Abstract
Riemerella anatipestifer infections cause major economic losses in the duck industry. In this study, a trivalent inactivated vaccine of R. anatipestifer, including strains CH3 (serotype 1), NJ3 (serotype 2), and HXb2 (serotype 10), was developed. Animal experiments showed that the ducks that received two immunizations with the vaccine were 100% protected from challenge with strains from any of the three serotypes (1, 2, or 10). No death or clinical signs of diarrhea, tremors, or limb swelling were shown in the protected ducks. Also, no R. anatipestifer bacteria were isolated from the livers or brains of the protected ducks. Furthermore, no histopathological changes were observed in the liver, spleen, or brain samples from the protected ducks during histological examination. The ducks that received two immunizations with the vaccine generated high antibody titers of 1:3,200 to 1:6,400 against the three serotypes of strains. The vaccine significantly enhanced the production of gamma interferon (IFN-γ) and interleukin 2 (IL-2) after one immunization and enhanced the production of IL-4 and IL-10 after two immunizations. In addition, real-time PCR indicated that the expression of major histocompatibility complex I (MHC-I), as well as that of CD40 and CD154 molecules, was significantly increased after one immunization, and the expressions of both MHC-I and MHC-II molecules were increased after two immunizations. Our study indicates that the vaccine can induce both humoral and cellular immunities in ducks and offer effective protection against R. anatipestifer infection.
INTRODUCTION
Riemerella anatipestifer is a Gram-negative, nonmotile, and non-spore-forming rod-shaped bacterium (1). R. anatipestifer infections cause major economic losses in the duck industry through high mortality rates, poor feed conversion, increased condemnations, and high treatment costs (2, 3). For ducks under about 8 weeks of age on infected farms, R. anatipestifer infection causes mortality rates between 10 and 75% (4). Up to now, 21 serotypes have been identified in the world, and there is poor cross-protection among these serotypes (1, 2). Infections from R. anatipestifer serotypes 1, 2, 3, 5, 6, 7, 8, 10, 11, 13, 14, and 15 have been reported in China; however, serotypes 1, 2, and 10 are responsible for most of the major outbreaks (5).
Various antibiotics are currently used to prevent and control R. anatipestifer infection in ducks, but they accelerate the emergence of drug-resistant strains (6, 7).The resistance of R. anatipestifer to many antibiotics has increased greatly, and antibiotic residues have been detected in duck-related products (3). Therefore, immunization should be the ideal way to control the disease. Vaccines based on a single serotype of inactivated bacterin or live or cell-free culture filtrate have not provided significant cross-protection among the serotypes (8). A trivalent formalin-inactivated bacterin containing Pasteurella anatipestifer serotype 1, 2, and 5 cells and a combination of Escherichia coli serotype O78 and P. anatipestifer bacterin were developed. However, the protection lasted only 2 weeks after the second inoculation (8, 9). Live attenuated vaccines stimulate protracted cell-mediated immunity and antibody responses, but their use proved to be unacceptable as a result of adverse effects on growth (10). In contrast, some attempts have been made toward developing a subunit vaccine against R. anatipestifer. The outer membrane protein A (OmpA) and a 41-kDa partial protein of R. anatipestifer have been reported to be immunogenic proteins; however, the established subunit vaccine did not provide effective protection against heterologous-serotype strain infection (11, 12). Recombinant R. anatipestifer GroEL showed cross-protection among serotypes 1, 2, and 10, but the protection was not adequate (13). Thus, the development of an effective R. anatipestifer vaccine, including prevalent serotypes of strains in the area, is important for providing effective protection against this disease.
The aim of this study was to develop a trivalent R. anatipestifer-inactivated vaccine and to evaluate both the protection of vaccinated ducks from challenge and their immune responses after vaccination. Strains from serotypes 1, 2, and 10 were selected for use in developing the vaccine because they are responsible for most of the major outbreaks in China.
MATERIALS AND METHODS
Animals.
One-day-old Cherry Valley Pekin ducks were obtained from Zhuanghang Duck Farm (Shanghai, China) and kept under a controlled temperature (28 to 30°C). The ducks were housed in cages with a 12-h light/dark cycle and free access to food and water during the study. The care and maintenance of all animals were in accordance with the Institutional Animal Care and Use Committee guidelines set by the Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Science. When needed, the ducks were euthanized humanely with an intravenous injection of sodium pentobarbital at a dose of 100 to 150 mg/kg. The ducks used for the experiments were grouped as shown in Table 1.
Table 1.
