Abstract
Interleukin-21 (IL-21) is a cytokine whose actions are closely related to B cell differentiation into plasma cells as well as to CD8+ cytolytic T cell effector and memory generation, influencing the T lymphocyte response to different viruses. X-linked lymphoproliferative syndrome type 1 (XLP-1) is a primary immunodeficiency syndrome that is characterized by a high susceptibility to Epstein-Barr virus. We observed in a pediatric patient with XLP-1 that IL-21 was expressed in nearly all peripheral blood CD4+ and CD8+ T cells. However, IL-21 could not be found in the lymph nodes, suggesting massive mobilization of activated cells toward the infection's target organs, where IL-21-producing cells were detected, resulting in large areas of tissue damage.
CASE REPORT
Our patient was a 10-month-old Caucasian male, born full-term to a nonconsanguineous couple, who had a medical record of bronchiolitis and ear, nose, and throat infections. The child was referred to our Pediatric Infectious Disease Unit because of fever (38.5°C to 39.5°C) lasting 11 days, associated with a nonpruritic erythematous rash, tonsillitis, cervical lymphadenopathies, and hepatomegaly (3 cm below the costal edge). Neither splenomegaly nor abdominal lymph node enlargement was detected on admission. At this point, the blood values showed a white blood cell count of 20,900/μl (lymphocytes, 72%), thrombocytopenia (72,000/μl), a raised C-reactive protein level, and a mild elevation of liver enzymes (aspartate aminotransferase [AST], 120 U/liter, alanine aminotransferase [ALT], 111 U/liter, and γ-glutamyl transferase [GGT], 324 U/liter). Specific IgM and IgG antibodies to cytomegalovirus (CMV) and Epstein-Barr virus (EBV) were found, with the latter at a high titer (anti-EBV IgG, >1/640). Viral loads were determined by means of PCRs. PCR values for EBV found in infectious mononucleosis (IMN) patients ranged from 6,541 copies/ml to 11,476 copies/ml. The value detected in the patient was as high as 50,368 copies/ml for EBV (human herpesvirus 4 [HHV-4]) but <600 copies/ml for CMV. EBV infection was diagnosed.
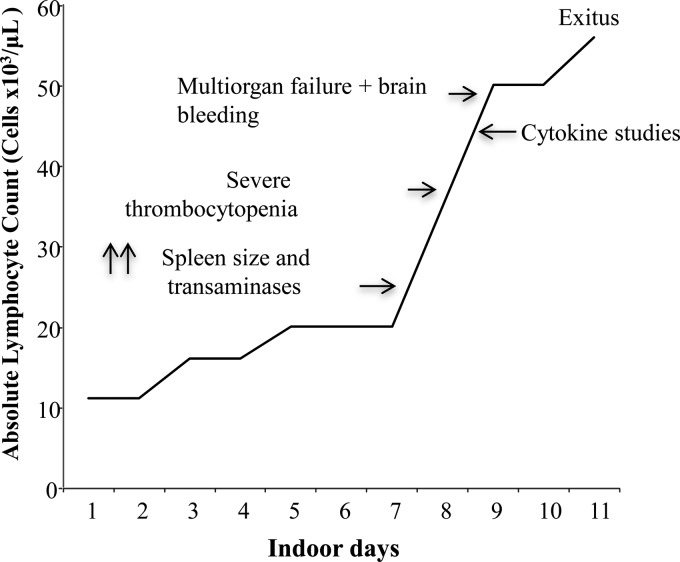
The patient's general condition worsened after 72 h, and he developed splenomegaly (14 cm) and basal right pneumonia, which was treated with 50 mg cefotaxime/kg of body weight intravenously every 12 h in the absence of microbiological culture data. The fever disappeared, and the patient's condition remained stable for a week, but the fever returned (39.5°C) and was accompanied by pronounced jaundice (bilirubin, >4 mg/dl; AST, 843 U/liter; ALT, 339 U/liter; and GGT, 1,233 U/liter). High levels of ferritin (2,682 ng/ml) and plasma triglycerides (240 mg/dl), low levels of hemoglobin (7.3 g/dl), and a lymphocyte count of >50,000/μl were detected. Within the next 24 h, severe thrombocytopenia occurred (<40,000 platelets/μl) along with general tonic-clonic seizures, brain front-lobe bleeding, and generalized cerebral edema (as observed on a computed tomography [CT] scan), which evolved in the subsequent 16 h to respiratory distress, hemodynamic shock, multiorgan failure, and exitus. The main clinical events that occurred in this X-linked lymphoproliferative syndrome type 1 (XLP-1) patient are represented in Fig. 1.
Fig 1.
Clinical course of the XLP-1 patient from hospital inception.
