Abstract
Hsp100 chaperones protect microorganisms and plants from environmental stress by cooperating with Hsp70 and its nucleotide exchange factor (NEF) and Hsp40 cochaperones to resolubilize proteins from aggregates. The Saccharomyces cerevisiae Hsp104 (Sc-Hsp104)-based disaggregation machinery also is essential for replication of amyloid-based prions. Escherichia coli ClpB can substitute for Hsp104 to propagate [PSI+] prions in yeast, but only if E. coli DnaK and GrpE (Hsp70 and NEF) are coexpressed. Here, we tested if the reported inability of Schizosaccharomyces pombe Hsp104 (Sp-Hsp104) to support [PSI+] propagation was due to similar species-specific chaperone requirements and find that Sp-Hsp104 alone supported propagation of three different yeast prions. Sp-Hsp70 and Sp-Fes1p (NEF) likewise functioned in place of their Sa. cerevisiae counterparts. Thus, chaperones of these long-diverged species possess conserved activities that function in processes essential for both cell growth and prion propagation, suggesting Sc. pombe can propagate its own prions. We show that curing by Hsp104 overexpression and inactivation can be distinguished and confirm the observation that, unlike Sc-Hsp104, Sp-Hsp104 cannot cure yeast of [PSI+] when it is overexpressed. These results are consistent with a view that mechanisms underlying prion replication and elimination are distinct.
INTRODUCTION
Microorganisms and plants encode Hsp100 family protein disaggregating chaperones that restore cell viability after exposure to environmental stresses by resolubilizing proteins from aggregates. Protein disaggregation by Hsp100 chaperones in vivo requires cooperation with the Hsp70 protein chaperone and its Hsp40 and nucleotide exchange factor (NEF) regulators (1, 2). In Saccharomyces cerevisiae, this machinery is also essential for replicating prions by fragmenting prion polymers, which consist of amyloid, a highly organized fibrous protein aggregate (3–5). The most studied yeast prions are [PSI+], [URE3], and [PIN+] (also known as [RNQ+]), which are composed of the proteins Sup35p, Ure2p, and Rnq1p, respectively (6–8).
Although yeast Hsp104 does not function in Escherichia coli and the 45% identical E. coli homolog ClpB does not function in yeast, swapping a region of these Hsp100 proteins that mediates interaction with their cognate Hsp70s overcomes these species-specific restrictions (1, 2). Additionally, coexpressing E. coli DnaK (Hsp70) and GrpE (NEF) with intact ClpB allows it to function in place of Hsp104 to provide thermotolerance and to promote prion replication (2). These findings show that amyloid remodeling activity of Hsp100 chaperone machinery is conserved across life kingdoms, and that specific interactions among these components of the disaggregation machinery are necessary and sufficient for it to function properly in different physiological contexts.
An earlier study reported that the 52% identical Schizosaccharomyces pombe Hsp104 (Sp-Hsp104) could function in place of Sa. cerevisiae Hsp104 (Sc-Hsp104) to provide thermotolerance but not propagation of [PSI+] prions (9). One suggested explanation for the failure of Sp-Hsp104 to substitute for Sc-Hsp104 in [PSI+] propagation was that it requires interaction with cognate cofactors, which we surmised would be its Hsp70/NEF system. Here, we used our system to test this hypothesis by exchanging Sa. cerevisiae Hsp104 (Sc-Hsp104), Hsp70 (Ssa1p and Ssa2p), and NEF (Fes1p) with their Sc. pombe counterparts. We find that these Sc. pombe disaggregation machinery components can act individually in place of their Sa. cerevisiae counterparts to provide functions required for propagation of not only [PSI+] but also [URE3] and [PIN+] prions. Our findings demonstrate conservation of the functions of these components and suggest the possibility that Sc. pombe can propagate prions.
MATERIALS AND METHODS
Yeast strains, plasmids, and media.
Sa. cerevisiae strains 1408 ([PSI+], [PIN+]) and 1410 ([URE3], [PIN+]), used to monitor HSP104 complementation, were described previously (2). They are isogenic to wild-type strain 779-6A (MATa kar1-01 SUQ5 ade2-1 his3Δ202 leu2Δ1 trp1Δ63 ura3-52), which was described previously (10). Both have hsp104::KANMX and have a URA3-based plasmid carrying HSP104. Strain 1410 has ADE2 regulated by the DAL5 promoter (PDAL5::ADE2) in place of ade2-1. Isogenic strains 1135 and 1161, used to monitor Hsp70 complementation, are the same as 1408 and 1410, respectively, but have ssa1::KANMX ssa2::HIS3 ssa3::TRP1 and ssa4::ura3-f2 and carry SSA1 or SSA2 on a plasmid to maintain viability (11). Isogenic strain SY346, used to monitor complementation of Fes1p, is strain 1410 with fes1::KANMX. Strain YKT12, which was described previously (12), is unrelated to our strains and carries the [PSI+]Sc4 prion (13). It is of the same genetic background as strain YJW532 used by Senechal et al. (9). Wild-type strain 74-D694 (3), widely used to study [PSI+] prions, is unrelated to YKT12 and our other strains. ATCC strain 38366 was the source of genomic DNA used as the PCR template to obtain the Sc. pombe chaperone genes.
