Abstract
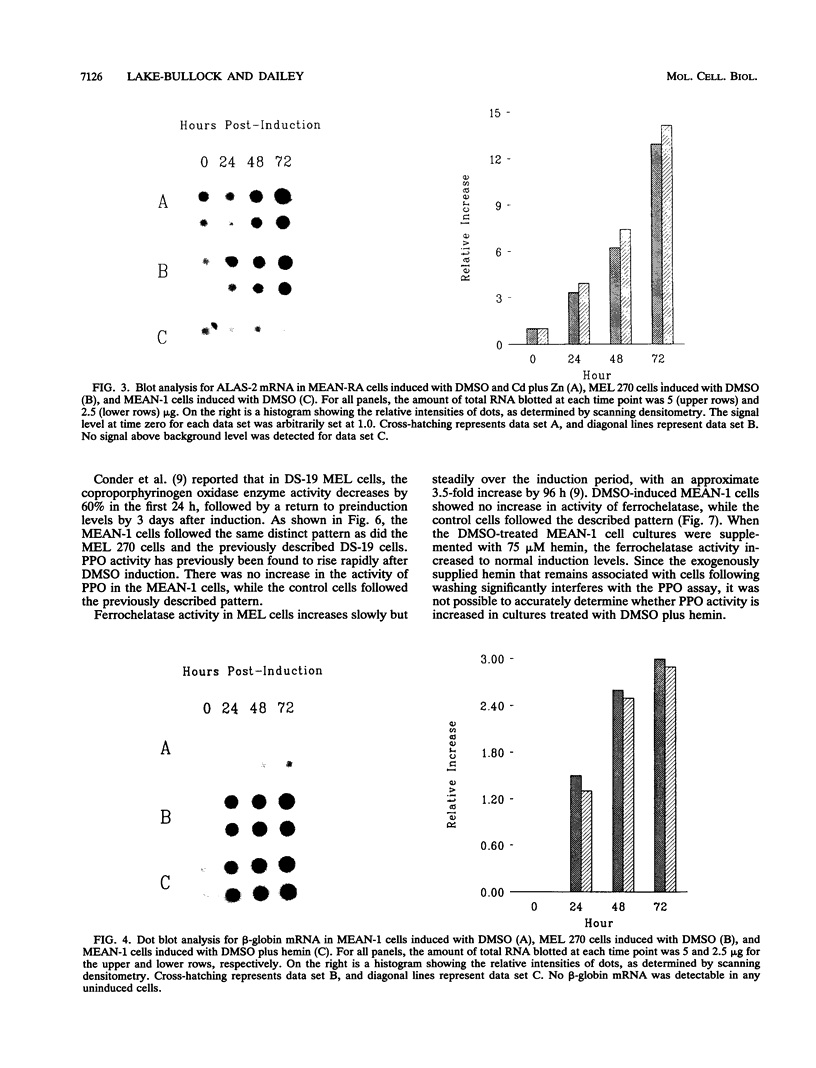
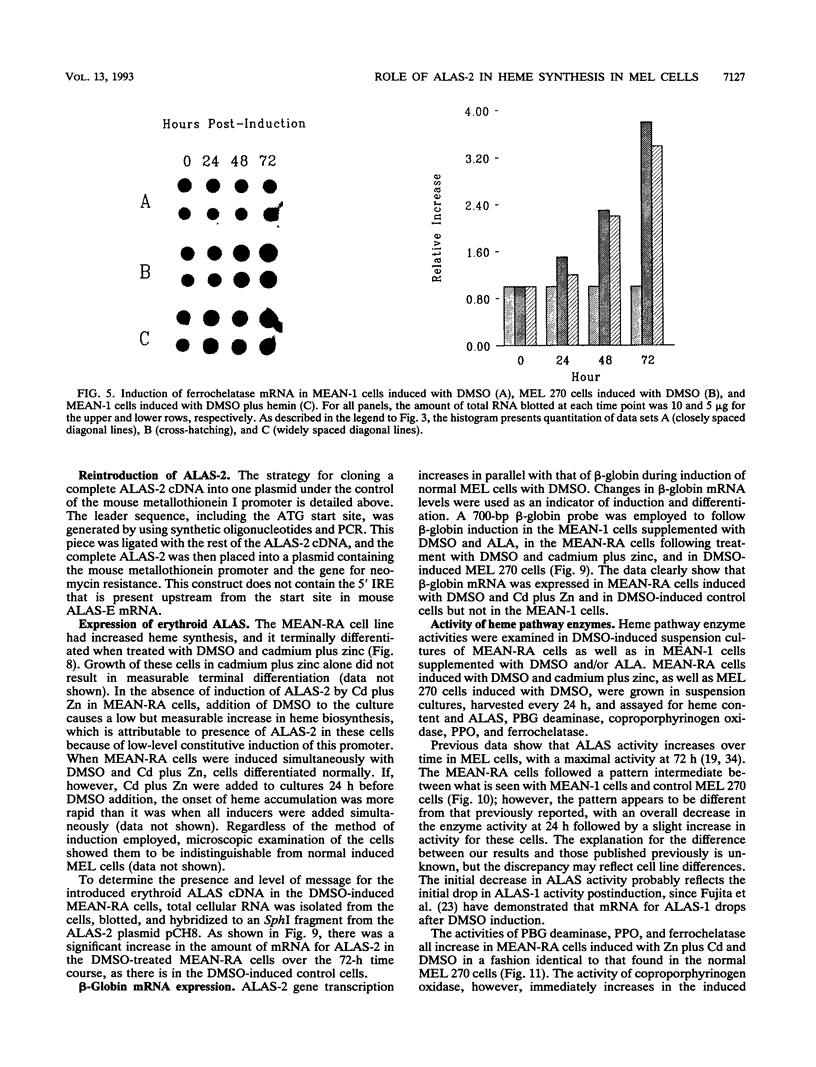
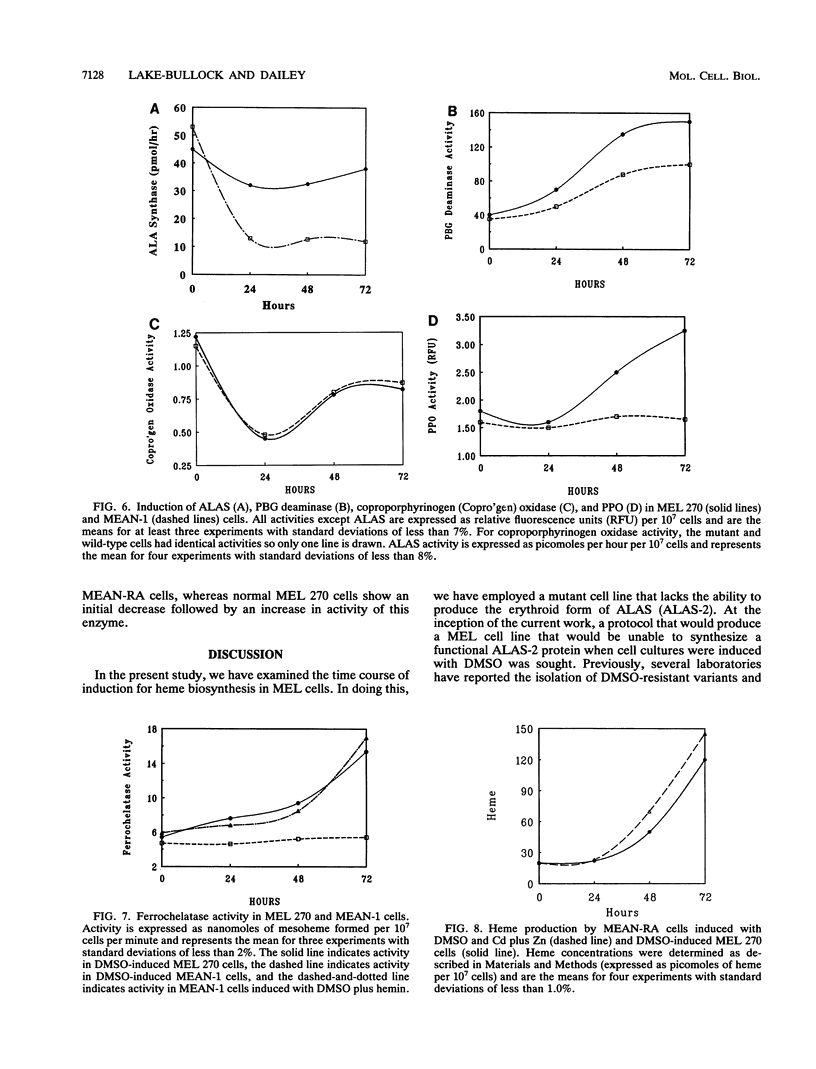
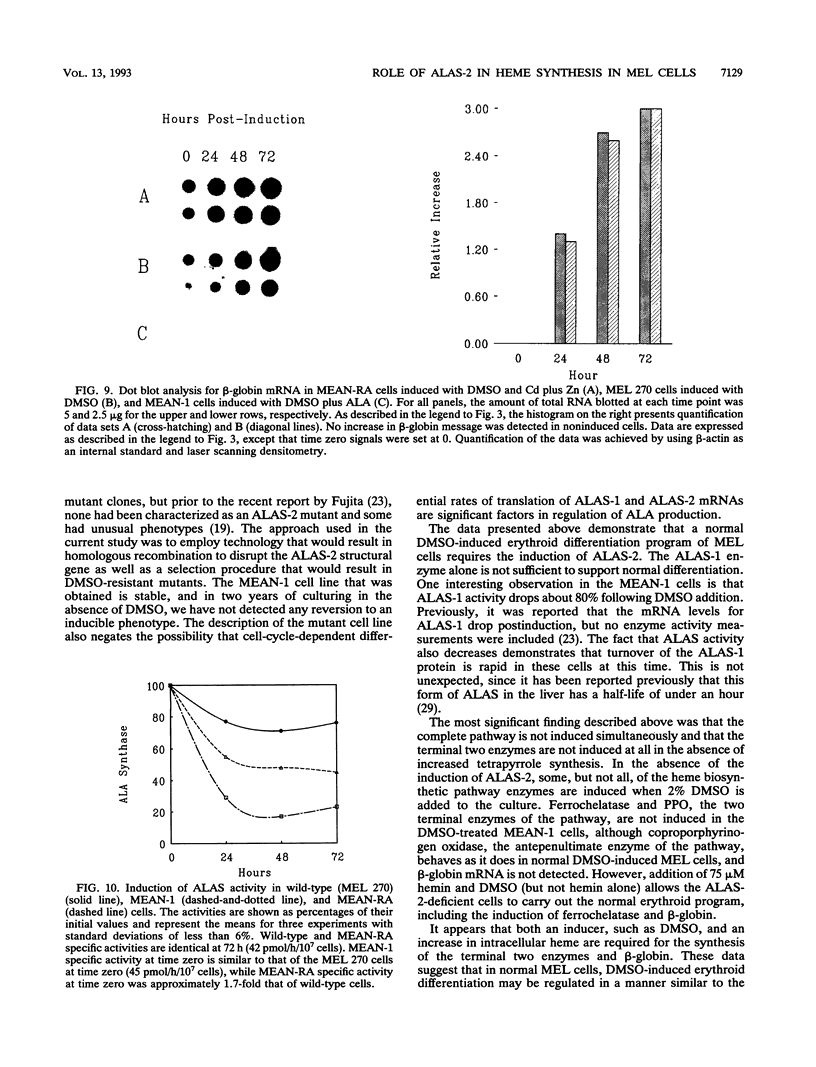
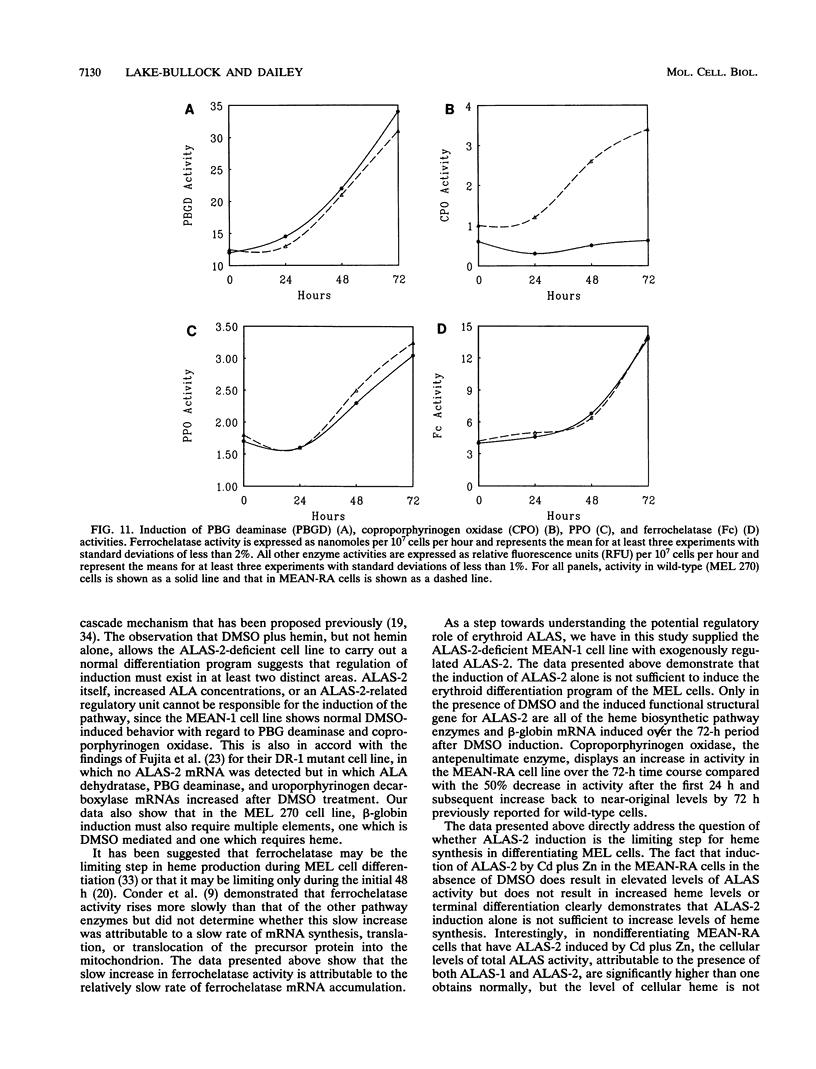
During dimethyl sulfoxide (DMSO)-stimulated differentiation of murine erythroleukemia (MEL) cells, one of the early events is the induction of the heme biosynthetic pathway. While recent reports have clearly demonstrated that GATA-1 is involved in the induction of erythroid cell-specific forms of 5-aminolevulinate synthase (ALAS-2) and porphobilinogen (PBG) deaminase and that cellular iron status plays a regulatory role for ALAS-2, little is known about regulation of the remainder of the pathway. In the current study, we have made use of a stable MEL cell mutant (MEAN-1) in which ALAS-2 enzyme activity is not induced by DMSO, hexamethylene bisacetamide (HMBA), or butyric acid. In this cell line, addition of 2% DMSO to growing cultures results in the normal induction of PBG deaminase and coproporphyrinogen oxidase but not in the induction of the terminal two enzymes, protoporphyrinogen oxidase and ferrochelatase. These DMSO-treated cells did not produce mRNA for beta-globin and do not terminally differentiate. In addition, the cellular level of ALAS activity declines rapidly after addition of DMSO, indicating that ALAS-1 must turn over rapidly at this time. Addition of 75 microM hemin alone to the cultures did not induce cells to terminally differentiate or induce any of the pathway enzymes. However, the simultaneous addition of 2% DMSO and 75 microM hemin caused the cells to carry out a normal program of terminal erythroid differentiation, including the induction of ferrochelatase and beta-globin. These data suggest that induction of the entire heme