XLP-1 is a primary immunodeficiency syndrome characterized by a high susceptibility to Epstein-Barr virus (EBV) (1–3). The disease is caused by germ line mutations in the SH2D1A gene, which encodes the adaptor molecule signaling lymphocytic activation molecule (SLAM)-associated protein (SAP) (4, 5). This protein modulates the signal transduction of SLAM family receptors in T lymphocytes, natural killer (NK) cells, and natural killer T (NKT) cells (6, 7), influencing their cytotoxic ability and cytokine regulation (8–10). The loss of a functional SAP results in both an impaired ability of cytotoxic cells to clear the EBV infection and overexpression of proinflammatory cytokines by T and NK cells (11).
Interleukin 21 (IL-21) is a cytokine that is produced mainly by CD4+ cells but also by CD8+ lymphocytes in different human diseases (12, 13). In EBV-infected B cells, IL-21 induces the expression of EBV genes, such as the latent membrane protein 1 (LMP1) gene, thus providing viral peptides that are recognizable by the immune system (14–16). Indeed, IL-21 is critical for CD8+ T cell survival and memory generation (17–21), as well as for promoting the activity of CD8+ T cell effectors during viral infections (22–24), enhancing the cytotoxic response to virally infected cells by NK cells and CD8+ T lymphocytes (25–27).
However, despite the well-known involvement of IL-21 in EBV infection, direct evidence of its participation in the mechanisms leading to, or maintaining, the lymphoproliferative response to EBV infection in SAP-deficient (SAPneg) patients is lacking. We report here the results for the expression, production, and function of IL-21 in a SAPneg pediatric patient with a fatal EBV infection.
Human subjects.
T cells from a SAPneg patient were analyzed during a fatal evolution of EBV infection. In this study, seven pediatric patients diagnosed with infectious mononucleosis and seven age- and sex-matched healthy donors were included to serve as controls. For this purpose, written informed consent from donors' and patients' parents as well as approval from the Institutional Review Board of The Reina Sofia University Hospital was obtained.
Gene analysis procedure.
Genomic DNA was extracted from whole peripheral blood using a Maxwell 16 blood DNA purification kit on a Maxwell DNA extraction device (Promega, USA). All coding exons and intronic boundaries of the PRF1, STX11, UNC13D, and SH2D1A genes were amplified by PCR. The PCR amplicons were purified with an illustra ExoStar one-step kit (GE Healthcare, USA); bidirectional fluorescence sequencing was performed with an ABI BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, USA), and samples were run on an automated ABI 3730 XL DNA analyzer.
Flow cytometry.
For surface-directed staining, cells were incubated with relevant fluorochrome-conjugated mouse anti-human monoclonal antibodies (MAb) on ice for 30 min in the dark and washed twice before analysis. Fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, PE-Cy7-, antigen-presenting cell (APC)-, or Alexa-Fluor 647-conjugated anti-CD3, -CD4, or -CD8 MAb and isotype-matched control mouse IgG1 and IgG2 MAb were used as isotype controls (all from BD Biosciences, San José, CA, USA). For intracellular staining, T cells from IMN patients and controls were activated with a lymphocyte activation cocktail consisting of 25 ng/ml phorbol myristate acetate (PMA) and 1 μg/ml ionomycin (Sigma-Aldrich Spain) for 6 h at 37°C, prior to being stained, fixed, and permeabilized with the Cytofix/Cytoperm kit and a BD Golgi plug (BD Pharmingen, USA), according to the manufacturer's protocol; an inactivated control was included in the assays and treated with brefeldin A (BD Pharmingen, San Diego, CA, USA) only. For analysis of the XLP-1 patient's T peripheral blood cells, the previous treatment was not performed, as they were found to be already activated. The following anti-human MAb were used: anti-gamma interferon (anti-IFN-γ) and anti-tumor necrosis factor alpha (TNF-α) (both from BD Biosciences, USA), anti-IL-22 (R&D Systems, Minneapolis, MN, USA), anti-IL-17 and anti-IL-21 (eBioscience, USA), and anti-IL-4, anti-IL-5, and anti-IL-10 (BD Pharmingen, USA). Mouse IgG1 and IgG2 were used as isotype controls. Flow cytometry was performed on a FACSCalibur flow cytometer (BD Biosciences, San José, CA, USA), and data were analyzed using the Cell Quest Pro software (BD Biosciences, San José, CA, USA).
Immune cell isolation and cultures.
