Abstract
Target identification of biologically active small-molecules is often the rate-determining step in forward chemical genetics. Photo-affinity labeling (PAL) represents a useful biochemical strategy for target identification in complex protein mixtures. This unit describes the use of alkyl diazirine-based photo-affinity probes and Cu(I)-catalyzed click chemistry to covalently label and visualize the targets of biologically active small-molecules. A general method for affinity purification of probe-modified proteins, useful for identification of protein targets, is also described.
Keywords: photo-affinity labeling, diazirine, click chemistry, target identification, affinity purification
Target identification of biologically active small-molecules is often the rate-determining step in forward chemical genetics. Photo-affinity labeling (PAL) represents a useful biochemical strategy for target identification in complex protein mixtures. PAL uses an analog of a biologically active small-molecule, known as a photo-affinity probe, that bears photo-reactive and reporter functional groups, to identify macromolecular binding partners. The photo-affinity probe is designed and synthesized based on SAR (structure-activity relationships) of a parent small-molecule having known biological activity. During PAL, the photo-affinity probe is incubated with a protein mixture and irradiated with UV light. Irradiation of the photo-reactive group generates a highly reactive chemical species (e.g. carbene, nitrene, or radical) that covalently crosslinks the photo-affinity probe to its macromolecular binding partner(s). Photo-crosslinked protein targets are then visualized by the reporter group (e.g. fluorophore, biotin, or radioactive label). Covalent bond formation between the probe and targets enable the subsequent purification and identification of the targets using techniques such as SDS-PAGE, immunoprecipitation, biotin-streptavidin affinity purification and mass spectrometry. Affinity purification of protein targets is often difficult with non-covalently bound small-molecules, especially those with low to moderate binding affinity for the target. The challenges are compounded with small-molecules that target integral membrane proteins, which often show decreased function after solubilization with detergents, a prerequisite for affinity purification.
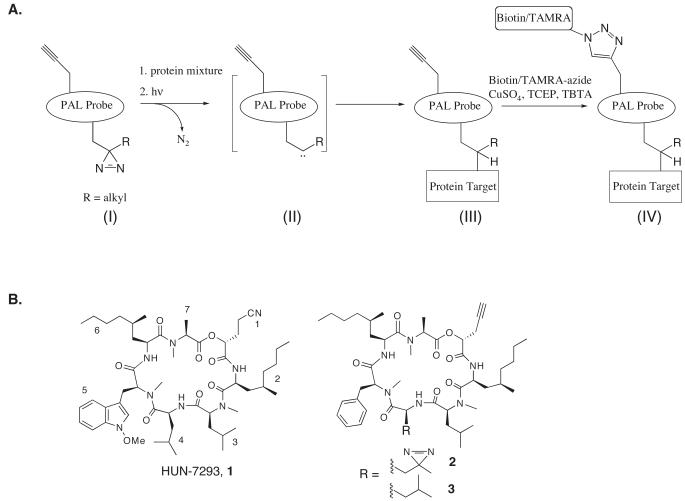
There are several photo-reactive functional groups frequently used in PAL (e.g. benzophenone, trifluoromethyl phenyl diazirine, aryl azide). Like most useful photo-affinity groups, the alkyl diazirine (Figure 1A, (I)) is activated at a wavelength of light (~355 nm) that is not damaging to protein(s). However, the alkyl diazirine holds unique advantages. First, it is compact in size, being nearly isosteric to a methyl group, and is accessed synthetically via an alkyl ketone. This allows installation of the diazirine at positions of a small-molecule that would not tolerate larger, aryl-based photo-reactive groups. Second, the carbene intermediate formed upon photo-activation of the diazirine (Figure 1A, (II)) rapidly inserts into X-H bonds (X = N, S, O), as well as C-H bonds, to form stable covalent insertion products (Brunner, 1993). When not poised for insertion into bonds of the macromolecular target, the alkyl carbene intermediate undergoes rapid quenching by solvent or internal rearrangement to a stable olefin side-product (Ford et al., 1998). The alkyl diazirine is stable toward acidic and basic conditions and toward ambient light encountered during routine chemical synthesis. Several improved methods for the synthesis of alkyl diazirines starting from alkyl ketone precursors have been recently reported (Bond et al., 2009; MacKinnon et al., 2007). Heterobifunctional amine-reactive alkyl diazirine crosslinkers, as well as alkyl diazirine-containing amino acid analogs are commercially available (Pierce, Thermo Scientific).
Figure 1.
(A) Generalized scheme for photo-affinity labeling and detection using a diazirine and alkyne-containing photo-affinity probe (I). UV irradiation of the diazirine generates a carbene intermediate (II) that covalently crosslinks to the protein target (III). The adduct is then detected by conjugation with an azide-containing reporter group under click chemistry conditions (IV). (B) Structures of the natural product HUN7293 (1), photo-affinity probe (2), and the photo-stable control compound (3).
Cu(I)-catalyzed click chemistry is an important method for bio-conjugation of probe-labeled proteins with reporter groups (Best, 2009). During the click reaction, Cu(I) catalyzes a highly selective, bio-orthogonal 1,3 dipolar cycloaddition reaction between a terminal alkyne group and an azide group to form a stable triazole product (Figure 1A, (III), (IV)). The terminal alkyne is typically present in the small-molecule probe, while the azide is present in a fluorescent or biotinylated reporter group. Alternatively, the azide can be incorporated into the probe and the alkyne incorporated into the reporter. However, this arrangement has been shown to produce higher background labeling of proteins (Speers and Cravatt, 2004). Following covalent labeling of protein targets via a (latent) chemically reactive moiety within the probe, probe-modified proteins are conjugated to the azide-bearing reporter under click chemistry conditions (Figure 1A, (IV)). The reporter group is thus introduced after covalent bond formation between the probe and target protein. This approach thereby avoids directly introducing a bulky reporter into the small-molecule probe, which could perturb the interaction between probe and target. The terminal alkyne (or azide) is extremely compact and therefore minimally perturbs the structure of the small-molecule, while providing the chemical functionality necessary for detection and affinity purification of targets. A variety of azide- and alkyne-reporters designed for use in bio-conjugate click reactions have been described (Speers and Cravatt, 2004) and many are commercially available (Invitrogen).
We recently utilized alkyl diazirine photo-activation and click chemistry methods to identify the molecular target of “cotransins”, a class of cyclic heptadepsipeptides derived from the fungal natural product HUN-7293 (1) (Figure 1B). Cotransins inhibit cotranslational translocation of nascent proteins across the endoplasmic reticulum (ER) membrane in a signal-sequence dependent manner (Garrison et al., 2005). Inhibition occurs at the level of insertion of the nascent protein into an ER membrane-embedded multiprotein complex, termed the translocon, which recognizes signal sequences and forms a pore through which substrate proteins traverse (Osborne et al., 2005). Utilizing an alkyl diazirine-based photo-affinity probe that bears an alkyne handle (2, Figure 1B), we identified an integral membrane protein subunit of the translocon complex, Sec61α, as the molecular target of cotransins (MacKinnon et al., 2007).