biosynthetic pathway is biphasic in nature and that induction of the terminal enzymes may be mediated by the end product of the pathway, heme. We have introduced mouse ALAS-2 cDNA into the ALAS-2 mutant cell line (MEAN-1) under the control of the mouse metallothionein promoter (MEAN-RA). When Cd and Zn are added to cultures of MEAN-RA in the absence of DMSO, ALAS-2 is induced but erythroid differentiation does not occur and cells continue to grow normally. In the presence of metallothionein inducers and DMSO, the MEAN-RA cells induce in a fashion similar to that found with the wild-type 270 MEL cells. Induction of the activities of ALAS, PBG deaminase, coproporphyrinogen oxidase, and ferrochelatase occurs. In cultures of MEAN-RA where ALAS-2 had been induced with Cd plus Zn 24 h prior to DMSO addition, onset of heme synthesis occurs more rapidly than when DMSO and Cd plus Zn are added simultaneously. This study reveals that induction of ALAS-2 alone is not sufficient to induce terminal differentiation of the MEAN-RA cells, and it does not appear that ALAS-2 alone is the rate-limiting enzyme of the heme biosynthetic pathway during MEL cell differentiation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Porphobilinogen deaminase: methods and principles of the enzymatic assay. Enzyme. 1982;28(2-3):146–157. doi: 10.1159/000459098. [DOI] [PubMed] [Google Scholar]
- Bishop D. F., Henderson A. S., Astrin K. H. Human delta-aminolevulinate synthase: assignment of the housekeeping gene to 3p21 and the erythroid-specific gene to the X chromosome. Genomics. 1990 Jun;7(2):207–214. doi: 10.1016/0888-7543(90)90542-3. [DOI] [PubMed] [Google Scholar]