Single-cell lymphocyte suspensions were isolated from peripheral blood mononuclear cells (PBMC), thymus, and lymph nodes by gradient centrifugation with Ficoll-Paque (Sigma-Aldrich, Spain). Cells were cultured in complete medium, consisting of RPMI 1640 medium containing 2 mM glutamine, 100 units/ml penicillin, and 100 mg/ml streptomycin and supplemented with 10% fetal bovine serum (FBS) (all from BioWhittaker, Belgium) at 37°C in a humidified atmosphere containing 5% CO2. For some experiments, peripheral blood lymphocytes (PBL) from IMN patients and controls were stimulated in the presence of anti-CD3/CD28-coated beads (1 bead/cell) (human T-Expander CD3/CD28; Invitrogen Dynal Biotech ASA, Norway) for 24 h. An EBV cell line was used as the stimulus in experiments addressing the composition of IL-21-producing T cells in response to prolonged exposure to EBV-infected cells. Briefly, 106 PBL/well were cocultured with 106 irradiated (3,500 rads) EBV-infected cells/well. Cells were plated at a final volume of 2 ml/well in 24-well culture plates (Nunc, Denmark) for 15 days. At day 7 of incubation, T cells were restimulated with the same number of EBV cells after careful removal of 1 ml of culture medium.
IL-21 production and proliferation assays.
The production and role of IL-21 in the proliferative response observed were studied as previously described (13). Briefly, carboxyfluorescein succinimidyl ester (CFSE; 5 μM; Sigma-Aldrich, Spain)-labeled T cells (5 × 104 cells/well) were stimulated with anti-CD3/CD28-coated beads (human T-Expander; 1 bead/cell) in the absence or presence of anti-IL-21 MAb (10 μg) in complete medium. After 3 days, proliferation was determined on a FACSCalibur flow cytometer with CellQuest Pro software. IL-21 and IFN-γ levels were determined in sera and 24-h culture supernatants from all studied subjects using enzyme-linked immunosorbent assays (ELISAs) according to manufacturer instructions (Med Systems, USA).
Immunohistochemistry.
Paraffin-embedded sections from brain and liver (4 μm) were deparaffinized and then hydrated with graded alcohol solutions. Endogenous peroxidase was blocked by incubation with 0.75% hydrogen peroxide for 15 min, followed by antigen unmasking with heated citrate buffer (pH 9). Sections were stained separately with anti-IL-21 at a 1/100 optimal dilution (Millipore, USA), mouse anti-human anti-CD4 MAb, or anti-CD8 MAb (Dako, United Kingdom) for 30 min. Slides were developed with the EnVision FLEX+, mouse, high-pH link kit (Dako, United Kingdom) by incubation for 30 min at room temperature with the horseradish peroxidase anti-rabbit/anti-mouse complex (Dako, United Kingdom) as described by the manufacturer (Autostainer Link 48; Dako, United Kingdom). Staining was performed by incubating slides with diaminobenzidine as the chromogenic substrate for 5 min. To counterstain, we used EnVision FLEX hematoxylin (Dako, United Kingdom). As negative controls for each staining, the same procedure as mentioned previously was followed except that isotype control MAb was used.
