Abstract
Candida albicans, a dimorphic fungus and an opportunistic pathogen, possesses a myriad of adherence factors, including members of the agglutinin-like sequence (Als) family of mannoproteins. The adhesin Als5p mediates adhesion to many substrates and is upregulated during commensal interactions but is downregulated during active C. albicans infections. An amyloid-forming core sequence at residues 325 to 331 is important for Als5p function, because a single-amino-acid substitution at position 326 (V326N) greatly reduces Als5p-mediated adherence. We evaluated the role of Als5p in host-microbe interactions by using Caenorhabditis elegans nematodes as a host model and feeding them Saccharomyces cerevisiae expressing Als5p on the surface. Als5p-expressing yeast had 8.5- and 3.5-fold-increased intestinal accumulation rates compared to Als5p-nonexpressing S. cerevisiae or yeast expressing amyloid-deficient Als5pV326N, respectively. Surprisingly, this accumulation delayed S. cerevisiae-induced killing of C. elegans. The median survival time was nearly twice as long as that of nematodes fed nonexpressing or non-amyloid-forming Als5pV326N-expressing S. cerevisiae. Treatment with the amyloid-inhibiting dye Congo red or repression of Als5p expression abrogated the protective effect of Als5p. Furthermore, Als5p had no effect on oocyte quantity or quality, since nematodes fed either empty vector (EV)- or Als5pV326N-expressing S. cerevisiae had similar egg-laying and egg-hatching rates. This study is the first, to our knowledge, to show that expression of an amyloid-forming protein can attenuate pathogenicity in C. elegans.
INTRODUCTION
Candida albicans is a dimorphic commensal yeast that normally colonizes human nasopharyngeal and urogenital tracts. Although C. albicans is one of the most commonly isolated causes of nosocomial fungal infections in immunocompromised patients, it is generally harmless to the healthy population. In the healthy population, the host has mechanisms such as the microbial flora, epithelial barriers, and the innate immune system that control its presence (1).
C. albicans adheres to and colonizes the host with the help of a myriad of adherence proteins which are important in both commensalism and pathogenesis. The agglutinin-like sequence (Als) family of proteins constitutes one prominent class of Candida adhesins. There are eight ALS genes in the Candida albicans genome, and their products are glycosylphosphatidylinositol (GPI)-anchored glycoproteins homologous to the α-agglutinin protein of Saccharomyces cerevisiae (2). Each Als protein has a similar domain structure that includes conserved tandem N-terminal Ig-like invasin domains that determine substrate specificity, a T-domain that allows for amyloid formation, a region with a variable number of 36-amino-acid tandem repeats (TR domains), and a glycosylated C-terminal serine-threonine-rich stalk domain that links to a modified GPI anchor that covalently attaches Als proteins to the cell wall of C. albicans (3, 4). When transgenically expressed in Saccharomyces cerevisiae, Als5p mediates fungal aggregation and also causes adherence to laminin, gelatin, fibronectin, and epithelial cells, as well as endothelial cells in vitro (5, 6).
The amyloid-forming region in Als5p and other adhesins is important for robust adherence and yeast aggregation onto a variety of surfaces (7–10). This region mediates clustering of adhesin molecules on the surface to form amyloid-like nanodomains that have high avidity for ligands (11, 12). Consequently, a single-amino-acid substitution, V326N, in the amyloid-forming region of Als5p abolishes its ability to form amyloids, cell surface nanodomains, cell aggregates, and biofilms on polystyrene (11–13). As a natural extension, we sought to assay the role of Als5p and its in vivo interactions with the host, as these functions are thought to play a role in both pathogenesis and commensalism.
Caenorhabditis elegans is a free-living microbivore nematode whose use as a model organism across various fields of biology has been expanding in recent years. C. elegans is an emerging model for the study of innate immunity and host-pathogen interactions (14–24). A variety of virulence factors that are important in mediating bacterial and fungal pathogenesis in humans also cause disease in nematodes. To date, however, C. elegans has never been used to study commensal-associated proteins that may be important in this dynamic interplay between host and microorganism. Our study represents the very first attempt, to our knowledge, to assay and demonstrate the role of the cell wall adhesin Als5p and its functional amyloid in commensalism and adherence in vivo.
MATERIALS AND METHODS
Strains and media.
Fluorescent Saccharomyces cerevisiae strains containing pJL1-Als5, pJL1-EV, and pJL1-Als5V326N (11) were generated by transformation with green fluorescent protein (GFP) and red fluorescent protein (RFP) expression plasmids pADH1-GFP and yEPGAP-Cherry, respectively, which were kindly provided by Neta Dean's laboratory (Stony Brook University, New York, NY) (25). The resulting strains were grown in complete synthetic medium lacking uracil and tryptophan (CSM−Ura−Trp) at 24°C to induce expression. Galactose (2%) was the carbon source unless otherwise stated. Candida albicans strain BWP17 expressing ADH1pyEmRFP (referred to as BWP17 throughout the study) was also gifted to us by the Dean laboratory and was grown in yeast extract-peptone-dextrose (YPD) broth at 24°C. Nematodes were propagated on modified nematode growth medium (NGM) (40 mg/liter adenine, 3g/liter NaCl, 17g/liter agar, 2.5g/liter peptone, 20g/liter galactose, 10g/liter yeast extract, 1 ml 1 M CaCl2, 1 ml 1 M MgSO4, 25 ml 1 M KPO4, and 1 ml of 5 mg/ml cholesterol in 95% ethyl alcohol [EtOH]) and fed Escherichia coli OP50. C. elegans nematodes were maintained at 18°C. E. coli OP50 was grown overnight in LB broth at 37°C. In Congo red (CR) experiments, plates were made as described above, with the addition of Congo red at a final concentration of 30 μM. All C. elegans N2 strains and E. coli OP50 were provided by the Caenorhabditis Genetics Center (CGC), which is funded by the NIH Office of Research Infrastructure Programs (under NIH grant no. P40 OD010440).
Intestinal accumulation assay.