- Bishop D. F., Kitchen H., Wood W. A. Evidence for erythroid and nonerythroid forms of delta-aminolevulinate synthetase. Arch Biochem Biophys. 1981 Feb;206(2):380–391. doi: 10.1016/0003-9861(81)90105-3. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., Bloomer J. R. A fluorometric assay for measurement of protoporphyrinogen oxidase activity in mammalian tissue. Clin Chim Acta. 1980 Jan 31;100(3):259–266. doi: 10.1016/0009-8981(80)90275-2. [DOI] [PubMed] [Google Scholar]
- Brooker J. D., Srivastava G., May B. K., Elliott W. H. Radiochemical assay for gamma-aminolevulinate synthase. Enzyme. 1982;28(2-3):109–119. doi: 10.1159/000459095. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chretien S., Dubart A., Beaupain D., Raich N., Grandchamp B., Rosa J., Goossens M., Romeo P. H. Alternative transcription and splicing of the human porphobilinogen deaminase gene result either in tissue-specific or in housekeeping expression. Proc Natl Acad Sci U S A. 1988 Jan;85(1):6–10. doi: 10.1073/pnas.85.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conder L. H., Woodard S. I., Dailey H. A. Multiple mechanisms for the regulation of haem synthesis during erythroid cell differentiation. Possible role for coproporphyrinogen oxidase. Biochem J. 1991 Apr 15;275(Pt 2):321–326. doi: 10.1042/bj2750321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter P. D., Baumann M., Bishop D. F. Enzymatic defect in "X-linked" sideroblastic anemia: molecular evidence for erythroid delta-aminolevulinate synthase deficiency. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4028–4032. doi: 10.1073/pnas.89.9.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. C., Bawden M. J., Abraham N. G., Bottomley S. S., May B. K., Baker E., Chen L. Z., Sutherland G. R. Erythroid 5-aminolevulinate synthase is located on the X chromosome. Am J Hum Genet. 1990 Jan;46(1):107–111. [PMC free article] [PubMed] [Google Scholar]