On the basis of the clinical evolution as a fatal infectious mononucleosis, mutational analysis of the XLP-1 (SH2D1A) gene and of familial hemophagocytic lymphohistiocytosis (FHLH)-related genes (PRF1, STX11, and UNC13D) was performed. No defects were detected in any of the FHLH-causative genes. However, at exon 2 of the SH2D1A gene, the nonsense mutation p.R55X was detected, a genetic variant previously reported as a disease-causing mutation (28).
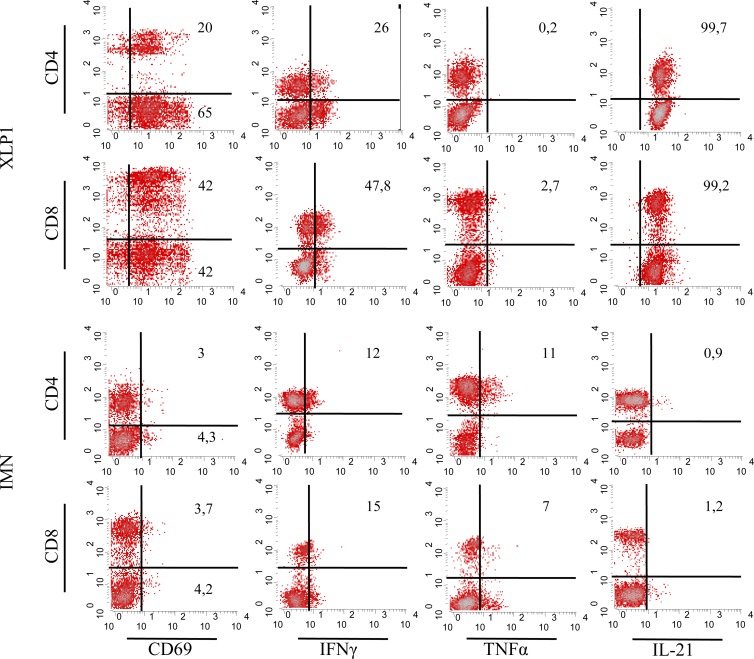
Ex vivo analysis of the T cell compartment of the SAPneg patient showed that, in addition to the number of the patient's circulating lymphocytes being increased, 85% of his T cells expressed CD69, denoting a T cell activation status that was not detected in EBV-infected SAP-positive (SAPpos) patients (Fig. 2, left column). To further analyze this massive activation of T cells, the production profiles of the Th1 (IFN-γ, TNF-α), Th2 (IL-4, IL-5, IL-10), and Th17 (IL-17, IL-21, IL-22) cytokine families were studied. Results showed that 26% of CD4+ cells and 47.8% of CD8+ T lymphocytes from the XLP-1 patient produced IFN-γ. Notably, T cells from the XLP-1 patient did not produce TNF-α, whereas CD4+ and CD8+ cells from IMN patients produced TNF-α and, to a lesser extent, IFN-γ (15% of CD8+ cells) (Fig. 2, two middle columns). Th2-related cytokine-producing cells were undetectable, except that IL-10 that was found in 7% of the total T cell population of the SAPneg patient (data not shown). Regarding the Th17 family, expression of IL-21 was detected in most of the peripheral blood T lymphocytes from the XLP-1 patient. IL-21 was expressed in only 2% of T cells from IMN patients (Fig. 2, right column). IL-17-producing T cells were absent in both the IMN patients and the SAP-deficient patient. IL-21 was found to be expressed in CD4+ and, remarkably, in CD8+ T cells. IL-21 and IFN-γ were coproduced by 26% of the CD4+ and 17% of the CD8+ T lymphocytes (Fig. 3A).
Fig 2.
T cell activation was assessed by expression of CD69 (left column). Intracellular IFN-γ, TNF-α, and IL-21 expression levels by CD4+ and CD8+ T cells from an XLP-1 patient (n = 1) and IMN patients (n = 7) are depicted in the middle and right columns, respectively. The results of a representative experiment are shown.
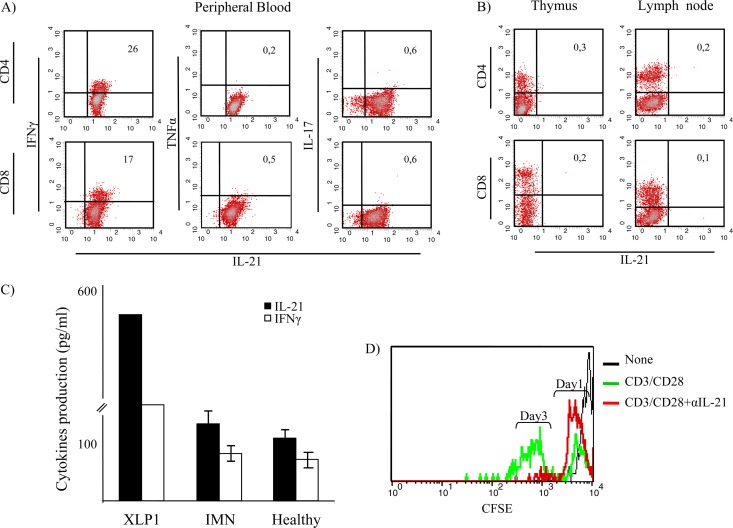
Fig 3.
Analysis of cytokine expression by T cell subtypes from the XLP-1 patient. IFN-γ was coexpressed by 26% of CD4+ T cells and 17% of CD8+ lymphocytes producing IL-21. (A) Peripheral blood T cells from the XLP-1 patient did not produce IL-17A. (B) Expression of IL-21 was not detected in single positive thymocytes or in lymph node T cells obtained from the XLP-1 patient in postmortem studies. (C) IL-21 and IFN-γ production levels by T cells from the XLP-1 patient, IMN patients (n = 7), and healthy individuals (n = 7) were measured in culture supernatants following stimulation with anti-CD3/CD28-coupled beads for 24 h. Results from the XLP-1 patient are expressed as the means of results from triplicate cultures; in the case of IMN patients and healthy individuals, results represent the means ± standard deviations (SD) obtained from 7 independent experiments. Neutralizing anti-IL-21 MAb inhibited T cell proliferation in all individuals tested. (D) Results obtained from the XLP-1 patient are shown.
