Abstract
Cryoglobulin characteristics in chronic hepatitis C (CHC) might be of importance for knowing more about the pathogenesis and treatment of the disease. We aimed to investigate the relationship between cryoglobulin types and their specificity against hepatitis C virus (HCV) antigenic epitopes in CHC patients. We analyzed samples from 43 patients with HCV-associated cryoglobulinemia, of whom 4 had concomitant lymphoma. Cryoglobulins were measured, purified, typed by immunofixation electrophoresis, and tested for IgG and IgM anti-HCV antibodies by immunoblot analysis and an enzyme-linked immunosorbent assay (ELISA). Clinical and other laboratory data were recorded. The median cryocrit level of the tested samples was 6%. Type I cryoglobulins were detected in 9.3% (4/43) of the cryoprecipitates, and type II cryoglobulins were detected in 48.8% (21/43) of the cryoprecipitates. IgM monoclonal protein, mainly IgM(κ), was found in 92% (23/25) of type I and II cryoprecipitates. Type III cryoglobulins were identified in 41.9% (18/43) of the patients and were associated with high blood serum IgG levels. In 81.3% (13/16) of type II and 92.3% (12/13) of type III cryoglobulins, there was IgG reactivity against the viral core region. Ninety-two percent and 32% of IgG anti-HCV core-positive cryoprecipitates had additional specificities against the NS3 and NS4 regions, respectively. Also, IgM anti-HCV antibodies were detected in 31% of the cryoprecipitates. In conclusion, all types of cryoglobulins were found in patients with HCV-associated cryoglobulinemia, with type II being the most frequently identified. Type III cryoglobulins were common and were associated with high serum IgG levels. HCV-related cryoglobulins demonstrated IgM, and particularly IgG, anti-HCV specificities, mainly against the core and NS3 epitopes.
INTRODUCTION
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease (1), with over 170 million people infected worldwide (2). HCV is an enveloped positive single-stranded RNA virus that belongs to the Flaviviridae family (1). Its genome is enclosed in an icosahedral capsid, itself enveloped by a lipid bilayer, where two different glycoproteins are anchored (3). An open reading frame of the genome encodes a polyprotein that is cleaved into structural proteins, consisting of the capsid protein core and the two envelope glycoproteins (E1 and E2), which are essential components for viral entry and fusion, and multiple nonstructural regulatory proteins, such as p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B (1).
HCV has the ability to infect and replicate in lymphocytes (lymphotropism) and other types of human cells, apart from liver cells, and it has been involved in extrahepatic manifestations of the disease, including mixed cryoglobulinemia (MC) syndrome (3). MC is the most common extrahepatic manifestation of chronic HCV infection and is associated with several clinical and laboratory autoimmune events (4, 5). MC might also be observed in the absence of liver disease, and it is characterized by cryoglobulin deposition on endothelial cells, eliciting vascular inflammation of small and medium vessels. The clinical spectrum of HCV cryoglobulinemia largely varies from an asymptomatic presentation to severe vasculitis and lymphoma. Even though there is observed variation in the presentation of overt symptoms, the basis of cryoglobulinemia consists of a clonal autoreactive B-lymphocyte expansion (2, 3). The survival of these immunocompetent cells might be related to the existence of many host factors, such as the B-lymphocyte activating factor (BAFF), which was found to be increased in the blood serum of chronically infected HCV patients with MC. Elevated levels of BAFF increase the stability of autoreactive B lymphocytes and might play a significant role in the pathogenesis of HCV MC (6). Another important factor on the B-cell surface is the cellular receptor CD81, which is upregulated in HCV-infected patients, particularly in those with MC (7).
It has been shown that in patients with chronic HCV infection, HCV-associated cryoglobulins, mainly types II and III and in rare cases type I, are frequently detected (8, 9, 10). The HCV-related cryoglobulin types are of clinical significance; this is especially the case in patients treated with rituximab and have monoclonal IgM(κ) cryoglobulin fraction. The chimeric monoclonal antibody directed against human CD20 (9, 11, 12, 13) may form complexes with IgM(κ) cryoglobulins, leading to increased levels of cryoprecipitation and adverse reactions (14, 15). The reactivities of IgG and IgM in cryoglobulins against specific HCV antigens has not been extensively studied.
In the present study, we analyzed HCV-related cryoglobulins in a cohort of patients with HCV-associated cryoglobulinemia. Our main target was to investigate the relationship between the cryoglobulin type and specificity against HCV antigens in order to better elucidate the complex relationship between HCV and autoreactivity.
MATERIALS AND METHODS
Study design and patients.
The study group consisted of 43 patients with chronic HCV infection who attended our liver unit and were found to have cryoglobulins in their blood serum. Screening for cryoglobulins was performed in all patients with clinical signs of cryoglobulinemia (i.e., vasculitis) or lymphoma and mostly at the time of chronic hepatitis C (CHC) diagnosis in those who were asymptomatic (n = 32). Patients with HIV or hepatitis B virus (HBV) coinfection were excluded.
