Abstract
Gamma-delta T cells are the most abundant of all epithelial-resident lymphocytes and are considered a first line of defense against pathogens in the mucosa. Our objective was to confirm the reduction in γδ T cell subsets and its relationship with mortality in patients with sepsis. We studied 135 patients with sepsis attended in the emergency department and intensive care unit of two hospitals and compared them with a similar control group of healthy subjects. The αβ and γδ T cell subsets were determined via flow cytometry according to the stage of the sepsis and its relationship with mortality. All the lymphocyte subsets were reduced with respect to the corresponding subsets in the control group. All the γδ T cell populations decreased significantly as the septic picture worsened. Furthermore, γδ T cells showed decreases at days 2, 3, and 4 from the start of sepsis. Twenty-six patients with sepsis died (19.3%). The γδ T cells, specifically, the CD3+ CD56+ γδ T cells, were significantly reduced in those septic patients who died. Our results indicate that, during sepsis, γδ T cells show the largest decrease and this reduction becomes more intense when the septic process becomes more severe. Mortality was associated with a significant decrease in γδ T cells.
INTRODUCTION
Sepsis is a serious public health problem, not only because of its constantly increasing incidence but also due to its high mortality and the associated high health care costs (1–3). Most patients with severe sepsis or septic shock are attended in hospital emergency departments or intensive care units (4, 5), so these are two key links in these patients' treatment chain.
Lymphocytes are fundamental cells in the immunological response to sepsis. Two distinct populations of T lymphocytes have currently been identified, depending on the type of antigen receptor expressed in the lymphocyte membrane: αβ T cells (T cell receptor-αβ [TCR-αβ]) and γδ T cells (TCR-γδ) (6–8).
αβ T cells are most abundant in the peripheral blood (90 to 95%), spleen, and lymphatic glands, where they mediate specific immune responses. Only 5 to 10% of γδ T cells are found in peripheral blood; they are mainly seen in epithelia, where they comprise 50% of the intraepithelial lymphocytes (IELs) in the mucosa (9, 10), playing a major role as the first line of defense.
There are two basic characteristics that differentiate γδ and αβ T cells. First, γδ T cells recognize proteins directly without any antigenic processing by the molecules in the major histocompatibility complex (MHC) (11–13). Second, they rarely, if ever, recognize peptides processed by the antigen-presenting cells (APC), but they recognize phosphorylated microbial metabolites and lipid-peptides. Thus, phosphate residues were the first ligands found to be related to the antigenic recognition of TCR-γδ (14, 15). Sepsis gives rise to an apoptotic phenomenon involving a reduction in the numbers of lymphocytes and epithelial and parenchymal cells. The first study to document apoptosis as an important mechanism of cellular death and depletion of immune cells in patients with sepsis was published just over a decade ago by Hotchkiss et al. (16) These findings are similar to those of studies performed on animals with experimental sepsis (17–21).
The immune cells in which apoptosis has been demonstrated include B cells, CD4+ T cells (22), and follicular and interdigitating dendritic cells but not macrophages (23). Not all the lymphocyte subsets have been studied with regard to their αβ and γδ surface receptors in human beings with sepsis.
Experimental studies in septic mice have shown that a reduction in the γδ T cells increases mortality (24, 25), but once again, no studies have demonstrated this relationship in human beings.
Therefore, the phenomenon of apoptosis described in sepsis would lead us to expect a reduction in lymphocyte subsets. Most pathogenic organisms enter the body through mucosas, and as γδ T cells are most abundant within the epithelial cell layer (9, 10) and are considered a first line of defense against pathogens in the mucosas, we hypothesized that septic patients might exhibit a reduction in their lymphocyte subsets, particularly that of the γδ T cells, and that this could have a relationship with mortality.
We therefore evaluated the frequencies of the B and T cell populations, both αβ and γδ, in the peripheral blood of a group of septic patients and compared them with the corresponding cell populations in a control group of healthy individuals. We also evaluated the frequencies of the lymphocyte populations with respect to the different stages of the disease (sepsis, severe sepsis, and septic shock), and we analyzed the relationship of these subsets with mortality.
MATERIALS AND METHODS
Study population.
In this prospective study of cases and controls, patients with sepsis (cases) were adults aged over 18 years who were attended in the emergency department (ED) or intensive care unit (ICU) of the Arnau de Vilanova or Doctor Peset Aleixandre Hospitals in Valencia (Spain) from 2009 to 2010. The patients with sepsis were recruited consecutively on admission to the ED/ICU of both hospitals. The controls were selected from a healthy population without any blood ties with the septic patients. They were companions of patients attending the outpatient clinic and family members of the hospital staff. Patients with previously defined criteria for sepsis (26) and its different stages of severity (27, 28) (sepsis, severe sepsis, and septic shock) were included. The exclusion criteria were autoimmune disease, vaccination in the previous 6 months, or immunosuppressive treatment. The members of the control group did not suffer from any acute infectious disease or any known immunodeficiency or autoimmune disease, nor had they been vaccinated in the previous 6 months or received immunosuppressive treatment. Both groups were paired according to sex and age (frequency matching) (29). Three age groups were considered for the grouping: 18 to 50 years, 51 to 70, and 71 and over.
All subjects, both patients and controls, were informed of the study objectives and gave authorization for their participation in the study. In the case of patients with a significantly altered state of awareness, consent was obtained from their relatives. The Research and Ethics Committee of both hospitals approved the study.
Variables studied.