Animal experiments
| Expt | Group | No. of injectionsa | Inoculation type | Challenge strainb | No. of ducks | Challenge or sampling time | Detection |
|---|---|---|---|---|---|---|---|
| A: immunization assay | 1 | 1 | Vaccine | YL4 | 24 | Each 8 ducks at days 14, 42, and 70 postvaccination | Protection |
| Yb2 | 24 | ||||||
| HXb2 | 24 | ||||||
| 2 | 1 | Saline in adjuvant | YL4 | 24 | Each 8 ducks at days 14, 42, and 70 postvaccination | Protection | |
| Yb2 | 24 | ||||||
| HXb2 | 24 | ||||||
| 3 | 2 | Vaccine | YL4 | 24 | Each 8 ducks at days 14, 42, and 70 postvaccination | Protection | |
| Yb2 | 24 | ||||||
| HXb2 | 24 | ||||||
| 4 | 2 | Saline in adjuvant | YL4 | 24 | Each 8 ducks at days 14, 42, and 70 postvaccination | Protection | |
| Yb2 | 24 | ||||||
| HXb2 | 24 | ||||||
| B: histological examinations | 5 | 2 | Vaccine | None | 3 | Day 21 after 2 immunizations | Pathology |
| 6 | 2 | Vaccine | YL4 | 3 | Day 14 postchallenge after 2 immunizations | Pathology | |
| Yb2 | 3 | ||||||
| HXb2 | 3 | ||||||
| C: detection of serum antibodies | 7 | 1 | Vaccine | None | 80 | Days 7 to 70 at 7-day intervals postimmunization | Serum antibodies |
| 8 | 2 | Vaccine | None | 80 | Days 7 to 70 at 7-day intervals after 2 immunizations | Serum antibodies | |
| D: detection of cytokines and MHC expression | 9 | 1 | Vaccine | None | 6 | Day 7 after 1 immunization | Cytokines; MHC-I, MHC-II, CD40, CD154 mRNA |
| 10 | 2 | Vaccine | None | 6 | Day 7 after 2 immunizations | Cytokines; MHC-I, MHC-II, CD40, CD154 mRNA | |
| Control: no vaccinationc | 11 | None | None | None | 179 | None | None |
Five-day-old ducks were given first injection. Two injections were given 2 weeks apart.
The challenge dose for all three strains was 3 LD50. The serotypes were 1 (YL4), 2 (Yb2), and 10 (HXb2).
No-vaccination controls. Ninety ducks were used for challenge, 6 for mRNA detection, 3 for histological examination, and 80 for antibody detection.
Bacterial strains and culture conditions.
R. anatipestifer strains CH3 (serotype 1), NJ3 (serotype 2), and HXb2 (serotype 10) were selected as the candidate strains for developing the trivalent R. anatipestifer vaccine. Strains YL4 (serotype 1), Yb2 (serotype 2), and HXb2 (serotype 10) were used as the challenge strains based on our previous studies (14, 15, 16). All of the strains were isolated in China (17) and cultured in tryptic soy agar (TSA) (Difco, Franklin Lakes, NJ) at 37°C for 24 h in 5% CO2 or tryptic soy broth (TSB) (Difco) at 37°C and 150 rpm for 8 to 12 h.
Preparation of the trivalent R. anatipestifer-inactivated vaccine.
Montanide ISA 70 VG adjuvant was purchased from Seppic Shanghai Special Chemical Corporation (Shanghai, China). Strains CH3, NJ3, and HXb2 were cultured separately in TSB at 37°C for 10 h with shaking. The number of bacterial CFU for each strain was measured by detecting the optical density at 600 nm (OD600) (an OD600 of 1 equals 2.5 × 109 CFU of bacteria according to our calculations) and adjusting to 3.0 × 1010 CFU/ml, 1.5 × 1010 CFU/ml, and 1.5 × 1010 CFU/ml for strains CH3, NJ3, and HXb2, respectively. The bacteria were then inactivated with 0.4% (vol/vol) formalin at 37°C for 16 h. The trivalent R. anatipestifer vaccine was made by blending 1 volume each of inactivated strains CH3, NJ3, and HXb2 and 7 volumes of Montanide ISA 70 VG adjuvant according to the manufacturer's protocol. Each duck was subcutaneously injected in the neck with 0.3 ml of the vaccine containing 109, 5 × 108, and 5 × 108 CFU bacterial cells for strains CH3, NJ3, and HXb2, respectively. These doses were based on the results from our previous experiments (14, 15, 16).
Vaccination and challenge studies.