Clinical and laboratory characteristics were recorded at the time of visit for all patients, including gender, age, level of HCV RNA, HCV genotype, and presence of cirrhosis. Liver cirrhosis was confirmed by either biopsy or a combination of clinical, laboratory, imaging, and fibroscan findings. Blood chemistry, rheumatoid factor, and quantitative immunoglobulin measurements were performed at the time of cryoglobulin detection.
The protocol for the clinical study was submitted and approved by the ethics committee of our center, and informed consent was obtained by all patients prior to their participation in the study.
Clinical laboratory assays.
Blood chemistry measurements, including liver enzyme and anti-HCV testing, were performed by commercially available assays (Abbott Laboratories, Abbot Park, IL). Rheumatoid factor and immunoglobulins were measured by nephelometry (Radim SpA, Rome, Italy). HCV RNA was detected by a PCR assay (Cobas Amplicor, Roche Molecular Systems Inc., Branchburg, NJ), and HCV genotype was determined by the line probe assay Versant HCV genotype LiPA 2.0 (Siemens Healthcare Diagnostics Inc., Tarrytown, NY). Blood serum HCV RNA levels were quantified by the Versant HCV RNA 3.0 branched DNA (bDNA) test (Siemens Healthcare Diagnostics Inc., Tarrytown, NY).
Isolation and characterization of cryoglobulins.
Blood samples of approximately 15 to 20 ml were collected into prewarmed tubes and clotted at 37°C for ≥1 h. The serum was separated from clots by centrifugation at 3,500 rpm for 20 min and was incubated for ≥7 days at 4°C. Cryoglobulin presence was first assessed by visual inspection of a precipitate that was formed after centrifugation and then by its resolubilization for 10 min in 37°C. The cryoglobulin concentration was measured as the cryocrit.
Cryoprecipitates were recovered from serum samples after four periodic cleaning cycles that included centrifugation at 3,500 rpm for 10 min at 4°C, supernatant removal, specimen reconstitution with saline (0.15 mmol/liter, 37°C [pH 7.4]), and overnight incubation at 4°C. After four successive washes, an appropriate amount of saline was added to the purified cryoprecipitate.
Complete purification, detailed characterization, and typing of the cryoprecipitate were assessed by gel electrophoresis and subsequent immunofixation electrophoresis (IFE) using SAS-3 (Helena Biosciences Europe, Tyne and Wear, United Kingdom), or Hydrasys (Sebia, France).
Immunoblotting for IgG antibodies directed against specific HCV antigens in the purified cryoglobulins was performed with the INNO-LIA HCV Score (Innogenetics NV, Ghent, Belgium). The presence of IgM anti-HCV antibodies (against core, recombinant NS3, NS4, and NS5 peptide mixture) was examined by enzyme-linked immunosorbent assay (ELISA) (Dia.Pro, Italy).
Statistical analysis.
All continuous variables are presented as the median accompanied by the range, and categorical variables are presented as absolute and relative frequencies. Student's t test was used for the comparison of continuous variables. Categorical variables were compared by the use of contingency tables and calculations of the chi-square test. Statistical significance was set at a P value of <0.05. All reported P values were based on two-sided tests. SPSS software v.20.0 (IBM SPSS Statistics) was used for all statistical calculations.
RESULTS
Forty-three patients, 18 males (41.9%) and 25 females (58.1%), with a median age of 48 years (range, 31 to 76 years) and who had HCV-related cryoglobulinemia (median cryocrit, 6%; range, 1 to 33%), were analyzed in this study. The median blood serum HCV RNA level was 6.2 × 105 IU/ml (range, 0.5 × 102 to 3.1 × 106 IU/ml). The HCV genotype distribution was 51.5% (17/33) for genotype 1, 3% (1/33) for genotype 2, 39.4% (13/33) for genotype 3, and 6.1% (2/33) for genotype 4. Twenty patients were diagnosed with liver cirrhosis, 2 patients with hepatocellular carcinoma, and 4 patients with concomitant lymphoma. Four patients were already on antiviral treatment with pegylated interferon (PEG-IFN) and ribavirin. Asymptomatic cryoglobulinemia was present in 32 of the 43 patients.
Type of HCV-related cryoglobulins.