This Basic Protocol describes a method for photo-affinity labeling and detection of photo-crosslinked proteins in complex protein mixtures. The method requires a photo-affinity probe that bears both an alkyl diazirine photo-reactive group and a terminal alkyne group. The scope and limitations of the method as well as essential controls, parameters and variables are discussed. Key design strategies that lead to the synthesis of photo-affinity probe 2, as well as a summary of pros and cons of commonly used photo-reactive groups are also discussed. The Basic Protocol describes the method applied on an analytical scale, followed by a Support Protocol that describes scale-up of the reactions, post-click chemistry work-up, and affinity purification of labeled proteins using monomeric avidin agarose or antibodies directed against carboxy-tetramethylrhodamine (TAMRA). The affinity purification protocol is useful for purifying and identifying unknown protein targets of biologically active small-molecules.
Strategic planning
Design and synthesis of a photo-affinity probe can be one of the major challenges of applying PAL to small-molecule target identification. Structure-activity-relationships (SAR) of the parent molecule typically guide the choice and placement of the photo-reactive or reporter groups within the parent scaffold. For example, in designing photo-affinity probe 2 (Figure 1B), a detailed SAR study of the HUN-7293 scaffold (Chen et al., 2002) revealed that the N-methoxy tryptophan side chain at position 5 (Figure 1B) could be replaced with a smaller, phenylalanine side chain without significantly altering its biological activity. While this suggested that a phenyl azide at this position might also preserve biological activity, photo-activation of the phenyl azide requires a wavelength of light (~260 nm) that is damaging to protein structures. The SAR study also suggested that a larger aromatic photo-reactive group at this position, such as benzophenone, would significantly reduce biological activity. To preserve biological activity, we therefore sought a compact, hydrophobic photo-reactive group that could be placed into one of the many hydrophobic alkyl side chains of the molecule (Figure 1B). The diazirine represented a suitable choice. Being nearly isosteric with a methyl group, the diazirine was intended to replace a terminal methyl group of a leucine residue in HUN-7293. To accomplish this, we synthesized a diazirine-containing leucine analog, known as photo-leucine (Suchanek et al., 2005), starting from an alkyl ketone precursor, and used this precursor in the total synthesis of photo-affinity probe 2 (MacKinnon et al., 2007).
We also required a method a detect photo-crosslinked proteins. The SAR indicated a tolerance for smaller side chains at position 1. We therefore installed a propargyl group at this position to enable detection of photo-crosslinked proteins using click chemistry. In parallel, we also synthesized a photo-stable control compound (3, Figure 1B) that was used in control experiments for distinguishing background from specific photo-crosslinks to protein targets (discussed in Critical Parameters). 2 and 3 were found to maintain nanomolar potency in biological assays, indistinguishable from the natural product 1.
Ideally, SAR-guided design of photo-affinity probes should involve take advantage of pre-existing found in the parent molecule with photo-reactive groups having similar chemical properties. Several types of photo-reactive groups that differ in size, hydrophobicity and ease of chemical synthesis are commonly used (see Table 1). Due to intrinsic differences in chemical and photo-physical properties between these groups, it is difficult to predict a priori which one will be best suited for a specific PAL application. In some cases it may be possible to test different photo-reactive groups in the same position of a probe, or the same photo-reactive group at different positions within the probe. In all cases, it is important to evaluate the biological activity of photo-affinity compounds.
Table 1.
Comparison of commonly used photo-reactive groups
| Photo-reactive group | Benefits | Downsides |
|---|---|---|
| Benzophenone | Photo-activation at ~350 nm is reversible, leading to high crosslinking yields with proteins. Selective for insertion into C-H σ bonds over bulk solvent (Dormán and Prestwich, 1994). Chemically stable. |
Large size. Reported to selectively react with methionine residues in proteins leading to inaccurate determination of probe-binding sites (Wittelsberger et al., 2006). |
| Trifluoromethyl phenyl diazirine | Generates a highly reactive carbene intermediate upon photo- activation at ~350 nm. Photo- insertion of the carbene into proteins can proceed in high (>70%) yield (Brunner, 1993). |
Relatively large size. Insertion products may be reversible under some conditions (Platz et al., 1991). Can undergo UV light- induced rearrangement to electrophilic diazo isomer (Brunner, 1993) leading to non- specific labeling. Challenging to synthesize. |
| Alkyl diazirine | Small size. Generates highly reactive carbene intermediate upon photo-activation at ~350 nm. Good yield of insertion into protein targets (~25%, MacKinnon et al., 2007). Synthesized directly from the ketone precursor. |
May undergo UV light-induced rearrangement to electrophilic linear diazo isomer (Brunner, 1993) leading to non-specific labeling. Intramolecular rearrangement of the alkyl carbene intermediate may compete with intermolecular insertion into proteins (Ford et al., 1998). |
| Phenyl azide | The singlet nitrene intermediate formed on photo-activation is highly reactive. Photo-activation of nitro-substituted aryl azides occurs at ~340 nm and is therefore not damaging to protein. Perfluoro phenyl azides react primarily via the singlet nitrene intermediate (Brunner, 1993). Easily synthesized. |
Un-substituted phenyl azides require activation at short wavelengths (~260 nm) that are damaging to protein. In non- perfluorinated phenyl azides, the singlet nitrene intermediate is prone to ring-expansion to a long- lived electrophilic species (Brunner, 1993), resulting in non- specific labeling. Phenyl azide is chemically less stable than other photo-reactive groups. |
A brief comparison of benefits and downsides of commonly used photo-reactive groups is presented in Table 1. For more detailed descriptions of these photo-reactive groups and their use in PAL, see Sadakane and Hatanaka (2006), Dormán (2000), Dormán and Prestwich (1994), and Brunner (1993).
Another important consideration in planning a PAL experiment is obtaining a photo-stable competitor compound. The competitor, which is often simply the parent compound or other competitive antagonist, is used in a critical competition control experiment to distinguish background from specific photo-crosslinks to protein targets (discussed in Critical Parameters).
Basic Protocol
Diazirine photo-activation and Cu(I)-catalyzed click chemistry for covalent labeling and detection of protein targets
The Basic Protocol describes the use of diazirine- and alkyne-containing photo-affinity probes for detection of protein targets in complex protein mixtures. Following diazirine photo-activation to covalently modify macromolecular binding partners, Cu(I)-catalyzed click chemistry is used to install a fluorescent- or biotin-reporter group for visualizing probe-modified proteins. While the method is described using photo-affinity probe 2 (Figure 1B) in a crude preparation of endoplasmic reticulum (ER) microsomes, it should serve as a general experimental guide for other PAL experiments. Critical experimental variables and essential controls are discussed.