The following variables were recorded: age and gender, stage of sepsis (sepsis, severe sepsis, and shock septic), time since onset of infection/sepsis (fever), mortality during the hospital stay, APACHE II (acute physiology and chronic health evaluation system) (30) and SOFA (sequential organ failure assessment) scores (31), positive cultures, complete blood count, and lymphocyte subsets, including CD3+, CD4+, CD8+, CD56+, CD19+, CD3+ αβ, CD4+ αβ, CD8+ αβ, CD56+ αβ, CD3+ γδ, CD4− CD8− γδ, CD8+ γδ, and CD56+ γδ cells.
Methods of blood sample analysis.
Blood samples were taken from patients at the time of admission and diagnosis in the ED/ICU and prior to any therapeutic action. Blood cell counts were performed using the Coulter LH750 automated hematology analyzer (Beckman Coulter, Fullerton, CA). The following monoclonal antibodies were used: CD45, CD4, CD8, CD3, and CD19 antibodies for the peripheral blood subsets and CD4, CD8, CD2, CD3, TCR-αβ, and TCR-γδ antibodies for the γδ T cell study.
Fluorescence analysis was performed using a Beckman-Coulter multiparameter flow cytometry analyzer, the Cytomics FC 500 (Beckman-Coulter, Miami, FL, USA), and later analyzed with CXP software. A minimum of 50,000 events was measured. Absolute counts of circulating cell subsets were calculated using the percentages obtained by flow cytometry, and the leukocyte count was obtained from the hematological analyzer using a dual-platform counting technology.
The γδ T lymphocyte subsets were analyzed with phycoerythrin-cyanine 5.1 (PC5)-conjugated anti-human TCR-γδ antibody (clone IMMU 510; Beckman Coulter, Miami, FL, USA). This is an antibody (mouse IgG1) that allows the identification and numeration of cell populations expressing the TCR-γδ antigen present in human biological samples using flow cytometry. IMMU 510 recognizes all γδ T cell populations regardless of the variable genes or junction regions they express, as assessed by immunofluorescence studies on polyclonal γδ T cell lines and γδ T cell clones.
The αβ T cell subsets were analyzed with PC5-conjugated anti-human TCR-αβ (clone IP26A; Beckman Coulter).
Statistical analysis.
Descriptive statistics were obtained using standard procedures. The assumption of a normal distribution for continuous variables was verified using graphic tests and the Kolmogorov-Smirnov test with a Lilliefor's significance correction. If normality was not reached, a logarithmic transformation was made. When normality was assumed, the Student t test was used to compare the means of the quantitative variables. When the hypothesis of normality of the quantitative variable was not accepted, the nonparametric Mann-Whitney U test was used. Contingency tables were drawn up for the qualitative variables (χ2 test or the Fisher exact test). Multivariate regression analysis, adjusting for age and sex, was built to analyze the relation between lymphocyte subpopulations (in logarithmic form) and the life status (dead or alive) among septic patients. The data were analyzed using the statistical software SPSS, version 19 (SPSS).
RESULTS
Demographic parameters and characteristics of patients with sepsis.
Two hundred seventy subjects were included in the study, of whom 135 were patients with sepsis (75 male and 60 female) and 135 were healthy controls with a similar sex distribution. The mean age of the patients with sepsis was 66.3 (95% confidence interval [CI], 62.8 to 69.8), and the mean age of the controls was 66.8 (95% CI, 66.4 to 70.2) (P = 0.84). Table 1 shows the characteristics of the patients with sepsis.
Table 1.
Characteristics of patients with sepsis
| Characteristic | Value [mean (95% CI)a or no. (%) of patients (n = 135)] |
|---|---|
| Age (yrs) | 66.3 (62.8–69.8) |
| APACHE II score | 14.1 (12.8–15.3) |
| SOFA score | 4.2 (3.6–4.8) |
| Sex | |
| Male | 75 (55.6) |
| Female | 60 (44.4) |
| Positive culture obtainedb | 48 (35.5) |
| Emergency department | 81 (60.0) |
| Intensive care unit | 54 (40.0) |
| In-hospital death | 26 (19.3) |
| Diagnosis | |
| Pneumonia | 65 (48.1) |
| Urinary tract infection | 26 (19.3) |
| Acute appendicitis | 6 (4.4) |
| Acute cholecystitis | 6 (4.4) |
| Undeterminedc | 5 (3.7) |
| Acute cholangitis | 4 (3.0) |
| Abscess | 4 (3.0) |
| Acute diverticulitis | 3 (2.2) |
| Cellulitis | 3 (2.2) |
| Meningitis | 3 (2.2) |
| Enterocolitis | 2 (1.5) |
| Otherd | 8 (5.9) |
| Stages of sepsis | |
| Sepsis | 48 (35.6) |
| Severe sepsis | 59 (43.7) |
| Septic shock | 28 (20.7) |
| Organ failure | |
| Acute respiratory failure | 49 (36.3) |
| Acute renal failure | 40 (29.6) |
| Neurologic | 36 (26.7) |
| Shock | 28 (20.7) |
| Metabolic | 24 (17.8) |
| Acute hepatic failure | 22 (16.3) |
| Hematologic | 21 (15.6) |
| No. of organs with failure | |
| 0 | 48 (35.6) |
| 1 | 23 (17.0) |
| 2 | 24 (17.8) |
| 3 | 21 (15.6) |
| 4 | 12 (8.9) |
| 5 | 4 (3.0) |
| 6 | 3 (2.2) |
Continuous variables are expressed as means (95% confidence intervals).
Positive cultures included blood (n = 28), exudate (n = 14), urine (n = 12), sputum (n = 8), stool (n = 1).
Undetermined sepsis represents the patient with fever and well-defined criteria of systemic inflammatory response without a well-defined septic focus. In our study, of the 5 patients with undetermined sepsis, 3 had positive blood cultures for E. Coli, one for S. Aureus, and one for S. hominis.