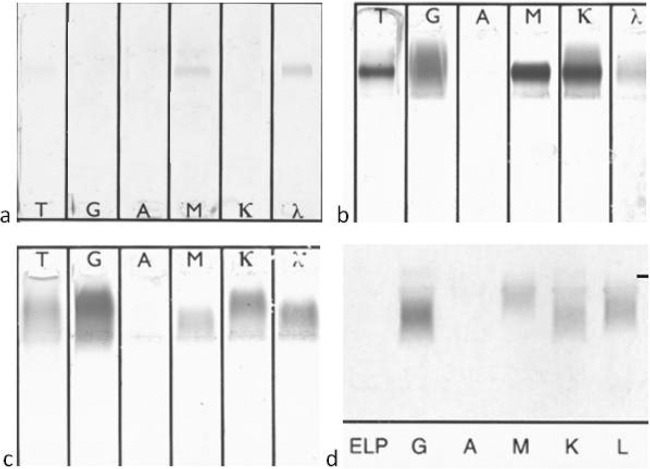
A monoclonal component was observed in 25/43 (58.1%) patients. Specifically, 4/43 (9.3%) patients had type I cryoglobulins and 21/43 (48.8%) had type II. Polyclonal type III cryoglobulins were found in 18/43 (41.9%) patients (Table 1, Fig. 1). The majority of the detected monoclonal proteins were of the IgM type (23/25, 92%); IgM(κ) was detected in 17/23 (74%) and IgM(λ) in 6/23 (26%) patients with an IgM monoclonal component in the cryoprecipitate. Monoclonal IgG(κ) immunoglobulin was present in 2 patients, both with type II cryoglobulinemia. All type I cryoglobulins contained monoclonal IgM protein [50% (2/4) IgM(κ) and 50% (2/4) IgM(λ)].
Table 1.
Types of cryoglobulins and the respective monoclonal component as detected by immunofixation electrophoresis (IFE) in patients with chronic hepatitis C and cryoglobulinemia
| Monoclonal component | No. of patients with cryoglobulin type: |
Total no. (%) of patients | ||
|---|---|---|---|---|
| I | II | III | ||
| Positive | 25 (58.1) | |||
| IgM(κ) | 2 | 15 | 0 | |
| IgM(λ) | 2 | 4 | 0 | |
| IgG(κ) | 0 | 2 | 0 | |
| Negative | 0 | 0 | 18 | 18 (41.9) |
| Total no. (%) of patients | 4 (9.3) | 21 (48.8) | 18 (41.9) | 43 |
Fig 1.
Representative results of immunofixation electrophoresis (IFE) of cryoglobulins. (a) Type I [monoclonal IgM(λ)]; (b) type II [monoclonal IgM(κ) and polyclonal immunoglobulins]; (c and d) type III (polyclonal immunoglobulins). The results shown were tested by IFE using SAS-3 (Helena Biosciences Europe, Tyne and Wear, United Kingdom) (a, b, and c) and Hydrasys (Sebia, France) (d).
The patients' median blood serum immunoglobulin levels were 1,700 mg/dl (range, 571 to 4,281 mg/dl) for IgG, 177 mg/dl (range, 73 to 1,020 mg/dl) for IgM, and 237 mg/dl (range, 63.8 to 844 mg/dl) for IgA, with upper limits of the normal of 1,600, 230, and 400 mg/dl, respectively. Patients with type III cryoglobulins had significantly higher blood serum IgG levels than those with types I and II (median, 2,016 mg/dl, and range, 1,050 to 4,281 mg/dl, versus median, 1,360 mg/dl, and range, 571 to 2,084; P = 0.011).
Out of the four patients with lymphoma, one had type I and three had type II cryoglobulinemia. Furthermore, these patients were infected by HCV genotype 1 or 4 (P = 0.001) and had higher levels of cryocrit (P < 0.05) than did patients without lymphoma.
Cryoglobulin anti-HCV specificity.
In 86.2% (25/29) of tested samples with type II or III cryoprecipitates, the IgG fraction of the cryoglobulins was found to react with HCV peptides. In detail, 81.3% (13/16) of type II and 92.3% (12/13) of type III cryoglobulins were found to have IgG anti-HCV activities, with specificity against the viral core region (C1 and/or C2). Additional IgG reactivity for the cryoprecipitates that were antibody positive for the HCV core region was found against the NS3 and NS4 regions in 92% (23/25) and 32% (8/25) of the samples, respectively. No reactions against the HCV E2 or NS5 region were detected (Fig. 2, Table 2).
Fig 2.
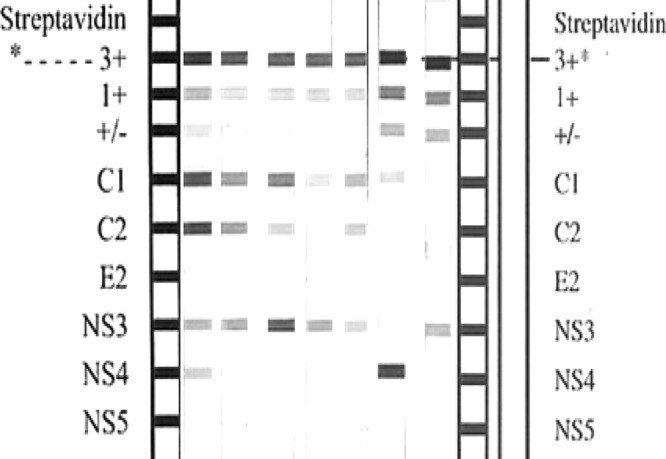
Examples of INNO-LIA HCV score results of cryoglobulins demonstrating anti-HCV core, NS3, and NS4 reactivity. No antibodies were detected against proteins in the E2 or NS5 regions.