C. elegans nematodes grown on modified NGM plates were inoculated with E. coli OP50 as previously described (26). For experiments with S. cerevisiae, the nematodes were fed on suspensions of S. cerevisiae transformed with pJL1, pJL1Als5V326N, or pJL1EV (denoted “Als5p-expressing,” “Als5pV326N-expressing,” and “EV” strains, respectively, below) (11). The agar plates were modified NGM plates spotted with 100 μl of a suspension of Als5p-expressing, Als5pV326N-expressing, or EV S. cerevisiae cells (optical density at 600 nm [OD600] = 2) or Candida albicans BWP17 (OD600 = 2). For mixing experiments, 100 μl of a 1:1 mixture of Als5p-expressing (OD600 = 2) and EV cells was spotted onto NGM plates. After 24 h, the plates were washed twice in 3 ml M9 buffer (5.8 g/liter Na2HPO4, 3.0 g/liter KH2PO4, 0.5 g/liter NaCl, 1.0 g/liter NH4Cl) to rinse off nematodes, which were collected into a 15-ml conical tube (∼5.5 ml total). The pooled suspensions were centrifuged for 1 min at 500 rpm, after which the supernatant was slowly decanted. This washing procedure was performed five times in order to ensure that residual yeast was removed. The nematode pellet was then resuspended in 500 μl of M9 buffer. To enumerate the number of nematodes in the pellet, we visually inspected 100-μl aliquots under a stereoscope (in triplicate). One hundred microliters of worm suspension was then transferred into a tube containing 900 μl 6 M urea and five glass beads (5 mm in diameter), vortex mixed at maximum speed for 5 min, and immediately diluted 1:10, 1:100, and 1:1,000 into M9 buffer. The yeast suspensions (100-μl aliquots) were then plated onto appropriate selective media (CSM−Ura−Trp+2% galactose and 40 mg/liter adenine for EV/Als5p/Als5pV326N and YPD for BWP17) and incubated at 30°C for 48 h.
C. elegans survival assays.
In preparation for survival assays, eggs were obtained by standard bleaching techniques according to methods described previously by Stiernagle (26). Briefly, C. elegans stock plates that had many gravid hermaphrodites were washed with sterile H2O. The suspension was collected into a sterile 15-ml conical centrifuge tube with a cap. Sterile H2O was added to a total of 3.5 ml. A total of 0.5 ml of 5 M NaOH was premixed fresh with 1 ml of 5% household bleach and added to this 3.5-ml solution. The solution was then vortex mixed and centrifuged, and the pellet was washed twice. The pellet was resuspended in 3 ml sterile M9 buffer. Eggs were placed onto lawns of E. coli OP50 at 24°C until they were L4 larvae to early adults. Modified NGM plates, supplemented with 2% galactose (unless otherwise stated), kanamycin, and ampicillin (to inhibit the growth of E. coli OP50), were spotted with yeast strains (OD600 = 2) and approximately 50 L4 larvae to early adult nematodes unless otherwise indicated. All survival assay plates were incubated at 18°C for 96 h. During the reproductive period, adults were transferred daily onto fresh plates. Worm mortality was scored over time, with a worm being considered dead when it failed to respond to touch. Nematodes that were not recovered from the plates were censored from the study. The percentage of C. elegans nematodes alive for each given day was calculated by using the following formula:
Microscopic analysis.
For visualization of yeast in the nematode intestines, nematodes were fed the yeast strains at 18°C, after which they were picked from experimental plates and mounted onto 2% agar pads and immobilized in 7 to 10 μl of 15 mM sodium azide. Slides were viewed under an Olympus BX51 microscope using an Olympus DP71 camera. Overlays of fluorescent and bright-field images were inspected by an evaluator blinded to the identity of the yeast in each experiment. Each sample was scored on a semiquantitative log2-based scale: <10 cells, 1 unit; 11 to 20 cells, 2 units; 21 to 40 cells, 3 units; 41 to 80 cells, 4 units; and >80 cells, 5 units.
Oocyte quality analysis.
In preparation for the oocyte quality analysis, early L4 larvae were obtained by techniques used for the survival assay described above. Modified NGM plates, supplemented with kanamycin and ampicillin, were spotted with Als5p-expressing, Als5pV326N-expressing, or EV S. cerevisiae cells as well as E. coli OP50, and a single nematode was placed onto each plate. Plates were then incubated at 18°C. Each independent assay was carried out four times, with a total population size of 5 to 7 nematodes for each microorganism strain, per experiment. During the reproductive period, adults were transferred daily onto fresh plates. Eggs laid and those which hatched were scored over time at 24-h intervals. The percentage of viable C. elegans eggs for each given day was calculated by using the following formula:
RESULTS
Als5p expression increases yeast occupancy within the C. elegans intestine.