Five-day-old Cherry Valley Pekin ducks (288 ducks, experiment A) were divided into four groups of 72. Each group received one subcutaneous injection in the neck with the vaccine (group 1), saline in adjuvant (groups 2 and 4), or two injections in the neck with the vaccine (group 3). The two injections were given 2 weeks apart. R. anatipestifer strain YL4 (serotype 1), strain Yb2 (serotype 2), and strain HXb2 (serotype 10) were used for the animal challenge experiment. Before the challenge, the median lethal dose (LD50) for each strain was measured as 4.74 × 106 CFU/ml, 1.07 × 105 CFU/ml, and 82 CFU/ml, respectively, as described previously (14, 15, 16). On days 14, 42, and 70 after immunization, eight ducks from each group were challenged with YL4, Yb2, or HXb2 by subcutaneous injection at a dose of 3 LD50 in 0.5 ml saline. We recorded the death of any ducks or ducks that were showing clinical signs daily for a period of 7 days. These data, combined with the data on bacterial isolates from the livers and brains of all live ducks at day 7 postchallenge, were used to evaluate the protection rate of the vaccine against the challenge. The live ducks with no clinical signs and no isolation of bacteria from the livers and brains during the observation period after challenge were considered protected.
Evaluation of vaccine safety and protection.
The habits, appetites, and injection sites of the vaccinated ducks were observed for 7 days postvaccination (p.v.) to evaluate the safety of the vaccine. Clinical observations of diarrhea, tremors, and limb swelling, the death rate, yellow-white exudate identified postmortem, and congestion throughout the body in a period of 7 days, as well as bacterial isolates from the livers and brains of all live ducks at day 7 postchallenge, were investigated to evaluate the protection rate of the vaccine against the challenge. Furthermore, histological examinations (experiment B) of the livers, spleens, and brains at day 21 after two immunizations (group 5) or at day 14 after the challenge of ducks with two immunizations (group 6) were performed to evaluate the protection rate of the vaccine against the challenge. For histological examination, the liver, spleen, and brain samples were collected, fixed in 15% neutral buffered formaldehyde for 24 to 48 h, sliced to 3- to 4-μm thicknesses with a rotary microtome (Leica BM2016, Germany), and stained with hematoxylin and eosin, as described previously (18). The samples from ducks with no immunization or no challenge were used as pathogen-negative controls, and those from ducks with no immunization plus a challenge were used as pathogen-positive controls.
Determination of serum antibody levels.
Eight ducks in groups 7 and 8 (experiment C) were bled at each time point from day 7 to 70 postimmunization(s) at 7-day intervals. The antisera were isolated to determine the antibody levels against R. anatipestifer strains CH3, NJ3, and HXb2 with an enzyme-linked immunosorbent assay (ELISA), as described previously (13) but with modification. Eight ducks in group 11 (control) were bled at each time point; the sera were isolated and used as negative controls. Specifically, 96-well ELISA plates were coated with 5 × 106 CFU/well of each strain in 50 μl bicarbonate buffer (pH 9.6) and heat-dried overnight at 50°C. The duck sera were diluted in 2-fold steps from 1:100 to 1:25,600 for the experiment. The resulting OD450 was obtained with a plate reader (Synergy 2; BioTek). The highest dilution of the sera with an OD450 value of >2.1 times that of the negative control wells was valued as the ELISA titers. All the samples were performed in triplicate. Data are presented as the means of three independent experiments.
Determination of cytokine production.
To determine the cytokine production, as well as the expression of major histocompatibility complex (MHC) and costimulatory molecules postvaccination, 12 ducks were divided into two groups of six and given one or two injections of the vaccine (experiment D, group 9 or 10, respectively), as performed in ducks in group 1 or group 3. The levels of gamma interferon (IFN-γ), interleukin 2 (IL-2), IL-4, and IL-10 in the serum samples from groups 9 and 10 were measured at day 7 after each immunization using ELISA kits (Hermes Criterion Biotechnology (HCB), Elixir Canada Medicine Company, Ltd., Vancouver, BC, Canada) according to the manufacturer's instructions. The levels of cytokines were determined with the help of standard curves and are expressed in picograms per milliliter. The serum samples were diluted in 5-fold steps in phosphate-buffered saline (PBS)–0.5% Tween 20 (PBST) for the detections. The OD value was read at 490 nm with a plate reader (BioTek) within 15 min. PBST was used as the negative control. All experiments on standard dilutions, controls, and experimental samples were carried out in triplicate on the same plate, and each reaction plate contained the standard curve for the cytokine in the same preparation.
Analysis of MHC and costimulatory molecule expression by real-time PCR.