Materials
Endoplasmic reticulum (ER) microsomes (~1 mg/mL total protein) or other soluble or membrane protein lysate containing the unknown macromolecular target
Photo-affinity probe (2) and photo-stable competitor compound (3) in concentrated stock solutions in DMSO
Phosphate buffered saline (PBS, 137 mM NaCl, 10 mM Na2HPO4, 2.7 mM KCl, pH 7.4) 10% sodium dodecyl-sulfate (SDS) in H2O
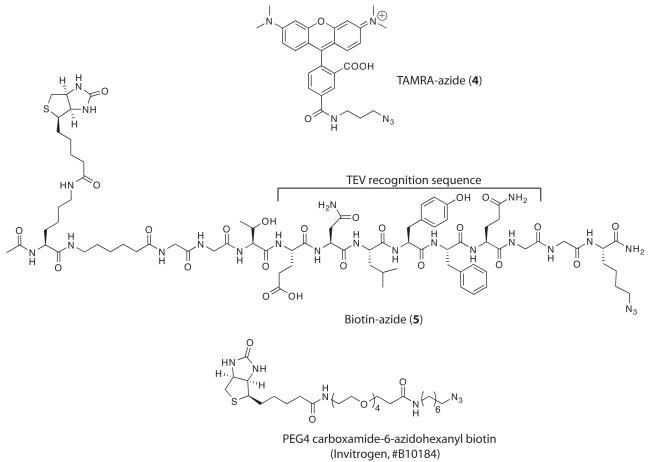
5 mM TAMRA-azide (4, Figure 2) or Biotin-azide (5, Figure 2), synthesized by published methods (Speers and Cravatt, 2004; Weerapana et al., 2007). Similar reagents are available commercially from Invitrogen.
Figure 2.
Structures of TAMRA-azide and Biotin-azide used in the protocol.
1.7 mM Tris(benzyltriazolylmethyl) amine (TBTA) in 80% tert-Butanol/20% DMSO (see recipe)
50 mM CuSO4 in H2O
50 mM tris-carboxyethyl phosphine (TCEP) in H2O adjusted to pH ~7 with 1M NaOH; prepare immediately before use
1M NaOH in H2O
6 × Laemmli sample buffer
Fluorescent molecular weight markers
1000W Hg(Xe) lamp (Oriel Instruments, model 66923) with bandpass filter for irradiation at ~355 nm (#59810, Oriel Instruments) and a filter to absorb heat (#59044, Oriel Instruments)
Typhoon 9400 phosphorimager (Amersham)
96-well plate or other open, shallow container
Additional reagents and equipment for SDS-PAGE and western blotting.
- In 0.5 mL polypropylene tubes, prepare 5 samples (labeled A-E), each containing 19 μL of ER microsomes at a total protein concentration of ~1 mg/mL in PBS.Other protein lysates (e.g. cytosolic proteins, subcellular fractions, crude plasma membrane fractions, or whole cell lysates) containing the unknown protein target of the small-molecule can also be tested. Prepare the lysate at a concentration of between 0.5-10 mg/mL total protein in a buffer compatible with the click chemistry (see Critical Parameters).Sample A is the experimental sample, B is the competition control, C is the negative PAL probe control, D is the negative UV irradiation control, and E is the negative click chemistry control.
- To sample B add 0.5 μL of the photo-stable competitor compound (3) to a final concentration of 20 μM from a 1.6 mM stock solution in DMSO. Add 0.5 μL of DMSO to samples A, C, D, and E and incubate all reactions for 15 minutes at 0 °C.Pre-incubation of sample B with a large excess of the photo-stable competitor (3) should pre-saturate relevant protein targets. This controls for non-specific photo-crosslinking during irradiation and is used to distinguish specifically photo-crosslinked proteins from non-specific background. Typically a 10-100-fold molar excess of competitor is used for this control. Optimal pre-incubation times and temperatures may vary depending on the kinetics of small-molecule binding to the protein target(s).
- To samples A, B, D, and E add 0.5 μL of the photo-affinity probe (2) to a final concentration of 500 nM from a 20 μM stock solution in DMSO. To sample C add 0.5 μL of DMSO. Incubate the reactions for an additional 15 minutes at 0 °C.The optimal concentration of the photo-affinity probe (2) and the optimal time and temperature of the incubation may depend on the particular system under study. The PAL probe is typically tested at between 0.1-10 μM. The concentration of DMSO in the final reaction should be kept as low as possible.
- Transfer each reaction to a well of a 96-well plate or other shallow container.A shallow dish serves to maximize the surface area of the liquid for good exposure during the irradiation step.
- Position the reactions ~6 cm from the source of a 1000W Hg(Xe) lamp equipped with a bandpass filter for irradiation at ~350 nm. Irradiate samples A, B, C, and E for 1 minute. Keep sample D protected from irradiation with aluminum foil.The samples can be irradiated simultaneously, provided they all lie within the boundaries of the incident light. Alternatively, samples can be irradiated sequentially.Irradiation of the diazirine releases N2(g) and generates a carbene intermediate that covalently crosslinks the photo-affinity probe to the protein target. The half-life of the diazirine (λmax ~355 nm), and thus the optimal irradiation time, depends on the wavelength of irradiation, the wattage of the UV light source, and the distance between the sample and the source (i.e. the power per unit area). A 1000W Hg(Xe) lamp provides an intense source of radiation and requires short (≤ 1 minute) irradiation times. A lower intensity long wavelength UV lamp that emits at ~365 nm is sufficient for diazirine photo-activation but usually requires longer irradiation times (~5-10 minutes for a 100W lamp).
Transfer 19.5 μL of samples A-E into new, labeled 0.5 mL polypropylene tubes for the click reaction.
- Add 2.5 μL of 10% SDS to each reaction and mix by vortexing.Addition of SDS denatures proteins and exposes the terminal alkyne to the click reagents.
- Add 0.5 μL of 5 mM TAMRA-azide (4) or 5 mM Biotin-azide (5) to each reaction.Other reporter-azide reagents are equally suitable and are commercially available (Invitrogen).Biotin-azide (5) has a TEV protease recognition sequence positioned between the biotin and azide groups (Figure 2). This feature can be useful for proteolytically cleaving biotinylated proteins or peptides from a streptavidin affinity matrix using TEV protease (Weerapana et al., 2007).
- Add 2.5 μL of catalyst mix (see below) to samples A, B, C, and D and mix by vortexing. Add 2.5 μL of mock catalyst mix (see below) to sample E and mix by vortexing.A master mix of the catalyst is prepared immediately before use by mixing 1.5 volumes 1.7 mM TBTA in 80% tert-butanol/20% DMSO, 0.5 volumes 50 mM CuSO4, and 0.5 volumes 50 mM TCEP followed by vortexing (2.5 μL of the catalyst mix for each 25 μL reaction). The catalyst master mix should have a faint blue color and be heterogeneous. Mix again before pipetting the catalyst into each separate reaction. For the mock catalyst, substitute de-ionized water for the CuSO4. In the absence of CuSO4, the click reaction should not proceed. Sample E therefore serves as a negative click chemistry control.
- Incubate the reactions for 30 minutes at 32 °C.Incubation for 1 hour at room temperature is also sufficient for labeling in the click reaction.Following the incubation, reactions can be diluted ~10-fold to reduce the concentration of SDS and immunoprecipitated using specific antibodies directed against candidate target proteins.
- After the incubation, add 5 μL of 6 × Laemmli sample buffer and mix.It is not necessary to heat the samples after addition of sample buffer. We have found that some proteins, including hydrophobic membrane proteins, irreversibly aggregate upon heating in the presence of the click reagents.
- Resolve 12.5 μL of samples A-E and 5-10 μL of fluorescent or broad molecular weight markers by SDS-PAGE. Run the gel until the dye front has completely exited the gel.Running the dye front (which also contains the click chemistry reagents) off the gel ensures less carryover of free TAMRA-azide (4) into the imaging step (step 14).Snap-freeze the remaining samples in liquid N2 and store at −80 °C. Storage of samples at −20 °C leads to an increase in non-specific background labeling due to the click chemistry reagents. The stored samples are stable for at least 1 week.
