Abstract
Having previously demonstrated the feasibility of administering A/H5N1 and seasonal influenza vaccine antigens in an MF59-adjuvanted tetravalent formulation, we now report on long-term antibody persistence and responses to a booster dose of a combined seasonal-pandemic, tetravalent influenza vaccine in adults. The primary objective was the evaluation of responses to a booster dose of tetravalent influenza vaccine containing seasonal (A/H1N1, A/H3N2, and B) and avian (A/H5N1, clade 2) influenza virus strains administered to 265 healthy 18- to 40-year-old volunteers 1 year after priming with one or two clade 1 A/H5N1 doses. Secondary objectives were assessment of reactogenicity, safety, and antibody persistence 1 year after priming with a combined seasonal-pandemic, tetravalent vaccine. Responses to seasonal strains met all European licensure criteria; seroprotection rates were 94 to 100%, 100%, and 61 to 90% for A/H1N1, A/H3N2, and B strains, respectively. Anamnestic responses were observed against homologous and heterologous A/H5N1 strains whether priming with one or two A/H5N1 doses, with a monovalent A/H5N1 vaccine, or with a tetravalent vaccine. A single dose of MF59-adjuvanted A/H5N1 vaccine given alone or as part of a fixed combination with a seasonal influenza vaccine was sufficient to prime adult subjects, resulting in robust antigen-specific and cross-reactive antibody responses to heterologous booster immunization 1 year later. These data support the feasibility of incorporating prepandemic priming into seasonal influenza vaccination programs. (This study has been registered at clinicaltrials.gov under registration no. NCT00481065.)
INTRODUCTION
Mass immunization is widely acknowledged to currently be the most effective method of minimizing the impact of pandemic influenza, being able to greatly reduce rates of infection, morbidity, and mortality and minimize levels of associated socioeconomic disruption. However, the A/H1N1 influenza outbreak of 2009 confirmed the shortcomings in the abilities of health authorities and vaccine manufacturers to rapidly meet the global demand for vaccine in the event of a pandemic. Prepandemic vaccination, i.e., preemptive vaccination with potentially pandemic influenza strains, would provide a degree of preexisting immunity against emerging pandemic strains, thereby reducing rates of transmission and severity of infection during the earliest stages of a pandemic (1).
The A/H5N1 (avian) influenza virus is recognized as having significant pandemic potential (2). To date, 596 confirmed human cases of avian influenza have been reported to the World Health Organization (WHO), with 350 (59%) of those cases resulting in death (3). Because the exact viral clade responsible for a future pandemic cannot be predicted accurately, prepandemic influenza vaccines must aim to induce cross-reactive antibodies and thereby provide a degree of cross-clade, heterologous immunity (4). The oil-in-water adjuvant, MF59 (Novartis Vaccines and Diagnostics), has a well-established safety profile (5, 6) and, in addition to heightening the antibody response to vaccination and enhancing long-term antibody persistence, has been shown to promote the production of broadly cross-reactive antibodies (7–11).
Prepandemic immunization against A/H5N1 influenza could be facilitated by the introduction of potentially pandemic influenza virus strains into seasonal influenza vaccines, which are routinely administered to large numbers of people on an annual basis (12). This clinical trial (registration no. NCT00481065 [www.clinicaltrials.gov]) was conducted to assess long-term antibody persistence after priming and homologous and cross-reactive antibody responses to a booster dose of tetravalent vaccine containing pandemic A/H5N1 (clade 2) and seasonal influenza virus strains, administered 1 year after priming with either one or two doses of a prepandemic (clade 1) A/H5N1 vaccine of a different clade, alone or in a fixed combination with a seasonal influenza vaccine (13).
MATERIALS AND METHODS
Study design and objectives.
This randomized, open-label, phase II study was conducted at the University of Cali in Colombia between May 2007 and November 2008 in two phases. The previously reported (13) first study phase consisted of a primary vaccination with one or two doses of A/H5N1 vaccine given either alone or concomitantly as separate injections or combined as an extemporaneous bedside mix with a seasonal influenza vaccine. The objective of the second study phase (reported here), conducted 1 year after priming, was to assess the anamnestic response to a booster dose of a preformulated tetravalent vaccine containing A/H5N1 (heterologous to the priming A/H5N1 strain) and seasonal A/H1N1, A/H3N2 and B strain antigens in the same study population. Long-term antibody persistence and long-term safety were also assessed. The second phase of the study was designed to answer two questions. First, is there a difference in the anamnestic response to a 1-year booster dose of tetravalent vaccine containing heterologous A/H5N1 and seasonal A/H1N1, A/H3N2, and B strains if subjects have been primed with either one or two doses of MF59-adjuvanted A/H5N1 vaccine (13)? Second, is there a difference in the anamnestic response if subjects have been primed with a standalone A/H5N1 vaccine or with a combination vaccine consisting of A/H5N1 and seasonal influenza virus antigens?