Real-time PCR was performed to measure the mRNA levels of MHC-I, MHC-II, CD40, and CD154 in the ducks in groups 9 and 10 using SYBR Premix Ex Taq reagents (TaKaRa, Dalian, China) and the Mastercycler ep realplex4 apparatus (Eppendorf, Hamburg, Germany) at day 7 after each immunization. Duck glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous control. Primers were designed using Primer Express software v3.0 (Applied Biosystems, Foster City, CA) and are listed in Table 2. Total RNA was extracted from spleen samples with a total RNA extraction kit (Tiangen, Beijing, China). cDNA synthesis was performed using an iScript cDNA synthesis kit (Promega) following the manufacturer's instructions. All of the real-time PCRs were conducted in a final volume of 20 μl of power SYBR green PCR master mix (Applied Biosystems, Foster City, CA) containing fast-start Taq DNA polymerase for a “hot start” and DNA-intercalating SYBR green 1 dye for the detection. The reaction program was 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min, followed by one cycle of 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s. All experiments on standard dilutions, controls, and experimental samples were carried out in triplicate on the same plate. The expression levels of the target genes were calculated relative to the expression of the GAPDH gene and presented as the fold expression relative to the control samples, as described previously (19).
Table 2.
Primers used for real-time PCR analysis of gene expression
| Gene | Direction | Primer sequence | Product size (bp) | GenBank accession no. |
|---|---|---|---|---|
| GAPDH | Forward | ATGTTCGTGATGGGTGTGAA | 176 | AY436595.1 |
| Reverse | CTGTCTTCGTGTGTGGCTGT | |||
| MHC-I | Forward | GAAGGAAGAGACTTCATTGCCTTGG | 196 | AB115246.1 |
| Reverse | CTCTCCTCTCCAGTACGTCCTTCC | |||
| MHC-II | Forward | CCACCTTTACCAGCTTCGAG | 229 | AY905540.1 |
| Reverse | CCGTTCTTCATCCAGGTGAT | |||
| CD40 | Forward | CTGAGAAGAGGCAGCCGATT | 132 | EU918729 |
| Reverse | TCAGCTTCCCTTTGTGGTAGGA | |||
| CD154 | Forward | CCACATGGCAGGACTGAAGAG | 110 | DQ267671 |
| Reverse | CCTTCAGCTTCCCTTTGTGGTA | |||
| IL-2 | Forward | TTCGCCAAGAGCTGACCAA | 161 | AF294323.1 |
| Reverse | AAGAGCATACAGTGGTCCCAAAG | |||
| IFN-γ | Forward | GACCTCGTGGAACTGTCAAACC | 168 | AJ012254.1 |
| Reverse | AAAGGCCGTATGATGTCATTAACA | |||
| IL-6 | Forward | CGGGCTTTTCACCTTTCAGA | 107 | AB191038 |
| Reverse | TCGTTGCCAGATGCTTTGTG |
Statistical analysis.
Significance was determined with the two-tailed independent Student t test, and a P value of <0.05 was considered statistically significant.
RESULTS
Vaccine safety.
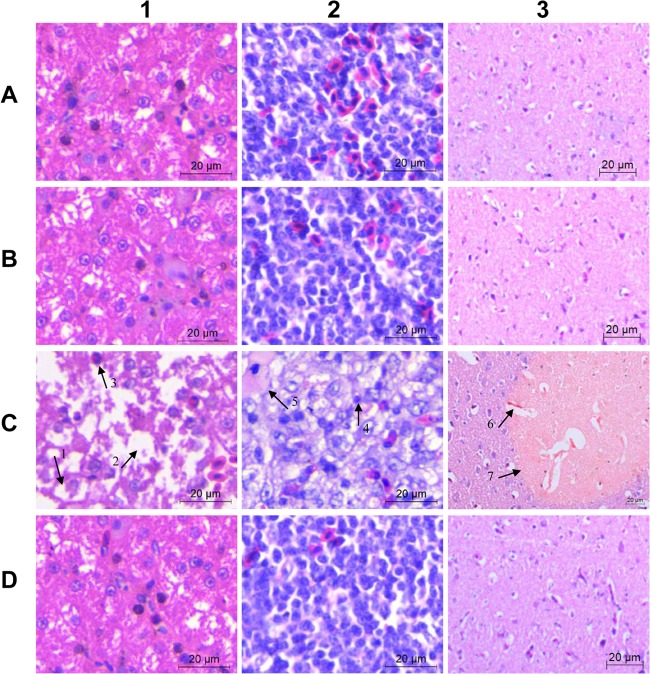
The vaccine was safe when given to 5-day-old ducks in three doses and was stable for over 12 months at room temperature without separation. The ducks injected with the vaccine showed no visible clinical signs of fever or coughing, and no pain or swelling problems were observed at the injection sites. Histological examinations revealed no visible pathological changes in the liver, spleen, or brain tissues when investigated at day 7 after vaccination (Fig. 1, B1, B2, and B3).