- Cox T. C., Bawden M. J., Martin A., May B. K. Human erythroid 5-aminolevulinate synthase: promoter analysis and identification of an iron-responsive element in the mRNA. EMBO J. 1991 Jul;10(7):1891–1902. doi: 10.1002/j.1460-2075.1991.tb07715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A., Fleming J. E. Bovine ferrochelatase. Kinetic analysis of inhibition by N-methylprotoporphyrin, manganese, and heme. J Biol Chem. 1983 Oct 10;258(19):11453–11459. [PubMed] [Google Scholar]
- Dailey H. A., Fleming J. E., Harbin B. M. Purification and characterization of mammalian and chicken ferrochelatase. Methods Enzymol. 1986;123:401–408. doi: 10.1016/s0076-6879(86)23049-9. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Karr S. W. Purification and characterization of murine protoporphyrinogen oxidase. Biochemistry. 1987 May 19;26(10):2697–2701. doi: 10.1021/bi00384a007. [DOI] [PubMed] [Google Scholar]
- Dandekar T., Stripecke R., Gray N. K., Goossen B., Constable A., Johansson H. E., Hentze M. W. Identification of a novel iron-responsive element in murine and human erythroid delta-aminolevulinic acid synthase mRNA. EMBO J. 1991 Jul;10(7):1903–1909. doi: 10.1002/j.1460-2075.1991.tb07716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnam D. M., Perrin F., Gannon F., Palmiter R. D. Isolation and characterization of the mouse metallothionein-I gene. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6511–6515. doi: 10.1073/pnas.77.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadigan A., Dailey H. A. Inhibition of ferrochelatase during differentiation of murine erythroleukaemia cells. Biochem J. 1987 Apr 15;243(2):419–424. doi: 10.1042/bj2430419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira G. C., Dailey H. A. Expression of mammalian 5-aminolevulinate synthase in Escherichia coli. Overproduction, purification, and characterization. J Biol Chem. 1993 Jan 5;268(1):584–590. [PubMed] [Google Scholar]