Subjects.
The study was approved by the local ethics committee of the University of Cali and was conducted in accordance with local regulations and the principles of the Declaration of Helsinki. The study was registered with the National Institutes of Health(see above). Healthy adults who had given their informed consent and participated in the first, priming phase of the study were eligible for inclusion in the second, booster study phase. Principal exclusion criteria included the following: vaccination with any seasonal or pandemic influenza vaccine during the period between the first and second study phases; administration of any investigational agent within 4 weeks before booster vaccination; acute disease or infection requiring systemic antibiotic or antiviral therapy within 7 days prior to enrollment; and fever within 3 days prior to booster vaccination. Subjects had been randomly assigned to one of eight vaccination groups during the first phase of the study. For the analysis of booster responses against A/H5N1 strains, the eight vaccination groups were pooled into two groups according to whether subjects received one or two priming doses of A/H5N1 vaccine (Table 1). Pooled group A consisted of groups 1, 4, 7, and 8 (subjects who received one priming dose of A/H5N1 vaccine alone or in combination with a seasonal vaccine). Pooled group B consisted of groups 2, 3, 5, and 6 (subjects who received two priming doses of A/H5N1 vaccine).
Table 1.
Vaccination groupsa
| Group | Vaccine dosing |
Pooled group | ||
|---|---|---|---|---|
| Day 1 A/H5N1 (clade 1) | Day 22 A/H5N1 (clade 1) | Day 382 A/H5N1 (clade 2) | ||
| 1 | Concomitant | No vaccine | Tetravalent | A |
| 2 | Concomitant | Tetravalent | Tetravalent | B |
| 3 | Concomitant | A/H5N1 | Tetravalent | B |
| 4 | Tetravalent | No vaccine | Tetravalent | A |
| 5 | Tetravalent | Tetravalent | Tetravalent | B |
| 6 | Tetravalent | A/H5N1 | Tetravalent | B |
| 7 | A/H5N1 | Seasonal | Tetravalent | A |
| 8 | Seasonal | A/H5N1 | Tetravalent | A |
“Concomitant” describes simultaneous but separate injections of A/H5N1 and seasonal influenza vaccines. “Tetravalent” describes the bedside mixing of A/H5N1 and seasonal influenza vaccines or the preformulated tetravalent vaccine.
Vaccines.
All subjects received one booster dose of tetravalent vaccine, comprised of A/H5N1 and seasonal influenza virus strains, approximately 1 year after priming (Table 1). One 0.5-ml dose of the MF59-adjuvanted booster vaccine contained 7.5 μg of A/turkey/Turkey/1/2005 (H5N1, clade 2) hemagglutinin surface antigen and 15 μg of antigen from each of the 2007-2008 WHO reference strains for the Southern Hemisphere: A/Solomon Islands/3/2006 (H1N1), A/Wisconsin/67/2005 (H3N2), and B/Malaysia/2506/2004. The clade 2 A/H5N1 antigen in the booster vaccine was heterologous to the clade 1 A/H5N1 strain used for primary immunization 1 year earlier. The tetravalent vaccine was given as a single injection of 0.5 ml in the deltoid muscle of the nondominant arm. One 0.5-ml dose of the nonadjuvanted, seasonal influenza vaccine used during the first study phase (13) contained 15 μg of antigen from each of the three WHO reference strains for the 2006-2007 influenza season in the Southern Hemisphere: A/New Caledonia/20/99 (H1N1), A/Wisconsin/67/2005 (H3N2), and B/Malaysia/2506/2004. One 0.5-ml dose of the MF59-adjuvanted, pandemic influenza vaccine used during the first study phase contained 7.5 μg of A/Vietnam/1194/2004 (H5N1, clade 1) surface antigen.
Immunogenicity analysis.