Fig 1.
Liver (column 1), spleen (column 2), and brain (column 3) tissues stained with hematoxylin and eosin (400× magnification). (Row A) Samples from nonvaccinated nonchallenged control ducks collected on day 21 postvaccination of the vaccinated group. (Row B) Samples from vaccinated nonchallenged control ducks collected day 21 postvaccination. (Row C) Samples from nonvaccinated challenged ducks collected 2 days after a YL4 challenge. (Row D) Samples from vaccinated challenged ducks collected 14 days after a YL4 challenge. In C1, arrow 1 points to hydropic degeneration in the liver, arrow 2 points to a large vacuole, most likely representing dropout of a hepatocyte, and arrow 3 points to a granulocyte. C2 shows diffuse vacuolation (edema) and decreased cellularity; arrow 4 points to a lymphocyte with a condensed nucleus, and arrow 5 points to a fibrin deposition. In C3, arrow 6 points to perivascular edema, and arrow 7 points to focal necrosis of a neuropile. Scale bars, 20 μm.
Animal challenge.
At day 14 postvaccination (p.v.), for the ducks in group 1 (one immunization), 8/8, 7/8, or 8/8 were protected, respectively, and all of the ducks in group 3 (two immunizations) were protected from challenges with virulent R. anatipestifer strains YL4, Yb2, and HXb2. At day 42 p.v., all of the ducks in groups 1 and 3 were protected from challenge with strain YL4, Yb2, or HXb2. In contrast, all of the ducks in groups 2 and 4 (control groups) were dead within 7 days after the challenge at day 14 or day 42 p.v. For ducks challenged at day 70 p.v., bacteria were isolated from blood at 24 h postchallenge, since the challenge might not have caused death in the nonvaccinated ducks of this age. The results showed that no bacteria were isolated from the ducks in groups 1 and 3; however, thousands of R. anatipestifer bacteria were counted per milliliter of blood from the ducks in groups 2 and 4. The protected ducks were in good health and had good appetites. They showed no clinical signs of diarrhea, tremors, or limb swelling, no postmortem yellow-white exudate or congestion throughout the body, and no death over a 7-day observation period. The livers, spleens, and brains from the protected ducks were examined histologically at day 14 after the challenge with YL4 and showed no pathological changes when compared with those from ducks that were not vaccinated or challenged with R. anatipestifer (Fig. 1, D1, D2, and D3). The samples from nonvaccinated ducks were collected at day 2 postchallenge and showed severe gross lesions of fibrinous exudate in the pericardial cavity, over the surface of the liver, and in the air sacs, as well as histopathological changes, such as vesicular degeneration, cell necrosis, and fibrinous exudation (Fig. 1, C1, C2, and C3). Challenges with Yb2 or HXb2 resulted in similar histopathological changes in liver, spleen, and brain tissues.
Determination of serum antibody levels.
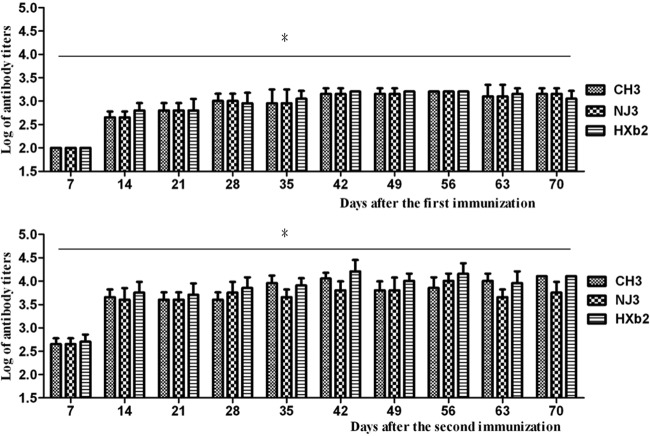
The antibodies against strains CH3, NJ3, and HXb2 were tested from day 7 to day 70 postimmunization at 7-day intervals. The sera isolated from normal ducks before immunization were used as negative controls. As shown in Fig. 2, the antibodies against the three strains were all significantly enhanced at day 7 and maintained at high levels up to day 70.
Fig 2.