Table 2.
Specificity of IgG cryoglobulins against HCV antigenic epitopes
| IgG fraction (n) | Specific antibodies (no./total no. [%]) against the following antigenic targets: |
||||
|---|---|---|---|---|---|
| Core | E2 | NS3 | NS4 | NS5 | |
| Type II (16) | 13/16 (81.3) | 0 | 11/16 (68.8) | 3/16 (18.8) | 0 |
| Type III (13) | 12/13 (92.3) | 0 | 12/13 (92.3) | 5/13 (38.5) | 0 |
| Total (29) | 25/29 (86.2) | 0 | 23/29 (79.3) | 8/29 (27.6) | 0 |
Further testing for the presence of IgM anti-HCV antibodies was performed with 29 cryoglobulin samples (2/29 type I, 15/29 type II, and 12/29 type III cryoglobulins), 31% (9/29) of which were positive. The majority of IgM anti-HCV-positive cryoprecipitates contained type II monoclonal IgM cryoglobulins (77.8%). The two cryoglobulin type I samples that were tested were found to be negative for IgM activity against HCV.
The presence of IgM or IgG antibodies against HCV in the cryoglobulins was not significantly associated with any clinical characteristics or laboratory data of the patients.
DISCUSSION
Cryoglobulins are detected in about 50% of patients with chronic HCV infection, which accounts for ≥90% of cases of MC (16). This is a result of complex virus-host interactions that are still under study. Mixed cryoglobulinemia in patients with chronic HCV infection is associated mainly with type II and III cryoglobulins (2). It has been postulated that type III cryoglobulins evolve over time to type II due to B-cell clonal expansion (17). In our study subjects, which consisted of symptomatic and asymptomatic patients, type II cryoglobulins were found in 48.8% (21/43) and type III in 41.9% (18/43) of cases. Type I cryoglobulinemia, which is associated with lymphoma, Waldenström's macroglobulinemia, and multiple myeloma (17), was observed in 9.3% (4/43) of the patients, of whom only one had B-cell non-Hodgkin's lymphoma. It was recently reported that in monoclonal type I cryoglobulinemic vasculitis, approximately half of the patients had only benign B-cell lymphoproliferation, i.e., monoclonal gammopathy of unknown significance (MGUS) (18). This might also be the case in our 3 patients with type I cryoglobulinemia who had no evidence of hematologic malignancy. In the present study, a monoclonal component was present in 58% (25/43) of the cryoprecipitates. The most frequently identified monoclonal protein was IgM(κ), and this observation has been confirmed by other studies that reported on the production of monoclonal IgM(κ) immunoglobulin with anti-IgG activity (rheumatoid factor [RF]) in MC (14). RF activity was detected in all patient serum samples that were included in our study.
Chronic HCV stimulation with the development of monoclonal cryoglobulins might rarely result in clinical overt lymphoma, although patients with cryoglobulinemic vasculitis develop B-cell malignancies at higher rates of up to 5 to 10%, compared with 0.2 to 2.6% of the overall HCV-infected population (19–24). In our study, four patients with chronic HCV infection had already developed overt lymphoma. One of these patients had type I and the other three had type II cryoglobulinemia. In all 4 patients, the monoclonal protein in the cryoprecipitate was IgM(κ). Most clonal B-cell expansions in HCV infection are associated with V(κ)-expressing B cells, as demonstrated by the highly increased κ:λ ratios and the usage of the V(κ) genes that belong to restricted subfamilies, such as Vκ3-15 and Vκ3-20 (25). The Vκ3-20 gene expression is often associated with VH1-69 expression in HCV-associated MC and lymphomas (25, 26, 27). Therefore, IgM(κ) is expected to be the predominant monoclonal component in HCV-related cryoglobulins, and especially in cases with associated lymphomas. Additionally, although the number of our patients with lymphoma was limited, we observed a genotype association, as all of them had either genotype 1 or 4 HCV infection. Moreover, patients with lymphoma had a higher quantity of cryoglobulins than the rest, suggesting a more vigorous immune or autoimmune reaction in those patients.
HCV-induced MC is considered to be a B-cell lymphoproliferative disorder resulting from B-cell clonal expansion, without the molecular characteristics of a true neoplastic process (28), caused by the constant immune stimulation by HCV, a virus with a high mutation frequency (2). Under poorly defined and complicated genetic and environmental conditions, the host's immune system produces polyclonal and monoclonal antibodies that bind to HCV proteins and also elicit a specific IgM RF response, causing large aggregates (cryoglobulins).
