Abstract
Vaccination reduces morbidity and mortality from pneumonia, but its effect on the tissue-level response to infection is still poorly understood. We evaluated pneumonia disease progression, acute-phase response, and lung gene expression profiles in mice inoculated intranasally with virulent Gram-positive Streptococcus pneumoniae serotype 3 (ST 3) with and without prior immunization with pneumococcal polysaccharide ST 3 (PPS3) or after coimmunization with PPS3 and a low dose of lipopolysaccharide (PPS3+LPS). Pneumonia severity was assessed in the acute phase at 5, 12, 24 and 48 h postinoculation (p.i.) and in the resolution phase at 7 days p.i. Primary PPS3-specific antibody production was upregulated, and IgM binding to pneumococci increased in PPS3-immunized mice. Immunizations with PPS3 or PPS3+LPS decreased bacterial recovery in the lung and blood at 24 and 48 h and increased survival. Microarray analysis of whole-lung RNA revealed significant changes in the acute-phase protein serum amyloid A (SAA) levels between noninfected and infected mice, and these changes were attenuated by immunization. SAA transcripts were higher in the liver and lungs of infected controls, and SAA protein was elevated in serum but decreased in PPS3-immunized mice. Thus, during a virulent pneumonia infection, prior immunization with PPS3 in an IgM-dependent manner as well as immunization with PPS3+LPS attenuated pneumonia severity and promoted resolution of infection, concomitant with significant regulation of cytokine gene expression levels in the lungs and acute-phase proteins in the lungs, liver, and serum.
INTRODUCTION
Pneumonia infection is a major public health concern and is responsible for 1.6 million deaths annually, with a greater incidence of mortality in populations most at risk, such as infants and children, the elderly, and the immunocompromised (1). Community-acquired bacterial pneumonia (CABP) or pneumonia infection caused by bacterial pathogens contracted outside the hospital setting claims 60,000 lives per year, with $17 billion spent on health care (2). Gram-positive Streptococcus pneumoniae is the most common bacterial isolate from patients with CABP and is the causative pathogen of noninvasive pneumococcal diseases such as sinusitis and otitis media, as well as being responsible for the invasive diseases bacteremic pneumonia and meningitis (2, 3). The introduction of pneumococcal polysaccharide-conjugated 7-valent and 23-valent vaccines has been highly successful in developed nations (1, 3, 4).
Pneumococcal polysaccharide specific for serotype 3 (ST 3) or pneumococcal polysaccharide ST 3 (PPS3) is derived from S. pneumoniae ST 3 and is composed biochemically of alternating glucose-glucuronic acid subunits (5). PPS3 has been utilized extensively to characterize the type II T-cell-independent (TI-2) immune response in which primary antibody immunoglobulin M (IgM) secretion is upregulated (6). S. pneumoniae ST 3 derives its virulence from a thick capsular polysaccharide wall that inhibits complement and antibody opsonization and phagocyte-mediated killing (7, 8). Prior investigations that have examined the virulence of ST 3 in murine models concluded that a more vigorous, early immune response characterized by upregulation of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) in the alveoli would promote macrophage function, opsonization, and phagocytic bacterial clearance (7, 8). Previously, coimmunization with PPS3 and lipopolysaccharide (LPS) in rats markedly stimulated the anti-PPS3 response, suggesting that the adjuvant-like properties of LPS might be beneficial in promoting a stronger protective response to pneumococcal pneumonia infection (9).
Owing to these findings, in the present report we have developed a murine model of pneumonia infection and immunization with PPS3 or LPS or combined immunization (coimmunization) with PPS3 and LPS (PPS3+LPS), given 5 days prior to infection with S. pneumoniae ST 3, to test overall outcome and specific changes in cytokines and immunoprotective factors in the lungs. We hypothesized that this immunization intervention would be protective against mortality from intranasal challenge with S. pneumoniae ST 3 due to upregulation of primary IgM production by PPS3 immunization as well as to the adjuvant effect of LPS. Additionally, we characterized the response of serum amyloid A (SAA) in a murine model of pneumonia in order to validate the use of SAA in humans as a serum marker of pneumonia disease severity and found that serum SAA was significantly upregulated in nonimmunized, infected mice as early as 48 h postinfection (p.i.). Here, we report that immunization with PPS3 as well as coimmunization with PPS3 and LPS attenuated pneumonia severity and promoted the resolution of infection.
MATERIALS AND METHODS
Animals.
Eight-week-old female BALB/c mice (Harlan, IN) were housed in a specific-pathogen-free environment and exposed to a 12-h light/12-h dark cycle with ad libitum access to food (rodent diet 5001; Purina Laboratory) and water. All animal experiments were performed with the approval of the Pennsylvania State University's institutional animal care and use committee (IACUC).
Pneumococcal pneumonia model.
S. pneumoniae ST 3 (ATCC 6303) was reconstituted with 1 ml of Todd-Hewitt broth (THB) and grown for 24 h to log phase in THB at 37°C with an atmosphere of 5% CO2. A loopful of bacteria was plated on Trypticase soy agar plates containing 5% sheep blood (BD Diagnostic Systems, Sparks, MD) for overnight growth. In the morning, a single colony of bacteria was selected, grown to mid-log phase in THB for 6 h, and frozen at −80°C in a final concentration of 25% glycerol (10).
Mice were immunized intraperitoneally (i.p.) with a 0.5-μg dose of PPS3 in 0.5 ml of phosphate-buffered saline (PBS), 0.1 μg/g of body weight of LPS purified from Pseudomonas aeruginosa (List Biological Laboratories, Inc. Campbell, CA), or a combination of PPS3 and LPS 5 days prior to infection. Mice injected with vehicle (PBS) served as controls. Prior to each immunization study, an aliquot of frozen bacteria at mid-log phase was thawed, washed, and centrifuged at 1,500 × g for 15 min twice in PBS. Mice were briefly anesthetized with isoflurane and intranasally (i.n.) inoculated by the application of an infectious dose of 5 × 103 to 1 × 105 CFU in 50 μl of PBS to the nares. The administered dose is noted for each experiment in the figure legends. Noninfected mice were mock infected with 50 μl of PBS. Inoculated mice were examined daily for weight loss, dehydration, and labored breathing and were euthanized if they appeared severely ill or lost over 20% of body weight. PPS3- and LPS-coimmunized mice and infected controls were assessed for pneumonia severity at the early acute time points of 5, 12, and 24 h p.i. Pneumonia disease was also examined at the acute time point of 48 h p.i. and at the resolution stage of 7 days p.i. Mice were euthanized by CO2 asphyxiation, and the lungs, liver, and blood from the vena cava were collected. The liver and left lobe of the lungs were snap-frozen in liquid nitrogen and later stored at −80°C for mRNA analysis. The remaining lobes of the lungs were placed in PBS on ice for determination of bacterial load. After centrifugation at 2,000 rpm for 10 min at room temperature, serum was collected and stored at −20°C. In a separate experiment characterizing lung histopathology at 48 h p.i., lungs were inflated by intratracheally instilling 1.5 ml of 10% formalin, placed in 7 ml of 10% formalin solution for 24 h at room temperature, and then transferred to 70% ethanol (10). After lung tissue paraffin embedding, sectioned slides were prepared with hematoxylin and eosin (H&E).
Antibody (PPS3-specific IgM and IgG), acute-phase protein (SAA), and proinflammatory cytokine (IL-6 and chemokine ligand 2 [CCL2]) ELISAs.
An enzyme-linked immunosorbent assay (ELISA) described previously (9) was used to measure IgM specific to PPS3 on serum samples collected from the mandibular facial vein 5 days after immunization and from the vena cava 2 days p.i. Briefly, polystyrene 96-well plates (Maxisorp-Nunc) were coated with PPS3 (15 μg/well) in 0.1 M glycine-HCl buffer at pH 3.5 and incubated overnight at 4°C. Plates were washed five times with washing buffer (0.5% Tween 20, 0.15 M Tris base, pH 7.6, 0.135 M NaCl) and blocked for 1 h with 1% bovine serum albumin (BSA) at room temperature. Serum samples were added and incubated overnight at 4°C. The following morning, plates were washed, and goat anti-mouse IgM or goat anti-mouse IgG conjugated to alkaline phosphatase (Sigma) was added and incubated for 1 h at room temperature. After plates were washed, phosphatase substrate (Sigma) was added, and the plates were incubated at 37°C for 30 min and read at 405 nm and 570 nm. SAA protein expression was determined by an SAA mouse ELISA (Invitrogen) as per the manufacturer's instructions using serum samples collected at 24 and 48 h p.i. An ELISA kit (Signosis) assessed IL-6 and CCL2 protein expression from serum samples collected at 24 and 48 h p.i.
IgM-binding assay.
The ability of IgM in serum to bind to pneumococci was determined by an in vitro IgM-binding assay as described previously (11). Bacteria frozen stock was washed twice in PBS and centrifuged at 4,000 × g for 6 min. For dispersion, bacteria were resuspended at a concentration of 1 × 106 CFU/50 μl of 1% BSA-PBS with 5 μl of mouse serum from day 2 p.i. and incubated at 37°C for 30 min. Samples were washed twice with 1% BSA-PBS, resuspended in 50 μl of goat anti-mouse IgM antibody (1:200 dilution in 1% BSA-PBS) (BD Biosciences), and incubated on ice for 30 min. Bacteria were then washed and fixed in 100 μl of 4% paraformaldehyde for flow cytometry. For a control, bacteria alone lacking mouse serum were incubated with antibody, and activity was used as a negative gate during flow cytometry analysis.