IL-21-producing cells were absent from the lymph nodes and thymus, as revealed by single-cell suspension flow cytometry postmortem analysis (Fig. 3B). The thymus did not contain IL-21-expressing cells, despite a prominent population of thymocytes producing IL-17A (data not shown). The levels of IL-21 and IFN-γ were measured to assess whether cytoplasmic cytokines found in T cells were secreted following adequate stimulation. Results confirmed that cytokines produced by T cells from both the XLP-1 patient and IMN patients were released into the culture supernatant in vitro (Fig. 3C). The concentrations of IFN-γ, TNF-α, and IL-21 were also determined in the patient's serum. Serum from the XLP-1 patient contained elevated levels of IL-21 compared with levels in IMN patients and healthy controls (Table 1). To elucidate whether IL-21 plays a role in the lymphoproliferation of this syndrome, we studied the ability of neutralizing anti-IL-21 MAb to inhibit T cell proliferation. Peripheral blood mononuclear cells (106/ml) from the SAPneg patient, IMN patients, and healthy donors were stimulated with anti-CD3/CD28-coupled beads in the presence or absence of the relevant MAb. Results showed that neutralization of IL-21 nearly abrogated the proliferation of T cells in all the subjects studied, including T cells from the XLP-1 patient (Fig. 3D), supporting a relevant autocrine role for the cytokine in the lymphoproliferative activity of this disease.
Table 1.
Serum cytokines in EBV-infected patients and healthy controls
| Patient(s) (no.) | Amt (pg/ml) of: |
||
|---|---|---|---|
| IFN-γ | TNF-α | IL-21 | |
| XLP-1 | 163.2 ± 21.4 | 17.3 ± 6.5 | 334.4 ± 24.1 |
| IMN (7) | 69.1 ± 14.3 | 11.5 ± 9.6 | 45.8 ± 12.6 |
| Control (7) | 36.3 ± 8.8 | 8.6 ± 3.3 | 34.7 ± 14.2 |
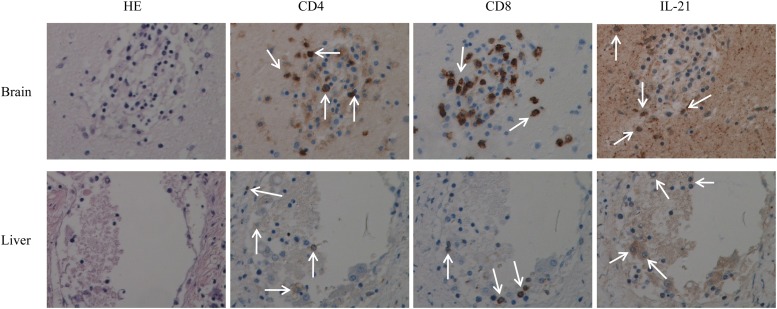
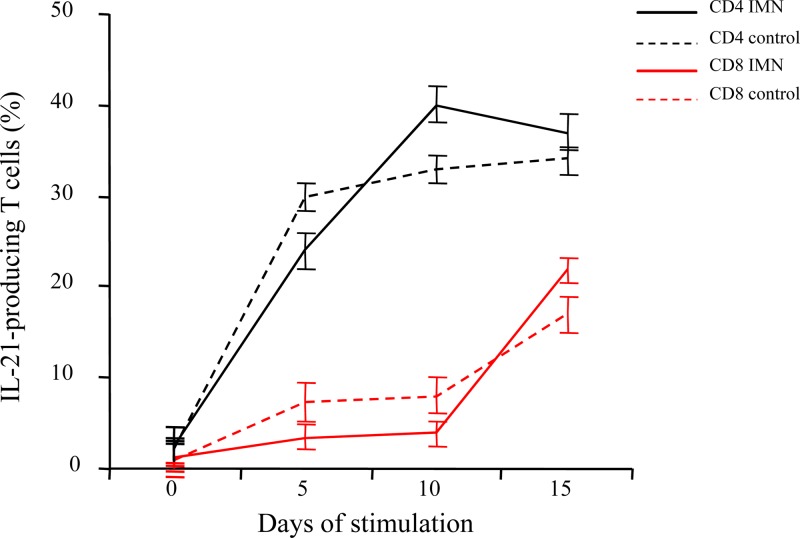
Immunochemistry studies demonstrated lymphocytic infiltration in the brain and liver, the attack-targeted organs in our patient. Infiltrating immune cell populations consisted of CD4+ and CD8+ T cells producing IL-21. These cells were found to occupy large areas of tissue destruction in both organs, strongly suggesting their participation in the mechanisms of organ damage of IL-21-producing cells in XLP-1 disease (Fig. 4). Finally, we studied the ability of a prolonged EBV cell line stimulation to generate IL-21-producing T cells and the compositions of the responding T cell populations in the IMN patient and controls. We found that in both groups, mainly CD4+ cells produced IL-21. Interestingly, the percentage of IL-21-producing CD8+ cells increased at the end of the culture period, reaching values of 17% ± 1.8% (controls) and 21% ± 2.2% (IMN) after 15 days in culture with EBV-infected cells (Fig. 5).
Fig 4.
Tissue sections from the brain (upper panels) and liver (lower panels) were used in immunochemistry studies to assess the presence of IL-21-producing T cells. CD4+ and CD8+ T lymphocytes were found to infiltrate brain and liver tissues. IL-21-producing T cell subtypes are shown (arrows point to stained cells for each marker). A representative immunohistochemistry (IHC) image (magnification, ×40) is shown. HE, hematoxylin and eosin.