Plasmid pMR117 (2) is a TRP1-based single-copy plasmid with the Sc-HSP104 gene and 500 bp of upstream and downstream flanking DNA. Plasmid pMR116 is the same but without the HSP104 coding region. Plasmid pMR170 is pMR117 with the Sp-HSP104 open reading frame (ORF) in place of the Sc-HSP104 coding region. It was created by PCR amplifying the Sc. pombe HSP104 ORF using primers to add SbfI and XhoI at the 5′ and 3′ ends, respectively. This product was then cloned into pMR116 digested with PstI and XhoI. Plasmids pMR171LA2 and pMR172LA2 are the Sc. pombe SSA1 and SSA2 ORFs, respectively, driven by the Sa. cerevisiae SSA2 promoter in the single-copy LEU2-based plasmid pRS415 (14). They were created by first replacing the GPD (glycerol-3-phosphate dehydrogenase) promoter in p415-GPD (15) with a PCR-generated fragment containing the 500-bp upstream sequence of Sa. cerevisiae SSA2, flanked by SacI and SpeI, to give p415-PSSA2. Sp-SSA1 was amplified by PCR and cloned into p415-PSSA2 via SpeI/XhoI. Sp-SSA2 was cloned similarly, except the restriction sites used were SpeI and PstI. Plasmid pMR173 is single-copy HIS3-based pRS313 (14) with the Sc. pombe FES1 ORF driven by the GPD promoter. It was created by replacing the GrpE ORF in pMR142H (2) with the Sc. pombe FES1 ORF via XbaI/XhoI. Plasmids p425GalSc104 and p425GalSp104 are Sc-HSP104 and Sp-HSP104, respectively, in p425-Gal1 (16). They were created by subcloning Sc-HSP104 and Sp-HSP104 PCR fragments cut with PstI/XhoI and SbfI/XhoI, respectively, into p425-Gal1 cut with PstI and XhoI. All cloned genes were verified by sequencing.
1/2YPD is 0.5% yeast extract, 2% peptone, and 2% glucose. It contains a limiting but undefined concentration of adenine. Synthetic media contain 2% glucose, 0.7% yeast nitrogen base, and the appropriate nutrients, except those required to maintain selection of plasmids or prions. Liquid media contain excess (400 mg/liter) adenine. Synthetic solid media contain 2% agar. When included in synthetic solid media, adenine was added at a concentration of 10 mg/liter, which is limiting, so that ade2 mutant cells form red colonies.
Monitoring prions.
Our strains use the white/red and adenine-independent/dependent phenotypes to monitor [PSI+] and [URE3] prions. In strains 1408 and 1135, the presence of [PSI+] suppresses the nonsense mutation in ade2-1, which allows growth in the absence of adenine and confers white/pink colony color on media containing a limiting concentration of adenine. [psi−] cells are adenine auxotrophs and appear red on limiting adenine. [PSI+] is monitored in strain YKT12 in a similar manner, except the prion suppresses a nonsense mutation in ade1-14. In strain 1410, a wild-type ADE2 gene is regulated by the DAL5 promoter, which is repressed by Ure2p under normal growth conditions. When [URE3] is present, Ure2p is depleted into prion aggregates, which activates the DAL5 promoter and expression of ADE2. The presence of [PIN+] prions was established by detection of fluorescent foci after transforming cells with a plasmid encoding an Rnq1-green fluorescent protein (GFP) fusion protein (17).
Curing of [PSI+] by overexpressing Hsp104.
Curing experiments were performed as described previously (18). Briefly, cells transformed by high-copy-number plasmids carrying various alleles of galactose-inducible Hsp104 were grown for several cell divisions in galactose medium, and at various times they were diluted and spread for 300 to 500 colonies on 1/2YPD plates. Entirely red colonies were scored as having arisen from cells cured of prions.
RESULTS
Sc. pombe Hsp104 supports propagation of three Saccharomyces cerevisiae prions.
We monitored [PSI+] and [URE3] prions by their effects on expression of Ade2p in strains 1408 and 1410, respectively, which lack chromosomal HSP104 and express Sc-Hsp104 from a URA3-based plasmid (see Materials and Methods). In the absence of prions, the cells do not express Ade2p and therefore require adenine for growth, and they accumulate red pigment when adenine is limiting. The presence of either prion causes enough expression of Ade2p to restore adenine prototrophy and white colony color.
To assess the ability of Sp-Hsp104 to propagate [PSI+] or [URE3] in place of Sc-Hsp104, strains 1408 and 1410 were first transformed by TRP1-based plasmids encoding Sp-Hsp104, Sc-Hsp104, or the empty vector. The transformants were grown on medium containing uracil to allow loss of the URA3 plasmid encoding Sc-Hsp104 and then replica plated onto medium containing 5-fluoro-orotic acid (FOA), which kills cells expressing Ura3p. Cells from the FOA plates, which express Hsp104 only from the TRP1 plasmids, were then checked for the presence of prions.
[PSI+] prions propagated in cells expressing either Sp-Hsp104 or Sc-Hsp104 (Fig. 1A). To determine if this unexpected finding was due to our use of a different strain background, we performed the same plasmid exchange using strain YKT12 (12), which has the same background as the strain used in the earlier study (9). All 20 Sp-Hsp104 transformants from three independent transformations of strain YKT12 that subsequently underwent the plasmid exchange propagated [PSI+]. Since [PSI+] suppresses ade1-14 in YKT12 less efficiently than ade2-1 in our strains, YKT12 [PSI+] cells have a pinker color when grown under the same conditions. Nevertheless, the prion phenotype of YKT12 cells expressing Sp-Hsp104 was similar to that of cells expressing Sc-Hsp104. In all instances, the presence of [PSI+] in these cells was confirmed using standard assays by its dominant phenotype in crosses and its elimination when cells were grown in the presence of 3 mM guanidine, which inactivates Hsp104 (19, 20).