- Fraser P. J., Curtis P. J. Specific pattern of gene expression during induction of mouse erythroleukemia cells. Genes Dev. 1987 Oct;1(8):855–861. doi: 10.1101/gad.1.8.855. [DOI] [PubMed] [Google Scholar]
- Fujita H., Yamamoto M., Yamagami T., Hayashi N., Sassa S. Erythroleukemia differentiation. Distinctive responses of the erythroid-specific and the nonspecific delta-aminolevulinate synthase mRNA. J Biol Chem. 1991 Sep 15;266(26):17494–17502. [PubMed] [Google Scholar]
- Lathrop J. T., Timko M. P. Regulation by heme of mitochondrial protein transport through a conserved amino acid motif. Science. 1993 Jan 22;259(5094):522–525. doi: 10.1126/science.8424176. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- May B. K., Bhasker C. R., Bawden M. J., Cox T. C. Molecular regulation of 5-aminolevulinate synthase. Diseases related to heme biosynthesis. Mol Biol Med. 1990 Oct;7(5):405–421. [PubMed] [Google Scholar]
- May B. K., Borthwick I. A., Srivastava G., Pirola B. A., Elliott W. H. Control of 5-aminolevulinate synthase in animals. Curr Top Cell Regul. 1986;28:233–262. doi: 10.1016/b978-0-12-152828-7.50008-1. [DOI] [PubMed] [Google Scholar]
- Ohashi A., Kikuchi G. Purification and some properties of two forms of delta-aminolevulinate synthase from rat liver cytosol. J Biochem. 1979 Jan;85(1):239–247. doi: 10.1093/oxfordjournals.jbchem.a132317. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Riddle R. D., Yamamoto M., Engel J. D. Expression of delta-aminolevulinate synthase in avian cells: separate genes encode erythroid-specific and nonspecific isozymes. Proc Natl Acad Sci U S A. 1989 Feb;86(3):792–796. doi: 10.1073/pnas.86.