It has been suggested that the presence of specific HCV proteins causes clonal B-cell expansion and enhanced immune complex generation (2, 29). Cold-precipitating immune complexes in HCV infection are thought to be primarily formed by IgM rheumatoid factor molecules bound to the Fc portion of IgG, whose main target is the HCV core protein. Moreover, the HCV core protein has been found in skin and renal biopsy samples of HCV-infected patients with MC-associated active vasculitis and nephropathy. In an eloquent study on patients with CHC cryoglobulinemia, nonenveloped core protein was found in the cryoprecipitate, in the form of immune complexes with specific anti-core IgG antibodies bound to IgM with RF activity. Precipitation at low temperature did not occur with the addition of any IgG immunoglobulin that was irrelevant to HCV (28). The cryoprecipitated complexes easily connect to the C1q protein, which subsequently binds to the specific C1q receptors located on the membranes of endothelial cells (17). In our study, we demonstrated that in 86.2% of our patients with type II or III MC, the IgG fraction of the cryoglobulins had strong reactivity against HCV viral proteins and particularly against the core protein (either C1 or C2). Possible formation of immune complexes or low levels of antibodies might have prohibited the demonstration of such activity in the other samples we analyzed. In any way, the presence of these antibodies confirms the pivotal role of core protein in cryoglobulin formation. It is of great interest that additional HCV IgG activity against NS3 and NS4 was observed in 92% (23/25) and 32% (8/25) of the anti-core-positive cryoprecipitates, respectively. Their presence cannot be attributed to entrapment from serum since albumin, the major serum protein, was absent in all of the tested cryoprecipitates. Even more, although the existence of IgG anti-E2 and anti-NS5 antibodies in the blood serum of CHC patients is well documented, we did not detect any IgG cryo-antibodies against these 2 regions (30). These antibodies in blood serum are usually detected at low levels, and this is probably related to weak antigenic activity due to their structural similarity to the human proteome, such as the immunoglobulin (Ig) variable domains and the T-cell receptor (TCR) (30, 31). While the role of anti-NS3 and anti-NS4 IgG antibodies in cryoglobulin formation needs to be investigated further, active viral replication that exposes the immune system to HCV structural proteins, such as the HCV core antigen (Ag), located in the inner surface of the virion, appears to be a prerequisite for cryoglobulin production.
Moreover, IgM anti-HCV antibodies were also detected in the cryoprecipitates from 31% of patients with HCV-related cryoglobulinemia, of whom the majority (78%) had type II MC. Even though we did not identify specific HCV epitopes for the IgM anti-HCV antibodies, previous studies have shown that autoreactive IgM might originate in response to core or even other HCV Ags, such as the NS3 or E2 proteins, with mimicry of Ig motifs. The binding of IgM with IgG Fc might be due to coincidental cross-reactivity (32, 33, 34). Interestingly, no IgM activity against HCV was detected in the small number of patients tested with type I cryoglobulinemia. This result might have occurred because of the low cryocrit levels (1 to 2%) in these samples or the proliferation of a different clonal B-cell population.
It is still a matter of debate whether a specific HCV genotype is involved in MC. A higher prevalence of cryoglobulinemia among patients infected with HCV genotypes 1 and 2a or 2c (35–41) has been reported, but several studies have shown all HCV genotypes to be related to MC (42). We did not observe any specific genotype association with the quantity of cryoglobulin or cryoglobulin type. Also, no association was observed between HCV genotype and anti-HCV antibodies in the cryoglobulins. This lack of association is further supported by a number of studies showing that the genotype distribution in patients with HCV-related MC simply reflects the epidemiology of genotypes that exists in HCV patients in a given geographical area (43, 44). Therefore, the differences in the prevalence of HCV genotypes in different geographical regions, which clearly exists, might bias previously observed genotype associations.
With the increased number of observed type I cryoglobulins, a technical concern was raised about the sensitivity of the methods used for the cryoglobulin type characterization, which is of great importance. In cases of type II cryoglobulinemia, one of the two components might be predominant (monoclonal or polyclonal component). The cryoglobulin component that is present in low levels can be missed, especially in low cryocrit levels, since the IFE assays that are widely used for cryoglobulin typing have been standardized for routine testing of blood serum or urine specimens. Thus, in cases with low levels of cryocrit, type II cryoglobulins might be misidentified as type I or III. Nevertheless, type II was the most frequently identified cryoglobulin associated with HCV. Another limitation of the study is the fact that due to an insufficient quantity of samples, we were not able to analyze all specimens for their activities against HCV antigens.