Fig 1.
Sc. pombe HSP104 supports propagation of prions in Sa. cerevisiae. (A) The hsp104Δ strains 1408 ([PSI+]), YKT12 ([PSI+]Sc4), and 1410 ([URE3]) carry empty URA3-based and TRP1-based plasmids (ev+ev) or Sc-HSP104 on a resident URA3-based plasmid plus Sc-HSP104 or Sp-HSP104 on TRP1-based plasmids, as indicated. Patches of cells grown on plates selecting for both plasmids (not shown) were replica plated onto medium lacking tryptophan, containing uracil and limiting adenine (upper panels). Cells from this plate were then replica plated onto FOA medium lacking adenine, where only cells that both express the indicated Hsp104 and carry prions will grow (lower panels). Six representative transformants of each from one of three independent experiments are shown. (B) Cells of strain 1408 from panel A, carrying empty vector (ev) only or expressing the indicated Hsp104, were transformed by a HIS3-based plasmid encoding an Rnq1-GFP fusion protein. Cells with diffuse fluorescence are [pin−]; the presence of [PIN+] prions is indicated by punctate fluorescence.
Using strain 1410, we found that Sp-Hsp104 also supported stable propagation of [URE3] (Fig. 1A). Since [PSI+] and [PIN+] are compatible and propagate stably in cells of strain 1408 (2), we also examined the [PSI+] cells expressing only Sp-Hsp104 to see if [PIN+] survived the plasmid exchange. We found that [PIN+] was present in all clones examined (Fig. 1B), showing that Sp-Hsp104 also supported stable propagation of [PIN+] prions. Therefore, Sp-Hsp104 functioned in place of Sc-Hsp104 to support propagation of three different yeast prions.
Sc. pombe Hsp70s support Sa. cerevisiae cell growth and propagation of [PSI+] and [URE3] prions.
Since our findings implied that Sc-Hsp70 cooperates with Sp-Hsp104 to fragment prion polymers, we anticipated that Sc. pombe Hsp70s would cooperate with Sa. cerevisiae Hsp104 to provide the Hsp70 activity required for prion propagation. To test this idea, we used strains 1135 (for monitoring [PSI+]) and 1161 (for monitoring [URE3]), which lack all four SSA subfamily genes and carry SSA1 or SSA2, respectively, on a URA3-based plasmid to support viability and prions. The different Hsp70s were used because our earlier characterization of [PSI+] and [URE3] prion phenotypes in cells expressing Ssa1p or Ssa2p as the only essential Ssa family Hsp70 showed that the [PSI+] phenotype is normal in cells expressing only Ssa1p but was slightly weaker when cells express only Ssa2p (11). Conversely, [URE3] is mitotically unstable in cells expressing only Ssa1p but propagates normally in cells expressing only Ssa2p.
After exchanging the resident URA3 plasmids encoding the Sc-Hsp70s in strains 1135 and 1161 with LEU2-based plasmids encoding Sp-Ssa1p or Sp-Ssa2p, we found that both of the Sp-Hsp70s supported robust growth of Sa. cerevisiae. In cells expressing the Sp-Hsp70s in place of Sc-Hsp70s, [PSI+] propagated normally but [URE3] was mitotically unstable, especially in cells expressing only Sp-Ssa2p (Fig. 2A). Therefore, both Sp-Hsp70s were able to cooperate with Sc-Hsp104 to promote prion replication, and the latter results suggest [URE3] requires specific nonessential Hsp70 functions that are incompletely complemented by the Sp-Hsp70s.
Fig 2.

Sc. pombe Hsp70s complement Sa. cerevisiae Hsp70s for growth and prion propagation. (A) Patches of cells of strains 1135 ([PSI+]; left) and 1161 ([URE3]; right) with URA3-based plasmids carrying Sc-SSA1 and Sc-SSA2, respectively, and the indicated SSA1 and SSA2 alleles on LEU2-based plasmids were transferred from plates selecting for both plasmids onto medium lacking leucine and containing uracil (upper). These were then replica plated onto medium containing FOA and limiting adenine (middle) or FOA without adenine (lower). Three individual transformants of each from one of three independent experiments are shown. (B) [URE3] cells expressing the indicated SSA allele as the only source of Ssa protein were taken from the plate containing FOA medium lacking adenine, streaked onto 1/2YPD, and grown for 3 days at 30°C.
Sp-Ssa1p and Sp-Ssa2p are functionally distinct.
In a manner similar to that seen with Sc-Ssa1p/Ssa2p, we observed that prion phenotypes were different in cells expressing the different Sc. pombe Hsp70s. For example, a slight but noticeable increase in pigmentation was seen in [PSI+] cells expressing Sp-Ssa1p and Sc-Ssa2p (Fig. 2A, left center), which indicates that Sp-Ssa1p behaves more like Sc-Ssa2p and Sp-Ssa2p functions more like Sc-Ssa1p. A more obvious reduction in efficiency of prion propagation was reflected by the [URE3] phenotypes. Here, [URE3] was clearly less stable in cells expressing Sp-Ssa2p than in cells expressing Sp-Ssa1p (Fig. 2B). In this respect, Sp-Ssa2p again functioned more like Sc-Ssa1p than Sc-Ssa2p.