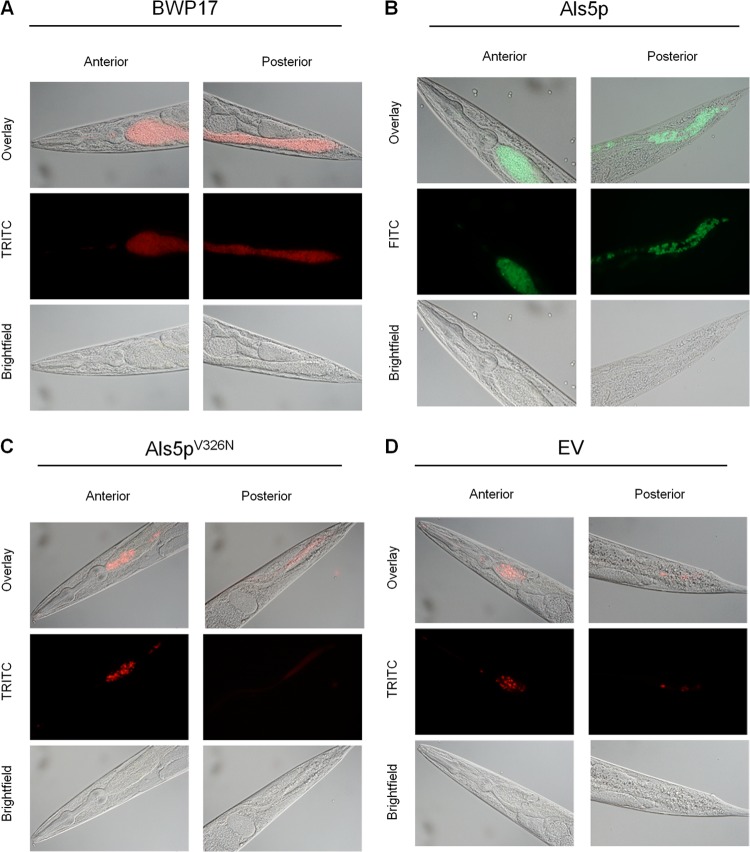
Accumulation of intact microbial cells in the intestine of C. elegans has been proposed to be an indicator of infection (20, 24, 27). We investigated whether Als5p mediates intestinal residence in C. elegans by using yeast cells expressing cytoplasmic fluorescent proteins. Candida albicans strain BWP17 accumulated within the nematode intestine in large numbers, with pronounced intestinal distention (Fig. 1A and 2A). We therefore hypothesized that if Als5p mediates binding to C. elegans, protein expression on the cell surface of S. cerevisiae would render this yeast pathogenic and cause yeast to accumulate in the nematode intestine. As expected, we observed Als5p-expressing yeast cells throughout the intestine of the nematode (Fig. 1B). In contrast, there were many fewer cells in worms harboring an empty vector (EV cells), with a distinct diffuse fluorescence throughout the intestine (Fig. 1D). Therefore, expression of Als5p in S. cerevisiae resulted in much greater fungal retention than in nonexpressing cells (Fig. 1 and 2A).
Fig 1.
Accumulation of Saccharomyces cerevisiae and Candida albicans strains in the intestine of Caenorhabditis elegans. Shown are micrographs of wild-type C. elegans strain N2 fed Candida albicans BWP17 (A) and Als5p-expressing (B), Als5pV326N-expressing (C), and EV-expressing (D) S. cerevisiae strains after 24 h. TRITC, tetramethyl rhodamine isocyanate; FITC, fluorescein isothiocyanate.
Fig 2.
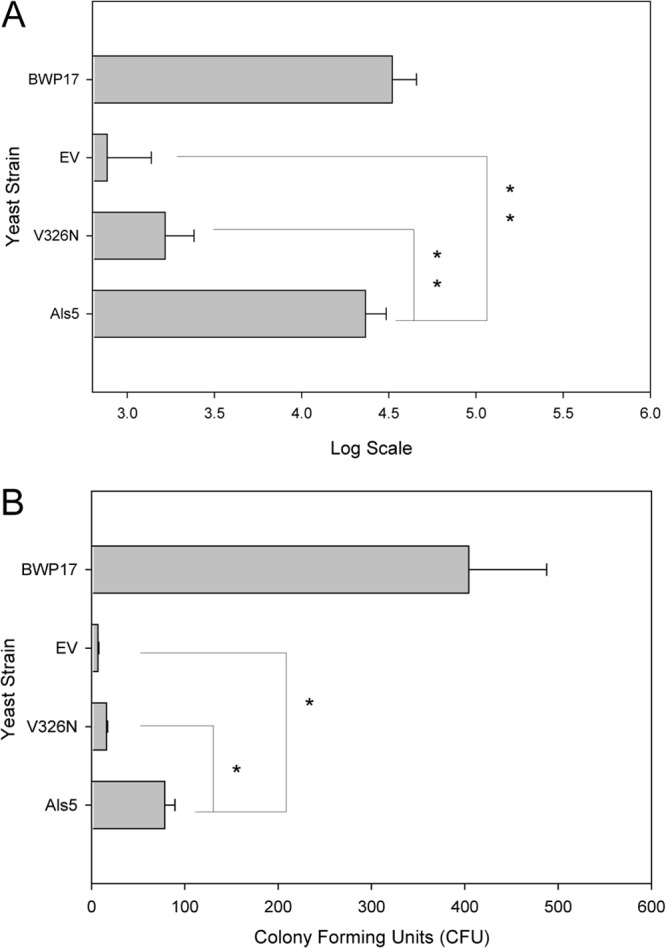
Effect of functional Als5p amyloid on intestinal occupation in C. elegans. (A) Accumulation of intact yeast cells counted in C. elegans alimentary tracts after 24 h (mean ± standard error of the mean for n ≥ 10 worms per food source). See Materials and Methods for details. (B) Live fungi recovered from C. elegans after 24 h of feeding. Shown are means ± standard errors of the means from two independent experiments where more than 50 animals were used in each trial. CFU are per nematode. ∗ indicates a Student t test P value of <0.05; ∗∗ indicates a Student t test P value of <0.005.
To support these results, we counted fungi in the intestine and represented the numbers using a log scale, as described in Materials and Methods. The counter was blinded to the identity of the ingested fungi. As expected, nematodes fed Als5p-expressing cells were occupied by fungal cells in significantly larger numbers than in those fed EV cells (Fig. 2A). Additionally, viable cells were recovered from C. elegans at 24 h postfeeding to quantify the number of cells occupying the nematode intestines. Fed nematodes were picked, washed, and lysed in urea, which dissociates aggregated yeast cells (5). The dissociation conditions (see Materials and Methods) had no effect on the viability of S. cerevisiae strains used in this study or Candida albicans BWP17 (data not shown). The mean fungal burden for S. cerevisiae expressing Als5p was 75 CFU per worm, 8.5-fold more than EV negative-control cells (Fig. 2B). This result confirmed the observation that Als5p expression rendered viable yeast more persistent in the intestine. C. albicans strain BWP17 was recovered from the intestine of the nematodes at rates higher than those for any of the other yeast strains (Fig. 2B), similar to what was observed microscopically (Fig. 1 and 2A).
The Als5p amyloid sequence is critical for intestinal accumulation.