Recovery of pneumococci from lungs and blood.
The right lobes of the lung were homogenized in 1 ml of PBS, and serial dilutions of homogenates were plated overnight at 37°C on Trypticase soy agar plates containing 5% sheep's blood (BD Biosciences). To determine septicemia, 10 μl of whole blood was plated for 12 h of incubation at 37°C (10). Bacterial colonies were counted the following morning.
Histologic evaluation.
Histopathological assessment of H&E-stained lung sections collected at 48 h p.i. was conducted by a veterinarian with training and experience in rodent pathology who was blinded to the experimental treatment. The incidence of neutrophils, lymphocytes, edema, necrosis, and lesions in each section was graded using a scale of 0 to 4. Sections with no inflammation were given a score of 0, sections with slight inflammation with few or scattered foci and <10% of the lung affected received a score of 1, sections with mild lesions with 10 to 20% of lung fields affected received a score of 2, sections with moderate lesions with 20 to 30% of lung fields affected received a score of 3, and sections with extensive lesions, inflammation, and >30% of the lung affected received a score of 4.
Q-PCR for determination of inflammatory mediator expression.
Gene expression was assessed by quantitative PCR (Q-PCR) in lung tissue for chemokine ligand 2 (CCL2), interleukin-10 (IL-10), IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α) and in liver tissue for the acute-phase proteins serum amyloid A1 (Saa1), serum amyloid protein (SAP), and lipopolysaccharide binding protein (LBP). Primer sets and annealing temperatures are provided in Table 1. Briefly, RNA was isolated from 0.5 mg of whole tissue with TRIzol (Invitrogen) and reverse transcribed into cDNA with reverse transcriptase (Promega), and Q-PCR was performed using iQ SYBR green Supermix (Bio-Rad, Hercules, CA). The level of mRNA expression is shown as ΔΔCT, the change in cycle threshold (CT) values compared to expression in noninfected controls, which was normalized to expression of the reference gene 18S rRNA (12, 13).
Table 1.
Primer pair details for Q-PCR
| Primer target | Accession no.a | Primer sequence | Melting temp (°C) | Product size (bp) |
|---|---|---|---|---|
| CCL2 | NM 011333 | 5′-AGGTCCCTGTCATGCTTCTG | 60 | 248 |
| 3′-TCTGGACCCATTCCTTCTTG | ||||
| IL-1β | NM 010548 | 5′-GCCCATCCTCTGTGACTCAT | 63 | 229 |
| 3′-AGGCCACAGGTATTTTGTCG | ||||
| IL-6 | NM 031168 | 5′-TTCCATCCAGTTGCCTTCTT | 60 | 493 |
| 3′-GAGCATTGGAAATTGGGGTA | ||||
| IL-10 | NM 008361 | 5′-ACGGAAACAACTCCTTGGAA | 60 | 485 |
| 3′-AACTGGCCACAGTTTTCAGG | ||||
| LBP | NM 008489 | 5′-GTGGCTGCTGAATCTCTTCC | 55 | 249 |
| 3′-GAGCGGTGATTCCGATTAAA | ||||
| Saa1 | NM 009117 | 5′-GAGGACATGAGGACACCATTGC | 63 | 196 |
| 3′-CCAGAGAGCATCTTCAGTGTTCC | ||||
| TNF-α | NM 013693 | 5′-AGCCCCCAGTCTGTATCCTT | 63 | 211 |
| 3′-CTCCCTTTGCAGAACTCAGG | ||||
| 18s rRNA | M35283 | 5′-AATGGTGCTACCGGTCATTC | 60 | 192 |
| 3′-ACCTCTCTTACCCGCTCTCC |
Accession numbers are for Mus musculus.
Microarray analysis.
Each lung RNA sample represented an individual mouse, with treatment groups as follows: noninfected (vehicle-immunized) control mice (n = 5), infected (vehicle-immunized) control mice at 48 h p.i. (n = 5), PPS3- and LPS-coimmunized mice at 48 h p.i. (n = 4), and PPS3- and LPS-coimmunized mice at 7 days p.i. (n = 4). Lung RNA was isolated with TRIzol (Invitrogen), purified using TRIzol LS (liquid sample) (Invitrogen), and diluted to 50 ng/10 μl. A mouse 430 2.0 array with an Affymetrix IVT (in vitro transcription) kit was performed at the National Cancer Institute (NCI) Microarray Facility (Frederick, MD), as described previously (14). The identities of RNA samples from groups were coded so that 4 or 5 arrays processed in proximity contained RNA from various immunization or infection conditions. Results were analyzed using NCI's mAdb software (http://nciarray.nci.nih.gov/) and transformed by centering the signal median and setting the signal floor to 1. We queried 18 total arrays containing 45,101 gene targets with the Statistical Analysis for Microarrays (SAM) program and set the delta value to 15.5 with a false-discovery rate of 0.0011, which resulted in 249 significantly regulated genes and a false-positive rate of 0.26 genes (less than 1). Table 2 lists gene information, including Entrez gene identifiers (IDs), and q values from SAM for Saa1, Saa2, Saa3, and ZBP1 that were further analyzed by expressing log2-transformed values, with the median intensity of a log2 value set to 0.
Table 2.
Gene information from Statistical Analysis for Microarrays
| Gene product | Description | Affymetrix feature ID | Entrez gene ID | q value |
|---|---|---|---|---|
| Saa1 | Serum amyloid A1 | 1450788_at | 20208 | <10−6 |
| Saa2 | Serum amyloid A2 | 1419075_s_at | 20209 | <10−6 |
| Saa3 | Serum amyloid A3 | 1450826_a_at | 20210 | 1.2−5 |
| ZBP1 | Z-DNA binding protein 1, transcript variant 1 | 1419604_at | 58203 | <10−6 |
Statistics.
One-way analysis of variance (ANOVA) with Tukey's posthoc test was used to analyze data from PPS3-specific IgM ELISAs, proinflammatory lung cytokine and hepatic acute-phase protein Q-PCRs, and lung bacterial recovery at 48 h p.i. Two-way ANOVA with Bonferroni's multiple comparison posthoc test was used to assess serum IgM levels as well as lung cytokine expression at early acute time points. A Student's t test was used to analyze infected samples versus noninfected baseline control values. Bacteremia data were analyzed by Fisher's exact test at the early time points of 5, 12, and 24 h p.i., and a chi-square (χ2) test was used for data from 48 h and 7 days p.i. Survival data (Kaplan-Meier curves) were analyzed using a log rank Mantel-Cox proportional hazards test. All statistical analyses were conducted using GraphPad Prism, version 5.0, software (La Jolla, CA). For all statistical tests, a P value of less than 0.05 was considered significant.
Microarray data accession number.
Microarray data have been deposited in the Gene Expression Omnibus (GEO) database under accession number GSE45644.
RESULTS
PPS3-specific primary antibody response and IgM-binding efficiency.
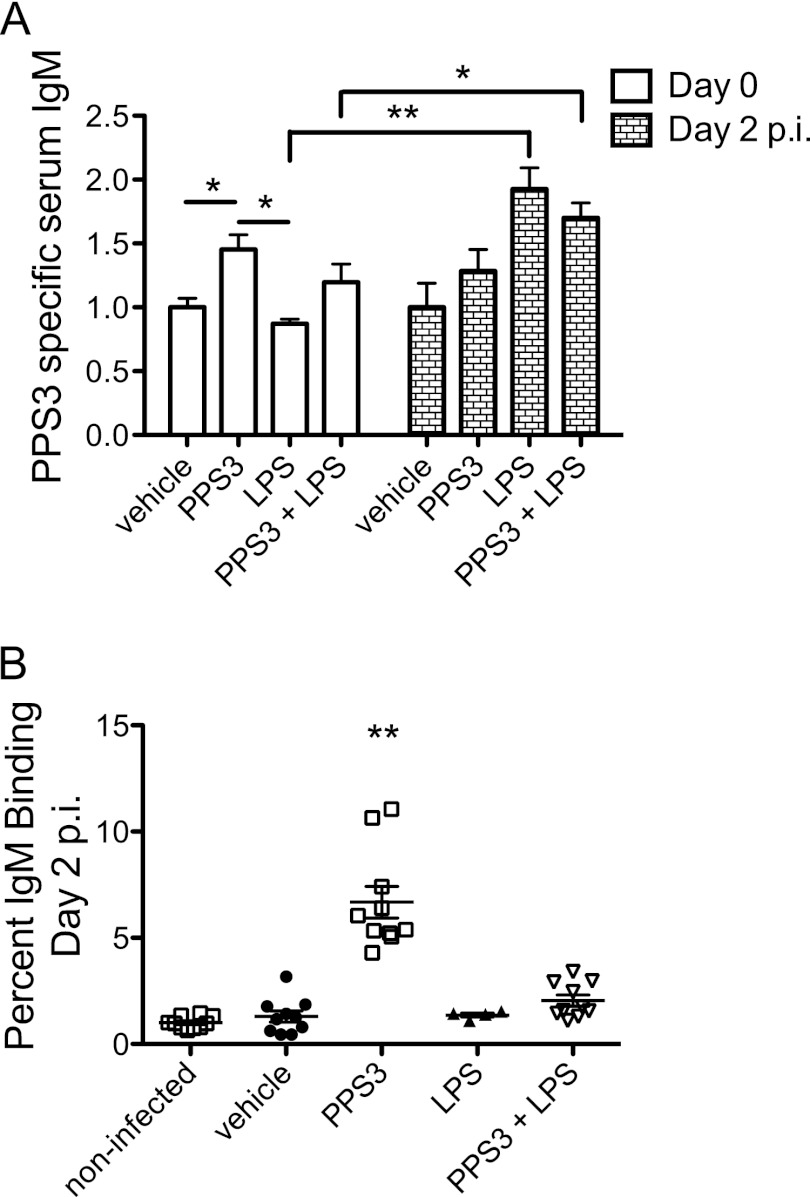
Mice were immunized with PPS3, LPS, or the combination of PPS3 and LPS 5 days prior to infection with S. pneumoniae ST 3, and serum anti-PPS3-specific IgM was measured 5 days later, on the day of infection (day 0), and 2 days p.i. Immunization with PPS3 significantly increased serum IgM on day 0 (P < 0.05 compared to vehicle) (Fig. 1A). Anti-PPS3 IgM levels did not change from day 0 to day 2 in PPS3-immunized mice but increased from day 0 to day 2 in LPS-immunized and coimmunized mice (P < 0.0001 for LPS at day 0 compared to day 2; P < 0.05 for PPS3+LPS at day 0 compared to day 2) (Fig. 1A). In order to further investigate how immunization was protective against infection, we performed in vitro IgM-binding assays from serum samples at 2 days p.i. in which IgM deposited on bacteria was measured by flow cytometry. IgM binding to pneumococci was significantly greater in PPS3-immunized mice than in LPS-immunized and coimmunized mice (P < 0.0001) (Fig. 1B).