Other diagnoses included one patient each with the following diagnoses: pelvic inflammatory disease, gangrene, malaria, intestinal ischemia, intestinal obstruction, acute pancreatitis, spontaneous bacterial peritonitis, and septic arthritis.
Hemogram and lymphocyte subsets.
The analysis of the hemogram and the lymphocyte subsets in septic patients and controls shows a significant increase in the total leukocytes in sepsis, depending on the neutrophils, whereas there was a very significant drop in lymphocytes and eosinophils in the patients with sepsis. Of the 135 patients with sepsis, 101 (74.8%) presented lymphopenia (<1.0 × 109), versus 4 of 135 (3.0%) in the control group (odds ratio [OR], 25.2; 95% CI, 9.6 to 66.6). However, all the lymphocyte subsets decreased significantly in the septic patients compared to the corresponding subsets in the control group (Table 2).
Table 2.
Hemogram and lymphocyte populations in septic patients and controls
| Cell population or hemogram parameter | No. [mean (95% CI)] of cells (×109/liter) or indicated measure in: |
P value | |
|---|---|---|---|
| Sepsis patients | Controls | ||
| Leukocytes | 15,291 (13,917–16,665) | 6,831 (6,481–7,181) | 0.001 |
| Neutrophils | 13,734 (12,375–15,094) | 3,901 (3,624–4,179) | 0.001 |
| Monocytes | 0.650 (0.553–0.747) | 0.561 (0.526–0.597) | 0.090 |
| Eosinophils | 0.044 (0.017–0.071) | 0.178 (0.155–0.200) | 0.001 |
| Basophils | 0.016 (0.009–0.024) | 0.028 (0.017–0.040) | 0.080 |
| Thrombocytes | 222.3 (206.4–238.3) | 234.0 (217.4–250.6) | 0.340 |
| Hemoglobin | 11.8 (11.4–12.1)a | 13.8 (13.5–14.2)a | 0.001 |
| Lymphocytes | 0.8490 (0.7560–0.9410) | 2,145 (2020–2270) | 0.001 |
| CD19+ B cells | 0.1367 (0.1135–0.1599) | 0.1852 (0.1632–0.2072) | 0.001 |
| CD3+ T cells | 0.5426 (0.4741–0.6111) | 1.5224 (1.4239–1.6210) | 0.001 |
| CD3+ CD4+ T cells | 0.3277 (0.2814–0.3738) | 0.9277 (0.8608–0.9945) | 0.001 |
| CD3+ CD8+ T cells | 0.2378 (0.1995–0.2761) | 0.6830 (0.6185–0.7475) | 0.001 |
| CD3+ CD56+ T cells | 0.1126 (0.0891–0.1362) | 0.3727 (0.3317–0.3148) | 0.001 |
| CD3+ αβ T cells | 0.4832 (0.4149–0.5514) | 1.4394 (1.3400–1.5389) | 0.001 |
| CD3+ CD4+ αβ T cells | 0.3026 (0.2550–0.3503) | 0.8948 (0.8230–0.9670) | 0.001 |
| CD3+ CD8+ αβ T cells | 0.1817 (0.1441–0.2192) | 0.5332 (0.4716–0.5947) | 0.001 |
| CD3+ CD56+ αβ T cells | 0.0283 (0.0210–0.0355) | 0.1110 (0.0878–0.1342) | 0.001 |
| CD3+ γδ T cells | 0.0159 (0.0114–0.0203) | 0.0620 (0.0512–0.0728) | 0.001 |
| CD3+ CD4− CD8− γδ T cells | 0.0081 (0.0056–0.0105) | 0.0294 (0.0229–0.0359) | 0.001 |
| CD3+ CD8+ γδ T cells | 0.0046 (0.0029–0.0062) | 0.0220 (0.0173–0.0266) | 0.001 |
| CD3+ CD56+ γδ T cells | 0.0032 (0.0020–0.0045) | 0.0141 (0.0103–0.0179) | 0.001 |
Values of hemoglobin are expressed as mg/dl.
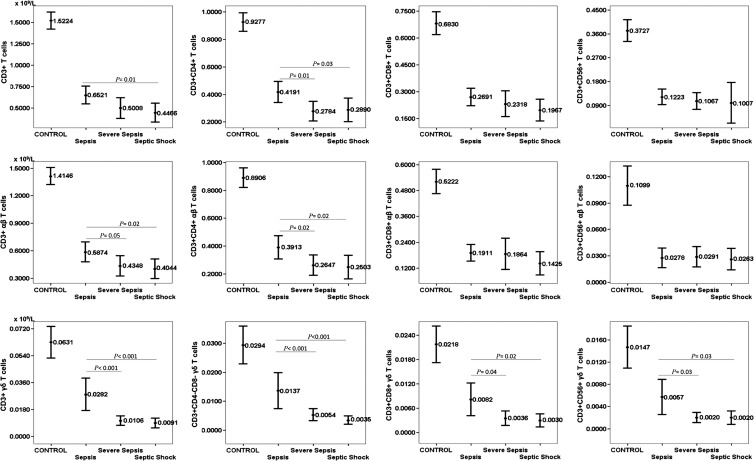
Figure 1 shows the T cell subsets, both the conventional ones and those associated with the αβ and γδ receptors, in relation to the stages of sepsis and the control group. In the conventional subsets, the total T cells are significantly reduced in septic patients with respect to the counts in the control group, and this reduction is greater when the sepsis worsens, depending on the CD4+ T cells. The same is true of the αβ T cell subsets. This reduction is much more intense, however, in the γδ T cell subsets than in the αβ T cell subsets when the septic process worsens. No differences were found in lymphocyte subsets in patients with positive blood cultures or with Gram-positive versus Gram-negative infections. Table 3 shows the organisms isolated from the different cultures of septic patients.