The amyloid-forming region of Als5p potentiates adherence in the S. cerevisiae expression model, and a V326N mutation is sufficient to abolish amyloid formation and severely inhibit adhesion in vitro (11). Therefore, we assayed the effect of the amyloid sequence in the C. elegans infection model. Intestinal accumulation of cells expressing Als5pV326N was less than that of Als5p-expressing cells and similar to or slightly more than that of EV cells (Fig. 1C versus B and D). Intact Als5p-expressing S. cerevisiae accumulated in the intestinal tract of C. elegans in larger numbers than Als5pV326N-expressing cells (Fig. 2A). CFU counts showed 3.5-fold fewer mutant Als5pV326N-expressing cells isolated from the C. elegans intestine than Als5p-expressing cells (Fig. 2B). These results suggested that Als5p amyloid function mediated adherence in C. elegans and led to decreased mortality of the nematodes.
Als5p decreases S. cerevisiae-induced mortality in C. elegans.
We assayed survival times by comparing nematodes fed C. albicans or S. cerevisiae expressing Als5p or Als5pV326N with those fed EV cells or E. coli OP50 to determine whether increased intestinal occupation of viable Als5p-expressing yeast cells was lethal to the host (Fig. 3A). Candida albicans BWP17 killed C. elegans more rapidly than did S. cerevisiae, killing most nematodes by day 2 and all of them by day 4 (Fig. 3A). Feeding on S. cerevisiae killed the nematodes, but it was less lethal than feeding on C. albicans. Interestingly, the worms fed S. cerevisiae Als5p survived longer than nematodes fed EV control yeast. Specifically, at 48 h postexposure, there were 20% more C. elegans worms alive on Als5p-expressing yeast cells (P < 0.005) than nematodes fed EV control yeast cells (Fig. 3A). This delayed lethality continued through days 3 (P < 0.005) and 4 (P < 0.05), with 20% and 14% more nematodes alive on Als5p-expressing cells than on EV control cells, respectively (Fig. 3A). Nematodes fed Als5p-expressing cells had a time to 50% death (TD50) value of 84.3 h, 35.6 h more (P < 0.005) than nematodes fed EV control cells (Fig. 3B). This mortality was similar to that observed for nematodes fed E. coli OP50 (Fig. 3C). Expression of fluorescent proteins had no effect on the mortality rates of C. elegans, since nonfluorescent strains exhibited survival rates similar to those of their fluorescent counterparts (data not shown). Together, these results indicated that the C. albicans adhesin Als5p increased colonization in the intestine of the nematodes and reduced the lethality of S. cerevisiae.
Fig 3.
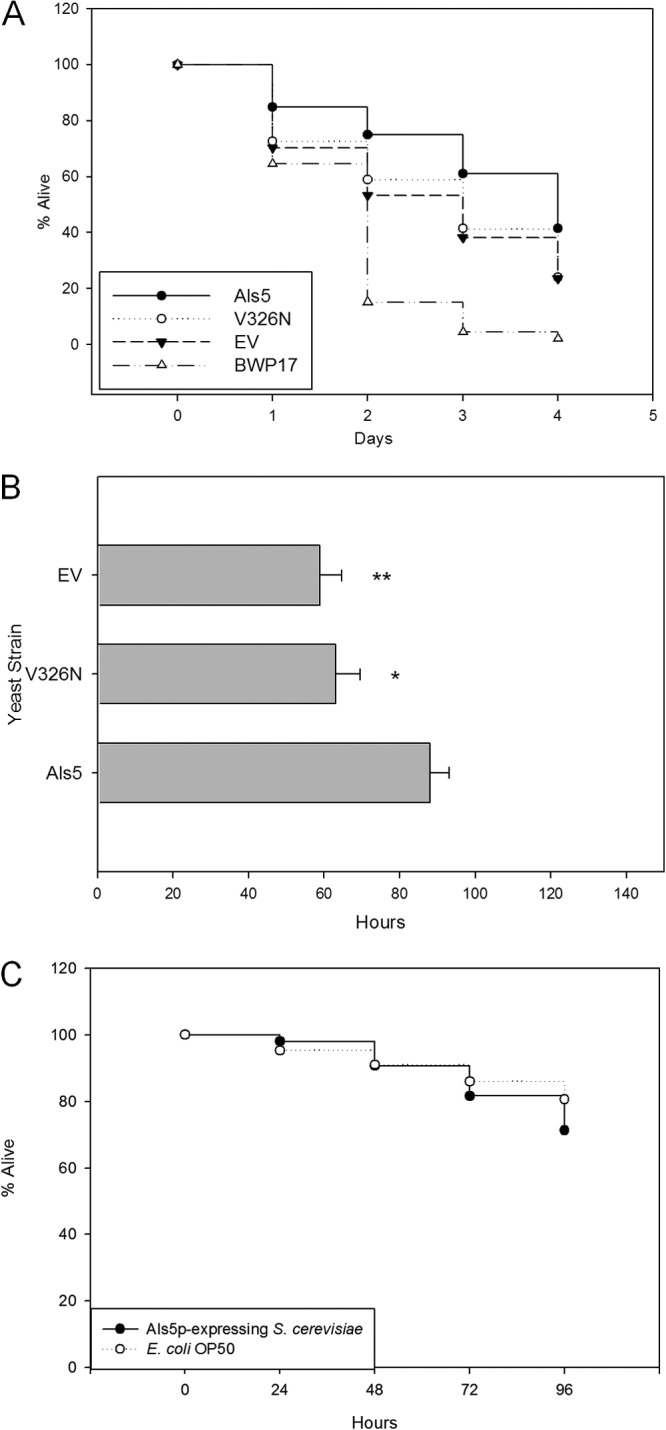
Survival of C. elegans challenged with Saccharomyces cerevisiae strains. (A) Percentage of C. elegans nematodes alive, starting with young adult animals fed on Als5p-expressing, Als5pV326N-expressing, or EV S. cerevisiae cells or C. albicans BWP17 over 4 days. Data are from eight independent experiments; more than 50 animals were used in each case. (B) Hours postexposure at which 50% of nematodes fed the indicated yeast strains died. Data are from eight independent experiments; more than 50 animals were used in each case. ∗ indicates a Student t test for difference from Als5p with a P value of <0.05; ∗∗ indicates a Student t test with a P value of <0.005. (C) Percentage of C. elegans nematodes alive, starting with young adult animals fed on Als5p-expressing cells or E. coli OP50 over 4 days. Data are from three independent experiments; more than 50 animals were used in each case.