Fig 1.
Serum IgM specific for ST 3 in immunized and infected mice and IgM-binding activity in mouse serum at day 2 p.i. (A) Mice (n = 8 per group) were immunized with vehicle (PBS), PPS3, or PPS3 and LPS and 5 days later challenged with 1 × 105 CFU of S. pneumoniae ST 3. Serum IgM is depicted as fold change in the optical density value relative to the noninfected control 5 days after immunization (vehicle, day 0) and to the infected control (vehicle, day 2). (B) IgM-binding activity was assessed by flow cytometry with serum from noninfected or immunized, infected mice at day 2 p.i. (n = 10/group; LPS-immunized, n = 4). The percentage of IgM binding is depicted as fold change normalized to noninfected control values. Asterisks denote P values of <0.05 (*) and <0.0001 (**) as detected by one-way ANOVA with Tukey's posthoc test (day 0) (A), (B) or two-way repeated-measures ANOVA with Bonferroni's multiple comparison post hoc test (A) (day 0 versus day 2). Data are shown combined from two independent experiments.
Bacterial recovery from the lung and blood.
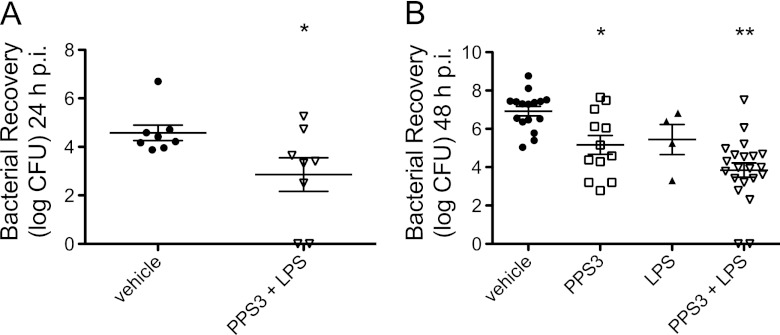
To determine bacterial burden at the infection site and systemically during the acute phase of infection, we measured bacterial colonies on agar plates from blood and lung tissue samples at 5, 12, 24, and 48 h p.i. No differences were observed in lung bacterial burden and bacteremia at the very earliest times of 5 and 12 h p.i. (data not shown). However, coimmunization with PPS3 and LPS significantly decreased lung CFU counts at 24 h p.i. (P < 0.05 compared to infected controls) (Fig. 2A). At 48 h p.i., either immunization with PPS3 alone or immunization with PPS3 and LPS resulted in lower bacterial loads in the lung (P < 0.05 for PPS3 and P < 0.001 for PPS3+LPS compared to infected controls) (Fig. 2B).
Fig 2.
Bacterial recovery from the lung during the acute phase of infection with S. pneumoniae ST 3. Immunized mice were inoculated with a dose of 5 × 104 CFU and euthanized at 24 h p.i. or inoculated with a dose of 5 × 104 CFU or 1 × 105 CFU and euthanized 48 h p.i. Lung homogenates were serially diluted and plated overnight to determine bacterial burden. Log CFU mean values and standard errors of the means are shown, with each point representing one mouse. (A) Lung bacterial recovery at 24 h p.i. An asterisk denotes a P value of <0.05 as determined by a Student's t test between vehicle and coimmunized mice (n = 8 per group). (B) Lung bacterial recovery at 48 h p.i. from mice immunized with vehicle (n = 16), PPS3 (n = 12), LPS (n = 4), and PPS3+LPS (n = 21). Asterisks denote P values of <0.05 (*) and <0.001 (**) as determined by one-way ANOVA with Tukey's multiple comparison posthoc test in comparison to vehicle-immunized infected controls. Data are shown combined from two separate in vivo infection experiments (A) or four independent infections (B).
Bacteremia was assessed at 48 h p.i., representing the acute phase, and at 7 days p.i., representing the resolution phase, in PPS3-immunized and PPS3+LPS-immunized mice compared to vehicle-immunized infected controls (Table 3). PPS3-immunized mice had lower bacterial counts than vehicle-immunized infected controls (P < 0.0001). LPS treatment alone provided slight protection from bacteremia at 48 h p.i. as Fisher's exact test revealed a significant difference between the LPS-immunized mice and the vehicle-immunized group (P < 0.05) (Table 3). These results were supported in an independent study (data not shown) in which bacteremia was also lower 24 h p.i. in PPS3+LPS-immunized mice (n = 8 per group) (P < 0.001) than in vehicle-immunized infected mice. By 7 days p.i., bacteremia was not detected in PPS3-immunized and coimmunized mice, thus indicating clearance by this time. Overall, by 48 h p.i. immunization with PPS3 alone or coimmunization with PPS3 and LPS decreased lung bacterial burden (Fig. 2) and bacteremia (Table 3), which are clinical measures of pneumonia severity at the site of infection and of systemic spread, respectively.
Table 3.
Bacteremia during the acute and resolution phases of S. pneumoniae ST3 infection
| Immunization | Infection duration | No. of infected mice | No. of mice with bacteremia | Proportion of mice with bacteremia (%)a |
|---|---|---|---|---|
| Vehicle | 48 h | 32 | 31 | 96.8 |
| 7 days | NDb | |||
| PPS3 | 48 h | 27 | 8 | 29.6** |
| 7 days | 10 | 0 | 0 | |
| LPS | 48 h | 10 | 7 | 70.0* |
| PPS3+LPS | 48 h | 32 | 12 | 37.5** |
| 7 days | 9 | 0 | 0 |
Bacteremia incidence after indicated infection duration with an infectious dose range of 5 × 104 CFU to 1 × 105 CFU of S. pneumoniae ST 3 was analyzed by a chi-square (χ2) test at 48 h and 7 days p.i. Whole blood was collected and plated overnight to determine the presence of bacteremia. *, P < 0.05; **, P = 0.0001 (in comparison to values for vehicle-immunized, infected mice).
ND, not detectable. Vehicle-immunized, infected mice did not survive past day 3 p.i.
Immunization with PPS3 and PPS3+LPS promotes survival.