Blood samples (∼20 ml per sample) were obtained by venipuncture and centrifuged at 1,500 × g for 10 min; sera were then stored at −18°C. Antibody titers against A/H5N1, A/H1N1, A/H3N2, and B strains were measured by hemagglutination inhibition (HI) assays; additionally, A/H5N1 antibody titers alone were assessed by microneutralization (MN) and single radial hemolysis (SRH) assays, as described previously (7). Homologous immunogenicity assays were performed using the H5N1 booster vaccine strain A/turkey/Turkey/1/2005 (clade 2). Immunogenicity assays to assess cross-reactive antibody responses were performed using the heterologous H5N1 strain A/Vietnam/1194/2004 (clade 1). HI assays against both the A/turkey/Turkey/1/2005 and A/Vietnam/1194/2004 H5N1 strains were performed using horse erythrocytes; HI assays against all seasonal influenza virus strains were performed using chicken erythrocytes. Laboratory staff was blinded to the participants' vaccination group. HI titer is expressed as the reciprocal of the highest dilution at which hemagglutination was totally inhibited. For MN assays, serial dilutions of serum started at 1:20; the reciprocals of 2-fold dilutions that achieved ≥50% neutralization of viral growth were considered a positive result. Seroconversion, as assessed by HI assay, is defined as a negative prevaccination antibody titer of <10 to a positive postvaccination titer of ≥40; as measured by MN assay, a titer of <20 to ≥40; by SRH, an area of ≤4 mm2 to ≥25 mm2. A significant increase in the antibody titer, as assessed by HI and MN assays, is defined as a ≥4-fold increase; by SRH, a ≥50% increase in area. HI and MN titers below the detection limits of 1:10 and 1:20, respectively, were arbitrarily assigned to half that limit for the purpose of analysis. All SRH areas below the lower limit of detection were set to 4 mm2 for analysis.
Safety analysis.
Reactogenicity and safety data were collected for all subjects who received vaccine. Subjects were observed for a minimum of 30 min after each vaccination to monitor for immediate adverse reactions. All vaccinees were provided with diary cards and asked to record any solicited local or systemic reactions occurring within 7 days of vaccination. Solicited local reactions included ecchymosis, erythema, induration, swelling, and pain at the site of injection. Solicited systemic reactions included chills, malaise, myalgia, arthralgia, headache, sweating, nausea, fatigue, and fever. Unsolicited adverse events (AEs) were recorded for 21 days after each booster vaccination. All serious adverse events (SAEs), AEs requiring the attention of a physician, and AEs leading to withdrawal from the study were recorded for 6 months after booster vaccination. The investigator used a standard scale to grade AEs, in which symptoms were defined as mild, moderate, or severe if they resulted in no limitation of, some limitation of, or an inability to perform normal daily activities, respectively.
Statistical analysis.
Sample sizes were estimated to provide sufficient power to examine the primary study objective. No formal statistical hypotheses associated with the immunogenicity objectives of this study were tested. Immunogenicity endpoints were based on licensure criteria established by the European Union Committee for Medicinal Products for Human Use (CHMP). The following adult CHMP licensure criteria applied: the number of subjects achieving seroconversion or significantly increased antibody titers should be >40%; GMR should be >2.5; and for seroprotection, the proportion of subjects achieving an HI titer of ≥1:40 or an SRH titer of >25 mm2 should be >70%. For each of the eight vaccination groups, geometric mean titers (GMTs), geometric mean ratios (GMRs), geometric mean areas (GMAs), and their associated two-sided 95% confidence intervals (CIs) were determined using analysis of variance (ANOVA) with one factor for vaccine group and one factor for subsite as a covariable. Percentages of subjects achieving seroconversion or a significant increase in HI or MN titers and SRH area and the associated two-sided 95% CIs were calculated using the Clopper-Pearson method. For the two pooled analysis groups (A and B), GMTs, GMRs, GMAs, and seroprotection rates were calculated and two-sided 95% CIs were determined using the Clopper-Pearson method. Statistical analyses were performed using the SAS 9.1 software program. Immunogenicity analyses were based on full analysis set (FAS) data. Safety data were evaluated descriptively and expressed as the percentage or number of subjects experiencing AEs within a group.
RESULTS
Study population.