Fig 5.
Peripheral blood lymphocytes from SAPpos IMN patients and healthy controls were stimulated in vitro with an EBV-transformed cell line. Numbers of T cells expressing IL-21 were determined at day 0 of culture and every 5 days afterwards. CD4+ T cells from IMN patients (solid black line) and controls (dashed black line) produced IL-21 in response to the EBV cell line. The percentages of IL-21+ cells within the CD8+ populations from patients (solid red line) and controls (dashed red line) were found to be increasing at day 15 of culture.
Discussion.
The clinical onset of XLP-1 is triggered by EBV infection. CD8+ T cells from patients with SH2D1A gene mutations show a defective lytic activity against EBV-infected cells that may have fatal consequences for the patient (29).
The main features of T lymphocytes from the XLP-1 patient in this study were an activated phenotype, a reduced proportion of cells producing IFN-γ, and the absence of TNF-α-positive cells, clearly pointing to T cell exhaustion (30, 31). Additionally, no IL-4 or other Th2 cytokines except for residual levels of IL-10 seemed to be present in the peripheral T lymphocytes of a patient with fatally evolved XLP-1. In the context of a productive anti-EBV humoral response (anti-EBV IgG being produced), the absence of IL-4 might reflect that this cytokine is no longer required for maintaining the ongoing antibody response (32). Instead, a strong polarization to IL-21 production was evidenced. Indeed, the large areas of tissue injury observed in the patient's brain and liver were strongly infiltrated by IL-21-producing lymphocytes, supporting their participation in tissue destruction. Given the cytotoxicity defect of the NK cells and specific cytolytic T lymphocytes, our observations raise the question of the mechanism leading to massive cell death and organ failure in SAP-deficient patients. A possible explanation suggesting that the defect of CD8+ T cell cytotoxicity from SAPneg individuals is restricted to antigens presented by B cells but not by other cell types has recently been offered (33). However, this hypothesis neither explains the role of HLA-I restriction of the EBV viral response nor provides the reason for the inability of NK cells to kill virus-infected cells (34). Our findings of IL-21-producing T lymphocytes within the affected organs suggest instead that once the EBV has gained access to organs containing non-B professional antigen-presenting cells (i.e., Kupffer cells, microglia), the EBV-specific T cells are attracted to them and organ tissue destruction is triggered.
The fact that CD8+ T cells from the XLP-1 patient also expressed and secreted IL-21 is in sharp contrast with the composition of the IL-21-producing population from SAPpos IMN patients and controls, which consisted mainly of CD4+ T cells. Interestingly, when EBV-infected cells were used in vitro as a persistent stimulus for T cells, a higher percentage of CD8+ cells expressed IL-21, pointing to sustained viral exposure as being responsible for the massive IL-21 production by SAPneg T lymphocytes. Notably, the population producing IL-21 consisted of either CD4+ or CD8+ cells, raising some points related to the fine-tuning of T cells involved in antiviral responses, among them, the mechanisms responsible for the regulation of IL-21 production in CD4+ and CD8+ cells and their requirements for antigen presentation and costimulation.
Previous studies have shown the importance of IL-21 in the generation, maintenance, and survival of virus-specific CD8+ T cells. It is known that IL-21 augments the proliferation of resting CD8+ T cells in vitro and promotes antigen-specific CD8+ T cell expansion and the response of CD8+ T cells to some viral antigens in vivo (35, 36). Therefore, direct IL-21 signaling on CD8+ T cells seems critical for the proliferation of antivirus-specific CD8+ T cells in vivo. On this basis, we investigated the role of autocrine IL-21 in the XLP-1 patient's T cell-proliferative activity. Our results showed a significant inhibition of T cell proliferation when the cells were cultured in the presence of anti-IL-21-neutralizing MAb. Importantly, the observation that the IL-21 serum levels were elevated in the XLP-1 patient is of interest, as it may, in a clinical setting, help to provide a simple and rapid diagnosis of a condition that must be considered a medical emergency, especially when a fatal evolution occurs, as in the case of the patient reported here. Consequently, studies on the suitability of anti-IL-21 strategies that might open new paths to finding tools for an earlier diagnosis, as well as be a more adequate therapeutic resource for XLP-1 patients, are on course. Nevertheless, we are aware of the limitations derived from the fact that data were obtained from a single XLP-1 patient and, therefore, should be cautiously interpreted. In this context, it is of importance that the patient did not receive any therapeutic agent known to increase IL-21 production by T cells. However, whether the patient in this study was idiosyncratic and did not represent a typical patient with XLP-1 should also be kept in mind. Indeed, organ failure secondary to a persistent, noncleared EBV infection may contribute to IL-21 serum elevation and IL-21-producing T cell bias in peripheral blood. However, the uniqueness of the clinical setting from which the data were obtained reinforces the interest of these results, which may help in expanding our understanding of the immunopathogenic mechanisms underlying XLP-1.