Sc. pombe Fes1p supports propagation of [URE3] in place of Sa. cerevisiae Fes1p.
Hsp70 NEFs are also important for propagation of yeast prions, and propagation of [URE3] depends upon the NEFs Sse1p and Fes1p (2, 21–23). To assess the ability of Sp-Fes1p to function in place of Sc-Fes1p, we first expressed Sp-Fes1p from a single-copy (CEN) plasmid in a diploid lacking one of its two chromosomal copies of FES1. We then induced the strain to undergo meiosis and sporulation and analyzed the haploid progeny. CEN plasmids typically segregate to two of the four spores.
Figure 3 shows the four meiotic progeny of a diploid cell in which all four of the possible combinations of FES1 genotypes are represented. Although our fes1Δ cells were able to maintain [URE3] when grown on medium without adenine (Fig. 3A), they grew more slowly without adenine. Also, under nonselective conditions, they accumulated red pigment and lost [URE3] at a very high frequency (Fig. 3B). These phenotypes reflect a strong impairment of [URE3] propagation in the absence of Fes1p, which is in line with earlier work showing that stable propagation of [URE3] requires Fes1p (23). Cells expressing Sc-Fes1p from the chromosome (FES1+) had a typical [URE3] phenotype, and when FES1+ cells expressed Sp-Fes1p from the plasmid they had a slight pink color (Fig. 3A, clones c and a, respectively). When subcultured, however, the clones expressing both Sc-Fes1p and Sp-Fes1p had a normal, stable [URE3] phenotype. Although cells expressing only Sp-Fes1p propagated [URE3], there was a consistent, infrequent loss of [URE3] upon subculturing under conditions where the prion is not required for growth (i.e., when adenine is present). However, [URE3] was much more stable in these cells than in those lacking Fes1p (Fig. 3B). Thus, Sp-Fes1p possessed a substantial amount of the Sc-Fes1p functions required for propagation of [URE3].
Fig 3.

Sc. pombe Fes1p possesses Sa. cerevisiae Fes1p function required for [URE3] propagation. (A) A [URE3] fes1Δ/FES1 diploid with a plasmid carrying Sp-FES1 was sporulated, and tetrads were dissected on rich medium. Spore clones were then replica plated onto the indicated media to monitor fes1Δ (G418), [URE3] (minus adenine, −Ade), and Sp-FES1 (minus histidine, −His). A tetrad with all four possible combinations of FES1 genotypes is shown. The presence of genomic Sc-FES1 and plasmid-borne Sp-FES1 is indicated at the bottom. (B) Spore clones lacking FES1 (fes1Δ) or expressing Sp-FES1 from the −Ade plate in panel A were streaked onto 1/2YPD plates and grown for 3 days at 30°C to assess the [URE3] phenotype. Cells expressing Sc-FES1 (lower panels) show wild-type [URE3] and [ure-o] phenotypes.
Plasmid-expressed Sp-Hsp104 does not cure cells of [PSI+].
Although Hsp104 is necessary for prion propagation, transiently overexpressing Sc-Hsp104 cures cells of [PSI+] prions (3). In the hsp104Δ strains where [PSI+] is supported by HSP104 on a single-copy plasmid, expression of Hsp104 is modestly elevated compared to wild-type levels (24), which causes [PSI+] to be weaker and mitotically unstable. In contrast, overexpressing Sp-Hsp104, even at higher levels, is ineffective at inhibiting [PSI+] propagation (9). The pinker color of strain 1408 [PSI+] cells expressing Sc-Hsp104 compared to the whiter color of those expressing Sp-Hsp104 from the plasmid (Fig. 1A, upper) is consistent with these observations. Additionally, when we streaked these cells for isolated colonies, we observed that although [PSI+] was clearly weakened and mitotically unstable in the two hsp104Δ strains expressing Sc-Hsp104, those expressing Sp-Hsp104 had strong and stable [PSI+] phenotypes (Fig. 4). These data are consistent with the finding that overexpression of Sp-Hsp104 does not inhibit [PSI+] propagation.
Fig 4.

Plasmid-expressed Sa. cerevisiae Hsp104, but not Sc. pombe Hsp104, cures cells of [PSI+] prions. Cells of hsp104Δ strains 1408 and YKT12, expressing the indicated Hsp104 proteins from single-copy plasmids, were streaked for individual colonies and grown on medium selecting for the plasmids and containing limiting adenine. Cells expressing Sp-Hsp104 (right) have normal prion phenotypes. Pinker color of [PSI+] colonies and presence of red [psi−] colonies in both strains expressing Sc-Hsp104 (center) reflects inhibition of [PSI+] propagation by Sc-Hsp104.
To test this conclusion more rigorously, we induced high-level expression of Hsp104 from galactose-inducible promoters on a high-copy-number plasmid in wild-type strains 779-6A and 74D-694, which are from different backgrounds and have [PSI+] prions derived from different sources. Sc-Hsp104 efficiently cured both strains of their [PSI+] prions, but Sp-Hsp104 did not (Fig. 5 and Table 1). Taken together, our data indicate that Sp-Hsp104 possessed Sc-Hsp104 activities required for propagation of [PSI+] but lacked an Sc-Hsp104 function necessary for curing cells of [PSI+] when overexpressed. These results are consistent with other data showing that Hsp104 activities important for replication and curing of [PSI+] prions are separable (17, 18, 24).
Fig 5.