We hypothesized that if Als5p contributed to the decreased mortality, then feeding nematodes on yeast grown on glucose would abrogate the observed reduction in mortality, because Als5p was expressed under a GAL1 promoter. Indeed, S. cerevisiae cells harboring pJL1 and pJL1-EV killed nematodes at rates similar to each other and to EV yeasts in galactose-containing medium (see Fig. S1 in the supplemental material). Thus, repression of Als5p expression led to more rapid death of the nematodes. These data support that Als5p is necessary for prevention of rapid S. cerevisiae-induced mortality.
The Als5p amyloid sequence is necessary for mortality reduction in C. elegans.
Because amyloid-forming Als5p increased both fungal burden and survival of the worms, we determined whether the amyloid sequence was responsible for the increased survival. We fed nematodes yeast cells expressing the non-amyloid-forming adhesin Als5pV326N. The amyloid-deficient mutant protein did not prolong nematode survival (Fig. 3A). More specifically, nematodes fed Als5pV326N-expressing cells had a TD50 value of 57.3 h, 27 h less (P ≤ 0.005) than that of nematodes fed Als5p-expressing cells (Fig. 3B) and similar to that of control EV yeasts. On day 2, there were 17% more nematodes alive on Als5p- than on Als5pV326N-expressing yeast cells, with that number going to 20% and 14% on days 3 and 4, respectively (Fig. 3A). The statistical significance of the difference between Als5p and Als5pV326N was a P value of <0.05 throughout the study. The viabilities of C. elegans fed Als5pV326N-expressing S. cerevisiae and EV negative-control cells were similar for days 1 through 4, strongly suggesting that Als5pV326N mutants behaved more like EV cells than Als5p-expressing yeast cells.
C. elegans nematodes fed Als5p-expressing S. cerevisiae cells exhibit normal physiological processes.
In order to determine if any of the nematodes fed Saccharomyces strains exhibited signs of infection, we assayed muscle function and movement in defecation (28, 29). Defecation is a highly regulated process that is vital to the nematode's constant fight against infection by ensuring that ingested microorganisms are not allowed to colonize the alimentary tract and persist for any period of time (28, 30). Nematodes fed Als5p-expressing S. cerevisiae cells defecated at rates similar to those of nematodes fed E. coli OP50, indicating that nematodes fed Als5p-expressing yeast exhibited normal physiological processes. Conversely, there was a significant difference between the defecation rates of nematodes fed Als5pV326N-expressing and those fed EV S. cerevisiae cells. The defecation rates of nematodes fed EV and Als5pV326N-expressing cells were lower by 13.2 s (P < 0.00001) and 6.7 s (P < 0.05), respectively, than those of nematodes fed Als5p-expressing cells (Fig. 4), indicating infection and constipation.
Fig 4.
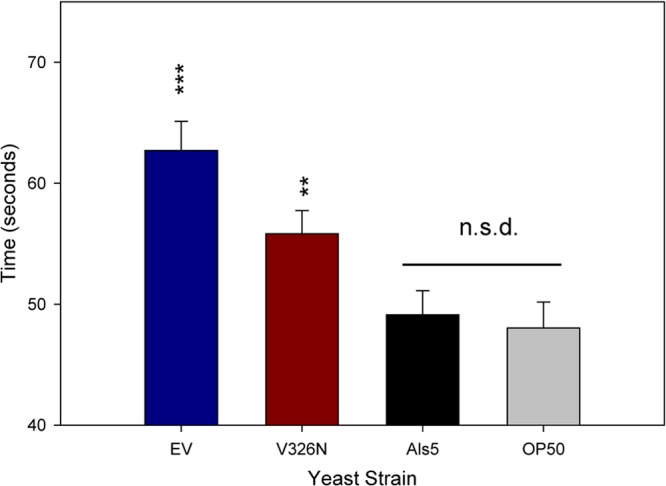
Defecation rates of C. elegans challenged with Als5p-, Als5pV326N-, or EV-expressing S. cerevisiae cells for 24 h. C. elegans nematodes were fed on Als5p-expressing, Als5pV326N-expressing, or EV S. cerevisiae or on C. albicans for 24 h. Data are from two independent experiments (n = 21). Asterisks show significance levels for deviations from Als5p-expressing S. cerevisiae. ∗∗ indicates a Student t test P value of <0.005; ∗∗∗ indicates a Student t test P value of <0.0005.
Oogenesis is a sensitive marker of reproductive changes resulting from starvation. Nematodes undergo an oogenic germ line starvation response, which results in reduced embryo viability and increased life span (31). Starvation can lead ultimately to matricide by eggs hatching within the hermaphrodite's uterus. To determine if nematodes were being starved in the presence of Als5p-expressing, Als5pV326N-expressing, and EV S. cerevisiae cells, we fed nematodes the above-mentioned fungal cells and counted the number of eggs laid and the number of eggs hatched over time. Nematodes fed Als5p-expressing cells laid a similar number of eggs as nematodes fed either Als5pV326N-expressing or EV cells at 24 to 72 h postfeeding (Fig. 5). The number of eggs laid was similar to those laid by worms fed E. coli OP50 for the first 24 h but was decreased at 48 and 72 h. In contrast, the fraction of eggs that hatched was not significantly different between nematodes fed any S. cerevisiae strains or OP50. These results, together with the differences in mortality rates of nematodes fed Als5p-expressing, Als5pV326N-expressing, or EV cells, suggest that nematodes are not undergoing starvation and that the decreased mortality rate observed in this study is Als5p dependent.
Fig 5.
