/− mice, and in AhR antagonist (CH-223191) treated mice while activation of AhR with biliverdin (endogenous ligand) up-regulates pancreatic IL-22. Consistent with the protective role of IL-22 and AhR ligand modulation of IL-22 in the pancreas, AhR inhibition and activation lead to worsening and ameliorating AP, respectively. Moreover, our findings of AhR modulatory effect in the pancreas during AP is mediated via IL-22 as confirmed using 2 independent experimental models showing the ability to reverse the effect of AhR inhibition and AhR activation in AP using exogenous IL-22 and anti-IL-22 antibody, respectively.
In conclusion, our findings delineate leukocyte source for pancreatic IL-22 and regulation of IL-22 by AhR under homeostasis and during AP. Our study as depicted and summarized in Figure 7E highlights the importance of AhR signaling and IL-22-mediated cross talk between leukocytes and epithelial cells in the pancreas in conferring protection against AP. Furthermore, modulation of AhR signaling offers a novel therapeutic target for treating AP.
Supplementary Material
Acknowledgments
The authors thank Jean Chen, Yi Wei, and Alexander Hanganu for their technical assistance; Luhua Zhang and John B. Sunwoo for providing AhR−/− mice; and Yujie Tang for assistance with qPCR experiments.
Funding
Supported by NIH Digestive Disease Center grants DK56339 to Stanford University, by National Institutes of Health Grant R01 DK092421, and by Robert Wood Johnson Foundation (to A.H.).
Abbreviations used in this paper
- AhR
aryl hydrocarbon receptor
- AP
acute pancreatitis
- CDE
choline-deficient diet supplemented with DL-ethionine
- ELISA
enzyme-linked immunosorbent assay
- IL
interleukin
- IL-22RA1
interleukin-22 receptor A1
- ILCs
innate lymphoid cells
- LTi
lymphoid tissue-inducer
- mAb
monoclonal antibodies
- mRNA
messenger RNA
- NK
natural killer
- PAP
pancreatitis-associated protein
- Reg
regenerating gene
- SAA
serum amyloid A
- STAT
signal transducers and activators of transcription
- rIL-22
recombinant IL-22
Footnotes
Supplementary Materials
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2012.08.051.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Gravante G, Garcea G, Ong SL, et al. Prediction of mortality in acute pancreatitis: a systematic review of the published evidence. Pancreatology. 2009;9:601–614. doi: 10.1159/000212097. [DOI] [PubMed] [Google Scholar]
- 2.Gaisano HY, Gorelick FS. New insights into the mechanisms of pancreatitis. Gastroenterology. 2009;136:2040–2044. doi: 10.1053/j.gastro.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zenewicz LA, Yancopoulos GD, Valenzuela DM, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie MH, Aggarwal S, Ho WH, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 6.Lejeune D, Dumoutier L, Constantinescu S, et al. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277:33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 7.Gurney AL. IL-22, a Th1 cytokine that targets the pancreas and select other peripheral tissues. Int Immunopharmacol. 2004;4:669–677. doi: 10.1016/j.intimp.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 9.Monteleone I, Rizzo A, Sarra M, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237–248. e1. doi: 10.1053/j.gastro.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Alam MS, Maekawa Y, Kitamura A, et al. Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2010;107:5943–5948. doi: 10.1073/pnas.0911755107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Habtezion A, Kwan R, Akhtar E, et al. Panhematin provides a therapeutic benefit in experimental pancreatitis. Gut. 2011;60:671–679. doi: 10.1136/gut.2010.217208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamichi I, Habtezion A, Zhong B, et al. Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction. J Clin Invest. 2005;115:3007–3014. doi: 10.1172/JCI24912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue J, Li X, Jiao S, et al. Prolyl hydroxylase-3 is down-regulated in colorectal cancer cells and inhibits IKKβ independent of hydroxylase activity. Gastroenterology. 2010;138:606–615. doi: 10.1053/j.gastro.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 14.Menozzi D, Jensen RT, Gardner JD. Dispersed pancreatic acinar cells and pancreatic acini. Methods Enzymol. 1990;192:271–279. doi: 10.1016/0076-6879(90)92076-p. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins TA, Gala RR, Dunbar JC. The lymphocyte and macrophage profile in the pancreas and spleen of NOD mice: percentage of interleukin-2 and prolactin receptors on immunocompetent cell subsets. J Reprod Immunol. 1996;32:55–71. doi: 10.1016/s0165-0378(96)00986-2. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal S, Xie MH, Maruoka M, et al. Acinar cells of the pancreas are a target of interleukin-22. J Interferon Cytokine Res. 2001;21:1047–1053. doi: 10.1089/107999001317205178. [DOI] [PubMed] [Google Scholar]
- 17.Graf R, Schiesser M, Lussi A, et al. Coordinate regulation of secretory stress proteins (PSP/reg, PAP I, PAP II, and PAP III) in the rat exocrine pancreas during experimental acute pancreatitis. J Surg Res. 2002;105:136–144. doi: 10.1006/jsre.2002.6387. [DOI] [PubMed] [Google Scholar]
- 18.Gironella M, Folch-Puy E, LeGoffic A, et al. Experimental acute pancreatitis in PAP/HIP knock-out mice. Gut. 2007;56:1091–1097. doi: 10.1136/gut.2006.116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin YY, Viterbo D, Mueller CM, et al. Small-interference RNA gene knockdown of pancreatitis-associated proteins in rat acute pancreatitis. Pancreas. 2008;36:402–410. doi: 10.1097/MPA.0b013e31815f3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonnenberg GF, Monticelli LA, Elloso MM, et al. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SH, Henry EC, Kim DK, et al. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol. 2006;69:1871–1878. doi: 10.1124/mol.105.021832. [DOI] [PubMed] [Google Scholar]
- 22.Braun L, Kardon T, El Koulali K, et al. Different induction of gulonolactone oxidase in aromatic hydrocarbon-responsive or -unresponsive mouse strains. FEBS Lett. 1999;463:345–349. doi: 10.1016/s0014-5793(99)01649-x. [DOI] [PubMed] [Google Scholar]
- 23.Phelan D, Winter GM, Rogers WJ, et al. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch Biochem Biophys. 1998;357:155–163. doi: 10.1006/abbi.1998.0814. [DOI] [PubMed] [Google Scholar]
- 24.Mayerle J. A novel role for leucocytes in determining the severity of acute pancreatitis. Gut. 2009;58:1440–1441. doi: 10.1136/gut.2009.186692. [DOI] [PubMed] [Google Scholar]
- 25.Feng D, Park O, Radaeva S, et al. Interleukin-22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway. Int J Biol Sci. 2012;8:249–257. doi: 10.7150/ijbs.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int Immunol. 2011;23:159–163. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- 27.Demols A, Le Moine O, Desalle F, et al. CD4(+)T cells play an important role in acute experimental pancreatitis in mice. Gastroenterology. 2000;118:582–590. doi: 10.1016/s0016-5085(00)70265-4. [DOI] [PubMed] [Google Scholar]
- 28.Radaeva S, Sun R, Pan HN, et al. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.