Fig 1.
Peripheral blood T cell subsets, both conventional and according to the αβ and γδ receptors, in relation to the stages of sepsis. Values are expressed as means (×109/liter), and I bars denote 95% confidence intervals. Significant differences between the control group and patients in the 3 stages of sepsis (P < 0.001) were determined. The numbers of patients in each group were as follows: sepsis, n = 48; severe sepsis, n = 59; and septic shock n = 28.
Table 3.
Organisms isolated from cultures of different sample types from septic patients
| Germ | Total no. (%) of isolates | No. (%) of isolates from: |
||||
|---|---|---|---|---|---|---|
| Blood | Exudate | Urine | Sputum | Feces | ||
| Gram-negative bacteria | ||||||
| Escherichia coli | 19 (30.5) | 9 | 2 | 8 | ||
| Pseudomonas | 5 (7.9) | 1 | 1 | 1 | 2 | |
| Proteus mirabilis | 3 (4.8) | 1 | 1 | 1 | ||
| Klebsiella pneumoniae | 2 (3.1) | 2 | ||||
| Salmonella | 1 (1.6) | 1 | ||||
| Acinetobacter baumannii | 1 (1.6) | 1 | ||||
| Haemophilus influenzae | 1 (1.6) | 1 | ||||
| Proteus vulgaris | 1 (1.6) | 1 | ||||
| Bacteroides fragilis | 1 (1.6) | 1 | ||||
| Enterobacter cloacae | 1 (1.6) | 1 | ||||
| Total | 35 (55.9) | 14 (22.2) | 6 (9.5) | 10 (15.9) | 4 (6.3) | 1 (1.6) |
| Gram-positive bacteria | ||||||
| Staphylococcus aureus | 10 (15.8) | 5 | 4 | 1 | ||
| Streptococcus pneumoniae | 6 (9.5) | 3 | 1 | 2 | ||
| Enterococcus faecalis | 4 (6.2) | 1 | 2 | 1 | ||
| Enterococcus faecium | 2 (3.1) | 1 | 1 | |||
| Streptococcus epidermidis | 1 (1.6) | 1 | ||||
| Streptococcus agalactiae | 1 (1.6) | 1 | ||||
| Staphylococcus haemolyticus | 1 (1.6) | 1 | ||||
| Total | 25 (39.4) | 13 (20.6) | 8 (12.7) | 1 (1.6) | 3 (4.8) | 0 (0.0) |
| Fungus | ||||||
| Candida albicans | 2 (3.1) | 1 | 1 | |||
| Parasite | ||||||
| Plasmodium falciparum | 1 (1.6) | 1 | ||||
| Total | 63 (100.0) | 28 (44.4) | 14 (22.2) | 12 (19.0) | 8 (12.7) | 1 (1.6) |
Time since onset of sepsis.
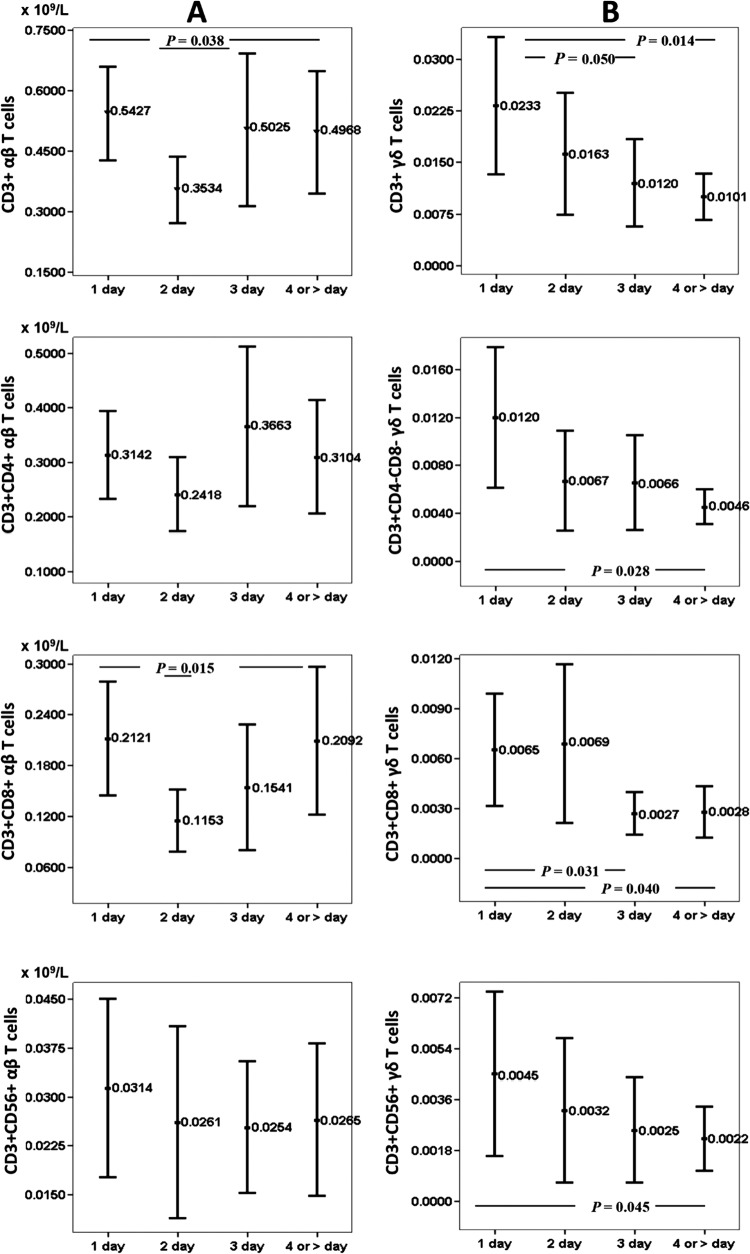
Figure 2 shows peripheral blood T cell subsets according to the αβ and γδ receptors in relation to time since onset of infection/sepsis at admission in ED/ICU departments. It may be seen that CD3+ αβ T cells and CD3+ CD8+ αβ T cells drop significantly at day 2 from diagnosis of sepsis, to recover at days 3 and 4. However, γδ T cells show a continuous decrease over time. This decrease is statistically significant for CD3+ γδ, CD3+ CD8+ γδ, and CD3+ CD56+ γδ T cells.