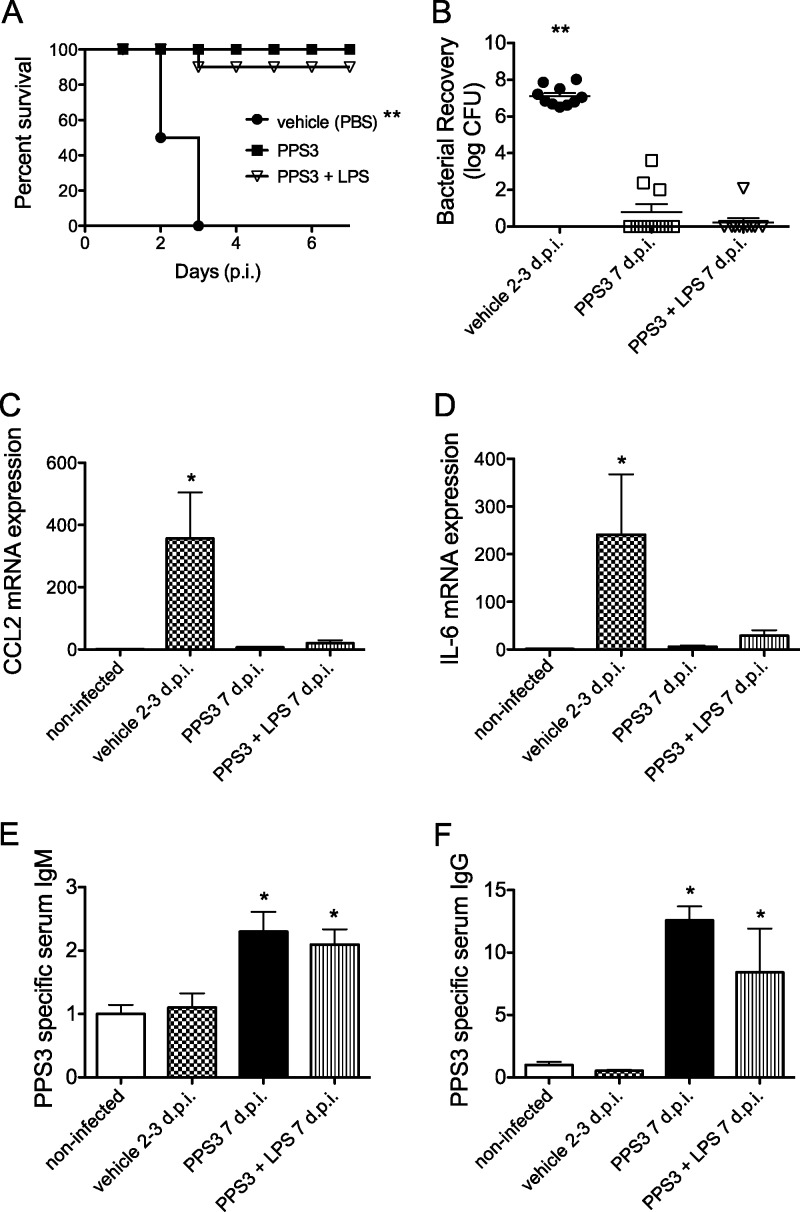
We next determined whether immunization could prevent mortality from infection with S. pneumoniae ST 3. Mice (n = 10 per group) were immunized with PPS3 or coimmunized with PPS3 and LPS, challenged 5 days later with 5 × 104 CFU of ST 3, and monitored for survival for 1 week. Mice were euthanized if their body weights decreased by 20% or if they appeared severely dehydrated or exhibited labored breathing. All infected control mice succumbed to infection by day 3 p.i., whereas all PPS3-immunized mice survived, and all but one coimmunized mouse survived to the resolution time point, 7 days p.i. By a log rank Mantel-Cox test, immunization with PPS3 or cotreatment with PPS3 and LPS increased survival in comparison to infected controls (P < 0.0001) (Fig. 3A). The median survival for the infected control mice was day 2.5 p.i. as the survival proportion on day 2 p.i. was 50% and on day 3 p.i. was 0% (Fig. 3A). Thus, in this model of pneumonia infection, bacteremia incidence was a strong predictor of death, and immunization with PPS3, with and without LPS, reduced both bacteremia and mortality. Since in previous studies LPS-immunized mice had exhibited a body condition similar to that of the infected control mice by 2 days p.i., this group was not included in survival study analysis.
Fig 3.
Immunization with PPS3 and PPS3+LPS promotes survival and resolution of pneumococcal challenge. Vehicle (PBS)-immunized, PPS3-immunized, and coimmunized mice (n = 10 per group) were inoculated with 5 × 104 CFU at 5 days after immunization and monitored for 1 week for survival. (A) Kaplan-Meier survival curve is shown. **, P < 0.0001 as determined by log-rank Mantel-Cox test. (B) Lungs (n = 10 per group) from vehicle-immunized mice at 2 to 3 days p.i. (d p.i.) and from PPS3- and PPS3+LPS-immunized mice at 7 days p.i. were homogenized, serially diluted, and plated to determine bacterial load. **, P < 0.0001, by a Student's t test in comparison to vehicle-immunized, infected controls. (C and D) Lung cytokine mRNA expression levels at 2 to 3 days (vehicle, n = 5) and 7 days (PPS3, n = 10; PPS3+LPS, n = 5) p.i. were assessed by Q-PCR, and results were analyzed by the ΔΔCT method. Expression levels are shown as fold change in comparison to baseline noninfected values. (E and F) Blood was collected from noninfected mice, from vehicle-immunized, infected mice at 2 to 3 days p.i. and from and PPS3- and PPS3+LPS-immunized, infected mice at 7 days p.i.; PPS3-specific IgM and IgG serum levels were determined by ELISA. Serum IgM and IgG (n = 7/group) levels are depicted as fold change in the optical density values relative to those of the noninfected controls. Asterisks denote P values of <0.05 (*) and <0.0001 (**) as determined by one-way ANOVA with Tukey's posthoc test in comparison to infected control values. Data from survival studies represent three separate in vivo pneumonia infections.
To ensure that resolution of infection was occurring by 1 week p.i., lung bacterial burden, bacteremia, and lung cytokine gene expression were examined during the survival study. Lung bacterial burden was very low or nil in PPS3-immunized and PPS3+LPS-immunized mice at 7 days p.i. in comparison to infected control mice at 2 to 3 days p.i. (P < 0.0001) (Fig. 3B). Furthermore, expression of the proinflammatory cytokines CCL2 and IL-6, measured in lung tissue by Q-PCR, decreased in the resolution phase by PPS3 immunization compared to the levels in infected control mice at 2 to 3 days p.i. (P < 0.05) (Fig. 3C and D). There was no significant difference between noninfected controls and PPS3-immunized and PPS3+LPS-immunized mice at 7 days p.i., which implies that the expression of these inflammatory cytokines in immunized, infected mice had been resolved at this stage of infection (Fig. 3C and D). Additionally, PPS3-immunized mice and PPS3+LPS-immunized mice did not exhibit bacteremia at day 7 p.i. (Table 3). Sera from infected control mice at 2 to 3 days p.i. and from PPS3-immunized mice and coimmunized mice at 7 days p.i. were tested by ELISA to determine antibody concentration. PPS3-specific serum IgM in both PPS3- and PPS3+LPS-immunized mice remained significantly elevated, while PPS3-specific IgG increased significantly by day 7 p.i. in comparison to the level in infected control mice (P < 0.05) (Fig. 3E and F). These results suggest that immunization with PPS3 and the combination of PPS3 with LPS promoted resolution of bacterial pneumonia and therefore improved survival.
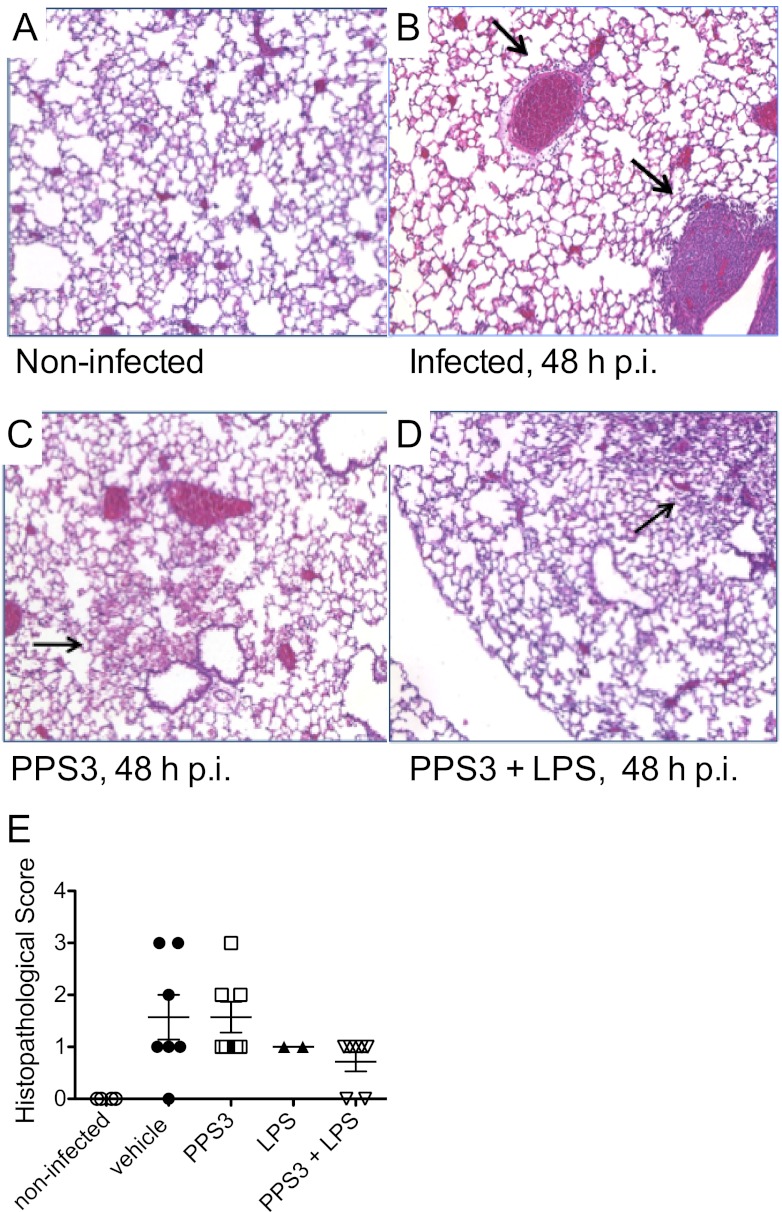
Lung histopathological scores reveal low to moderate inflammation in all immunization groups.