- Wash the gel 3 × 10 minutes with de-ionized H2O.The gel is thoroughly washed with several changes of de-ionized water to help remove residual traces of free TAMRA-azide from the gel.
If the click reaction was performed with TAMRA-azide (4), scan the gel using a Typhoon fluorescent gel scanner (Amersham, excitation wavelength 532 nm, emission wavelength 580 nm). If the click reaction was performed with Biotin-azide (5) or other biotin-azide, transfer proteins to nitrocellulose or PVDF membrane with a Western Blot transfer apparatus and probe for biotinylated proteins using streptavidin-HRP.
Support Protocol
Affinity purification of probe-modified proteins
In this Support Protocol, probe-modified proteins labeled with TAMRA-azide (4) or Biotin-azide (5) under click chemistry conditions are affinity purified. This is accomplished using monomeric avidin agarose (Pierce) for biotin-labeled proteins, or antibodies directed against TAMRA (Invitrogen) for TAMRA-labeled proteins. The use of monomeric avidin agarose or anti-TAMRA antibodies enables mild elution of labeled proteins. The protocol can be followed after optimizing the photo-labeling and click reaction steps described in the Basic Protocol. The Support Protocol is useful for ultimately identifying the protein target(s) using techniques such as Edman sequencing or mass spectrometry.
Additional Materials
Acetone cooled to −20 °C
1% SDS in PBS
Affinity purification buffer (50 mM Hepes, pH 7.4; 100 mM NaCl; 1% NP-40)
Monomeric avidin agarose beads (Pierce)
Anti-TAMRA antibody (Invitrogen, product number A6397)
Protein-A sepharose beads (GE Healthcare)
Wash buffer (50mM Hepes, pH 7.4; 500 mM NaCl; 1% NP-40)
Elution buffer (2 mM d-biotin; 1% NP-40; PBS)
Streptavidin-conjugated horseradish peroxidase (strep-HRP)
Polyallomer 1.5 mL microcentrifuge tubes (Beckman-Coulter)
Benchtop ultracentrifuge
Sonicating water bath
Refrigerated microcentrifuge
Rotating tube mixer
1. Complete steps 1-5 of the Basic Protocol on a 25-50 × scale, (e.g. 0.7 mL of 1 mg/mL total protein).
The complete set of control experiments described in the Basic Protocol are not necessary here if they were previously performed in analytical scale experiments.
During scale-up, a larger vessel, such as a well of a 12- or 24-well tissue culture dish can be used during the irradiation step. For proper irradiation, it is important that the entire sample lies within the bounds of the incident light (a circle of ~6 cm in diameter using the lamp setup described here). A 1 minute irradiation of a 0.7 mL of sample in a well of a 24 well dish, as used here, is sufficient for complete photo-activation of the diazirine. For sample volumes significantly larger than 0.7 mL, longer irradiation times, coincident sample mixing, or irradiation in batches may be required. A small aliquot of the mixture can be removed and analyzed by SDS-PAGE following click chemistry to determine the extent of crosslinking.
2. Following irradiation, transfer the mixture containing the ER microsomes to a 1.5 mL polyallomer microcentrifuge tube and sediment the microsomes in a benchtop ultracentrifuge at 50,000 × g for 10 minutes.
Sedimentation concentrates the microsomes and allows the click reaction to be conducted on a smaller volume. For other types of protein mixtures that cannot be concentrated by sedimentation, other protein precipitation methods can be tested in pilot experiments, or the mixture can be directly subjected to the click chemistry.
3. Aspirate the supernatant and re-suspend the microsomes in 97.5 μL of PBS.
4. Follow steps 7-10 of the Basic Protocol, scaling up the click reagent volumes according to the volume of the re-suspended microsome pellet. For 97.5 μL of re-suspended microsomes, add 12.5 μL 10% SDS, 2.5 μL TAMRA-azide (4) or Biotin-azide (5), and 12.5 μL of the catalyst mix (see steps 9 and 10 in the Basic Protocol).
5. Following the click reaction, remove a 5 μL aliquot, add Laemmli sample buffer, snap-freeze in liquid nitrogen and store the sample at −80 °C. The saved sample should be stable for at least one week at −80 °C.
This saved aliquot represents the “input” into the affinity purification. At this stage, detection of specific photo-crosslinked proteins can be determined by SDS-PAGE (step 14 of Basic Protocol).
An aliquot of the sample can also be diluted ~10-fold to reduce the concentration of SDS, and immunoprecipitated using specific antibodies directed against candidate target proteins.
6. To the remaining sample (120 μL), add 0.5 mL of acetone, cooled to −20 °C, to ~80% (v/v). Vortex briefly and place at −20 °C for 0.5 hours.
A white precipitate should form which contains precipitated proteins. Cold acetone precipitation removes the large molar excess of free TAMRA-azide (4) or Biotin-azide (5) that would interfere with the binding to the TAMRA-antibody or monomeric avidin beads, respectively. The azide label is soluble in acetone while the proteins are not, permitting their separation.
Following addition of cold acetone, the sample can also be stored overnight at −20 °C
7. Sediment the precipitated protein in a microcentrifuge at 20,000 × g for 10 minutes at 4 °C.
A white pellet containing precipitated protein should be observed in the bottom of the tube.
8. Aspirate the supernatant and add 0.5 mL of cold acetone.
10. Use a sonicating water-bath to breakup and disperse the precipitated protein pellet in the acetone.
Avoid heating the sample during the sonication by taking rests.
11. Return the sample to −20 °C for 10 minutes.
Longer incubation times at −20 °C may improve the recovery of precipitated protein.
12. Repeat steps 7-11 two more times.
It is essential to remove all traces of free TAMRA-azide (4) or Biotin-azide (5) for maximum yield of affinity purified proteins.
13. Aspirate the supernatant and air-dry the pellet briefly for ~10 minutes at room temperature.
Air drying removes residual acetone from the protein pellet. Do not over-dry the pellet as it will become difficult to re-solubilize.
14. Add 50 μL of 1% SDS in PBS to the pellet and gently dislodge the pellet from the side of the tube by vortexing and/or sonication.
Do not use a pipette tip to dislodge the pellet as the pellet may stick to the tip.
The SDS aids in re-solubilizing the protein pellet.
15. Once the pellet has completely dissolved, dilute the sample with 0.5 mL of affinity purification buffer.
The SDS must be diluted to ≤ 0.1% for efficient purification in steps 16-19. The NP-40 detergent helps “mask” the SDS in mixed micelles.
15. Remove a 20 μL aliquot of the mixture, add Laemmli sample buffer, snap-freeze in liquid nitrogen and store at −80 °C. The sample should be stable for at least one week at −80 °C.
This sample can be used to evaluate the efficiency of the precipitation and re-solubilization (steps 6-14) by comparison to an aliquot of the “input” click reaction (saved in step 5).
16. Follow steps 16a-17a and 24a for TAMRA-labeled proteins, follow steps 16b-17b and 24b for biotin-labeled proteins.
16a. Equilibrate 30 μL of Protein A sepharose in affinity purification buffer and prepare a 50% slurry in the same buffer.
16b. Prepare the monomeric avidin beads (Pierce) according to the manufacturer’s direction, equilibrate with affinity purification buffer, and prepare a 50% slurry in affinity purification buffer.