Although an association between the quantity of cryoglobulins and symptomatic HCV-related MC has been reported (45), it is generally thought that the levels of cryoglobulin do not correlate with the severity of symptoms. In this study, the majority of the patients examined were asymptomatic and only 11/43 (26%) had MC vasculitis and/or lymphoma. This low number might have prohibited us from detecting more significant clinical correlations. Nevertheless, higher cryocrit levels were seen in patients with lymphoma and patients with vasculitis than in asymptomatic patients, but this result reached statistical significance only for lymphoma.
In conclusion, type II and III cryoglobulins were predominantly found in HCV-related MC, although rare cases of type I were seen, even in the absence of lymphoma. Type III cryoglobulinemia was associated with high blood serum IgG levels, but no other significant clinical associations were found. The HCV-related cryoglobulins had mainly IgG anti-core activity, but also total IgM anti-HCV and specific IgG anti-NS3 and anti-NS4 activities were present. The complex nature of HCV-related cryoglobulins needs to be further investigated, particularly in association with clinical findings.
Footnotes
Published ahead of print 6 March 2013
REFERENCES
- 1. Suzuki T, Ishii K, Aizaki H, Wakita T. 2007. Hepatitis C viral life cycle. Adv. Drug Deliv. Rev. 59: 1200–1212 [DOI] [PubMed] [Google Scholar]
- 2. Charles ED, Dustin LB. 2009. Hepatitis C virus-induced cryoglobulinemia. Kidney Int. 76: 818–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pozzato G, Zorat F, Bonetto S, Mazzaro C. 2011. Hepatitis C virus and non-Hodgkin's lymphoma: biology, epidemiology and therapy. Oncol. Rev. 5: 249–260 [Google Scholar]
- 4. Pawlotsky JM. 2004. Pathophysiology of hepatitis C virus infection and related liver disease. Trends Microbiol. 12: 96–102 [DOI] [PubMed] [Google Scholar]
- 5. Dore MP, Fattovich G, Sepulveda AR, Realdi G. 2006. Cryoglobulinemia related to hepatitis C virus infection. Dig. Dis. Sci. 52: 897–907 [DOI] [PubMed] [Google Scholar]
- 6. Lake-Bakaar G, Jacobson I, Talal A. 2012. B cell activating factor (BAFF) in the natural history of chronic hepatitis C virus liver disease and mixed cryoglobulinaemia. Clin. Exp. Immunol. 170: 231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schamberg NJ, Lake-Bakaar GV. 2007. Hepatitis C virus-related mixed cryoglobulinemia: pathogenesis, clinical manifestations, and new therapies. Gastroenterol. Hepatol. (N Y) 3: 695–703 [PMC free article] [PubMed] [Google Scholar]
- 8. Sargur R, White P, Egner W. 2010. Cryoglobulin evaluation: best practice? Ann. Clin. Biochem. 47: 8–16 [DOI] [PubMed] [Google Scholar]
- 9. Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. 1974. Biological and clinical significance of cryoglobulins: a report of 86 cases. Am. J. Med. 57: 775–788 [DOI] [PubMed] [Google Scholar]
- 10. Dammacco F. 2012. HCV infection and cryoglobulinemia. Springer-Verlag Italia, Milan, Italy [Google Scholar]
- 11. Ferri C, Cacoub P, Mazzaro C, Roccatello D, Scaini P, Sebastiani M, Tavoni A, Zignego AL, De Vita S. 2011. Treatment with rituximab in patients with mixed cryoglobulinemia syndrome: results of multicenter cohort study and review of the literature. Autoimmun. Rev. 11: 48–55 [DOI] [PubMed] [Google Scholar]
- 12. Sneller MC, Hu Z, Langford CA. 2012. A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitis. Arthritis Rheum. 64: 835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Vita S, Quartuccio L, Isola M, Mazzaro C, Scaini P, Lenzi M, Campanini M, Naclerio C, Tavoni A, Pietrogrande M, Ferri C, Mascia MT, Masolini P, Zabotti A, Maset M, Roccatello D, Zignego AL, Pioltelli P, Gabrielli A, Filippini D, Perrella O, Migliaresi S, Galli M, Bombardieri S, Monti G. 2012. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum. 64: 843–853 [DOI] [PubMed] [Google Scholar]
- 14. Clair St. EW. 2012. Hepatitis C virus-related cryoglobulinemic vasculitis: emerging trends in therapy. Arthritis Rheum. 64: 604–608 [DOI] [PubMed] [Google Scholar]