Of the 405 subjects that participated in the priming phase of the study, 265 subjects (65%) proceeded to the booster study phase. All 265 subjects received a single booster vaccine dose on day 382. Of the 140 subjects that did not proceed to the booster phase, 85 subjects no longer wished to participate in the study, 26 subjects were lost to follow-up, 9 subjects experienced AEs, and there were 18 cases of protocol deviation. Three of the nine AEs were classed as serious (fatal bronchial aspiration, non-Hodgkin lymphoma, and ependymoma). The remaining six AEs were not serious and were reactions common to vaccination (i.e., pain at the site of injection, malaise, headache, myalgia, arthralgia, and mild fever). No AEs were considered to be related to vaccination. The demographic data for the pooled study populations are shown in Table 2. The mean ages of subjects in each pooled group were similar, ranging between 29.8 and 30.1 years. There were approximately twice as many women than men in each pooled analysis group.
Table 2.
Demographics of pooled study populations A and B
| Characteristic | Value for pooled group (na)b: |
|
|---|---|---|
| A (137) | B (129) | |
| Age (yr) (±SD) | 30.1 ± 6.3 | 29.8 ± 6.0 |
| Male (%) | 37 | 36 |
| Height (cm) (±SD) | 164.5 ± 8.0 | 163.9 ± 9.0 |
| Weight (kg) (±SD) | 64.5 ± 11.3 | 64.6 ± 12.6 |
| Hispanic (%) | 94 | 92 |
| Black (%) | 6 | 8 |
n, no. of subjects.
Pooled group A, groups 1, 4, 7, and 8 (subjects receiving one priming dose of A/H5N1 vaccine); pooled group B, groups 2, 3, 5, and 6 (subjects receiving two priming doses of A/H5N1 vaccine).
Immunogenicity analysis.
For seasonal vaccine strains, HI data for the seasonal strains are shown in Table 3 (percentages of subjects with HI titer of ≥40) and Table 4 (GMTs). For all eight study groups, seroprotection rates 3 weeks after tetravalent vaccination (day 403) were between 94 and 100% for A/H1N1, 100% for A/H3N2, and 61 to 90% for the B strain. Seroconversion rates and mean increases showed similar results (data not shown); CHMP licensure criteria were met in all eight priming groups.
Table 3.
Seroprotection rates by hemagglutination inhibition assay
| Strain and day | % (range) of subjects achieving seroprotection in group (na)b: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 (31) | 2 (33) | 3 (36) | 4 (39) | 5 (31) | 6 (29) | 7 (33) | 8 (32) | |
| A/Solomon Islands/3/2006 (H1N1) | ||||||||
| Day 1 | 28 (16–43) | 38 (24–53) | 34 (21–49) | 46 (32–61) | 31 (18–45) | 40 (26–55) | 50 (35–65) | 45 (30–61) |
| Day 22 | 91 (79–98) | 85 (72–94) | 87 (74–95) | 82 (69–91) | 92 (80–98) | 85 (72–94) | 65 (49–78) | 89 (75–96) |
| Day 382 | 63 (45–81) | 70 (51–84) | 69 (52–84) | 56 (40–72) | 71 (52–86) | 69 (49–85) | 70 (51–84) | 69 (50–84) |
| Day 403 | 100 (86–100) | 100 (86–100) | 100 (88–100) | 94 (81–99) | 96 (80–100) | 95 (75–100) | 100 (88–100) | 100 (86–100) |
| A/Wisconsin/67/2005 (H3N2) | ||||||||
| Day 1 | 43 (29–59) | 46 (31–61) | 30 (17–45) | 46 (32–61) | 53 (38–67) | 46 (31–61) | 25 (14–40) | 50 (35–65) |
| Day 22 | 89 (76–96) | 90 (77–97) | 94 (82–99) | 98 (89–100) | 96 (86–100) | 100 (93–100) | 42 (28–57) | 93 (81–99) |
| Day 382 | 84 (66–95) | 94 (80–99) | 89 (74–97) | 90 (76–97) | 84 (66–95) | 83 (64–94) | 85 (68–95) | 84 (67–95) |
| Day 403 | 100 (86–100) | 100 (86–100) | 100 (88–100) | 100 (90–100) | 100 (86–100) | 100 (83–100) | 100 (88–100) | 100 (86–100) |
| B/Malaysia/2506/2004 | ||||||||
| Day 1 | 11 (4–24) | 8 (2–20) | 13 (5–26) | 20 (10–34) | 4 (0–14) | 19 (9–33) | 25 (14–40) | 11 (4–25) |
| Day 22 | 87 (74–95) | 88 (75–95) | 91 (80–98) | 96 (86–100) | 90 (78–97) | 96 (86–99) | 31 (19–46) | 80 (65–90) |
| Day 382 | 61 (42–78) | 61 (42–77) | 42 (26–59) | 67 (50–81) | 32 (17–51) | 48 (29–67) | 48 (31–66) | 31 (16–50) |
| Day 403 | 88 (69–97) | 84 (64–95) | 79 (59–92) | 89 (73–97) | 72 (51–88) | 90 (68–99) | 61 (41–78) | 80 (59–93) |
n, no. of subjects.