Having observed that immunization with PPS3 and the with combination of PPS3 and LPS conferred protection against pneumococcal challenge, with a significantly lower bacterial burden both in the immediate lung microenvironment and systemically, we next examined lung histology to determine if immunization reduced lung damage. As illustrated in Fig. 4A to D, we did not observe a dramatic difference in lung inflammation between PPS3-immunized mice and infected controls at 48 h p.i. The type of cellular infiltrates was not recorded due to the lack of significant differences between immunization treatments, but lymphocytes were more commonly observed than neutrophils. This acute-phase time point was selected to characterize lung histopathological scoring because in previous experiments vehicle-immunized, infected control mice had succumbed to infection by day 3 p.i. (Fig. 3A), and thus we would expect to observe differences in lung histology, if evident, by this time. Challenge with either a moderate infectious dose (5 × 104 CFU) or a low infectious dose (5 × 103 CFU) produced similar inflammation patterns, as determined by histopathological severity scores (Fig. 4E). Thus, PPS3-immunized or coimmunized mice did not have significantly less lung inflammation in the acute phase after pneumococcal infection.
Fig 4.
Lung histopathology and severity scores at 48 h p.i. A representative H&E-stained lung section is shown at ×10 magnification from noninfected control mice (A) and at 48 h p.i. after administration of an infectious dose of 5 × 104 CFU of S. pneumoniae ST 3 from vehicle-immunized (B), PPS3-immunized (C), and coimmunized (D) mice. Black arrows indicate sites of inflammatory infiltrates or hemorrhaging. (E) Mean histopathological severity scores ± standard errors of the means are shown for each group, with one point representing one mouse at an infectious dose of 5 × 104 CFU (n = 4 per group) or 5 × 103 CFU (n = 2 per group). Severity scores were analyzed by one-way ANOVA, and a P value of <0.05 was considered significant.
Lung cytokines levels are attenuated by immunization with PPS3 and the PPS3+LPS combination.
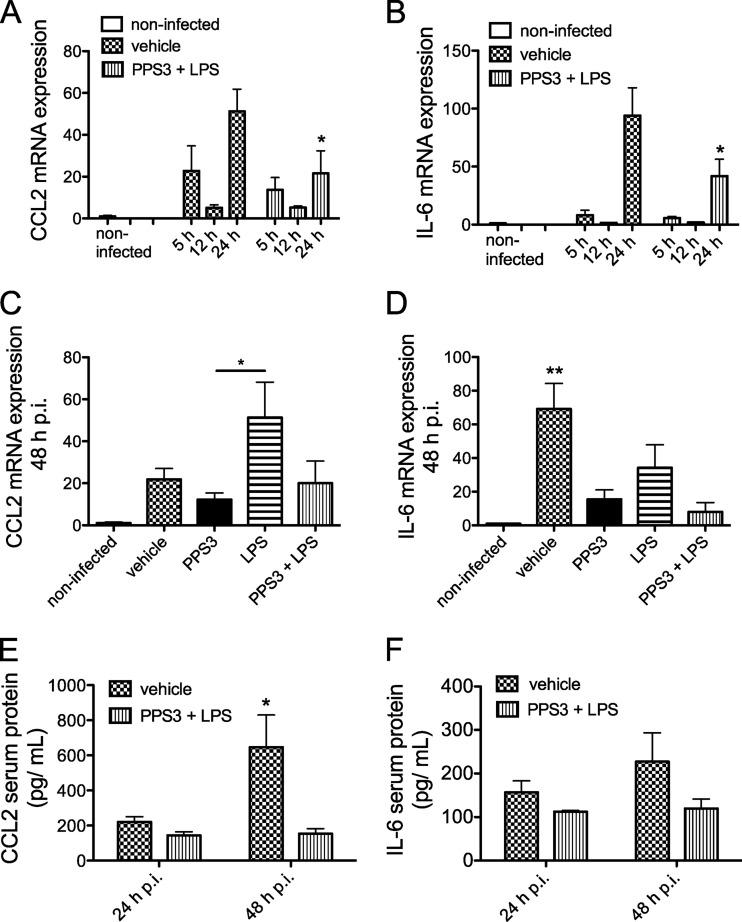
In agreement with the observed lung bacterial burden, a decrease in pulmonary proinflammatory cytokine expression was also observed in immunized mice during the acute phase of infection. For both CCL2 (Fig. 5A) and IL-6 (Fig. 5B), transcripts were highest at 24 h p.i. in the vehicle-treated infected group. A significant attenuation of both CCL2 and IL-6 transcripts was observed in PPS3+LPS-immunized mice in comparison to vehicle-immunized infected mice at 24 h p.i. at an infectious dose of 5 × 104 CFU (P < 0.05) (Fig. 5A and B). In a separate study 48 h after infection, CCL2 transcript levels were lower in PPS3-immunized mice than in LPS-immunized mice (P < 0.05) (Fig. 5C). Furthermore, both the PPS3 immunization and the coimmunization resulted in attenuation of IL-6 mRNA expression (P < 0.01 versus infected controls) (Fig. 5D). Forty-eight hours after challenge with a slightly lower infectious dose of 5 × 103 CFU, the expression patterns for TNF-α and CCL2 were similar and did not differ significantly between immunization groups (see Fig. S1A and E in the supplemental material). Lung IL-6 mRNA expression at 48 h p.i. after a lower infectious dose was very similar to that after a slightly greater dose as PPS3-immunized and coimmunized mice both had significantly less transcript accumulation than the vehicle-immunized, infected controls (P < 0.01). Additionally, the increase in IL-1β mRNA expression due to infection was reduced by both PPS3 immunization and the combined PPS3+LPS immunization at 48 h p.i. with the lower infectious dose (P < 0.01 in comparison to infected controls) (see Fig. S1C). IL-10 expression was also lower in the lungs of PPS3+LPS-immunized infected mice than in infected controls (P < 0.05) (see Fig. S1D). In serum, CCL2 protein decreased significantly in coimmunized mice by 48 h p.i. in comparison to infected controls (P < 0.05), and the biological trend of attenuated IL-6 expression was also observed at this time (Fig. 5E and F). Thus, during the acute phase of infection, immunization with PPS3+LPS decreased pulmonary IL-6 transcript expression and serum CCL2 protein expression in comparison to levels in infected controls.
Fig 5.
Lung proinflammatory cytokine mRNA and protein expression levels during the acute phase of infection with S. pneumoniae ST 3. (A and B) Mice were immunized with vehicle (PBS) or PPS3+LPS 5 days prior to inoculation with 5 × 104 CFU S. pneumoniae ST 3. At 5 h (n = 4), 12 h (n = 4), and 24 h (n = 8) p.i., gene expression levels of CCL2 (A) and IL-6 (B) were assessed by Q-PCR. Results are shown as fold change in comparison to baseline noninfected values (n = 6). (C and D) At 48 h p.i. after inoculation with 1 × 105 CFU of S. pneumoniae ST 3, lung mRNA expression of CCL2 (C) and IL-6 (D) was assessed by Q-PCR in mice immunized with vehicle (n = 8), PPS3 (n = 8), LPS (n = 4), or PPS3+LPS (n = 8) 5 days prior to infection. Results are shown as fold change in comparison to baseline noninfected values (n = 5). Noninfected baseline control values are shown on the left of each bar graph for comparison but were not included in statistical analyses. (E and F) CCL2 and IL-6 protein levels in serum were measured by ELISA at 24 and 48 h p.i. (n = 4 to 5/group). Asterisks denote P values of <0.05 (*) and <0.01 (**) as determined by two-way ANOVA with Bonferroni's posthoc test (A, B, E, and F) or by one-way ANOVA with Tukey's posthoc test (C and D).
Hepatic acute-phase protein mRNA expression and serum Saa increased during infection but was attenuated by immunization.