Fig 2.
Peripheral blood T cell subsets according to the αβ and γδ receptors in relation to time since onset of infection/sepsis at admission in ED/ICU departments. The numbers of patients in each group were as follows: 1 day, n = 48; 2 days, n = 32; 3 days, n = 22; and four or more days, n = 33. Values are expressed as means (×109/liter), and I bars denote 95% confidence intervals.
Lymphocyte subsets and organ failure.
We analyzed the correlation of lymphocyte subsets with the number of organs that failed in septic patients. We found an inverse relationship between the αβ T cells and γδ T cells; this correlation was more significant for CD3+ γδ T cells (Spearman's rho, −0.309; P < 0.001) and CD3+ CD56+ γδ T cells (Spearman's rho, −0.215; P < 0.001).
We also examined the association between frequency of T cells and failure of specific organs. We found a significant decrease in γδ T cells when there was any organ failure, except for neurological failure. Thus, in patients with shock, the frequencies of CD3+ αβ T cells and CD3+ CD4+ αβ T cells were lower than in patients without shock (P = 0.007 and P = 0.038), although the decrease was stronger for CD3+ γδ T cells (P < 0.001). There were significant decreases in γδ T cells in patients with the other organ failures, as follows: acute hepatic failure, CD3+ γδ T cells, CD3+ CD4− CD8− γδ T cells, CD3+ CD8+ γδ T cells, and CD3+ CD56+ γδ T cells (P < 0.001); hematologic failure, CD3+ γδ T cells, CD3+ CD4− CD8− γδ T cells, and CD3+ CD8+ γδ T cells (P < 0.001); metabolic failure, CD3+ γδ T cells (P < 0.003), CD3+ CD4− CD8− γδ T cells (P = 0.003), and CD3+ CD8+ γδ T cells (P = 0.008); acute respiratory failure, CD3+ γδ T cells (P = 0.001), CD3+ CD4− CD8− γδ T cells (P = 0.010), and CD3+ CD56+ γδ T cells (P = 0.016); and acute renal failure, CD3+ γδ T cells (P = 0.015). In summary, the most-intense γδ T cell reductions occurred in acute hepatic failure, followed by hematologic and metabolic failure.
Mortality.
Twenty-six patients with sepsis died (19.3%), including 10 of 75 males (13.3%) and 16 of 60 females (26.7%) (P = 0.08). The mean age of the patients with sepsis who died was 79.0 (95% CI, 74.4 to 83.6) years, versus 63.3 (95% CI, 59.2 to 67.3) years in those who survived (P < 0.001).
The patients that died had a mean of 3.1 (95% CI, 2.5 to 3.6) affected organs, versus 1.3 (95% CI, 1.0 to 1.5) in surviving patients (P < 0.001). The organ failures significantly associated with mortality were respiratory, neurologic, renal, and metabolic (P < 0.001).
We evaluated the duration of symptoms of patients with sepsis up to the time of assistance at the hospital. The patients who died came to the hospital at 3.6 (95% CI, 2.5 to 4.8) days from the start of symptoms versus 2.4 (95% CI, 1.9 to 2.8) days for patients who did not die (P = 0.023). This indicates that patients who subsequently died were attended at the hospital almost 2 days later than patients who survived. Septic patients died in the hospital at a mean of 6.2 (95% CI, 3.4 to 9.1) days, whereas patients who survived were discharged from the hospital at 11.2 (95% CI, 9.0 to 13.4) days (P < 0.001). Fifty percent of patients who died did so in the first 2 days of admission.
From the first symptoms to the resolution of the process in patients who survived, there was a mean of 13.6 (95% CI, 11.4 to 15.9) days, while the time between first symptoms and death in patients who died was 9.9 (95% CI, 6.6 to 13.1) days (P = 0.036).