Seroprotection (HI titer ≥ 1:40) against seasonal influenza vaccine strains was determined at baseline (day 1), 3 weeks (day 22), and 1 year (day 382) after primary immunization and 3 weeks (day 403) after booster vaccination.
Table 4.
GMTs by hemagglutination inhibition assaya
| Strain and day | GMT (range) [nb] against strain |
|||||||
|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | |
| A/Solomon Islands/3/2006 (H1N1) | ||||||||
| Day 382 | 62 (34–111) [31] | 100 (56–176) [33] | 66 (38–116) [36] | 47 (28–80) [39] | 82 (45–147) [31] | 58 (31–106) [29] | 67 (38–119) [33] | 101 (56–180) [32] |
| Day 403 | 299 (199–449) [25] | 308 (206–461) [25] | 300 (203–442) [28] | 233 (165–329) [35] | 177 (117–265) [25] | 249 (158–391) [20] | 289 (196–426) [28] | 312 (209–467) [25] |
| A/Wisconsin/67/2005 (H3N2) | ||||||||
| Day 382 | 77 (51–117) [32] | 122 (81–184) [33] | 90 (61–134) [36] | 117 (80–172) [39] | 119 (78–182) [31] | 77 (50–119) [29] | 80 (53–120) [34] | 87 (57–132) [32] |
| Day 403 | 252 (181–351) [25] | 252 (182–350) [25] | 285 (208–392) [28] | 301 (227–399) [35] | 259 (186–361) [25] | 226 (156–326) [20] | 231 (168–317) [28] | 235 (170–327) [25] |
| B/Malaysia/2506/2004 | ||||||||
| Day 382 | 38 (26–55) [32] | 36 (25–52) [33] | 23 (16–33) [36] | 34 (24–48) [39] | 19 (13–28) [31] | 26 (18–39) [29] | 28 (20–41) [34] | 17 (11–24) [32] |
| Day 403 | 76 (54–106) [25] | 45 (32–63) [25] | 58 (42–80) [28] | 67 (50–89) [35] | 37 (26–52) [25] | 52 (36–76) [20] | 45 (33–62) [28] | 38 (27–53) [25] |
GMTs against seasonal influenza vaccine strains 1 year after primary immunization (day 382) and 3 weeks after booster vaccination (day 403).
n, no. of subjects.
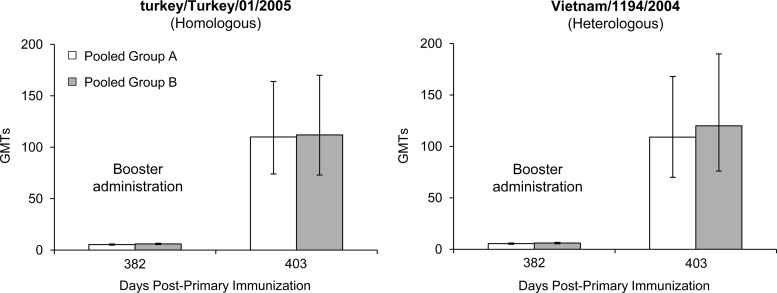
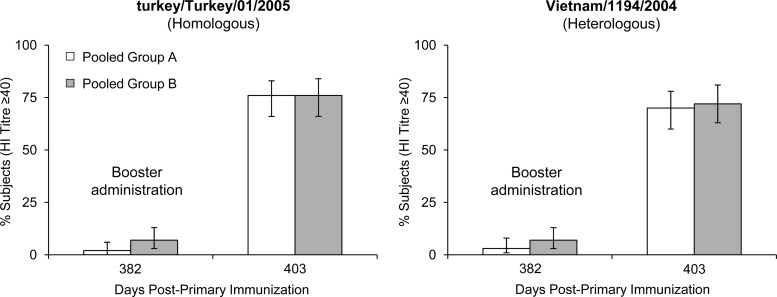
For the A/H5N1/turkey/Turkey/1/2005 (homologous) strain, at 12 months after priming (day 382), GMTs had declined to pre-priming levels independent of whether the subjects had received one or two priming doses of A/H5N1 vaccine or whether the pandemic vaccine was given alone, concomitantly, or combined with the seasonal vaccine (Fig. 1). Following a single booster dose of tetravalent vaccine containing A/H5N1 (clade 2), A/H1N1, A/H3N2, and B strains, GMTs (HI) against the vaccine strain increased from undetectable titers to a maximum of 112 in both the one- or two-dose primed groups (Fig. 1), resulting in a 76% seroprotection rate in both groups (Fig. 2). Again, independent of the priming schedule, both groups responded in similar strengths and speeds to the booster dose. SRH results confirmed these findings (data not shown).