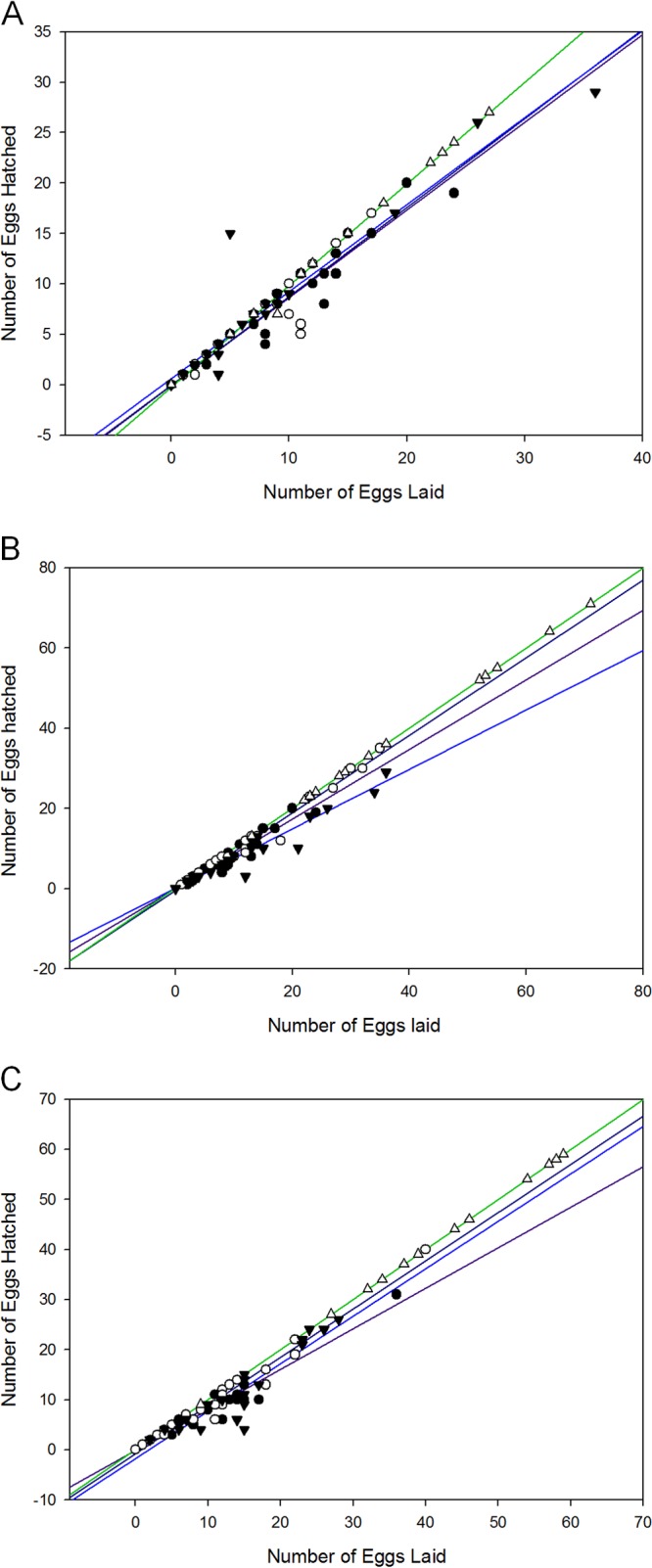
Oocyte quality of eggs laid by C. elegans when challenged with Als5p-expressing, Als5pV326N-expressing, or EV S. cerevisiae. Shown are numbers of eggs laid and hatched by C. elegans fed at 24 h (A), 48 h (B), and 72 h (C) on Als5p-expressing (●), Als5pV326N-expressing (○), and EV (▼) S. cerevisiae strains or on E. coli OP50 (△) over 3 days. Data are from six independent experiments; more than 5 animals were used in each case.
Amyloid binding dye Congo red negates Als5p effects.
Amyloid binding dyes are used for visualization of these insoluble fibrils, but at higher concentrations, their presence can also prevent amyloid formation (32). Accordingly, Congo red (CR) decreases aggregation of Als5p-expressing S. cerevisiae cells to each other (11). Therefore, we hypothesized that performing our survival assays on plates containing CR would prevent amyloid formation and negate the Als5p-mediated protective effect. In fact, the presence of 30 μM CR inhibited Als5p amyloid-mediated life extension (Fig. 6A). In detail, addition of 30 μM CR to the agar plates led to death of the nematodes fed Als5p-expressing yeast at rates similar to those of worms fed EV cells (Fig. 6A versus C). The mortality of nematodes fed Als5p strains in the presence of CR increased at statistically significant amounts at 48 h and 72 h postexposure compared to those in the absence of CR. Specifically, there were about 20% more nematodes alive that were fed Als5p-expressing S. cerevisiae in the absence of CR than in its presence during this time (P ≤ 0.05). CR did not affect the viability of the yeast cells or nematodes (data not shown). The addition of CR did not affect the survival of worms fed amyloid mutant protein-expressing cells or EV nonexpressing cells (Fig. 6B and C). Together, these data strongly suggest that the diminished lethality observed for nematodes fed Als5p-expressing yeast cells was not only dependent on Als5p but also specifically mediated by the amyloid-forming ability of this protein.
Fig 6.