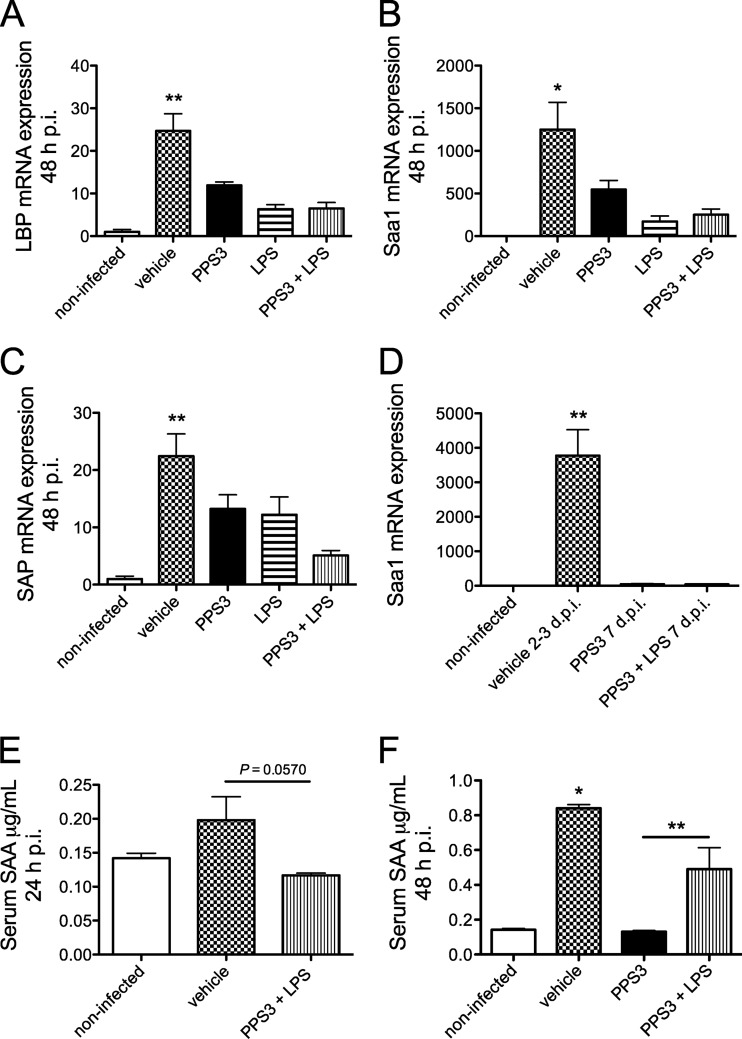
Since IL-6 is a major inducer of hepatic acute-phase proteins, we next hypothesized that IL-6 may be regulated systemically, in the liver, and that the expression of downstream targets of IL-6 may also be controlled by immunization with PPS3 and PPS3+LPS. Thus, we examined the liver for the expression of LBP, Saa1, and SAP during both the acute and resolution phases of infection. We did not observe a significant difference in the transcript expression levels of acute-phase proteins between infected controls and PPS+LPS-immunized mice at the early acute time points of 5, 12, and 24 h p.i. (data not shown). However, by 48 h p.i., expression of each of these acute-phase proteins in infected controls was upregulated significantly (Fig. 6A to C). LBP and Saa1 transcript levels were both significantly lower in the liver of mice immunized with PPS3, treated with LPS, and coimmunized with PPS3 and LPS (all P < 0.05 in comparison to infected controls) (Fig. 6 A and B). SAP mRNA expression was reduced only by PPS3+LPS immunization (P < 0.01 in comparison to infected controls) (Fig. 6C) although there was a nonsignificant trend toward lower levels with PPS3 immunization and LPS treatment alone. In comparison to vehicle-immunized mice at 2 to 3 days p.i., by 7 days p.i. Saa1 expression in coimmunized mice remained low, thus denoting resolution of systemic inflammation (Fig. 6D). Overall, expression of acute-phase proteins in the liver of infected controls was highly upregulated by 48 h p.i., while PPS3 immunization with or without LPS cotreatment attenuated LBP, Saa1, and SAP gene expression in the liver.
Fig 6.
Hepatic acute-phase protein mRNA and Saa protein expression levels in serum during the acute and resolution phases of infection with S. pneumoniae ST 3. Mice were immunized with vehicle (PBS) (n = 8), PPS3 (n = 8), LPS (n = 4), and PPS3+LPS (n = 8) 5 days prior to inoculation with 1 × 105 CFU (A to C) or 5 × 104 CFU (D) of S. pneumoniae ST 3. Liver mRNA expression was assessed at 48 h p.i. (A to C) and 7 days p.i. (D) by Q-PCR, and results were analyzed by the ΔΔCT method. Expression levels are shown as fold change in comparison to baseline noninfected values for LBP (A), Saa1 (B), and SAP (C) at 2 days p.i. and for Saa1 at 7 days p.i. (D). An ELISA examining serum SAA expression was conducted on blood samples collected via the vena cava at 24 h p.i. after inoculation with 5 × 104 CFU (E) and at 48 h p.i. after inoculation with 1 × 105 CFU (F) of S. pneumoniae ST 3. A Student's t test and one-way ANOVA were used for statistical analysis at 24 and 48 h p.i., respectively. Bars denote means plus standard errors of the means; asterisks denote P values of <0.05 (*) and <0.01 (**), as determined by one-way ANOVA with Tukey's posthoc test in comparison to infected control values.
To determine if upregulated Saa gene expression in the infected control mouse lung is also observed at the protein level, serum Saa was measured by ELISA in samples collected at 24 and 48 h p.i. (Fig. 6E and F). By 24 h p.i., serum SAA was increased a nonsignificant ∼30% in infected mice, while PPS3+LPS-immunized mice had lower levels of serum SAA which, however, just missed statistical significance (t test, P = 0.0570) (Fig. 6E). By 48 h p.i., serum SAA was increased significantly in infected control mice (P < 0.0001 compared to noninfected controls). Serum SAA was significantly reduced in PPS3-immunized, infected mice (P < 0.01 in comparison to infected controls) and in PPS3+LPS-immunized, infected mice (P < 0.05 in comparison to infected controls) (Fig. 6F). Thus, in addition to the transcript level of expression, the concentration of circulating SAA protein is also upregulated by infection and significantly attenuated by immunization.
Lung microarray analysis detects an upregulation of the acute-phase protein Saa and Z-DNA binding protein-1 (ZBP1) in infected controls.
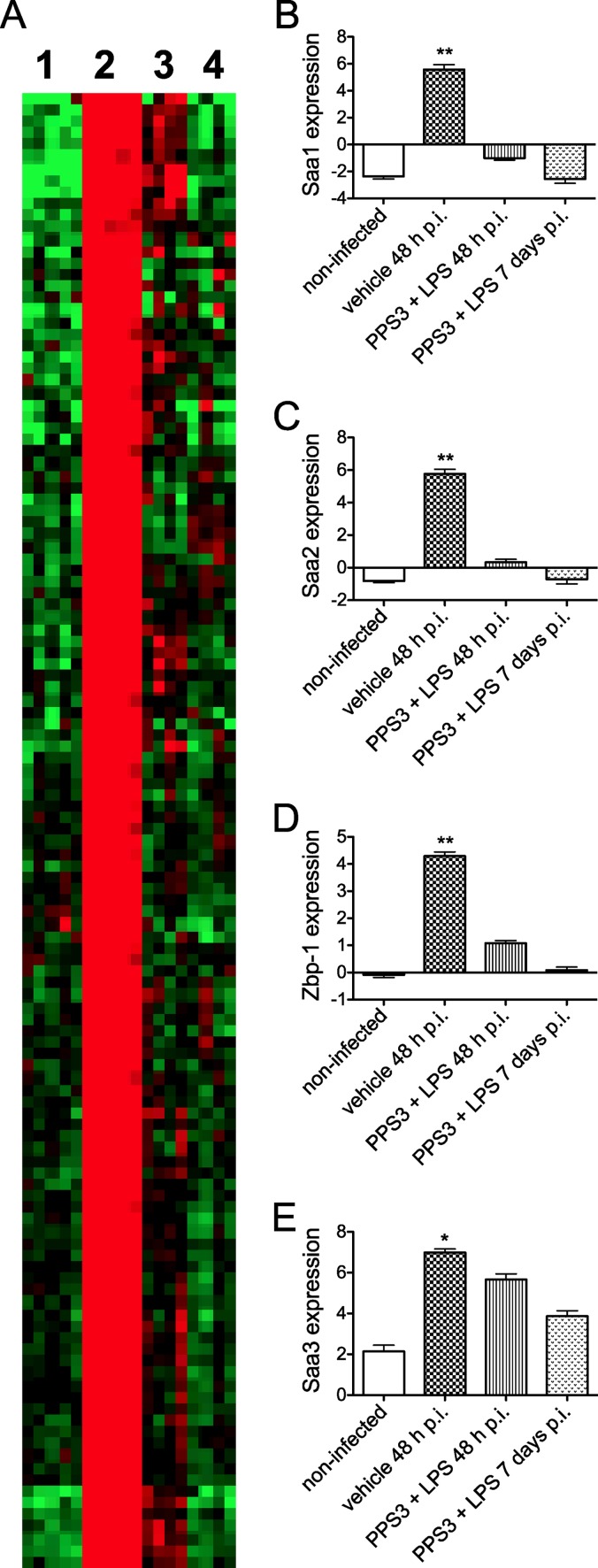
To further characterize specific gene changes during S. pneumoniae infection, we subjected RNA isolated from whole-lung tissue to microarray analysis. Figure 7A illustrates a heat map representing noninfected mice (Fig. 7A, lane 1) (n = 5), vehicle-immunized mice at 48 h p.i. mice (Fig. 7A, lane 2) (n = 5), PPS3+LPS-immunized mice at 48 h p.i. (Fig. 7A, lane 3) (n = 4), and PPS3+LPS-immunized mice at 7 days p.i. (Fig. 7A, lane 4) (n = 4). For simplification, only the upregulated portion of the heat map is shown; the downregulated portion was omitted. Genes elevated by pneumonia infection are indicated in red for the most significantly upregulated of these genes (Fig. 7A, compare lanes 1 and 2). In marked contrast, lungs from PPS3+LPS-immunized, infected mice, both at 48 h and 7 days p.i., revealed a global pattern that was very similar to that of the noninfected controls, denoting unchanged and/or downregulated expression, respectively (Fig. 7A, compare lanes 3 and 4 to lane 1). Bar graphs to the right of the heat map depict log2 values for four of the most significantly regulated transcripts (shown in descending order of most to least significant difference as determined by a t test), three of which represent the acute-phase protein serum amyloid a (SAA) variants Saa1 (Fig. 7B), Saa2 (Fig. 7C), and Saa3 (Fig. 7E). A fourth highly regulated gene was Z-DNA binding protein-1 (ZBP1) (Fig. 7D), of interest as a putative sensor of infection. Each of these genes was highly upregulated in infected control mice in comparison to levels in noninfected mice, as well as in comparison to levels in immunized, infected mice at 48 h p.i. (P < 0.05). Expression levels of cotreated infected mice at 7 days p.i. were not included in the statistical analysis. Thus, microarray analyses revealed a dramatic upregulation of gene expression in the lungs caused by virulent pneumonia infection and demonstrated that coimmunization inhibits this increase, producing gene expression patterns very similar to those of noninfected controls.