17a. Add 30 μL of the protein A sepharose slurry and 5 μL of anti-TAMRA antibody to the re-solubilized protein pellet.
17b. Add 50 μL of the monomeric avidin agarose slurry to the re-solubilized protein pellet.
18. Incubate samples on a rotating tube mixer for 3 hours at 4 °C.
Samples can also be incubated overnight at 4 °C.
19. Sediment the agarose beads at 10,000 × g for 1 minute in a microcentrifuge and remove the supernatant.
20. Save a 20 μL aliquot of the supernatant, add Laemmli sample buffer, snap-freeze in liquid nitrogen and store at −80 °C. The saved sample should be stable for at least one week at −80 °C.
The saved aliquot of the supernatant can be used to evaluate the efficiency of the affinity purification step by comparison to an equal aliquot of the re-solubilized pellet (saved in step 15).
21. Add 1 mL of affinity purification buffer to the sedimented agarose beads and rotate on a tube mixer for 10 minutes at 4 °C.
Longer mixing times may be more effective at removing non-specifically bound proteins from the agarose resin.
22. Repeat steps 19-21 (the supernatants from the washes can be discarded).
23. Repeat step 22 twice but replace the affinity purification buffer with wash buffer.
24a. After the final wash, elute bound proteins with 50 μL of 1 × Laemmli sample buffer for 20 minutes at room temperature.
24b. After the final wash, elute bound proteins with 50 μL of elution buffer for 20 minutes at room temperature, or as described by the manufacturer.
The NP-40 detergent in the elution buffer is included to help maintain proteins in solution after elution. The detergent may not be required when eluting soluble proteins.
25. Resolve equivalent amounts of the “input” sample (saved in step 5, 4.5 μL), the re-solubilized protein pellet (saved in step 15, 20 μL), the post-affinity purified supernatant (saved in step 20, 20 μL), and eluent (saved in step 24, 1.8 μL) by SDS-PAGE. Scan the gel for fluorescence (step 14 of Basic Protocol) or transfer proteins to a nitrocellulose or PVDF membrane and probe with Streptavidin-HRP.
Comparison of the signal intensity of the “input” and re-solubilized pellet samples indicates the efficiency of the acetone precipitation and re-solubilization steps (steps 6-15). Comparison of the signal intensity of the re-solubilized pellet with post-affinity purified supernatant indicates the efficiency of the pull down (steps 16-19). Comparison of the signal intensity of the re-solubilized pellet with the eluent indicates the recovery of labeled proteins (steps 21-24).
Reagents and Solutions
1.7 mM Tris(benzyltriazolylmethyl) amine (TBTA) in 80% tert-Butanol/20% DMSO
Solid TBTA is commercially available or can be synthesized by published methods (Chan et al., 2004). The working stock is prepared by mixing one volume 8.5 mM TBTA stock in DMSO with four volumes tert-Butanol. This solution is stable for months when stored at −20 °C.
Commentary
Background Information
Identifying the target of a biologically active small-molecule is a major step toward understanding its underlying mechanism of action. A traditional biochemical method for small-molecule target identification employs affinity chromatography of the target followed by identification by mass spectrometry or Edman degradation (Ding et al., 2003; Taunton et al., 1996; Harding et al., 1989). In this method, a complex protein mixture is passed over a resin matrix that has been covalently modified with the small-molecule of interest. The affinity matrix is stringently washed and specifically-bound proteins are eluted, resolved by SDS-PAGE and identified. The success of this approach requires that the target and small-molecule have a sufficiently strong binding affinity (typically in the nM range) to survive the extensive washing steps required to reduce non-specific binding of proteins to the affinity matrix. In a recent variation of this technique, less stringent washing conditions coupled with highly sensitive quantitative mass spectrometry were used to identify specific protein targets of inhibitors with μM affinity (Ong et al., 2009). The approach works best with soluble protein targets since integral membrane proteins require detergent-solubilization prior to chromatography, which often prevents binding to the immobilized small-molecule.
PAL has several features that distinguish it from the traditional affinity chromatography approach. First, since photo-activation is performed under native conditions, PAL provides the opportunity for detection and identification of integral membrane protein targets (Colca et al., 2004; Saghatelian et al., 2004), an important class of proteins targeted by a large number of small-molecule drugs. PAL can also be used to characterize and map the ligand binding sites of known integral membrane proteins or other targets that lack high resolution structural information (Al-Mawsawi et al., 2006; Xi et al., 2006). Furthermore, since PAL establishes a stable, covalent bond between the small-molecule probe and the target, the targets of even moderately potent small-molecules can, in principle, be identified.
Several different click reaction conditions have been described in the literature. In the version described here, Cu(II)SO4 serves as the precursor to the Cu(I) species that catalyzes triazole formation between the terminal alkyne and azide. Tris-carboxyethyl phosphine (TCEP) presumably reduces Cu(II) to Cu(I) in situ during preparation of the catalyst mix (Basic Protocol step 9). Tris(benzyltriazolylmethyl) amine (TBTA) is a polytriazole ligand that stabilizes the Cu(I) ion and enhances its catalytic activity in solution (Chan et al. 2004). Highly pure Cu(I)Br (99.999%) (Dieterich et al., 2007) or Cu(I) triflate (Strable et al., 2008) have also been used to effect the click reaction. However, we prefer in situ generation of Cu(I) since Cu(I)Br is sparingly soluble and aqueous Cu(I) solutions are prone to oxidation by dissolved oxygen.
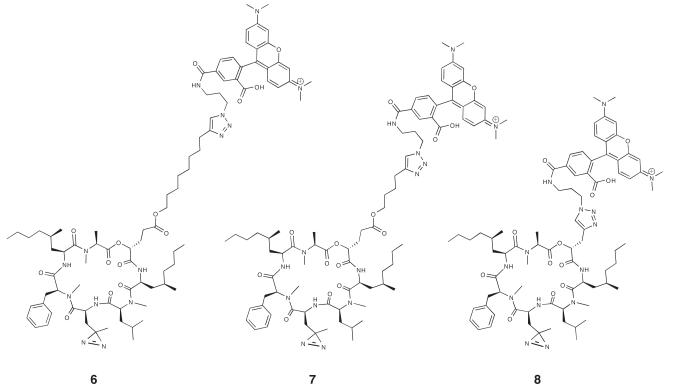
Tagging of probe-modified proteins with reporter groups by click chemistry requires the presence of only a small alkyne or azide group in the probe. This avoids introducing a bulky reporter directly into the small-molecule scaffold. While there are examples of successful target identification using chemically reactive probes that have been directly modified with a biotin reporter (Kwok et al., 2001; Sin et al., 1997), such bulky groups can perturb the interaction between the probe and protein targets. This point is exemplified in our own study of compounds 6, 7, and 8 (Figure 3), where the rhodamine reporter is conjugated directly to the natural product scaffold via a triazole linkage and variable length alkyl spacer arm. Photo-crosslinking to the target, Sec61α, in ER microsomes was only ~5-10% as efficient using 6 compared to photo-crosslinking followed by click chemistry using 2. Compound 7 crosslinked even less efficiently than 6, and specific photo-crosslinks to Sec61α were undetectable using compound 8 (data not shown). The reduced photo-crosslinking yield presumably reflects a reduction in binding affinity after conjugating the molecule with a bulky rhodamine reporter. Introduction of the alkyne group preserved the nanomolar potency of the compound, while providing the chemical functionality needed to detect photo-crosslinked proteins in a second, click chemistry step.