Fig 1.
Hemagglutination inhibition assay. GMTs (95% CI) for pooled vaccination groups A (n = 202) and B (n = 203) against the homologous A/H5N1 vaccine strain, turkey/Turkey/01/2005, and the heterologous A/H5N1 strain, Vietnam/1194/2004, 1 year after primary immunization (day 382) and 3 weeks (day 403) after booster vaccination.
Fig 2.
Hemagglutination inhibition assay. Percentages (95% CI) of subjects in pooled vaccination groups A (n = 202) and B (n = 203) with seroprotective antibody titers (≥40) against the homologous A/H5N1 vaccine strain, turkey/Turkey/01/2005, and the heterologous A/H5N1 strain, Vietnam/1194/2004, 1 year after primary immunization (day 382) and 3 weeks (day 403) after booster vaccination.
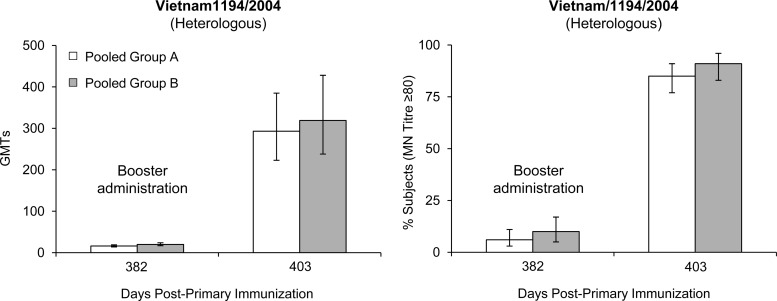
For the A/H5N1/Vietnam/1194/2004 (heterologous) strain, GMTs (HI) against the Vietnam A/H5N1 strain (clade 1) also increased from undetectable titers prebooster to GMTs of 136 in group A and 132 in group B 3 weeks after the booster dose (Fig. 1). Seroprotection rates were 70% in group A and 72% in group B (Fig. 2). A degree of long-term antibody persistence was demonstrated by MN assay on day 382 (Fig. 3). Booster vaccination resulted in a 22-fold GMT (MN) increase (16 to 351) in group A subjects between days 382 and 403 and a 19-fold increase (20 to 387) in group B subjects (Fig. 3). High antibody titers were observed as soon as 7 days after booster administration (day 389); peak GMTs were detected on day 403, 3 weeks after booster administration. Antibody (MN) titers of >1:80 were achieved by 85% and 91% of group A and group B subjects, respectively (Fig. 3). HI and MN results were confirmed by SRH assay (data not shown).
Fig 3.
Microneutralization assay. GMTs and percentages (95% CI) of subjects in pooled vaccination groups A (n = 202) and B (n = 203) with antibody titers of ≥80 against the heterologous A/H5N1 strain, Vietnam/1194/2004, 1 year after primary immunization (day 382), and 3 weeks (day 403) after booster vaccination.
Safety analysis.
In the 1-year period between primary and booster vaccinations, 13 subjects reported SAEs, none of which were considered to be related to vaccination. Nine subjects experienced SAEs leading to withdrawal: two cases of trauma due to car and motorbike accidents, one case of ependymoma, one case of non-Hodgkin lymphoma, one case of periorbital cellulitis, one case of pneumonia, one case of gastrointestinal infection, one case of adenomyomatosis, and one case of appendicitis. One subject died due to bronchial aspiration and left ventricular dilatation compatible with myocardial infarction (occurring 102 days after vaccination), and three subjects experienced spontaneous abortion. Importantly, no cases of autoimmune disease or narcolepsy occurred during the 12-month period after primary vaccination. After the booster vaccination, 68% of subjects reported local reactions, including erythema, induration, and pain at the site of injection; systemic reactions, including fever, headache, fatigue, malaise, myalgia, and arthralgia, occurred in 49% of subjects. The vast majority of reactions were transient and mild, with only 2% classified as severe. The use of analgesic and antipyretic medication was required by 12% of subjects. No SAEs or vaccine-related AEs occurred during the 6-month follow-up period after booster administration.