Fig 7.
Microarray analysis of lung RNA samples. A mouse 430 2.0 array was conducted using an Affymetrix IVT express labeling kit platform. (A) TreeView heat map shows downregulated (green), unchanged (black), and upregulated (red) gene expression in the lungs of mice, as follows: lane 1, noninfected (n = 5); lane 2, infected at 48 h p.i. (n = 5); lane 3, coimmunized and infected at 48 h p.i. (n = 4); and lane 4, coimmunized and infected at 7 days p.i. (n = 4) with 5 × 104 CFU of S. pneumoniae ST 3. Bar graphs for Saa1, Saa2, ZBP1, and Saa3 show log2 comparisons of the differences after the median expression level was set to 0 and are in descending order from most significant to least significant difference as determined by the Significance Analysis of Microarrays program. Bars denote means plus standard errors of the means; asterisks denote P values of <0.01 (*) and <0.0001 (**) as determined by a Student's t test comparing noninfected and coimmunized, infected groups to infected control values.
DISCUSSION
Although the effects of pneumococcal polysaccharide have been investigated previously in mice as an immunization strategy (6, 9), our work is novel in that we explored the adjuvant effects of LPS against pneumonia infection with a more virulent ST of S. pneumoniae, and we examined the response of the lung tissue to infection, with and without prior immunization, to better understand how immunization may exert its protective effects. Our model of immunization with PPS3 or LPS or with a combination of the two and infection with a low dose of 5 × 104 to 1 × 105 CFU of S. pneumoniae ST 3 5 days after treatment allowed us to examine the response to immunization and LPS adjuvanticity in terms of antibody production, lung infection, bacteremia, lung cytokine expression, and hepatic acute-phase proteins that are known or considered very likely to play a role in the resolution of infection. Immunization with PPS3 and coimmunization with PPS3 and LPS prior to infection allowed mice to survive. This immunization thus enabled us to characterize pneumonia infection beyond the time (∼3 days) when all vehicle-immunized, infected mice had succumbed to infection. Both of the infectious doses we used, 5 × 104 and 1 × 105 CFU, are considered low doses (7, 10), and they elicited similar pneumonia pathogeneses in adult female mice. The importance of primary serum IgM in promoting bacterial clearance is demonstrated by the data in Fig. 1 as baseline day 0 levels in PPS3-immunized mice increased 5 days after immunization but slightly declined by day 2, which corresponds to increased IgM binding to bacteria in the presence of PPS3-immunized serum (Fig. 1B). Additionally, PPS3-specific IgM serum levels in PPS3- and PPS3+LPS-immunized mice that survived to 7 days p.i. remained significantly elevated in comparison to levels in infected control mice, and IgG increased in immunized mice by this time point (Fig. 3E and F). IgM deposited on pneumococci may have been cleared by phagocytic cells during the early acute phase of infection and thus may explain one mechanism by which PPS3-immunized mice are protected against pneumonia. Unexpectedly, treatment with PPS3+LPS before infection did not result in increased IgM binding to S. pneumoniae in vitro (Fig. 1B), which is not explainable at this time but may suggest a different trafficking of antibody due to LPS. Notably, and most importantly, mice treated in this manner were protected in the survival study.
Immunization with PPS3 and the combined immunization of PPS3 and LPS promoted clearance of the bacteria at the tissue site of infection and inhibited systemic spread in the blood (Fig. 2 and Table 3). Lung bacterial burden was measured at the early acute time points of 5 and 12 h p.i. with no significant differences observed, which most likely is due to the exponential growth phase of bacteria in the lung tissue at this time (data not shown). By 24 h p.i., lung CFU counts were lower in coimmunized mice than in infected controls, and this trend persisted to 48 h p.i. as complete clearance of bacteria from the lungs of PPS3-immunized or coimmunized mice was observed by day 7 p.i. (Fig. 2 and 3B). Immunization with PPS3 or the combination also inhibited systemic bacterial dissemination because immunized mice had significantly lower bacteremia counts than infected controls by 24 and 48 h p.i. (Table 3). Other investigators have reported bacteremia in a mouse model of pneumonia at 24 h p.i. using a slightly greater infectious dose (4 × 106 CFU) of S. pneumoniae ST 3, thus noting the virulence of ST 3 (10).
Although immunization with either PPS3 or the combination of PPS3 and LPS conferred protection against pneumococcal challenge by significantly decreasing bacterial burden in the immediate lung microenvironment, a significant difference in lung inflammatory scores between PPS3-immunized mice and infected controls was not observed at 48 h p.i. (Fig. 4). Bacteremia may be a more significant cause of death because pneumonia pathology was not severe in the present model, but infection was lethal in all infected control mice by 3 days p.i. (Fig. 4 and Table 3). At 48 h p.i., proinflammatory cytokine mRNA expression was examined in the lung to further characterize inflammation, and this better reflects the pattern of lung bacterial burden than the histopathological score. A decrease in pulmonary IL-6 mRNA level coincided with lower lung CFU counts in PPS3-immunized mice, and transcript expression returned to the baseline level at the resolution stage of infection in immunized mice, which did not differ from the level in noninfected controls (Fig. 2B, 3D, and 5D). The proinflammatory lung cytokines IL-1β, IL-6, and CCL2 and the hepatic acute-phase protein Saa1 transcripts also returned to the levels of noninfected control mice by 7 days p.i., which further suggests resolution of inflammation consistent with evidence for resolution of infection (Fig. 3C and D; data not shown for IL-1β and Saa1). Previously, a decrease in proinflammatory cytokine mRNA expression of MIP-2, IL-12, monocyte chemotactic protein-1 (MCP-1)/JE, and TNF-α at 24 h p.i. with S. pneumoniae ST 8 infection was observed after immunization with human IgM specific for ST 8 pneumococcal capsular polysaccharide D11 (15). We noted a decrease in IL-6 and CCL2 mRNA expression levels in the lungs of PPS3+LPS-immunized mice at 24 and 48 h p.i. and in serum CCL2 protein levels at 48 h p.i. (Fig. 5), suggesting that increased IgM may provide protection against acute pneumonia by aiding in early resolution of infection.
LPS, a component of the cell wall of Gram-negative bacteria, binds to Toll-like receptor 4 (TLR4), an endotoxin receptor, promoting both TRIF and MyD88 signaling, which results in the upregulation of early-immediate response gene programs such as proinflammatory cytokines, especially TNF-α (16, 17). The adjuvant, AS04, which is comprised of monophosphoryl lipid A (MPL), a detoxified moiety of LPS and alum, produces a robust Th1 response (18). Although signaling by LPS through TLR4 produces a pyrogenic effect, MPL signals exclusively though the TRIF adaptor and is not a pyrogen (19). Our reasoning in immunizing with PPS3 in conjunction with LPS was that the combination may upregulate cytokine production (IL-6 and TNF-α), thus activating macrophages and promoting opsonization and complement-mediated clearance in the lung microenvironment. Moreover, significantly higher serum anti-PPS3 IgM has been reported in rats coimmunized with PPS3 and LPS (9). Although LPS immunization did not decrease lung bacterial burden, we did observe a 26.8% decrease in bacteremia and an upregulation in pulmonary CCL2 at the mRNA level at 48 h p.i. (Table 3 and Fig. 5C). LPS immunization could be upregulating protective proinflammatory cytokine expression before infection, which may be attenuating bacteremia (16, 17). In a murine model of pneumonia infection, Winter and colleagues (10) detailed the importance of CCL2, which is homologous to human monocyte chemotactic protein-1 (MCP-1), and observed that ablation of CCL2 by genetic deficiency resulted in a 50% decrease in survival from infection with a less virulent strain of S. pneumoniae, ST 19 (10). In their studies, the absence of CCL2 decreased macrophage and dendritic cell accumulation in lung exudates. Interestingly, both the wild-type and CCL2-deficient mice were unable to resolve infection from ST 3 and died by day 4 p.i., which is consistent with our survival study results with ST 3 as all infected control mice died by day 4 p.i. (10) (Fig. 3A). In future studies, the protective effect of the adjuvant MPL could be investigated with less virulent STs of S. pneumoniae, such as ST 19, as MPL will provide nonpyrogenic immune stimulation, is a licensed adjuvant, and thus has been approved for use in humans (19).
In congruence with the decreased bacteremia observed at the 48-h acute time point in our studies, mRNA expression levels of the hepatic acute-phase proteins LBP, SAP, and Saa1 were also lower in immunized mice than in infected control mice, indicating that systemic inflammation due to pneumococcal infection is less severe in immunized mice by 48 h p.i. (Fig. 6). In another murine model of pneumonia infection, SAP was proven to be vital to pneumococcal clearance as SAP−/− mice did not survive infection from S. pneumoniae serotype 2, 4, or 23F (20). Quinton et al. (21) reported different kinetics of acute-phase protein mRNA expression than determined in our pneumonia model, such as a significant upregulation of Saa1, SAP, and LBP by 24 h p.i., while we observed an increase in these acute-phase proteins at the gene level by 48 h p.i. This difference may be in part due to the greater inoculating dose of ST 3 used in their study and/or the route of administration as the bolus dose used by Quinton et al. was inserted into the left lobe of the lung by an angiocatheter (21). Administering a greater dose to the site of pneumonia infection may have allowed for a more rapid systemic dissemination than our model of intranasal challenge with a 10-fold-lower infectious dose of ST 3.