Figure 3.
Structures of compounds 6, 7, and 8 which have a fluorescent reporter group (TAMRA) directly incorporated into the natural product scaffold.
Introduction of a radiolabel into the small-molecule probe is a widely used approach to detect probe-modified proteins. While radiolabels are small in size, extremely sensitive, and offer a high ratio of signal to noise, radio-labeled probes can be costly to synthesize and radioactive materials require special handling and dedicated equipment. Click chemistry provides a non-radioactive alternative which is also highly sensitive, and has the added advantage of coincidently installing a chemical handle (biotin or TAMRA) that can be used to affinity purify and identify probe-modified proteins. In some cases, this method can afford the precise site of probe modification at the amino acid level (Weerapana et al., 2007; Speers and Cravatt, 2005; Adam et al., 2004).
Affinity purification of probe-modified targets is an essential step in target identification. Purification of biotinylated molecules with matrix-immobilized tetrameric streptavidin is a widely used technique that takes advantage of the extremely tight interaction between biotin and streptavidin (Kd ~10−15 M). While this tight interaction permits stringent washing conditions resulting in low background, elution of specifically bound material requires harsh conditions, typically boiling in SDS-PAGE sample buffer. Such conditions may not be suitable for some proteins and SDS present in sample buffer is not compatible with many downstream applications including LC/MS. Several novel cleavable biotin reagents have been described (Verlhelst et al., 2007; Weerapana et al., 2007) and others are commercially available (Pierce). These reagents allow elution of streptavidin-bound material chemically or enzymatically without disrupting the strong biotin-streptavidin interaction. However, many of these cleavable reagents still suffer from low elution efficiencies. For example, Biotin-azide (5) used in the present protocol, has a TEV protease recognition sequence positioned between the biotin and azide groups (Figure 2). This feature is designed to permit elution of biotinylated proteins by incubation with TEV protease. However, in our case, we were unable to efficiently elute bound Sec61α by incubation with TEV protease, possibly due to steric occlusion of the protease recognition sequence.
To circumvent problems associated with tetrameric streptavidin and cleavable biotin-azide reagents, the Support Protocol utilizes monomeric avidin (Pierce) for affinity purification of biotinylated targets (Figure 4B). Monomeric avidin has a lower affinity for biotin (Kd ~10−8 M), permitting elution of bound material with 2 mM biotin in PBS, a condition more suitable for diverse downstream applications. We also present a mild capture and elution method for TAMRA-labeled proteins utilizing anti-TAMRA antibodies (Invitrogen) and protein A sepharose. Affinity purification with anti-TAMRA antibodies has the advantage that, following affinity purification, purified protein targets can be proteolytically digested and probe-labeled peptide fragments visualized by fluorescence detection (Okerberg et al., 2005; Adam et al., 2004). Affinity purification with monomeric avidin or anti-TAMRA antibodies both provide ~25% yield of labeled proteins.
Figure 4.
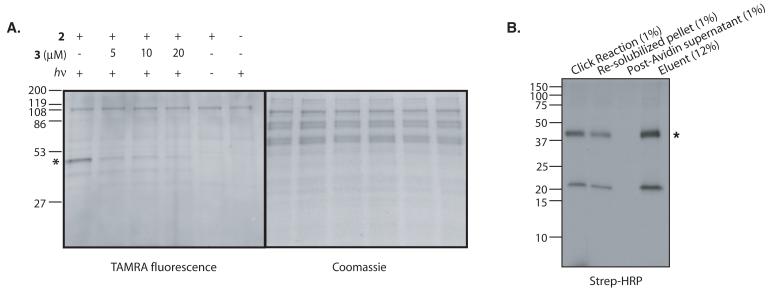
(A) Photo-crosslinking in ER microsomes with 2 followed by click chemistry with TAMRA-azide (4) as described in the Basic Protocol. Sec61α is marked with an asterisk (figure adapted with permission). (B) Photo-crosslinking in ER microsomes with 2 followed by click chemistry with Biotin-azide (5) and affinity purification using monomeric avidin as described in the Support Protocol. Samples representing the click reaction (Lane 1), the re-solubilized protein pellet following acetone precipitation (Lane 2), the post-monomeric avidin supernatant (Lane 3), and the eluent (Lane 4), were resolved by SDS-PAGE, transferred to nitrocellulose, and probed for biotinylated proteins with streptavidin-HRP (Strep-HRP). Percentages indicate the fraction of the total sample that was loaded in each lane. The position of Sec61α is marked with an asterisk. The biotinylated protein at ~21 kDa is a background band.
Critical Parameters
Non-specific photo-crosslinking to highly abundant or “sticky” proteins in a complex protein mixture can be problematic in PAL. High background can obscure detection of specific crosslinks to less abundant proteins. Detection of specific crosslinks therefore depends on the relative abundance of the protein target (i.e. the amount of target protein per total protein). Subcellular or biochemical fractionation that enriches the sample for a putative target can be employed to improve the ratio of specific signal to background noise. For example, while we could not detect specific photo-crosslinks to Sec61α in crude mammalian cell extract (data not shown), robust crosslinking was observed in purified ER microsomes (Figure 4). Even in the presence of high background, valuable information on specific photo-crosslinks to targets can be determined by immunoprecipitation against candidate proteins (Kukar et al., 2008). Extremely low abundant targets may be difficult or impossible to detect even in fractionated lysates. In such cases, it may be possible to detect these targets following an affinity purification step after the click reaction. Ultimately, detection of targets in a crude mixture of proteins depends on a favorable confluence of variables, including the relative abundance of the target, the photo-crosslinking specificity and yield, and the click chemistry yield (discussed below).
Several controls are essential for distinguishing background from specific photo-crosslinking. First, it is important to demonstrate that labeling of a putative target depends on the presence of both the photo-affinity probe and on UV irradiation. When labeling is observed in the absence of the probe or UV light, it may indicate background labeling due to the click reaction (discussed below). Secondly, it is important to conduct a competition experiment to control for the specificity of photo-crosslinking. This is done by incubating the protein sample with a large excess of a photo-stable competitor compound prior to UV irradiation. Crosslinks to specifically labeled proteins are dose-dependently competed by the photo-stable compound whereas background crosslinks are weakly competed or not competed at all (Figure 4A).
Background labeling that is independent of the photo-affinity probe or UV irradiation is due to non-specific labeling during the click reaction. The level of background appears to be strongly dependent on the total protein concentration with lower concentrations of total protein yielding less background. The optimal concentration of total protein that provides the best ratio of specific signal to background noise should be determined empirically. Reducing the concentration of the TAMRA-azide (4) or Biotin-azide (5) (we’ve gone as low as ~25 μM) in the reaction can also help reduce non-specific background labeling. We have found that background increases when click reactions are stored at −20 °C, even after addition of Laemmli sample buffer. Quick-freezing samples in liquid N2 and storing at −80 °C prevents this. A low concentration of SDS (0.1-1%) in the click reaction also reduces non-specific background labeling.