ACKNOWLEDGMENTS
This work was supported by grants (to M.S.) SAF2006-09991 from the Spanish Ministry of Education and 0156/05 from the Consejería de Salud, Junta de Andalucía, Spain.
Footnotes
Published ahead of print 6 March 2013
REFERENCES
- 1.Bar RS, DeLor CJ, Clausen KP, Hurtubise P, Henle W, Hewetson JF. 1974. Fatal infectious mononucleosis in a family. N. Engl. J. Med. 290:363–367 [DOI] [PubMed] [Google Scholar]
- 2.Provisor AJ, Iacuone JJ, Chilcote RR, Neiburger RG, Crussi FG. 1975. Acquired agammaglobulinemia after a life-threatening illness with clinical and laboratory features of infectious mononucleosis in three related male children. N. Engl. J. Med. 293:62–65 [DOI] [PubMed] [Google Scholar]
- 3.Purtilo DT, Cassel CK, Yang JP, Harper R. 1975. X-linked recessive progressive combined variable immunodeficiency (Duncan's disease). Lancet i:935–940 [DOI] [PubMed] [Google Scholar]
- 4.Coffey AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, Cahn AP, Durham J, Heath P, Wray P, Pavitt R, Wilkinson J, Leversha M, Huckle E, Shaw-Smith CJ, Dunham A, Rhodes S, Schuster V, Porta G, Yin L, Serafini P, Sylla B, Zollo M, Franco B, Bolino A, Seri M, Lanyi A, Davis JR, Webster D, Harris A, Lenoir G, de St Basile G, Jones A, Behloradsky BH, Achatz H, Murken J, Fassler R, Sumegi J, Romeo G, Vaudin M, Ross MT, Meindl A, Bentley DR. 1998. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat. Genet. 20:129–135 [DOI] [PubMed] [Google Scholar]
- 5.Nichols KE, Harkin DP, Levitz S, Krainer M, Kolquist KA, Genovese C, Bernard A, Ferguson M, Zuo L, Snyder E, Buckler AJ, Wise C, Ashley J, Lovett M, Valentine MB, Look AT, Gerald W, Housman DE, Haber DA. 1998. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl. Acad. Sci. U. S. A. 95:13765–13770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichols KE, Hom J, Gong SY, Ganguly A, Ma CS, Cannons JL, Tangye SG, Schwartzberg PL, Koretzky GA, Stein PL. 2005. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat. Med. 11:340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veillette A. 2003. SAP: a molecular switch regulating the immune response through a unique signaling mechanism. Eur. J. Immunol. 33:1141–1144 [DOI] [PubMed] [Google Scholar]
- 8.Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D, Nichols KE, Antonellis A, Koretzky GA, Gardner K, Schwartzberg PL. 2004. SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-kappaB1. Immunity 21:693–706 [DOI] [PubMed] [Google Scholar]
- 9.Dupre L, Andolfi G, Tangye SG, Clementi R, Locatelli F, Arico M, Aiuti A, Roncarolo MG. 2005. SAP controls the cytolytic activity of CD8+ T cells against EBV-infected cells. Blood 105:4383–4389 [DOI] [PubMed] [Google Scholar]
- 10.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. 2008. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature 455:764–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benoit L, Wang X, Pabst HF, Dutz J, Tan R. 2000. Defective NK cell activation in X-linked lymphoproliferative disease. J. Immunol. 165:3549–3553 [DOI] [PubMed] [Google Scholar]
- 12.Ortega C, Fernandez AS, Carrillo JM, Romero P, Molina IJ, Moreno JC, Santamaria M. 2009. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J. Leukoc. Biol. 86:435–443 [DOI] [PubMed] [Google Scholar]
- 13.Fernandez S, Molina IJ, Romero P, Gonzalez R, Pena J, Sanchez F, Reynoso FR, Perez-Navero JL, Estevez O, Ortega C, Santamaria M. 2011. Characterization of gliadin-specific Th17 cells from the mucosa of celiac disease patients. Am. J. Gastroenterol. 106:528–538 [DOI] [PubMed] [Google Scholar]
- 14.Kis LL, Salamon D, Persson EK, Nagy N, Scheeren FA, Spits H, Klein G, Klein E. 2010. IL-21 imposes a type II EBV gene expression on type III and type I B cells by the repression of C- and activation of LMP-1-promoter. Proc. Natl. Acad. Sci. U. S. A. 107:872–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konforte D, Simard N, Paige CJ. 2008. Interleukin-21 regulates expression of key Epstein-Barr virus oncoproteins, EBNA2 and LMP1, in infected human B cells. Virology 374:100–113 [DOI] [PubMed] [Google Scholar]
- 16.Konforte D, Paige CJ. 2009. Interleukin-21 regulates expression of the immediate-early lytic cycle genes and proteins in Epstein-Barr Virus infected B cells. Virus Res. 144:339–343 [DOI] [PubMed] [Google Scholar]