DISCUSSION
The A/H1N1 influenza pandemic of 2009 confirmed several concerns regarding the provision of adequate vaccination coverage in the event of any future pandemic. Pandemic preparedness strategies must ensure the widest possible population coverage in the fastest possible time. However, the logistics and general practicalities of manufacturing and administering such massive quantities of vaccine across the globe remain a significant hurdle to achieving adequate levels of coverage in the very short time frame required. The logistical problems of mass pandemic immunization could be reduced by simply incorporating potentially pandemic influenza virus strains into seasonal influenza vaccines, which are routinely received by many people on an annual basis, providing that such a combination is well tolerated and does not result in a diminished response to any of the individual component strains (12, 13).
The levels of long-term antibody persistence observed in the present study were low, independent of whether subjects were primed with one or two doses and independent of whether those doses were given as a monovalent vaccine or combined with a seasonal influenza vaccine. Rapid and profound cross-reactive anamnestic responses were observed in response to 1-year heterologous A/H5N1 booster vaccination (tetravalent formulation), regardless of whether individuals were primed with one or two doses of monovalent or combined vaccine. The data presented in this report demonstrate that priming subjects with a combined A/H5N1-seasonal influenza vaccine is a feasible prepandemic priming concept and furthermore that one-dose priming is as good as two-dose priming. The height and kinetics of antibody responses to A/H5N1 were not affected by combining A/H5N1 with seasonal antigens and were independent of one or two dose priming schedules. Likewise, responses to seasonal vaccine strains were not impaired by either of the priming vaccines or the tetravalent booster vaccine. Larger phase III studies with subjects of all ages are required to support and extend the findings of this trial. Analysis of booster responses following a 2- or 3-year interval between primary and booster immunizations may also be beneficial. This study series has two shortcomings: first, the trial did not include a comparator vaccination group who only received seasonal vaccine, because the focus of this study was on A/H5N1 responses. Second, the volume of the tetravalent combination vaccine injected per dose in the first part of the study was 1.0 ml, rather than the usual 0.5 ml per dose. Further investigation is required to assess how immunogenicity and safety profiles in non-Hispanic, pediatric, and elderly populations compare to those reported in this article.
The present study is the first clinical trial conducted to evaluate immunogenicity and safety profiles in response to a combined formulation of trivalent seasonal and MF59-adjuvanted A/H5N1 vaccines (13). The magnitude of antibody responses to MF59-adjuvanted A/H5N1 vaccine observed during this study are consistent with that of previously published data (7) and compare favorably with findings for other adjuvanted A/H5N1 vaccines (14, 15). The frequency and nature of adverse events occurring during this trial are in keeping with the well-established safety profile of MF59-adjuvanted influenza vaccines (5, 6, 16). No vaccine-related serious adverse events occurred during the 1-year observational period between primary and booster immunizations.
The findings of this study provide strong evidence that the MF59-adjuvanted A/H5N1 vaccine can be administered safely in combination with trivalent seasonal influenza vaccine without impairing the antibody response to any of the component seasonal or A/H5N1 strains. A single priming dose of MF59-adjuvanted A/H5N1 or of the tetravalent vaccine was demonstrated to be highly immunogenic and adequate to prime for robust homologous and heterologous booster responses 1 year later. These data support the feasibility of prepandemic immunization against A/H5N1 influenza by incorporating potentially pandemic strains into annual seasonal influenza vaccination programs.
ACKNOWLEDGMENTS
We acknowledge the support of Elena Fragapane, Nicola Groth, and Victor Sales (all of Novartis Vaccines and Diagnostics) during the course of the study. We are also grateful to Keith Veitch and Jamie Stirling (both of Novartis Vaccines and Diagnostics) for editorial assistance in the preparation of the manuscript.
P.L. was the principal investigator; Y.C. and A.S. were coinvestigators; S.T. was the biostatistician; R.C. and A.B. were responsible for the study design and analysis. All authors contributed to the development of the manuscript and approved the final draft for submission.