TAMRA-azide (4) or other commercially available fluorescent-azides (Invitrogen) used during the click reaction can trail though the gel lanes when reactions are resolved by SDS-PAGE. Trailing fluorophore contributes to background fluorescence in the gel and reduces the sensitivity of in-gel fluorescent scanning. To mitigate this problem, the dye front containing the fluorophore should be run completely off the bottom of the gel during electrophoresis. Removal of excess free TAMRA-azide by gel filtration, dialysis, or protein precipitation (e.g. acetone precipitation as used in this protocol) prior to SDS-PAGE can also significantly reduce the background due to trailing fluorophore. Gels should be thoroughly washed with several changes of de-ionized water prior to in-gel fluorescent scanning to remove traces of residual fluorophore.
The click reaction tolerates a fairly broad range of salt, buffer, and detergent concentrations, as well as a broad range of pH and temperatures. Metal chelators such as EDTA or EGTA should be avoided during preparation of the protein lysate as these sequester the Cu(II) ions required for the reaction. We have also found (unpublished data) that β-mercaptoethanol and dithiothreitol (DTT) inhibit the reaction at fairly low concentrations (~100 μM). Labeling of some probe-modified proteins requires or is improved by the presence of a low concentration of SDS (0.1-1%) or other detergent including deoxy-BigChaps, TX-100, NP-40, or sodium cholate.
Trouble shooting
A trouble shooting guide is presented in Table 2.
Table 2.
Troubleshooting guide.
| Problem | Cause | Solution |
|---|---|---|
| No signal observed on gel/blot following the click reaction |
Incorrect wavelength for diazirine photoactivation; insufficient time for irradiation |
Check the wavelength settings on the lamp; perform an irradiation time-course |
| Concentration of the photo- affinity probe is too low |
Titrate the photo-affinity probe into a fixed amount of protein lysate and conduct PAL and click reactions |
|
| The photo-affinity probe does not bind the target of the parent molecule |
Confirm that the photo-affinity probe is biologically active |
|
| The target’s relative abundance is too low |
Enrich the sample for the target by biochemical or subcellular fractionation; affinity purify following the click reaction |
|
| The click reaction failed | Prepare new reagent stocks; use freshly prepared TCEP solution |
|
| High background observed on gel/blot following the click reaction |
Non-specific photo-crosslinking | Reduce the concentration of the photo-affinity probe or the concentration of total protein; enrich the sample for putative targets prior to PAL |
| Non-specific background due to click reaction |
Reduce the total protein concentration; reduce the concentration of the azide used during the click reaction; store reactions at −80 °C |
|
| Non-specific and specific bands overlap on SDS-PAGE |
Change SDS-PAGE conditions, for example test different acrylamide concentrations, different buffer systems (e.g. Tris-Tricine), or 2-dimensional gel electrophoresis |
|
| Labeled proteins are not depleted during affinity purification |
Contaminating free TAMRA- azide (4) or Biotin-azide (5) |
Repeat the acetone precipitation steps to remove contaminating TAMRA-azide or Biotin-azide and repeat the affinity purification |
Anticipated Results
Figure 4A shows a gel resulting from following the Basic Protocol in crude ER microsomes using photo-affinity probe 2 and click chemistry with TAMRA-azide (4) (adapted from MacKinnon et al., 2007). Three proteins were labeled in the presence of the PAL probe (Lane 1), including one major band at ~45 kDa (marked with an asterisk). Labeling of the major band was dependent on UV light (Lane 5), the PAL probe 2 (Lane 6), and was dose-dependently competed by pre-incubation with the photo-stable competitor 3 (Lanes 2-4), indicating specific photo-crosslinking to this protein. Labeling of two other proteins at ~60 kDa and ~40 kDa also depended on the PAL probe and UV light, but was not competed by excess 3, indicating non-specific (i.e. non-saturable) photo-crosslinking to these proteins. Coomassie staining indicated equal loading of protein across all samples. The ~45 kDa protein, previously identified as Sec61α (MacKinnon et al., 2007), is present at about 1% of total ER proteins and represented the major labeled protein. However, Sec61α did not represent a major Coomassie-stainable band, indicating the specificity of the reaction and the ability to detect a protein target of moderate abundance in a complex mixture of ER proteins. Labeling that was independent of 2 and UV light was background due to the click reaction (Lanes 5 and 6).
Figure 4B shows affinity purification of biotinylated proteins using monomeric avidin following photo-crosslinking with 2 and click chemistry with Biotin-azide (5), as described in the Support Protocol. The position of Sec61α is marked with an asterisk. Comparison of equivalent aliquots of the starting click reaction (1% of total reaction, Lane 1) with the re-solubilized protein pellet (1% of total sample, Lane 2), showed ~50% protein recovery following the acetone precipitation protocol. Comparison of equivalent aliquots of the re-solubilized protein pellet (Lane 2) with the post-monomeric avidin supernatant (1% of total sample, Lane 3), showed quantitative depletion of biotinylated proteins from the sample using monomeric avidin. Recovery of biotinylated proteins by mild elution with 2 mM biotin proceeded in ~25% yield, as determined by comparison of Lanes 2 and 4.
Time Consideration
A significant investment of time and resources is required for the design and synthesis of a photo-affinity probe that retains potent biological activity. Once a suitable probe is in hand, it can be rapidly tested in the photo-crosslinking and click reactions in < 4 hours when using TAMRA-azide (4) in the click reaction. The time required for optimization of the photo-crosslinking and click chemistry will vary but may be completed in < 1 week. Affinity purification and analysis of samples takes 1-2 days.
Acknowledgments
This work was supported by the NIH (GM81644) and the Howard Hughes Medical Institute.