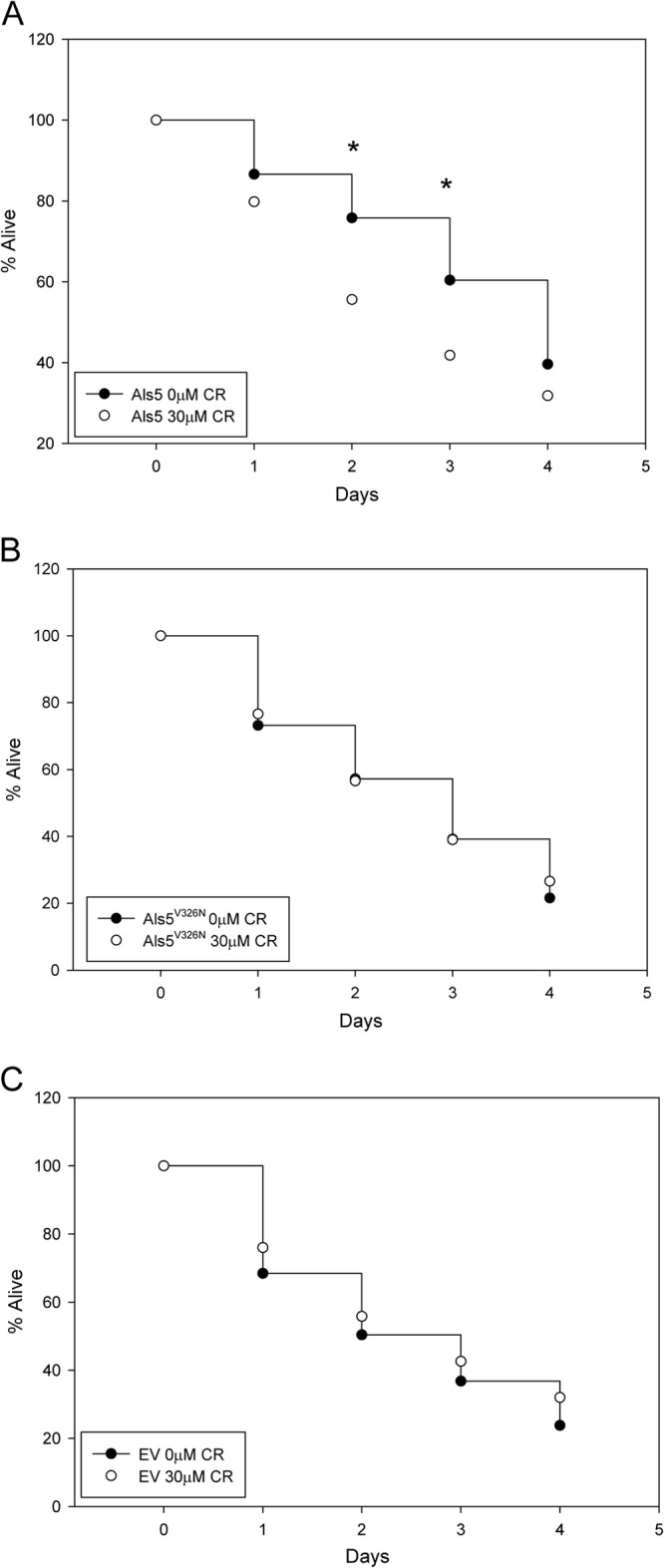
Effect of Congo red (CR) on S. cerevisiae-mediated killing of C. elegans. Shown are percentages of C. elegans nematodes alive, starting with young adult animals fed on Als5p-expressing S. cerevisiae over 4 days in the presence and absence of 30 μM CR. Data are from five independent experiments with at least 50 animals per experiment. (A) Nematodes fed Als5p-expressing S. cerevisiae. ∗ indicates a Student t test P value of <0.05. (B) Nematodes fed Als5pV326N-expressing S. cerevisiae. (C) Nematodes fed EV S. cerevisiae.
Als5p can attenuate mortality due to S. cerevisiae.
In order to evaluate if Als5p-expressing and -nonexpressing S. cerevisiae strains can cohabit the intestine of the C. elegans host, we fed nematodes a 1:1 mixture of Als5p-expressing and EV cells. We found that many nematodes retained a large number of EV cells along with a similar number of Als5p-expressing cells (Fig. 7C and D). Similarly, in nematodes whose intestines retained few Als5p-expressing cells, there were few EV cells present (data not shown). We also evaluated the mortality rates of these nematodes. Nematodes fed a mixture of Als5p-exppressing and EV cells had mortality rates similar to those fed only Als5p-expressing cells (Fig. 7A). Both of these experimental groups had decreased mortality rates compared to those of nematodes fed solely EV control cells.
Fig 7.
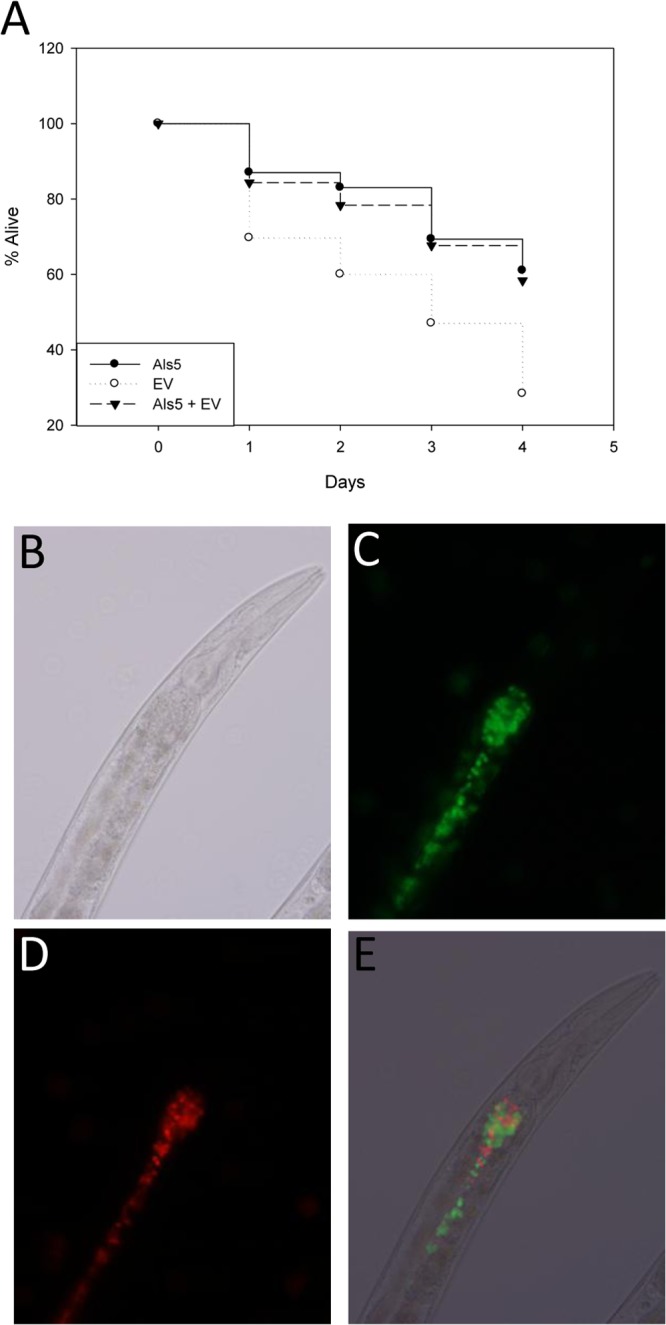
Mixed-feeding effects of Als5p-expressing and -nonexpressing strains on Caenorhabditis elegans. (A) Percentages of C. elegans nematodes alive, starting with young adult animals fed on Als5p-expressing or EV S. cerevisiae or a 1:1 mixture of the two strains over 4 days. Data are from three independent experiments; more than 50 animals were used in each case. (B to D) Micrographs of wild-type C. elegans strain N2 fed a 1:1 mixture of Als5p-expressing (green) (C) and EV (red) (D) S. cerevisiae cells after 24 h. (E) Overlay of the FITC and TRITC channels.