- 17.Yi JS, Du M, Zajac AJ. 2009. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324:1572–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi JS, Ingram JT, Zajac AJ. 2010. IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J. Immunol. 185:4835–4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues L, Nandakumar S, Bonorino C, Rouse BT, Kumaraguru U. 2009. IL-21 and IL-15 cytokine DNA augments HSV specific effector and memory CD8+ T cell response. Mol. Immunol. 46:1494–1504 [DOI] [PubMed] [Google Scholar]
- 20.Novy P, Huang X, Leonard WJ, Yang Y. 2011. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J. Immunol. 186:2729–2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elsaesser H, Sauer K, Brooks DG. 2009. IL-21 is required to control chronic viral infection. Science 324:1569–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spolski R, Leonard WJ. 2008. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 26:57–79 [DOI] [PubMed] [Google Scholar]
- 23.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. 2000. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408:57–63 [DOI] [PubMed] [Google Scholar]
- 24.Nutt SL, Brady J, Hayakawa Y, Smyth MJ. 2004. Interleukin 21: a key player in lymphocyte maturation. Crit. Rev. Immunol. 24:239–250 [DOI] [PubMed] [Google Scholar]
- 25.Parrish-Novak J, Foster DC, Holly RD, Clegg CH. 2002. Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J. Leukoc. Biol. 72:856–863 [PubMed] [Google Scholar]
- 26.Leonard WJ, Zeng R, Spolski R. 2008. Interleukin 21: a cytokine/cytokine receptor system that has come of age. J. Leukoc. Biol. 84:348–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spolski R, Wang L, Wan CK, Bonville CA, Domachowske JB, Kim HP, Yu Z, Leonard WJ. 2012. IL-21 promotes the pathologic immune response to pneumovirus infection. J. Immunol. 188:1924–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, van Schaik S, Notarangelo L, Geha R, Roncarolo MG, Oettgen H, De Vries JE, Aversa G, Terhorst C. 1998. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature 395:462–469 [DOI] [PubMed] [Google Scholar]
- 29.Filipovich AH, Zhang K, Snow AL, Marsh RA. 2010. X-linked lymphoproliferative syndromes: brothers or distant cousins? Blood 116:3398–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palendira U, Low C, Chan A, Hislop AD, Ho E, Phan TG, Deenick E, Cook MC, Riminton DS, Choo S, Loh R, Alvaro F, Booth C, Gaspar HB, Moretta A, Khanna R, Rickinson AB, Tangye SG. 2011. Molecular pathogenesis of EBV susceptibility in XLP as revealed by analysis of female carriers with heterozygous expression of SAP. PLoS Biol. 9:e1001187 doi:10.1371/journal.pbio.1001187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackerness KJ, Cox MA, Lilly LM, Weaver CT, Harrington LE, Zajac AJ. 2010. Pronounced virus-dependent activation drives exhaustion but sustains IFN-gamma transcript levels. J. Immunol. 185:3643–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Recher M, Berglund LJ, Avery DT, Cowan MJ, Gennery AR, Smart J, Peake J, Wong M, Pai SY, Baxi S, Walter JE, Palendira U, Tangye GA, Rice M, Brothers S, Al-Herz W, Oettgen H, Eibel H, Puck JM, Cattaneo F, Ziegler JB, Giliani S, Tangye SG, Notarangelo LD. 2011. IL-21 is the primary common gamma chain-binding cytokine required for human B-cell differentiation in vivo. Blood 118:6824–6835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hislop AD, Palendira U, Leese AM, Arkwright PD, Rohrlich PS, Tangye SG, Gaspar HB, Lankester AC, Moretta A, Rickinson AB. 2010. Impaired Epstein-Barr virus-specific CD8+ T-cell function in X-linked lymphoproliferative disease is restricted to SLAM family-positive B-cell targets. Blood 116:3249–3257 [DOI] [PubMed] [Google Scholar]
- 34.Zuo J, Quinn LL, Tamblyn J, Thomas WA, Feederle R, Delecluse HJ, Hislop AD, Rowe M. 2011. The Epstein-Barr virus-encoded BILF1 protein modulates immune recognition of endogenously processed antigen by targeting major histocompatibility complex class I molecules trafficking on both the exocytic and endocytic pathways. J. Virol. 85:1604–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. 2007. The molecular basis of IL-21-mediated proliferation. Blood 109:4135–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ. 2005. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med. 201:139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]