- 15. Sène D, Ghillani-Dalbin P, Amoura Z, Musset L, Cacoub P. 2009. Rituximab may form a complex with IgMkappa mixed cryoglobulin and induce severe systemic reactions in patients with hepatitis C virus-induced vasculitis. Arthritis Rheum. 60: 3848–3855 [DOI] [PubMed] [Google Scholar]
- 16. Ramos-Casals M, Stone JH, Cid MC, Bosch X. 2012. The cryoglobulinaemias. Lancet 379: 348–360 [DOI] [PubMed] [Google Scholar]
- 17. Sansonno D, Dammacco F. 2005. Hepatitis C virus, cryoglobulinaemia, and vasculitis: immune complex relations. Lancet Infect. Dis. 5: 227–236 [DOI] [PubMed] [Google Scholar]
- 18. Terrier B, Cacoub P. 2013. Cryoglobulinemia vasculitis: an update. Curr. Opin. Rheumatol. 25: 10–18 [DOI] [PubMed] [Google Scholar]
- 19. Sautto G, Mancini N, Solforosi L, Diotti RA, Clementi M, Burioni R. 2012. HCV proteins and immunoglobulin variable gene (IgV) subfamilies in HCV-induced type II mixed cryoglobulinemia: a concurrent pathogenetic role. Clin. Dev. Immunol. 2012: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marcucci F, Mele A. 2011. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood 117: 1792–1798 [DOI] [PubMed] [Google Scholar]
- 21. Invernizzi F, Galli M, Serino G, Monti G, Meroni PL, Granatieri C, Zanussi C. 1983. Secondary and essential cryoglobulinemias. Frequency, nosological classification, and long-term follow-up. Acta Haematol. 70: 73–82 [DOI] [PubMed] [Google Scholar]
- 22. Ohsawa M, Shingu N, Miwa H, Yoshihara H, Kubo M, Tsukuma H, Teshima H, Hashimoto M, Aozasa K. 1999. Risk of non-Hodgkin's lymphoma in patients with hepatitis C virus infection. Int. J. Cancer 80: 237–239 [DOI] [PubMed] [Google Scholar]
- 23. Giordano TP, Henderson L, Landgren O, Chiao EY, Kramer JR, El-Serag H, Engels EA. 2007. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA 297: 2010–2017 [DOI] [PubMed] [Google Scholar]
- 24. Kawamura Y, Ikeda K, Arase Y, Yatsuji H, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Suzuki F, Suzuki Y, Kumada H. 2007. Viral elimination reduces incidence of malignant lymphoma in patients with hepatitis C. Am. J. Med. 120: 1034–1041 [DOI] [PubMed] [Google Scholar]
- 25. Ohtsubo K, Sata M, Kawaguchi T, Morishige S, Takata Y, Oku E, Imamura R, Seki R, Hashiguchi M, Osaki K, Yakushiji K, Kanaji T, Yoshimoto K, Ueno T, Okamura T. 2009. Characterization of the light chain-restricted clonal B cells in peripheral blood of HCV-positive patients. Int. J. Hematol. 89: 452–459 [DOI] [PubMed] [Google Scholar]
- 26. De Re V, De Vita S, Sansonno D, Gasparotto D, Simula MP, Tucci FA, Marzotto A, Fabris M, Gloghini A, Carbone A, Dammacco F, Boiocchi M. 2006. Type II mixed cryoglobulinaemia as an oligo rather than a mono B-cell disorder: evidence from GeneScan and MALDI-TOF analyses. Rheumatology (Oxford) 45: 685–693 [DOI] [PubMed] [Google Scholar]
- 27. de Re V, Simula MP, Pavan A, Garziera M, Marin D, Dolcetti R, de Vita S, Sansonno D, Geremia S, Toffoli G. 2009. Characterization of antibodies directed against the immunoglobulin light kappa chain variable chain region (VK) of hepatitis C virus-related type-II mixed cryoglobulinemia and B-cell proliferations. Ann. N. Y. Acad. Sci. 1173: 152–160 [DOI] [PubMed] [Google Scholar]
- 28. Sansonno D, Lauletta G, Nisi L, Gatti P, Pesola F, Pansini N, Dammacco F. 2003. Non-enveloped HCV core protein as constitutive antigen of cold-precipitable immune complexes in type II mixed cryoglobulinaemia. Clin. Exp. Immunol. 133: 275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maillard P, Lavergne JP, Sibéril S, Faure G, Roohvand F, Petres S, Teillaud JL, Budkowska A. 2009. Fcgamma receptor-like activity of hepatitis C virus core protein. J. Biol. Chem. 279: 2430–2437 [DOI] [PubMed] [Google Scholar]
- 30. Kanduc D, Tessitore L, Lucchese G, Kusalik A, Farber E, Marincola FM. 2008. Sequence uniqueness and sequence variability as modulating factors of human anti-HCV humoral immune response. Cancer Immunol. Immunother. 57: 1215–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu YW, Rocheleau L, Larke B, Chui L, Lee B, Ma M, Liu S, Omlin T, Pelchat M, Brown EG. 2005. Immunoglobulin mimicry by hepatitis C virus envelope protein E2. Virology 332: 538–549 [DOI] [PubMed] [Google Scholar]