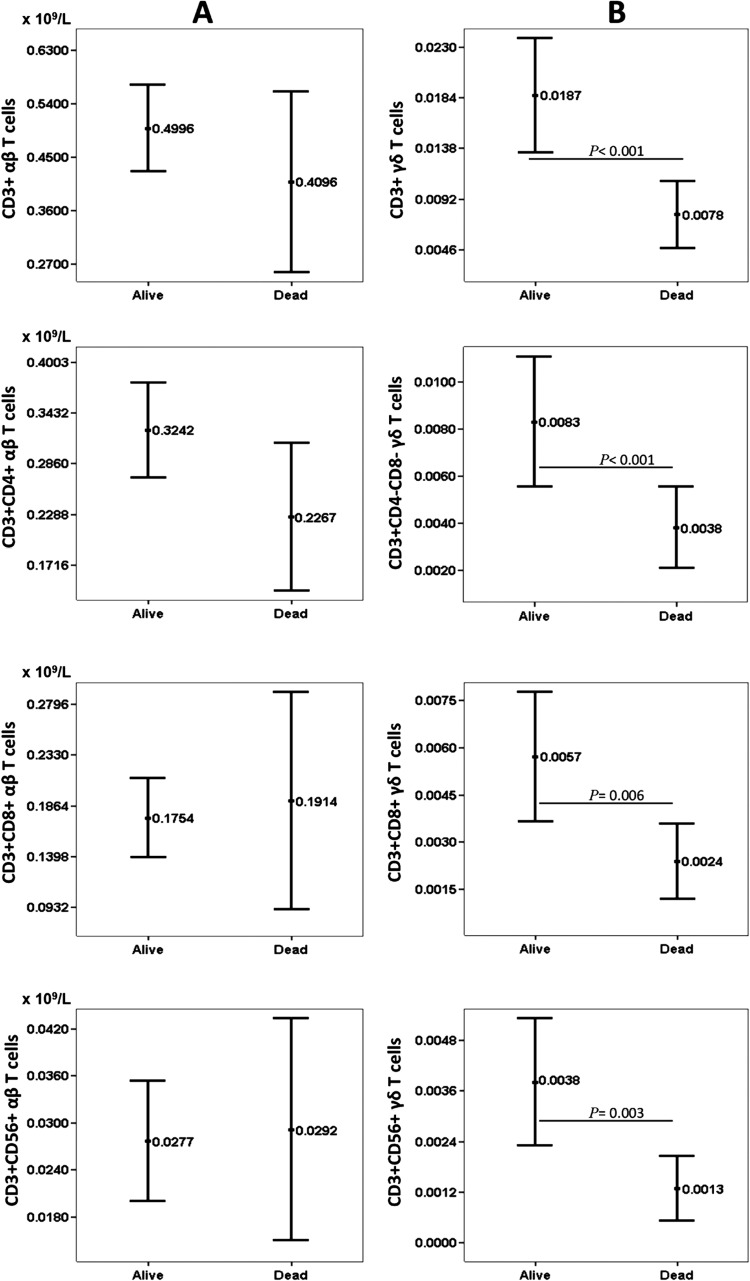
Figure 3 shows the differences in the conventional T subsets between the septic patients who died and those who survived, according to the expression of the αβ and γδ receptors. As can be seen, mortality in the patients with sepsis was associated with a reduction in CD3+ γδ T cells, as well as reductions in CD3+ CD8+ γδ T cells, CD3+ CD4− CD8− γδ T cells, and CD3+ CD56+ γδ T cells.
Fig 3.
Peripheral blood T cell subsets according to the αβ and γδ receptors in relation to mortality (n = 26) after hospital admission for sepsis. Values are expressed as means (×109/liter), and I bars denote 95% confidence intervals.
However, in the multivariate linear regression analysis, after adjusting for the variables of age and sex, only the association of mortality with CD3+ CD56+ γδ T cells retained statistical significance (P = 0.028). For the other three groups of γδ T cell subsets, there was also a reduction in the group that died, but this relation was not statistically significant.
DISCUSSION
This is the first study to evaluate all the αβ and γδ lymphocyte subsets in a significant number of septic patients and their corresponding controls.
The results from the hemograms show that 74.8% of the septic patients presented lymphopenia. It is interesting to note that there was also a drop in eosinophils, although this was not the case with monocytes, the precursors of macrophages, which is in keeping with the results of a previous study (23). The reason for these discrepancies between the decrease in some cells and stability of others is of enormous interest and deserves to be studied in depth in the future.
It is essential to analyze the contribution of the different lymphocyte populations to the transitory immunodeficiency that seems to affect patients with sepsis. Both the B and the T cells present reductions, but this is more marked in the T cells. Both the αβ and the γδ Τ cell subsets decrease, but the biggest decreases in patients with sepsis occur in the γδ T cells. This is a crucial finding if we consider the importance of the γδ T cells as a first line of defense against pathogenic attacks on the mucosa and, also, as facilitators of the activation and development of the abundant αβ T cells in the blood. Matsushima et al. (32) studied αβ and γδ T cells in a population of patients with systemic inflammatory response syndrome that included 23 patients with sepsis and 14 with traumatisms; these were compared with a control group of healthy subjects. They found a significant reduction in both subsets, although this was more intense in the γδ T cells. This study did not, however, analyze the lymphocyte subsets or the associated mortality.
Venet et al. (33) also demonstrated a marked decrease in the γδ T cells in peripheral blood in 21 septic patients compared to the corresponding cell population in a control group, while the percentage of αβ T cells went up. The results, however, were expressed in percentage values and not in absolute values, and furthermore, the patients were not paired with the controls by sex and age.
In our study, the moment of the lymphocyte decrease varies in the different lymphocyte subsets. So, αβ T cells show an immediate decrease that recovers in the following days, whereas the decrease is progressive after the onset of sepsis for the γδ T cells. It is clear that patients that die have disease durations of more than 3 days before admission to hospital; this time coincides with the lowest γδ T cell levels.
The different stages of sepsis, severe sepsis, and septic shock are clearly associated with a progressive increase in the probability of dying in an intensive care unit (34). In a 10-year study that we undertook in the Valencian community, including patients both inside and outside intensive care units, we found that the probability of death increased 5-fold in severe sepsis (3). In the present study, the progressive reduction of lymphocytes, specifically, the CD3+ CD56+ γδ T cells, is also associated with the severity of the sepsis and mortality. We know that lymphopenia has been associated with mortality in patients with sepsis of all ages (35, 36), but no study to date has investigated which specific cell subsets are related to mortality.