Antibody titers after immunization with trivalent R. anatipestifer-inactivated vaccine. The antibody titers against R. anatipestifer strains CH3, NJ3, and HXb2 in immunized duck sera from day 14 after one immunization and day 7 after two immunizations were significantly enhanced compared with those in negative control sera (antibody titers were <1:100). Data were converted and expressed as log titers. The logarithmic mean antibody titers (mean ± SD) are from one representative experiment with eight animals (n = 8). Asterisks (*) indicate significant differences compared to nonvaccinated controls (P < 0.05, Student's t test).
Cytokine production.
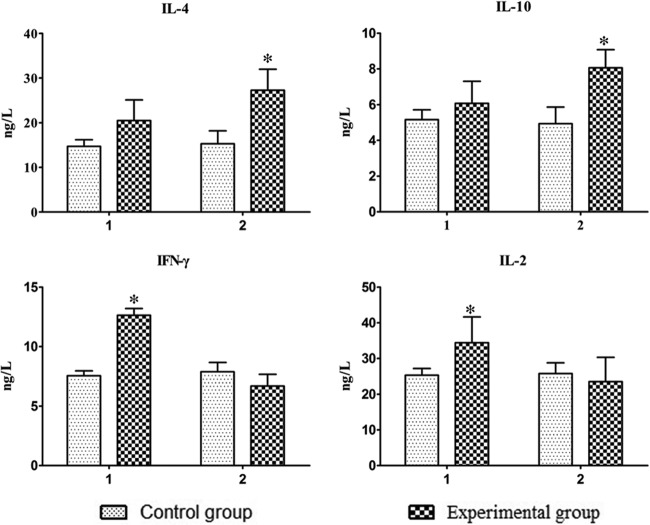
Cytokine production in the sera of vaccinated ducks was detected by an ELISA kit at day 7 after each immunization. As shown in Fig. 3, the vaccinated ducks produced significantly larger amounts of IFN-γ and IL-2 (the Th1 cytokines) after one immunization and larger amounts of IL-4 and IL-10 (the Th2 cytokines) after two immunizations than those in the control group (P < 0.05).
Fig 3.
Cytokines induced by immunization with the vaccine. Serum IL-4, IL-10, IFN-γ, and IL-2 were detected by an ELISA kit. Shown are the levels of the cytokines at day 7 after one immunization (1) or two immunizations (2). The bars show the means ± SD from six animals (n = 6). Asterisks (*) indicate significant differences compared to nonvaccinated controls (P < 0.05, Student's t test).
Expression of MHC and costimulatory molecules.
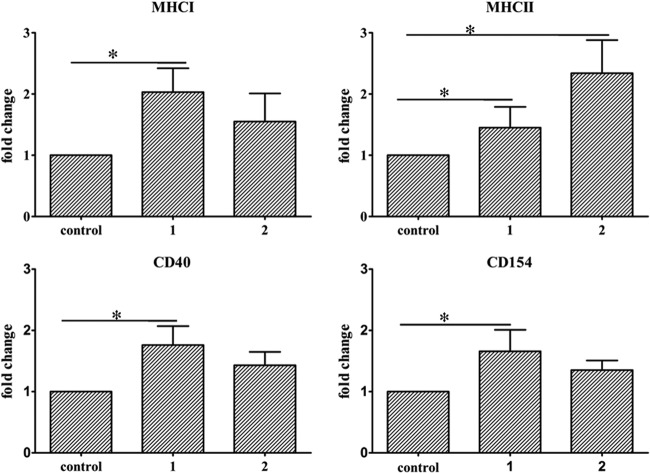
Differences in gene expression were measured as fold change using the GAPDH gene for normalization. As shown in Fig. 4, expressions of MHC-I and MHC-II molecules were significantly increased after the first immunization compared with that in the control group (P < 0.05). After two immunizations, only the expression of the MHC-II molecule was significantly increased (P < 0.05).
Fig 4.
Expression levels of MHC-I, MHC-II, CD40, and CD154 genes were determined by real-time quantitative PCR. Total RNA was extracted from the duck spleens. Duck GAPDH was used as an endogenous control. Total RNA from duck spleens after the injection of saline in adjuvant were used as a negative control. The expression levels are shown as the fold increase over those of the control groups. Control, negative control; 1, expression levels at day 7 after one immunization; 2, expression levels at day 7 after two immunizations. The error bars represent the means ± SD from six animals (n = 6). Asterisks (*) indicate significant differences compared to nonvaccinated controls (P < 0.05, Student's t test).
The expression of CD40 and its ligand CD154 genes were further examined by real-time PCR. The expression levels of CD40 and CD154 molecules were also upregulated significantly after the first immunization (P < 0.05) compared with those in the control group (Fig. 4).