- 32. Ferri S, Dal Pero F, Bortoletto G, Bianchi FB, Lenzi M, Alberti A, Gerotto M. 2006. Detailed analysis of the E2-IgM complex in hepatitis C-related type II mixed cryoglobulinaemia. J. Viral Hepat. 13: 166–176 [DOI] [PubMed] [Google Scholar]
- 33. Hartmann H, Schott P, Polzien F, Mihm S, Uy A, Kaboth U, Pardowitz I, Ramadori G. 1995. Cryoglobulinemia in chronic hepatitis C virus infection: prevalence, clinical manifestations, response to interferon treatment and analysis of cryoprecipitates. Z. Gastroenterol. 33: 643–650 [PubMed] [Google Scholar]
- 34. De Re V, Sansonno D, Simula MP, Caggiari L, Gasparotto D, Fabris M, Tucci FA, Racanelli V, Talamini R, Campagnolo M, Geremia S, Dammacco F, De Vita S. 2006. HCV-NS3 and IgG-Fc crossreactive IgM in patients with type II mixed cryoglobulinemia and B-cell clonal proliferations. Leukemia 20: 1145–1154 [DOI] [PubMed] [Google Scholar]
- 35. Zignego AL, Ferri C, Giannini C, Monti M, La Civita L, Careccia G, Longombardo G, Lombardini F, Bombardieri S, Gentilini P. 1996. Hepatitis C virus genotype analysis in patients with type II mixed cryoglobulinemia. Ann. Intern. Med. 124: 31–34 [DOI] [PubMed] [Google Scholar]
- 36. Ramos-Casals M, Forns X, Brito-Zerón P, Vargas A, Ruiz M, Laguno M, Yagüe J, Sánchez-Tapias JM, Gatell JM, Font J. 2007. Cryoglobulinaemia associated with hepatitis C virus: influence of HCV genotypes, HCV-RNA viraemia and HIV coinfection. J. Viral Hepat. 14: 736–742 [DOI] [PubMed] [Google Scholar]
- 37. Crovatto M, Ceselli S, Mazzaro C, Modolo ML, Martelli P, Mazzi G, Pozzato G, Giannini F, Barbisin M, Chiarotto B, Santini GF. 1995. HCV genotypes and cryoglobulinemia. Clin. Exp. Rheumatol. 13(Suppl 13): S79–S82 [PubMed] [Google Scholar]
- 38. Sinico RA, Ribero ML, Fornasieri A, Renoldi P, Zhou J, Fasola M, Portera G, Arrigo G, Gibelli A, D'Amico G, Taggera A. 1995. Hepatitis C virus genotype in patients with essential mixed cryoglobulinaemia. QJM 88: 805–810 [PubMed] [Google Scholar]
- 39. Zehender G, de Maddalena C, Monti G, Ballare M, Saccardo F, Piconi S, Invernizzi F, Monteverde A, Galli M. 1995. HCV genotypes in bone marrow and peripheral blood mononuclear cells of patients with mixed cryoglobulinemia. Clin. Exp. Rheumatol. 13(Suppl 13): S87–S90 [PubMed] [Google Scholar]
- 40. Monteverde A, Ballarè M, Pileri S. 1997. Hepatic lymphoid aggregates in chronic hepatitis C and mixed cryoglobulinemia. Springer Semin. Immunopathol. 19: 99–110 [DOI] [PubMed] [Google Scholar]
- 41. Sansonno D, De Vita S, Iacobelli AR, Cornacchiulo V, Boiocchi M, Dammacco F. 1998. Clonal analysis of intrahepatic B cells from HCV-infected patients with and without mixed cryoglobulinemia. J. Immunol. 160: 3594–3601 [PubMed] [Google Scholar]
- 42. Sautto G, Mancini N, Clementi M, Burioni R. 2012. Molecular signatures of hepatitis C virus (HCV)-induced type II mixed cryoglobulinemia (MCII). Viruses 4: 2924–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zuckerman E, Zuckerman T, Levine AM, Douer D, Gutekunst K, Mizokami M, Qian DG, Velankar M, Nathwani BN, Fong TL. 1997. Hepatitis C virus infection in patients with B-cell non-Hodgkin lymphoma. Ann. Intern. Med. 127: 423–428 [DOI] [PubMed] [Google Scholar]
- 44. Nguyen QT, Leruez-Ville M, Ferrière F, Cohen P, Roulot-Marullo D, Coste T, Dény P, Guillevin L. 1998. Hepatitis C virus genotypes implicated in mixed cryoglobulinemia. J. Med. Virol. 54: 20–25 [DOI] [PubMed] [Google Scholar]
- 45. Vassilopoulos D, Younossi ZM, Hadziyannis E, Boparai N, Yen-Lieberman B, Hsi E, Villa-Forte A, Ball E, Kimberly RP, Calabrese LH. 2003. Study of host and virological factors of patients with chronic HCV infection and associated laboratory or clinical autoimmune manifestations. Clin. Exp. Rheumatol. 21(6 Suppl 32): S101–S111 [PubMed] [Google Scholar]