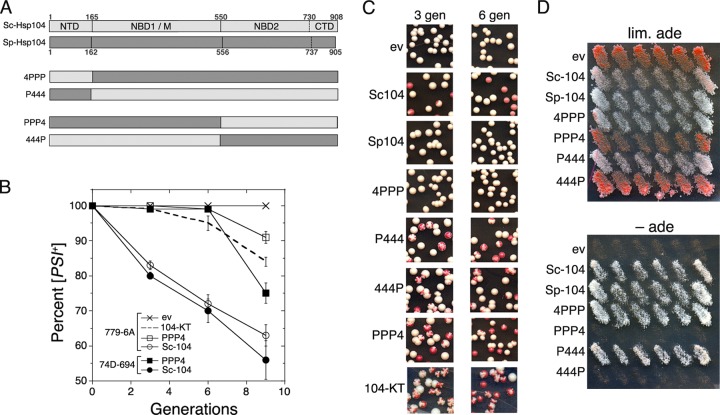
Curing of [PSI+] by overexpressing alleles of Hsp104. (A) Domain organization of Sa. cerevisiae and Sc. pombe Hsp104s and their hybrids. Numbers indicate amino acid residues that delineate domains: NTD, N-terminal domain; NBD1/M, nucleotide-binding domain 1/M region; NBD2, nucleotide-binding domain 2; CTD, C-terminal domain. (B) Kinetics of curing of [PSI+] by overexpression of Sc-Hsp104 versus its inactivation. Aliquots of cultures grown in galactose medium were removed at three, six, and nine generations, plated on 1/2YPD, and incubated at 30°C for 3 days to determine the proportion of [PSI+] cells. The dashed line is 779-6A, expressing dominant-negative Hsp104-KT. Data are from 1,200 to 1,500 colonies counted for each time point in at least three independent experiments. Error bars show standard deviations. (C) Representative images of colonies from the experiment described for panel B. Sp-Hsp104 and the hybrid 4PPP did not affect [PSI+] stability. (D) Hybrids 4PPP and P444 support prion propagation. Strain 1408 was transformed by single-copy TRP1 plasmids expressing the indicated Hsp104 proteins from the HSP104 promoter and processed as described in the legend to Fig. 1. Shown are cells on −Trp plates, with and without limiting adenine, replica plated from FOA.
Table 1.
Curing of [PSI+] by Hsp104 overexpressiona
| Overexpressed Hsp104 | Percent [PSI+] cells in strain: |
|||||
|---|---|---|---|---|---|---|
| 779-6A |
74D-694 |
|||||
| 3 gen | 6 gen | 9 gen | 3 gen | 6 gen | 9 gen | |
| ev | 100 | 100 | 100 | 100 | 100 | 100 |
| Sc-Hsp104 | 84 (1.2) | 72 (2.6) | 63 (3.1) | 80 (1.0) | 69 (3.4) | 56 (5.5) |
| Sp-Hsp104 | 100 | 100 | 100 | 100 | 100 | 100 |
| 4PPP | 100 | 100 | ND | 100 | 100 | ND |
| P444 | 99 (0.4) | 93 (1.1) | ND | 99 (0.02) | 98 (1.2) | ND |
| PPP4 | 99 (0.2) | 99 (0.2) | 91 (1.7) | 99 (0.3) | 99 (0.4) | 75 (2.9) |
| 444P | 99 (0.1) | 94 (0.8) | ND | 99 (0.5) | 99 (0.3) | ND |
| Hsp104-KT | 99 (0.3) | 95 (2.0) | 84 (1.3) | ND | ND | ND |
Strains carrying plasmids encoding galactose-inducible HSP104 alleles were grown to mid-log phase in −Trp dextrose medium without adenine to maintain selection for prions and then transferred to −Trp galactose medium containing adenine to induce Hsp104 expression and allow prion loss. When cultures reached the indicated number of generations (gen), aliquots were removed and spread at a concentration of 300 to 500 cells per plate onto 1/2YPD. Only entirely red colonies were scored as [psi−]. Values are percent [PSI+] among 1,200 to 1,500 colonies, with standard deviations in parentheses. ND, not determined. 4PPP and P444 are NTD-swapped hybrids, PPP4 and 444P are CTD-swapped hybrids (Fig. 5A), ev is empty vector, and Hsp104-KT is the dominant-negative Hsp104 mutant.
Overexpressing Sc-Hsp104–Sp-Hsp104 hybrids cures [PSI+] by impairing prion replication.
Since Sc-Hsp104 lacking its amino-terminal domain (NTD) behaves like Sp-Hsp104, in that it propagates [PSI+] but does not cure [PSI+] when overexpressed (18), we considered that Sp-Hsp104 lacked an NTD activity required for curing by overexpression. We therefore assessed the curing ability of Sp-Hsp104 after replacing its NTD with that of Sc-Hsp104. Overexpressing this hybrid protein, named 4PPP, did not cure [PSI+] (Fig. 5 and Table 1). These results suggest that a nonconserved Hsp104 function separate from that in the NTD is necessary for the curing.