DISCUSSION
In the present study, a trivalent R. anatipestifer-inactivated vaccine, including strains CH3 (serotype 1), NJ3 (serotype 2), and HXb2 (serotype 10), was successfully developed. Animal experiments demonstrated that the trivalent vaccine was effective in protecting the ducks from challenge with serotypes 1, 2, and 10 of the strains. The humoral and cellular immunities induced by the vaccination were also evaluated. The results showed that the vaccine induced high levels of antibodies and cytokine production after immunization. Moreover, MHC and costimulatory molecules were upregulated after immunization with the vaccine. These results indicate that the effective immune responses were induced by vaccination of the host.
Cytokines play a central role in the modulation of immune responses, which are important in directing the adaptive immune response toward a Th1- or Th2-type response. Cytokines might play a role in the protection conferred by vaccines (20). Our results show that the production of IFN-γ and IL-2 was increased after one immunization, and the production of IL-4 and IL-10 was increased after two immunizations, suggesting that both humoral and cellular immunities were induced after vaccination.
The expression of MHC molecules is very important for the antigen processing and presentation that lead to T cell activation. MHC expression by professional antigen-presenting cells is necessary for the effective activation of antigen-specific T cells (21). In addition to the MHC molecules, costimulatory molecules also play an essential role in the activation of T cells or antigen-presenting cells. In this study, we demonstrated that the mRNA levels of MHC-I and MHC-II were significantly upregulated in ducks vaccinated with the inactivated trivalent oil emulsion vaccine. Furthermore, the expression levels of costimulation molecules CD40 and CD154 were also increased by the vaccination. Involvement by the costimulation molecules, such as CD40, CD80, and CD86, is needed for T cell activation in mammals (22, 23). The expression patterns of MHC and costimulation molecules in the spleen were associated with immune responses in vaccinated ducks. Our data indicate that the vaccine can effectively mobilize the antigen presentation and costimulation pathways leading to the immune response. The results suggest that the vaccine might induce strong immune responses by upregulating antigen presentation and costimulation.
In summary, a safe trivalent R. anatipestifer-inactivated vaccine was developed successfully. The vaccine provided effective protection of ducks from challenges with R. anatipestifer serotypes 1, 2, and 10. Our study demonstrated the efficacy of the vaccine against serotypes 1, 2, and 10 of R. anatipestifer infection. This study has important implications for the effective control of R. anatipestifer infections in China.
ACKNOWLEDGMENTS
We thank Huoying Shi at Yangzhou University in China for technical support with the histological examinations.
This work was supported by the Special Fund for Agro-Scientific Research in the Public Interest (grant no. 201003012), the National Natural Science Foundation of China (31072161), the Shanghai Key Project on Agricultural Development through Science and Technology (2009HNG5-3), and the National Basic Fund for Research Institutes, which is supported by the Chinese Academy of Agricultural Sciences (2012JB15).
Footnotes
Published ahead of print 6 March 2013
REFERENCES
- 1. Sandhu TS. 2008. Riemerella anatipestifer infection, p 758–764 In Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE. (ed), Diseases of poultry, 12th ed. Blackwell Publishing, Hoboken, NJ [Google Scholar]
- 2. Kardos G, Nagy J, Antal M, Bistyak A, Tenk M, Kiss I. 2007. Development of a novel PCR assay specific for Riemerella anatipestifer. Lett. Appl. Microbiol. 44: 145–148 [DOI] [PubMed] [Google Scholar]
- 3. Sun N, Liu JH, Yang F, Lin DC, Li GH, Chen ZL, Zeng ZL. 2012. Molecular characterization of the antimicrobial resistance of Riemerella anatipestifer isolated from ducks. Vet. Microbiol. 158: 376–383 [DOI] [PubMed] [Google Scholar]