Literature Cited
- Adam GC, Burbaum J, Kozarich JW, Patricelli MP, Cravatt BF. Mapping enzyme active sites in complex proteomes. J. Am. Chem. Soc. 2004;126:1363–1368. doi: 10.1021/ja038441g. [DOI] [PubMed] [Google Scholar]
- Al-Mawsawi LQ, Fikkert V, Dayam R, Witvrouw M, Burke TR, Jr., Borchers CH, Neamati N. Discovery of a small-molecule HIV-1 integrase inhibitor-binding site. Proc. Natl. Acad. Sci. USA. 2006;103:10080–10085. doi: 10.1073/pnas.0511254103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best MD. Click chemistry and bioorthogonal reactions: unprecedented selectivity in the labeling of biological molecules. Biochemistry. 2009;48:6571–6584. doi: 10.1021/bi9007726. [DOI] [PubMed] [Google Scholar]
- Bond MR, Zhang H, Vu PD, Kohler JJ. Photocrosslinking of glycoconjugates using metabolically incorporated diazirine-containing sugars. Nat. Protoc. 2009;4:1044–1063. doi: 10.1038/nprot.2009.85. [DOI] [PubMed] [Google Scholar]
- Brunner J. New photolabeling and crosslinking methods. Annu. Rev. Biochem. 1993;62:483–514. doi: 10.1146/annurev.bi.62.070193.002411. [DOI] [PubMed] [Google Scholar]
- Chan TR, Hilgraf R, Sharpless KB, Fokin VV. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org. Lett. 2004;6:2853–2855. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bilban M, Foster CA, Boger DL. Solution-phase parallel synthesis of a pharmacophore library of HUN-7293 analogues: a general chemical mutagenesis approach to defining structure-function properties of naturally occurring cyclic (depsi)peptides. J. Am. Chem. Soc. 2002;124:5431–5440. doi: 10.1021/ja020166v. [DOI] [PubMed] [Google Scholar]
- Colca JR, McDonald WG, Waldon DJ, Leone JW, Lull JM, Bannow CA, Lund ET, Mathews WR. Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am. J. Physiol. Endocrinol. Metab. 2004;286:E252–E260. doi: 10.1152/ajpendo.00424.2003. [DOI] [PubMed] [Google Scholar]
- Dieterich DC, Lee JJ, Link AJ, Graumann J, Tirrell DA, Schuman EM. Labeling, detection and identification of newly synthesized proteomes with bioorthogonal non-canonical amino-acid tagging. Nat. Protoc. 2007;2:532–540. doi: 10.1038/nprot.2007.52. [DOI] [PubMed] [Google Scholar]
- Ding S, Wu TYH, Brinker A, Peters EC, Hur W, Gray NS, Schultz PG. Synthetic small molecules that control stem cell fate. Proc. Natl. Acad. Sci USA. 2004;100:856–861. doi: 10.1073/pnas.0732087100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormán G. Photoaffinity labeling in biological signal transduction. Top. Curr. Chem. 2000;211:169–225. [Google Scholar]
- Dormán G, Prestwich GD. Benzophenone photophores in biochemistry. Biochemistry. 1994;33:5661–5673. doi: 10.1021/bi00185a001. [DOI] [PubMed] [Google Scholar]
- Ford F, Yuzawa T, Platz MS, Matzinger S, Fulscher M. Rearrangement of dimethylcarbene to propene: study by laser flash photolysis and ab initio molecular orbital theory. J. Am. Chem. Soc. 1998;120:4430–4438. [Google Scholar]
- Harding MW, Galat A, Uehling DE, Schreiber SL. A receptor for the immuno-suppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989;341:758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- Kukar TL, Ladd TB, Bann MA, Fraering PC, Narlawar R, Maharvi GM, Healy B, Chapman R, Welzel AT, Price RW, Moore B, Rangachari V, Cusack B, Eriksen J, Jansen-West K, Verbeeck C, Yager D, Eckman C, Ye W, Sagi S, Cottrell BA, Torpey J, Rosenberry TL, Fauq A, Wolfe MS, Schmidt B, Walsh DM, Koo EH, Golde TE. Substrate-targeting γ-secretase modulators. Nature. 2008;453:925–930. doi: 10.1038/nature07055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok BHB, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverdew directly binds to and inhibits IκB kinase. Chem. Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- MacKinnon AL, Garrison JL, Hegde RS, Taunton J. Photo-leucine incorporation reveals the target of a cyclodepsipeptide inhibitor of cotranslational translocation. J. Am. Chem. Soc. 2007;129:14560–14561. doi: 10.1021/ja076250y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okerberg ES, Wu J, Zhang B, Samii B, Blackford K, Winn DT, Shreder KR, Burbaum JJ, Patricelli MP. High-resolution functional proteomics by active-site peptide profiling. Proc. Natl. Acad. Sci. USA. 2005;102:4996–5001. doi: 10.1073/pnas.0501205102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S, Schenone M, Margolin AA, Li X, Do K, Doud MK, Mani DR, Kuai L, Wang X, Wood JL, Tolliday NJ, Koehler AN, Marcaurelle LA, Golub TR, Gould RJ, Schreiber SL, Carr SA. Identifying the proteins to which small-molecule probes and drugs bind in cells. Proc. Natl. Acad. Sci. USA. 2009;106:4617–4622. doi: 10.1073/pnas.0900191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne AR, Rapoport TA, van den Berg B. Protein translocation by the Sec61/SecY channel. Annu. Rev. Cell Dev. Biol. 2005;21:529–550. doi: 10.1146/annurev.cellbio.21.012704.133214. [DOI] [PubMed] [Google Scholar]
- Platz M, Admasu AS, Kwiatkowski S, Crocker PJ, Imai N, Watt DS. Photolysis of 3-aryl-3-(trifluoromethyl) diazirines: a caveat regarding their use in photoaffinity probes. Bioconjug. Chem. 1991;2:337–341. doi: 10.1021/bc00011a008. [DOI] [PubMed] [Google Scholar]
- Sadakane Y, Hatanaka Y. Photochemical fishing approaches for identifying target proteins and elucidating the structure of a ligand-binding region using carbene-generating photoreactive probes. Anal. Sci. 2006;22:209–218. doi: 10.2116/analsci.22.209. [DOI] [PubMed] [Google Scholar]
- Saghatelian A, Jessani N, Joseph A, Humphrey M, Cravatt BF. Activity-based probes for the proteomic profiling of metaloproteases. Proc. Natl. Acad. Sci. USA. 2004;101:1000–1005. doi: 10.1073/pnas.0402784101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin N, Meng L, Wang MQW, Wen JJ, Bornmann WG, Crews CM. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc. Natl. Acad. Sci. USA. 1997;94:6099–6103. doi: 10.1073/pnas.94.12.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speers AE, Cravatt BF. Profiling enzyme activities in vivo using click chemistry methods. Chem. Biol. 2004;11:535–546. doi: 10.1016/j.chembiol.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Speers AE, Cravatt BF. A tandem orthogonal proteolysis strategy for high-content chemical proteomics. J. Am. Chem. Soc. 2005;127:10018–10019. doi: 10.1021/ja0532842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strable E, Prasuhn DE, Jr., Udit AK, Brown S, Link AJ, Ngo JT, Lander G, Quispe J, Potter CS, Carragher B, Tirrell DA, Finn MG. Unnatural amino acid incorporation into virus-like particles. Bioconjug. Chem. 2008;19:866–875. doi: 10.1021/bc700390r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchanek M, Radzikowska A, Thiele C. Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells. Nat. Methods. 2005;2:261–268. doi: 10.1038/nmeth752. [DOI] [PubMed] [Google Scholar]
- Taunton J, Hassig CA, Schreiber SL. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- Verhelst SHL, Fonovic M, Bogyo M. A mild chemically cleavable linker system for functional proteomic applications. Angew. Chem. Int. Ed. 2007;46:1284–1286. doi: 10.1002/anie.200603811. [DOI] [PubMed] [Google Scholar]
- Weerapana E, Speers AE, Cravatt BF. Tandem orthogonal proteolysis-activity-based protein profiling (TOP-ABPP)− a general method for mapping sites of probe modification in proteomes. Nat. Protoc. 2007;2:1414–1425. doi: 10.1038/nprot.2007.194. [DOI] [PubMed] [Google Scholar]
- Wittelsberger A, Thomas BE, Mierke DF, Rosenblatt M. Methionine acts as a “magnet” in photoaffinity crosslinking experiments. FEBS Lett. 2006;580:1872–1876. doi: 10.1016/j.febslet.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Xi J, Liu R, Rossi MJ, Yang J, Loll PJ, Dailey WP, Eckenhoff RG. Photoactive analogues of the haloether anesthetics provide high-resolution features from low-affinity interactions. ACS Chem. Biol. 2006;1:377–384. doi: 10.1021/cb600207d. [DOI] [PubMed] [Google Scholar]