Overexpressing Hsp104 cures cells of [PSI+] by a mechanism clearly distinct from that of inactivating Hsp104. The processes differ by the kinetics of curing and by the phenotypes of colonies arising from cells of cultures undergoing curing. Inhibiting Hsp104 by chemical means or by expressing dominant-negative Hsp104 mutants arrests replication of heritable prion particles, or propagons (10, 25–28). [PSI+] cells typically have about 100 to 300 propagons per cell (29), so after inactivating Hsp104 there is a lag of 4 to 6 cell divisions before [psi−] cells appear, while the nonreplicating propagons are diluted among the dividing cells until there are too few of them to be inherited by all the progeny. Beyond that point, the number of [psi−] cells roughly doubles after each cell division. When assessed during the curing, many [PSI+] cells recovering on plates where Hsp104 regains activity grow into colonies with several red sectors containing [psi−] progeny. In contrast, overexpressing wild-type Hsp104 causes [psi−] cells to arise shortly after inducing expression and at a linear rate of roughly 10% per cell division (24). [PSI+] cells recovering on plates where Hsp104 abundance returns to normal give rise to colonies that are entirely white or that have one or very few red sectors.
We tested other Hsp104 hybrids with swapped domains (P444, PPP4, and 444P) and found that, unlike 4PPP, they all cured [PSI+] when they were overexpressed. However, both a lag in the kinetics of curing and a highly sectored colony phenotype from cells overexpressing these hybrids indicated that the curing was caused by arrested prion replication followed by dilution of propagons among dividing cells (Fig. 5 and Table 1). These phenotypes are characteristic of cells in which Hsp104 function has been inhibited, as in cells expressing known dominant-negative Hsp104 mutants, such as Hsp104-KT (Fig. 5 and Table 1) (3, 24, 26, 30). Thus, the hybrid proteins cured [PSI+] by a dominant-negative mechanism that is unlike curing by overexpression of wild-type Sc-Hsp104.
Using the plasmid shuffle described above, we found that P444 and 4PPP were able to propagate [PSI+] in place of Sc-Hsp104, again showing that NTD function is not critical for [PSI+] propagation (Fig. 5D). The ability of hybrid P444 to support [PSI+] propagation was unexpected, because overexpressing it had a dominant inhibitory effect on the ability of Sc-Hsp104 to propagate [PSI+]. More work will be necessary to explain this unusual combination of traits. Neither 444P nor PPP4 was able to propagate [PSI+]. This lack of function is consistent with their curing of [PSI+] by a dominant-negative effect.
DISCUSSION
In contrast to earlier work (9), we find that Sp-Hsp104 supports stable propagation of [PSI+] prions in Sa. cerevisiae. We ruled out the possibility that this finding was due to differences in yeast strain background. Additionally, the variants of [PSI+] prions propagating in the 1408 and YKT12 strains used here are distinct, indicating that Sp-Hsp104 can act on different conformational variants of prions composed of the same Sup35p. Moreover, Sp-Hsp104 also supported stable propagation of [URE3] and [PIN+] prions, which shows that it possesses general Sc-Hsp104 activities required for replication of prions composed of different proteins. We cannot explain the discrepancy with the earlier study, but it is possible that the earlier work was done using a different Sc. pombe HSP104 allele.
We recently showed that the E. coli homolog of Hsp104 could promote propagation of yeast prions only if its cognate Hsp70 and NEF are coexpressed, or if it is modified to interact with yeast Hsp70. This specificity of cooperation between Hsp100 and Hsp70 indicates that the ability of Sp-Hsp104 to propagate prions in place of Sc-Hsp104 must reflect its ability to cooperate functionally with the Sa. cerevisiae Hsp70 system. Consequently, we were not surprised to find that both Sp-Hsp70s, which are 94% identical to each other and 80% identical to Sc-Ssa1/2p, functioned reciprocally as components of the Sa. cerevisiae Hsp104 disaggregation machinery to promote prion replication. Additionally, our results showing that Sp-Ssa1p and Sp-Ssa2p were much like Sc-Ssa1p and Sc-Ssa2p in their distinct effects on prions suggest that certain differences in the functions of nearly identical Hsp70s within species are conserved across long-diverged species.
Our finding that Sc. pombe Hsp70s supported propagation of prions in budding yeast would not have been possible if they did not also provide the essential Hsp70 functions required to support viability of Sa. cerevisiae. The strong growth complementation by the Sp-Hsp70s reflects a high level of conservation of essential Hsp70 functions among these Sc. pombe and Sa. cerevisiae chaperones. Essential cellular processes that require Hsp70, such as translation and transport of proteins across membranes, depend on cooperation of Hsp70 with many regulatory cofactors. For example, protein transport relies on complexes that include Hsp40s, NEFs, and tetratricopeptide repeat (TPR)-containing cochaperones that recruit Hsp70 and regulate its activity (31). Therefore, in addition to being able to function with the disaggregation machinery, the ability of Sp-Hsp70s to support such robust growth shows that they interact functionally and efficiently with these Sa. cerevisiae Hsp70 system components. While it can be presumed that Hsp70 interactions with its obligate Hsp40 cochaperones occur promiscuously, our finding that Sp-Fes1p largely compensates for the loss of Sc-Fes1p demonstrates a specific aspect of this conservation.
Although overexpressing Hsp104 was shown to cure cells of [PSI+] prions many years ago, the molecular mechanism underlying the curing has not been resolved, because many factors strongly influence the curing. Some Hsp70s (Ssb) enhance curing (32), while others (Ssa) antagonize it (33, 34). Moreover, Hsp40s, NEFs, Hsp90 machinery components, ubiquitin, actin, and factors involved in anchoring membrane proteins all influence the curing (17, 24, 35–38). Here, we confirm the earlier findings that Sp-Hsp104 does not cure cells of [PSI+], and that swapping the C-terminal regions of Sp-Hsp104 and Sc-Hsp104 produced hybrids that cured [PSI+] when overexpressed. However, we find that all our hybrid proteins that cure do so by dominant-negative effects. Therefore, these hybrids are not useful for identifying the regions of Sc-Hsp104 that confer functions specifically required for curing of [PSI+] by Hsp104 overexpression. Nevertheless, the reciprocal compatibility between the interspecies Hsp100 and Hsp70 systems implies that the distinction is not related to differences in interactions among these disaggregation machinery components.