A decrease has been reported in γδ T cells and natural killer T cells after viral infection in rhesus macaques (37). Little is known about the implications of these T cell subsets in humans, and even less in septic patients. NKTγδ subsets have been described in the thymus of mice, which subsequently migrate to the liver and spleen, producing a variety of effector cytokines soon (minutes or hours) after antigenic stimulation, including with interleukin-4 (IL-4) and gamma interferon (IFN-γ). Unlike NKTαβ cells that recognize glycolipids presented by the nonclassical MHC class I molecule CD1d on cortical thymocytes, NKTγδ cells do not bind to CD1d-αGC (α-galactosylceramide) complexes and they develop in the absence of CD1d (38). This allows them to modulate immunity in a broad spectrum of diseases, including cancer, autoimmunity, and infection, and they have been given an important role between innate and acquired immunity (39). Therefore, and even without specific studies of NKTγδ cells in humans, it would be logical to think that the significant deficiency observed in our study was associated with a poor prognosis of sepsis.
Our findings concur with those of the experimental studies performed on septic mice by Chung et al. (24) and Tschöp et al. (25), where there was an increased early mortality in mice lacking T cells (γδ−/− mice) after sepsis. Tschöp et al. (25) found that mice deficient in γδ T cells had decreased survival times and increased tissue damage after cecal ligation and puncture compared with these parameters in wild-type mice. Furthermore, recruitment of neutrophils and myeloid suppressor cells to the site of infection was decreased in γδ T cell-deficient mice, and the bacterial load increased significantly in γδ T cell-deficient mice. However, antibiotic treatment did not significantly change mortality. In our study, no differences were found in lymphocyte subsets or mortality among patients with or without previous antibiotic treatment at the time of blood collection (data not shown). The results of our study suggest the vital importance of γδ T cells in the body's defense against infection and a relation of their collapse with the poor prognosis of sepsis.
The reduction of circulating gamma-delta T cells is probably associated with a similar reduction of γδ T cells in the mucosa. Demonstration of this hypothesis would require additional studies that are difficult to perform from technical and ethical standpoints.
We believe that the data obtained in the present study support the idea of a central role for γδ T cells in physiological and pathological conditions (i.e., sepsis) and open up the possibility of initial explorations of new therapeutic strategies. The capacity of γδ T cells to participate in antibody-dependent cell-mediated cytotoxicity in the absence of an allogeneic response and the discovery that γδ T cells can be selectively activated by natural or synthetic phosphoantigens opens up new paths for the development of immunotherapy based on γδ T cells (15, 40).
In summary, there is a major reduction in circulating lymphocytes in sepsis, affecting both the B and the T cells, but the biggest reduction is in the γδ T cells. This reduction becomes more intense as the septic process becomes more severe. Mortality in patients with sepsis is associated with a reduction in γδ T cells, specifically, the CD3+ CD56+ γδ T cells.
ACKNOWLEDGMENTS
Our thanks go to Anselmo Villar-Grimalt, Head of the Internal Medicine Department, and José Mayans, Head of the Hematology Department, for their unconditional support for our work, as well as Alejandro Hueso Zarandieta, nurse laboratory technologist.
All authors report no conflicts of interest relevant to this article.
The work received no financial support.
Footnotes
Published ahead of print 20 March 2013
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 2.Martín GS, Mannino DM, Eaton S, Moss M. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348:1546–1554 [DOI] [PubMed] [Google Scholar]
- 3.Andreu-Ballester JC, Ballester F, González Sánchez A, Almela Quilis A, Colomer Rubio E, Peñarroja Otero C. 2008. Epidemiology of sepsis in the Valencian Community (Spain), 1995-2004. Infect. Control Hosp. Epidemiol. 29:630–634 [DOI] [PubMed] [Google Scholar]
- 4.Rivers EP, Nguyen HB, Amponsah D. 2003. Sepsis: a landscape from the emergency department to the intensive care unit. Crit. Care Med. 31:968–969 [DOI] [PubMed] [Google Scholar]
- 5.Strehlow MC, Emond SD, Shapiro NI, Pelletier AJ, Camargo CA., Jr 2006. National study of emergency department visits for sepsis, 1992 to 2001. Ann. Emerg. Med. 48:326–331 [DOI] [PubMed] [Google Scholar]
- 6.Saito H, Kranz DM, Takagaki Y, Hayday A, Eisen H, Tonegawa S. 1984. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. Nature 309:757–762 [DOI] [PubMed] [Google Scholar]
- 7.Raulet DH. 1989. The structure, function, and molecular genetics of the gamma/delta T cell receptor. Annu. Rev. Immunol. 7:175–207 [DOI] [PubMed] [Google Scholar]
- 8.Allison JP, Havran WL. 1991. The immunobiology of T cells with invariant gamma delta antigen receptors. Annu. Rev. Immunol. 9:679–705 [DOI] [PubMed] [Google Scholar]
- 9.Goodman T, Lefrançois L. 1988. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature 333:855–858 [DOI] [PubMed] [Google Scholar]
- 10.Bonneville M, Janeway CA, Jr, Ito K, Haser W, Ishida I, Nakanishi N, Tonegawa S. 1988. Intestinal intraepithelial lymphocytes are a distinct set of gamma delta T cells. Nature 336:479–481 [DOI] [PubMed] [Google Scholar]
- 11.Schild H, Mavaddat N, Litzenberger C, Ehrich EW, Davis MM, Bluestone JA, Matis L, Draper RK, Chien YH. 1994. The nature of major histocompatibility complex recognition by γδ T-cells. Cell 76:29–37 [DOI] [PubMed] [Google Scholar]