To our knowledge, SAA has not been reported to be expressed in the lung during pneumonia infection. Although acute-phase proteins generally increase more dramatically at the gene level than at the protein level, our results are still novel in showing that two acutely expressed SAA variants, Saa1 and Saa2, and one constitutively expressed transcript, Saa3, were the genes most significantly affected by infection or immunization, as detected by lung RNA microarray analysis (Fig. 7) (22–24). Additionally, SAA protein in serum reflects the same trend as the mRNA level: upregulated in infected controls and decreased in response to immunization (Fig. 6F). We have yet to confirm at the protein level the presence of other genes detected by microarray analysis, but our results support the use of SAA as a marker of pneumonia disease severity (25, 26) and suggest a correlation between serum and lung tissue SAA expression. Furthermore, SAA has been reported to increase the secretion of IL-1β, IL-1 receptor α (IL-1Rα), and TNF receptor II (TNFR-II) proteins by THP-1 cells and to promote monocyte chemotaxis as well as hypersensitivity reactions after subcutaneous injection (27). Thus, SAA has been demonstrated to be a strong immune activator both in vitro and in vivo, and its relevance as a biomarker of pneumonia severity is further supported by our study.
An interesting and unanticipated result of the microarray analysis was the identification of Z-DNA binding protein-1 (ZBP1, first splice variant) as a highly upregulated gene in the lungs of the pneumococcus-infected mice and a gene whose expression was also downregulated by PPS3 and LPS coimmunization after infection (Fig. 7). ZBP1 (also known as DAI/DLM-1) has been implicated in the response to or sensing of viruses as a cytoplasmic DNA sensor (28) and during tumor formation (29). However, we believe that we are the first to report ZBP1 (first splice variant) transcript upregulation in response to bacterial pneumonia infection. Due to its structure, ZBP1 can associate with Z-configured DNA or double-stranded RNA (dsRNA), which then binds to promyelocytic leukemia protein (PML) oncogenic domains, thus promoting an antiviral interferon (IFN) response (29). Our gene expression results suggest that ZBP1 may play a broader role in pattern recognition and response than for viruses only, and additional studies of its regulation and functions during bacterial infection are now warranted.
In conclusion, we have developed a murine model of pneumonia infection and immunization with PPS3, LPS, and the combination of PPS3 and LPS 5 days prior to infection with S. pneumoniae ST 3. Whereas this infection is highly virulent, immunization with PPS3 in an IgM-dependent manner as well as coimmunization with PPS3 and LPS greatly attenuated pneumonia severity and promoted resolution of infection, concomitant with significant regulation of cytokine gene expression in the lungs, acute-phase proteins in the lungs, liver, and serum, and regulation of novel factors such as ZBP1 that may potentially contribute to bacterial sensing, response, and resolution of infection.
Supplementary Material
ACKNOWLEDGMENTS
Financial support for this work was provided by grant DK41479 and CA90214 BANGEO funds from the National Institutes of Health.
Pneumococcal polysaccharide ST 3 was kindly provided by Christopher E. Taylor.
Footnotes
Published ahead of print 6 February 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00593-12.
REFERENCES
- 1. World Health Organization 2007. Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly. Epidemiol. Rec. 82:93–104 [PubMed] [Google Scholar]
- 2. Haessler S, Schimmel JJ. 2012. Managing community-acquired pneumonia during flu season. Cleve. Clin. J. Med. 79:67–78 [DOI] [PubMed] [Google Scholar]
- 3. Pitsiou GG, Kioumis IP. 2011. Pneumococcal vaccination in adults: does it really work? Respir. Med. 105:1776–1783 [DOI] [PubMed] [Google Scholar]
- 4. Schranz J. 2009. Pneumoccoal conjugate vaccines: what do we know and what do we need? Procedia Vaccinol. 1:189–205 [Google Scholar]
- 5. How MJ, Brimacombe JS, Stacey M. 1964. The pneumococcal polysaccharides. Adv. Carbohydr. Chem. 19:303–358 [DOI] [PubMed] [Google Scholar]
- 6. Baker PJ, Amsbaugh DF, Stashak PW, Caldes G, Prescott B. 1981. Regulation of the antibody response to pneumococcal polysaccharide by thymus-derived cells. Rev. Infect. Dis. 3:332–341 [DOI] [PubMed] [Google Scholar]
- 7. Ferreira DM, Moreno AT, Cianciarullo AM, Ho PL, Oliveira ML, Miyaji EN. 2009. Comparison of the pulmonary response against lethal and non-lethal intranasal challenges with two different pneumococcal strains. Microb. Pathog. 47:157–163 [DOI] [PubMed] [Google Scholar]
- 8. Mizrachi-Nebenzahl Y, Lifshiz S, Teitelbaum R, Novick S, Levi A, Benharroch D, Ling E, Dagan R. 2003. Differential activation of the immune system by virulent Streptococcus pneumoniae strains determines recovery or death of the host. Clin. Exp. Immunol. 134:23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pasatiempo AM, Kinoshita M, Foulke DT, Ross AC. 1994. The antibody response of vitamin A-deficient rats to pneumococcal polysaccharide is enhanced though coimmunization with lipopolysaccharide. J. Infect. Dis. 169:441–444 [DOI] [PubMed] [Google Scholar]
- 10. Winter C, Herbold W, Maus R, Länger F, Briles DE, Paton JC, Welte T, Maus UA. 2009. Important role for CC chemokine ligand 2-dependent lung mononuclear phagocyte recruitment to inhibit sepsis in mice infected with Streptococcus pneumoniae. J. Immunol. 182:4931–4937 [DOI] [PubMed] [Google Scholar]
- 11. Li J, Szalai AJ, Hollingshead SK, Nahm MH, Briles DE. 2009. Antibody to the type 3 capsular facilitates immune adherence of pneumococci to erythrocytes and augments their transfer to macrophages. Infect. Immun. 77:464–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 13. Pfaffl MW. 2001. A new mathematical model for relative quantification of real-time RT-PCR. Nucleic Acids Res. 29:2001–2007 [Google Scholar]
- 14. Londhe KB, Borlak J. 2012. A cross-platform comparison of genome-wide expression changes of laser microdissected lung tissue of c-Raf. PLoS One 7:e40778 doi:10.1371/journal.pone.0040778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burns T, Abadi M, Pirofski LA. 2005. Modulation of the lung inflammatory response to serotype 8 pneumococcal infection by a human immunoglobulin M monoclonal antibody to serotype 8 capsular polysaccharide. Infect. Immun. 73:4530–4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu YC, Yeh WC, Ohashi PS. 2008. LPS/TLR4 signal transduction pathway. Cytokine 42:145–151 [DOI] [PubMed] [Google Scholar]
- 17. Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. 2007. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 316:1628–1632 [DOI] [PubMed] [Google Scholar]
- 18. Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, Larocque D, Van Mechelen M, Garçon N. 2009. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 183:6186–6197 [DOI] [PubMed] [Google Scholar]
- 19. Casella CR, Mitchell TC. 2008. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell. Mol. Life. Sci. 65:3231–3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yuste J, Botto M, Bottoms SE, Brown JS. 2007. Serum amyloid P aids complement-mediated immunity to Streptococcus pneumoniae. PLoS Pathog. 3:e120 doi:10.1371/journal.ppat.0030120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quinton LJ, Jones MR, Robson BE, Mizgerd JP. 2009. Mechanisms of the hepatic acute-phase response during bacterial pneumonia. Infect. Immun. 77:2417–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coetzee GA, Strachan AF, van der Westhuyzen DR, Hoppe HC, Jeenah MS, de Beer FC. 1986. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J. Biol. Chem. 261:9644–9651 [PubMed] [Google Scholar]
- 23. Eriksen N, Benditt EP. 1980. Isolation and characterization of the amyloid-related apoprotein (SAA) from human high density lipoprotein. Proc. Natl. Acad. Sci. U. S. A. 77:6860–6864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uhlar CM, Whitehead AS. 1999. Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 265:501–523 [DOI] [PubMed] [Google Scholar]
- 25. Huttunen T, Teppo AM, Lupisan S, Ruutu P, Nohynek H. 2003. Correlation between the severity of infectious disease in children and the ratio of serum amyloid A protein and C-reactive protein. Scand. J. Infect. Dis. 35:488–490 [DOI] [PubMed] [Google Scholar]
- 26. Lannergård A, Larsson A, Kragsbjerg P, Friman G. 2003. Correlations between serum amyloid A protein and C-reactive protein in infectious diseases. Scand. J. Clin. Lab. Invest. 63:267–272 [PubMed] [Google Scholar]
- 27. Patel H, Fellowes R, Coade S, Woo P. 1998. Human serum amyloid A has cytokine-like properties. Scand. J. Immunol. 48:410–418 [DOI] [PubMed] [Google Scholar]
- 28. Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and activator of innate immune response. Nature 448:501–506 [DOI] [PubMed] [Google Scholar]
- 29. Pham HT, Park MY, Kim KK, Kim YG, Ahn JH. 2006. Intracellular localization of human ZBP1: differential regulation by the Z-DNA binding domain, Zα, in splice variants. Biochem. Biophys. Res. Commun. 348:145–152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.