Our C-terminal domain (CTD) hybrids differed from those described earlier, in that the region we swapped was extended to include NBD2. Thus, it is possible that curing by the Hsp104 hybrids with the smaller CTD occurs by the overexpression mechanism rather than by a dominant-negative effect. In line with this possibility, the inability of the 4PPP hybrid to cure [PSI+] prions suggests that an Sc-Hsp104-specific function residing outside the NTD is important for curing. Nevertheless, further work with the alleles containing swaps of the smaller CTD regions will be necessary to determine the mechanism by which they cure. The ways we show to distinguish curing by overexpression or dominant-negative effects will be useful for such work and for other studies that address how overexpressing Hsp104 cures [PSI+]. For example, recent work led to a suggestion that overexpressing Hsp104 cures cells of [PSI+] by inhibiting the fragmentation of prion fibers that produces propagons, or, in other words, by inhibiting prion replication (39). Blocking prion replication, however, is the way that inhibiting Hsp104 causes curing, which is distinct from that of overexpressing wild-type Hsp104.
Taken together, our results show a very high degree of conservation of protein disaggregation machinery functions between Sc. pombe and Sa. cerevisiae, in particular those required for propagation of different prions in Sa. cerevisiae. These findings imply that Sc. pombe possesses the chaperone machinery functions required to propagate its own prions. Such a capability would provide another useful model system to study prion/amyloid behavior and might have important implications with regard to Sc. pombe phenotypes.
ACKNOWLEDGMENTS
We thank Jonathan Weissman (UCSF) for strain YKT12 and our NIH colleagues for insightful discussion and critical reading of the manuscript.
This work was supported by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Published ahead of print 15 March 2013
REFERENCES
- 1. Miot M, Reidy M, Doyle SM, Hoskins JR, Johnston DM, Genest O, Vitery MC, Masison DC, Wickner S. 2011. Species-specific collaboration of heat shock proteins (Hsp) 70 and 100 in thermotolerance and protein disaggregation. Proc. Natl. Acad. Sci. U. S. A. 108:6915–6920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reidy M, Miot M, Masison DC. 2012. Prokaryotic chaperones support yeast prions and thermotolerance and define disaggregation machinery interactions. Genetics 192:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chernoff YO, Lindquist SL, Ono B, Inge-Vechtomov SG, Liebman SW. 1995. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+]. Science 268:880–884 [DOI] [PubMed] [Google Scholar]
- 4. Paushkin SV, Kushnirov VV, Smirnov VN, Ter-Avanesyan MD. 1996. Propagation of the yeast prion-like [psi+] determinant is mediated by oligomerization of the SUP35-encoded polypeptide chain release factor. EMBO J. 15:3127–3134 [PMC free article] [PubMed] [Google Scholar]
- 5. Kryndushkin DS, Alexandrov IM, Ter-Avanesyan MD, Kushnirov VV. 2003. Yeast [PSI+] prion aggregates are formed by small Sup35 polymers fragmented by Hsp104. J. Biol. Chem. 278:49636–49643 [DOI] [PubMed] [Google Scholar]
- 6. Wickner RB. 1994. Evidence for a prion analog in Saccharomyces cerevisiae: the [URE3] non-Mendelian genetic element as an altered URE2 protein. Science 264:566–569 [DOI] [PubMed] [Google Scholar]
- 7. Derkatch IL, Bradley ME, Zhou P, Chernoff YO, Liebman SW. 1997. Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae. Genetics 147:507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sondheimer N, Lindquist S. 2000. Rnq1: an epigenetic modifier of protein function in yeast. Mol. Cell 5:163–172 [DOI] [PubMed] [Google Scholar]
- 9. Senechal P, Arseneault G, Leroux A, Lindquist S, Rokeach LA. 2009. The Schizosaccharomyces pombe Hsp104 disaggregase is unable to propagate the [PSI+] prion. PLoS One 4:e6939 doi:10.1371/journal.pone.0006939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jung G, Masison DC. 2001. Guanidine hydrochloride inhibits Hsp104 activity in vivo: a possible explanation for its effect in curing yeast prions. Curr. Microbiol. 43:7–10 [DOI] [PubMed] [Google Scholar]
- 11. Sharma D, Masison DC. 2008. Functionally redundant isoforms of a yeast Hsp70 chaperone subfamily have different antiprion effects. Genetics 179:1301–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tipton KA, Verges KJ, Weissman JS. 2008. In vivo monitoring of the prion replication cycle reveals a critical role for Sis1 in delivering substrates to Hsp104. Mol. Cell 32:584–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tanaka M, Chien P, Naber N, Cooke R, Weissman JS. 2004. Conformational variations in an infectious protein determine prion strain differences. Nature 428:323–328 [DOI] [PubMed] [Google Scholar]
- 14. Sikorski RS, Hieter P. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mumberg D, Muller R, Funk M. 1995. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156:119–122 [DOI] [PubMed] [Google Scholar]