- 4. Subramaniam S, Huang B, Loh H, Tan H-M, Chua K-L, Frey J, Kwang J. 2000. Characterization of a predominant immunogenic outer membrane protein of Riemerella anatipestifer. Clin. Diagn. Lab. Immunol. 7: 168–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu Q, Liu X, Miao J, Zhao D, Zhang L, Ding C. 2001. The epidemiology study of Riemerella anatipestifer infection in Jiangsu and Anhui provinces. Guide Chin. Poult. 18: 18–19 [Google Scholar]
- 6. Chen YP, Lee SH, Chou CH, Tsai HJ. 2012. Detection of florfenicol resistance genes in Riemerella anatipestifer isolated from ducks and geese. Vet. Microbiol. 154: 325–331 [DOI] [PubMed] [Google Scholar]
- 7. Chen YP, Tsao MY, Lee SH, Chou CH, Tsai HJ. 2010. Prevalence and molecular characterization of chloramphenicol resistance in Riemerella anatipestifer isolated from ducks and geese in Taiwan. Avian Pathol. 39: 333–338 [DOI] [PubMed] [Google Scholar]
- 8. Sandhu T. 1979. Immunization of white Pekin ducklings against Pasteurella anatipestifer infection. Avian Dis. 23: 662–669 [PubMed] [Google Scholar]
- 9. Sandhu T, Layton HW. 1985. Laboratory and field trials with formalin-inactivated Escherichia coli (O78) Pasteurella anatipestifer bacterin in white Pekin ducks. Avian Dis. 29: 128–135 [PubMed] [Google Scholar]
- 10. Higgins DA, Henry RR, Kounev ZV. 2000. Duck immune responses to Riemerella anatipestifer vaccines. Dev. Comp. Immunol. 24: 153–167 [DOI] [PubMed] [Google Scholar]
- 11. Hu Q, Ding C, Tu J, Wang X, Han X, Duan Y, Yu S. 2012. Immunoproteomics analysis of whole cell bacterial proteins of Riemerella anatipestifer. Vet. Microbiol. 157: 428–438 [DOI] [PubMed] [Google Scholar]
- 12. Huang B, Subramaniam S, Frey J, Loh H, Tan HM, Fernandez CJ, Kwang J, Chua KL. 2002. Vaccination of ducks with recombinant outer membrane protein (OmpA) and a 41 kDa partial protein (P45N′) of Riemerella anatipestifer. Vet. Microbiol. 84: 219–230 [DOI] [PubMed] [Google Scholar]
- 13. Han X, Hu Q, Ding S, Chen W, Ding C, He L, Wang X, Ding J, Yu S. 2012. Identification and immunological characteristics of chaperonin GroEL in Riemerella anatipestifer. Appl. Microbiol. Biotechnol. 93: 1197–1205 [DOI] [PubMed] [Google Scholar]
- 14. Wang X, Hu QH, Han XG, Ding C, Yu SQ. 2012. Screening of serotype 2 Riemerella anatipestifer candidate strains for the production of inactivated oil-emulsion vaccine. Chin. J. Anim. Infect. Dis. 20: 54–58 [Google Scholar]
- 15. Wang X, Hu QH, Tong YL, Han XG, Ding C, Yu SQ. 2012. Study on the inactivated oil-emulsion vaccine of serotype 1 Riemerella anatipestifer. Chin. J. Prev. Vet. Med. 34: 313–316 [Google Scholar]
- 16. Wang X, Liu L, Liu HW, Mao S, Hu QH, Han XG, Ding C, Yu SQ. 2012. Study on inactivated oil-emulsion vaccine of serotype 10 Riemerella anatipestifer. China Poult. 34: 29–32 [Google Scholar]
- 17. Hu Q, Han X, Zhou X, Ding S, Ding C, Yu S. 2010. Characterization of biofilm formation by Riemerella anatipestifer. Vet. Microbiol. 144: 429–436 [DOI] [PubMed] [Google Scholar]
- 18. He S, Chen H, Jiao F. 2011. Improved preparation method for animal histopathological section. Prog. Vet. Med. 32: 130–132 [Google Scholar]
- 19. Liu H, Zhang M, Han H, Yuan J, Li Z. 2010. Comparison of the expression of cytokine genes in the bursal tissues of the chickens following challenge with infectious bursal disease viruses of varying virulence. Virol. J. 7: 364 doi:10.1186/1743-422X-7-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abdul-Careem, Hunter DB, Lambourne MD, Read LR, Parvizi P, Sharif S. 2008. Expression of cytokine genes following pre- and post-hatch immunization of chickens with herpesvirus of turkeys. Vaccine 26: 2369–2377 [DOI] [PubMed] [Google Scholar]
- 21. Byrne SN, Halliday GM. 2003. High levels of Fas ligand and MHC class II in the absence of CD80 or CD86 expression and a decreased CD4+ T cell infiltration, enables murine skin tumours to progress. Cancer Immunol. Immunother. 52: 396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang Y, Jin H, Zheng G, Xie Q, Yin J, Yu Y, Xiao C, Zhang X, Chen A, Wang B. 2005. The adjuvant effect of levamisole on killed viral vaccines. Vaccine 23: 5543–5550 [DOI] [PubMed] [Google Scholar]
- 23. Tan P, Anasetti C, Hansen JA, Melrose J, Brunvand M, Bradshaw J, Ledbetter JA, Linsley PS. 1993. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1. J. Exp. Med. 177: 165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]