A.B. and S.T. are permanent employees of Novartis Vaccines and Diagnostics. At the time of the study, R.C. was an employee of Novartis Vaccines and Diagnostics. Pio Lopez received a grant from Novartis Vaccines and Diagnostics. All other authors declare no potential conflicts of interest. The study sponsor had no role in on-site data collection.
This study was sponsored by Novartis Vaccines and Diagnostics.
Footnotes
Published ahead of print 27 March 2013
REFERENCES
- 1.Stohr K. 2010. Vaccinate before the next pandemic? Nature 465:161. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization April 2011. Media centre. Avian influenza. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/avian_influenza/en/ [Google Scholar]
- 3.World Health Organization 12 March 2012. Influenza. Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO. World Health Organization, Geneva, Switzerland: http://www.who.int/influenza/human_animal_interface/EN_GIP_20120312uCumulativeNumberH5N1cases.pdf [Google Scholar]
- 4.World Health Organization 2 September 2005. Global Alert and Response (GAR): responding to the avian influenza pandemic threat. Recommended strategic actions. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/resources/publications/influenza/WHO_CDS_CSR_GIP_2005_8/en/index.html [Google Scholar]
- 5.Banzhoff A, Haertel S, Praus M. 2011. Passive surveillance of adverse events of an MF59-adjuvanted H1N1v vaccine during the pandemic mass vaccinations. Hum. Vaccin. 7:539–548 [DOI] [PubMed] [Google Scholar]
- 6.Tsai TF, Crucitti A, Nacci P, Nicolay U, Della Cioppa G, Ferguson J, Clemens R. 2011. Explorations of clinical trials and pharmacovigilance databases of MF59(R)-adjuvanted influenza vaccines for associated cases of narcolepsy. Scand. J. Infect. Dis. 43:702–706 [DOI] [PubMed] [Google Scholar]
- 7.Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, Capecchi PL, di Giovanni P, Sticchi L, Gentile C, Hilbert A, Brauer V, Tilman S, Podda A. 2009. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One 4:e4384 doi:10.1371/journal.pone.0004384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fragapane E, Gasparini R, Schioppa F, Laghi-Pasini F, Montomoli E, Banzhoff A. 2010. A heterologous MF59-adjuvanted H5N1 prepandemic influenza booster vaccine induces a robust, cross-reactive immune response in adults and the elderly. Clin. Vaccine Immunol. 17:1817–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, Malzone C, Castellino F, Gentile C, McNally T, Del Giudice G, Banzhoff A, Brauer V, Montomoli E, Zambon M, Katz J, Nicholson K, Stephenson I. 2009. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc. Natl. Acad. Sci. U. S. A. 106:7962–7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khurana S, Chearwae W, Castellino F, Manischewitz J, King LR, Honorkiewicz A, Rock MT, Edwards KM, Del Giudice G, Rappuoli R, Golding H. 2010. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci. Transl. Med. 2:15ra5 doi:10.1126/scitranslmed.3000624 [DOI] [PubMed] [Google Scholar]
- 11.Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del Giudice G, Rappuoli R, Golding H. 2011. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci. Transl. Med. 3:85ra48 doi:10.1126/scitranslmed.3002336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. 2006. Strategies for mitigating an influenza pandemic. Nature 442:448–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez P, Caicedo Y, Sierra A, Tilman S, Banzhoff A, Clemens R. 2011. Combined, concurrent, and sequential administration of seasonal influenza and MF59-adjuvanted A/H5N1 vaccines: a phase II randomized, controlled trial of immunogenicity and safety in healthy adults. J. Infect. Dis. 203:1719–1728 [DOI] [PubMed] [Google Scholar]
- 14.Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, Devaster JM, Leroux-Roels G. 2007. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 370:580–589 [DOI] [PubMed] [Google Scholar]
- 15.Lin J, Zhang J, Dong X, Fang H, Chen J, Su N, Gao Q, Zhang Z, Liu Y, Wang Z, Yang M, Sun R, Li C, Lin S, Ji M, Wang X, Wood J, Feng Z, Wang Y, Yin W. 2006. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet 368:991–997 [DOI] [PubMed] [Google Scholar]
- 16.Banzhoff A, Pellegrini M, Del Giudice G, Fragapane E, Groth N, Podda A. 2008. MF59-adjuvanted vaccines for seasonal and pandemic influenza prophylaxis. Influenza Other Respi. Viruses 2:243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]