- 16. Mumberg D, Muller R, Funk M. 1994. Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 22:5767–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kirkland PA, Reidy M, Masison DC. 2011. Functions of yeast Hsp40 chaperone Sis1p dispensable for prion propagation but important for prion curing and protection from prion toxicity. Genetics 188:565–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hung GC, Masison DC. 2006. N-terminal domain of yeast Hsp104 chaperone is dispensable for thermotolerance and prion propagation but necessary for curing prions by Hsp104 overexpression. Genetics 173:611–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glover JR, Lindquist S. 1998. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94:73–82 [DOI] [PubMed] [Google Scholar]
- 20. Grimminger V, Richter K, Imhof A, Buchner J, Walter S. 2004. The prion curing agent guanidinium chloride specifically inhibits ATP hydrolysis by Hsp104. J. Biol. Chem. 279:7378–7383 [DOI] [PubMed] [Google Scholar]
- 21. Jones G, Song Y, Chung S, Masison DC. 2004. Propagation of Saccharomyces cerevisiae [PSI+] prion is impaired by factors that regulate Hsp70 substrate binding. Mol. Cell. Biol. 24:3928–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan Q, Park KW, Du Z, Morano KA, Li L. 2007. The role of Sse1 in the de novo formation and variant determination of the [PSI+] prion. Genetics 177:1583–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kryndushkin D, Wickner RB. 2007. Nucleotide exchange factors for Hsp70s are required for [URE3] prion propagation in Saccharomyces cerevisiae. Mol. Biol. Cell 18:2149–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reidy M, Masison DC. 2010. Sti1 regulation of Hsp70 and Hsp90 is critical for curing of Saccharomyces cerevisiae [PSI+] prions by Hsp104. Mol. Cell. Biol. 30:3542–3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eaglestone SS, Ruddock LW, Cox BS, Tuite MF. 2000. Guanidine hydrochloride blocks a critical step in the propagation of the prion-like determinant [PSI+] of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 97:240–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferreira PC, Ness F, Edwards SR, Cox BS, Tuite MF. 2001. The elimination of the yeast [PSI+] prion by guanidine hydrochloride is the result of Hsp104 inactivation. Mol. Microbiol. 40:1357–1369 [DOI] [PubMed] [Google Scholar]
- 27. Ness F, Ferreira P, Cox BS, Tuite MF. 2002. Guanidine hydrochloride inhibits the generation of prion “seeds” but not prion protein aggregation in yeast. Mol. Cell. Biol. 22:5593–5605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cox B, Ness F, Tuite M. 2003. Analysis of the generation and segregation of propagons: entities that propagate the [PSI+] prion in yeast. Genetics 165:23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Byrne LJ, Cole DJ, Cox BS, Ridout MS, Morgan BJ, Tuite MF. 2009. The number and transmission of [PSI+] prion seeds (propagons) in the yeast Saccharomyces cerevisiae. PLoS One 4:e4670 doi:10.1371/journal.pone.0004670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park YN, Morales D, Rubinson EH, Masison D, Eisenberg E, Greene LE. 2012. Differences in the curing of [PSI+] prion by various methods of Hsp104 inactivation. PLoS One 7:e37692 doi:10.1371/journal.pone.0037692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fan AC, Young JC. 2011. Function of cytosolic chaperones in Tom70-mediated mitochondrial import. Prot. Pept. Lett. 18:122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chernoff YO, Newnam GP, Kumar J, Allen K, Zink AD. 1999. Evidence for a protein mutator in yeast: role of the Hsp70-related chaperone ssb in formation, stability, and toxicity of the [PSI] prion. Mol. Cell. Biol. 19:8103–8112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Newnam GP, Wegrzyn RD, Lindquist SL, Chernoff YO. 1999. Antagonistic interactions between yeast chaperones Hsp104 and Hsp70 in prion curing. Mol. Cell. Biol. 19:1325–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allen KD, Wegrzyn RD, Chernova TA, Muller S, Newnam GP, Winslett PA, Wittich KB, Wilkinson KD, Chernoff YO. 2005. Hsp70 chaperones as modulators of prion life cycle: novel effects of Ssa and Ssb on the Saccharomyces cerevisiae prion [PSI+]. Genetics 169:1227–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ganusova EE, Ozolins LN, Bhagat S, Newnam GP, Wegrzyn RD, Sherman MY, Chernoff YO. 2006. Modulation of prion formation, aggregation, and toxicity by the actin cytoskeleton in yeast. Mol. Cell. Biol. 26:617–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Allen KD, Chernova TA, Tennant EP, Wilkinson KD, Chernoff YO. 2007. Effects of ubiquitin system alterations on the formation and loss of a yeast prion. J. Biol. Chem. 282:3004–3013 [DOI] [PubMed] [Google Scholar]
- 37. Sadlish H, Rampelt H, Shorter J, Wegrzyn RD, Andreasson C, Lindquist S, Bukau B. 2008. Hsp110 chaperones regulate prion formation and propagation in S. cerevisiae by two discrete activities. PLoS One 3:e1763 doi:10.1371/journal.pone.0001763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kiktev DA, Patterson JC, Muller S, Bariar B, Pan T, Chernoff YO. 2012. Regulation of chaperone effects on a yeast prion by cochaperone Sgt2. Mol. Cell. Biol. 32:4960–4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Winkler J, Tyedmers J, Bukau B, Mogk A. 2012. Hsp70 targets Hsp100 chaperones to substrates for protein disaggregation and prion fragmentation. J. Cell Biol. 198:387–404 [DOI] [PMC free article] [PubMed] [Google Scholar]