- 12.Chien YH, Jores R, Crowley MP. 1996. Recognition by γδ T cells. Annu. Rev. Immunol. 14:511–532 [DOI] [PubMed] [Google Scholar]
- 13.Crowley MP, Reich Z, Mavaddat N, Altman JD, Chien Y. 1997. The recognition of the nonclassical major histocompatibility complex (MHC) class I molecule, T10, by the γδ T cell, G8. J. Exp. Med. 185:1223–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeffer K, Schoel B, Gulle H, Kaufmann SHE, Wagner H. 1990. Primary responses of human T cells to mycobacteria: a frequent set of γδ T cells are stimulated by protease resistant ligands. Eur. J. Immunol. 20:1175–1180 [DOI] [PubMed] [Google Scholar]
- 15.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournié JJ. 1994. Stimulation of human γδ T-cells by nonpeptidic mycobacterial ligands. Science 264:267–270 [DOI] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. 1999. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 27:1230–1251 [DOI] [PubMed] [Google Scholar]
- 17.Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. 1997. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit. Care Med. 25:1298–1307 [DOI] [PubMed] [Google Scholar]
- 18.Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. 1997. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock 7:247–253 [DOI] [PubMed] [Google Scholar]
- 19.Chung CS, Xu YX, Wang W, Chaudry IH, Ayala A. 1998. Is Fas ligand or endotoxin responsible for mucosal lymphocyte apoptosis in sepsis? Arch. Surg. 133:1213–1220 [DOI] [PubMed] [Google Scholar]
- 20.Oberholzer C, Oberholzer A, Bahjat FR, Minter RM, Tannahill CL, Abouhamze A, LaFace D, Hutchins B, Clare-Salzler MJ, Moldawer LL. 2001. Targeted adenovirus-induced expression of IL-10 decreases thymic apoptosis and improves survival in murine sepsis. Proc. Natl. Acad. Sci. U. S. A. 98:11503–11508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, Jr, Buchman TG, Karl IE, Hotchkiss RS. 2002. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA 287:1716–1721 [DOI] [PubMed] [Google Scholar]
- 22.Hotchkiss RS, Tinsley KW, Swanson PE. 2001. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 166:6952–6963 [DOI] [PubMed] [Google Scholar]
- 23.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE. 2002. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J. Immunol. 168:2493–2500 [DOI] [PubMed] [Google Scholar]
- 24.Chung CS, Watkins L, Funches A, Lomas-Neira J, Cioffi WG, Ayala A. 2006. Deficiency of γδ T-lymphocytes contributes to mortality and immunosuppression in sepsis. Am. J. Physiol. Regul Integr. Comp. Physiol. 291:R1338–R1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tschöp J, Martignoni A, Goetzman HS, Choi LG, Wang Q, Noel JG, Ogle CK, Pritts TA, Johannigman JA, Lentsch AB, Caldwell CC. 2008. γδ T cells mitigate the organ injury and mortality of sepsis. J. Leukoc. Biol. 83:581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, SCCM/ESICM/ACCP/ATS/ SIS. 2003. SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit. Care Med. 31:1250–1256 [DOI] [PubMed] [Google Scholar]
- 27.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM. 2004. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 30:536–555 [DOI] [PubMed] [Google Scholar]
- 28.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. 2009. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–1655 [DOI] [PubMed] [Google Scholar]
- 29.Szklo M, Nieto FJ. 2007. Epidemiology: beyond the basics, 2nd ed Jones & Bartlett Learning, Sudbury, MA [Google Scholar]
- 30.Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. 1981. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit. Care Med. 9:591–597 [DOI] [PubMed] [Google Scholar]
- 31.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. 1998. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 26:1793–1800 [DOI] [PubMed] [Google Scholar]
- 32.Matsushima A, Ogura H, Fujita K, Koh T, Tanaka H, Sumi Y, Yoshiya K, Hosotsubo H, Kuwagata Y, Shimazu T, Sugimoto H. 2004. Early activation of γδ T lymphocytes in patients with severe systemic inflammatory response syndrome. Shock 22:11–15 [DOI] [PubMed] [Google Scholar]
- 33.Venet F, Bohé J, Debard AL, Bienvenu J, Lepape A, Monneret G. 2005. Both percentage of γδ T lymphocytes and CD3 expression are reduced during septic shock. Crit. Care Med. 33:2836–2840 [DOI] [PubMed] [Google Scholar]
- 34.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D. 2006. Sepsis in European intensive care units: results of the SOAP study. Crit. Care Med. 34:344–353 [DOI] [PubMed] [Google Scholar]
- 35.Le Tulzo Y, Pangault C, Gacouin A, Guilloux V, Tribut O, Amiot L, Tattevin P, Thomas R, Fauchet R, Drénou B. 2002. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock 18:487–494 [DOI] [PubMed] [Google Scholar]
- 36.Felmet KA, Hall MW, Clark RS, Jaffe R, Carcillo JA. 2005. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J. Immunol. 174:3765–3772 [DOI] [PubMed] [Google Scholar]
- 37.Rodas JD, Cairo C, Djavani M, Zapata JC, Ruckwardt T, Bryant J, Pauza CD, Lukashevich IS, Salvato MS. 2009. Circulating natural killer and gammadelta T cells decrease soon after infection of rhesus macaques with lymphocytic choriomeningitis virus. Mem. Inst. Oswaldo Cruz 104:583–591 [DOI] [PubMed] [Google Scholar]
- 38.Pereira P, Boucontet L. 2012. Innate NKTγδ and NKTαβ cells exert similar functions and compete for a thymic niche. Eur. J. Immunol. 42:1272–1281 [DOI] [PubMed] [Google Scholar]
- 39.Godfrey DI, Stankovic S, Baxter AG. 2010. Raising the NKT cell family. Nat. Immunol. 11:197–206 [DOI] [PubMed] [Google Scholar]
- 40.Lamb LS, Jr, Musk P, Ye Z, van Rhee F, Geier SS, Tong JJ, King KM, Henslee-Downey PJ. 2001. Human γδ (+) T lymphocytes have in vitro graft vs leukaemia activity in the absence of an allogeneic response. Bone Marrow Transplant. 27:601–606 [DOI] [PubMed] [Google Scholar]