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford T., Thompson G. G., Moore M. R. Heme biosynthesis in Friend erythroleukemia cells: control by ferrochelatase. Proc Natl Acad Sci U S A. 1979 Feb;76(2):833–836. doi: 10.1073/pnas.76.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J Exp Med. 1976 Feb 1;143(2):305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenhaut D. S., Curtis P. J. Nucleotide sequence of mouse 5-aminolevulinic acid synthase cDNA and expression of its gene in hepatic and erythroid tissues. Gene. 1986;48(1):55–63. doi: 10.1016/0378-1119(86)90351-3. [DOI] [PubMed] [Google Scholar]
- Schoenhaut D. S., Curtis P. J. Structure of a mouse erythroid 5-aminolevulinate synthase gene and mapping of erythroid-specific DNAse I hypersensitive sites. Nucleic Acids Res. 1989 Sep 12;17(17):7013–7028. doi: 10.1093/nar/17.17.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Hayashi N., Kikuchi G. delta-Aminolevulinate synthase isozymes in the liver and erythroid cells of chicken. Biochem Biophys Res Commun. 1983 Jun 15;113(2):377–383. doi: 10.1016/0006-291x(83)91737-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Fujita H., Watanabe N., Hayashi N., Kikuchi G. An immunochemical study of delta-aminolevulinate synthase and delta-aminolevulinate dehydratase in liver and erythroid cells of rat. Arch Biochem Biophys. 1986 Feb 15;245(1):76–83. doi: 10.1016/0003-9861(86)90191-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Yew N. S., Federspiel M., Dodgson J. B., Hayashi N., Engel J. D. Isolation of recombinant cDNAs encoding chicken erythroid delta-aminolevulinate synthase. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3702–3706. doi: 10.1073/pnas.82.11.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]