DISCUSSION
Our data suggest that Als5p is not critical for pathogenesis but rather that it can move the relationship toward a commensal-like state. The expression of Als5p moved the yeast-host interaction toward each of the hallmarks of commensalism: (i) increased occupancy of the host nematode by Als5p-expressing cells, (ii) maintenance of a nondiseased physiological state in the host, and (iii) possible fungal evasion of the nematode innate immune response to allow survival of the microbe. For each of these criteria, the amyloid-forming sequence in Als5p was necessary for activity.
Als5p mediates a commensal-like state.
Als5p was sufficient to enable S. cerevisiae cells to accumulate within the intestine of the nematode host. Furthermore, the yeast in the alimentary tract remained viable. In CFU assays, 8-fold more Als5p-expressing yeast cells were isolated from nematodes than EV yeast cells (Fig. 2). Thus, expression of Als5p led to fulfillment of the first two criteria of commensalism: increased occupancy and viability of the yeast. The expression of Als5p on the yeast surface also led to a nondiseased state in the host, the third condition. The defecation rates were indistinguishable from those of worms fed E. coli, whereas worms fed EV yeast had a significant decrease in the rate (Fig. 4), a sign of disease (28, 29). In addition, feeding on C. elegans on S. cerevisiae did not affect egg viability. The fraction of eggs hatched was constant and similar to that with E. coli for the yeast strains tested. Most remarkably, there was increased survival of the nematodes, even in the presence of increased fungal occupancy. The viability of worms feeding on Als5p-expressing yeast was in fact similar to that of worms fed E. coli (Fig. 3C), whereas nematodes fed EV yeasts died significantly faster (Fig. 3A). The increased survival was dependent on expression of the Als5p protein (see Fig. S1 in the supplemental material). Therefore, by measures or nematode viability, defecation rates, and egg viability, the host organism was in a nondisease state after feeding on Als5p-expressing yeast (the third criterion of commensalism).
Is the amyloid sequence in Als5p a commensal-associated molecular pattern?
The amyloid-forming sequence in Als5p was necessary for each of the changes in the yeast-host response. The amyloid-forming sequence was key in both gut occupancy and viability: numbers of Als5p-expressing yeast cells were visually greatly increased in the gut over yeast cells expressing the nonamyloid mutant form of the protein, Als5pV326N (Fig. 1), with a 3-fold increase in the number of viable yeast cells (Fig. 2). Similarly, yeast cells expressing Als5pV326N, a sequence that does not form amyloid, showed decreased defecation rates (Fig. 4), a symptom of a diseased state (29).
To us, the most remarkable result was that feeding with yeast expressing the amyloid-forming wild-type version of Als5p led to longer nematode survival than did feeding with yeast expressing the Als5pV326N protein, whose sequence was mutated to inhibit amyloid formation (Fig. 2). Indeed, the average life span of the worms fed yeast with the nonamyloid Als5pV326N protein was statistically similar to that of the worms feeding on EV yeast, which did not express Als5p at all.
Therefore, the amyloid-forming sequence in Als5p was necessary for accumulation of viable yeasts in the nematode gut and for increased survival of the worms. These results support the hypothesis that Als5p can function in modulating innate immunity to promote a more commensal-like state in the relationship between nematodes and S. cerevisiae. The Als5p amyloid-forming sequence is necessary for this activity. In this model, the Als5p amyloid sequence functions as a commensal-associated molecular pattern (CAMP), analogous to a pathogen-associated molecular pattern (PAMP) in pathogenesis (33). This concept helps to define the differences and similarities between commensalism and pathogenicity, with both states being outcomes of host-microbe interactions linked to characteristics of both the host and microbe (34). Indeed, our results identify a particular protein, region, and indeed a single-amino-acid residue that can shift the host-microbe relationship between pathogenicity and commensalism.
Our mixing experiments support this hypothesis. Als5p-expressing cells can enhance the retention of otherwise nonretained EV-expressing S. cerevisiae cells within the intestine of C. elegans. Furthermore, this retention of EV control cells was not accompanied by an increase in the mortality rate. On the contrary, nematodes fed the mixture of Als5p-expressing and EV S. cerevisiae cells succumbed at rates similar to those of nematodes fed only Als5p-expressing cells. This observation is directly in line with our hypothesis that Als5p may possess CAMP-like properties, perhaps downregulating innate immune functions in the nematode to promote or enhance survival of both yeast and host. This proposed ability may help explain why EV cells are found in larger numbers within the intestine of the nematodes fed the S. cerevisiae mixture. We hypothesize that Als5p prevents robust activation of host immune functions, which allows for the microbe's survival in this host niche.
The results of our study do not rule out other explanations. For instance, amyloid-forming Als5p might function to sequester S. cerevisiae toxins, thereby promoting tolerance. Alternately, surface amyloids may strengthen the yeast cell wall, preventing disruption by the grinder and allowing persistence in the gut with disease (for C. albicans) or without disease (S. cerevisiae). Nevertheless, the commensalism model appears to be supported by previous work including the finding that Als5p is upregulated in nonpathogenic states (1).
Amyloids in clinically important interactions.
Our study adds to the clinical relevance of amyloids since they are naturally present in any host, including human. There are many examples of host interactions with amyloids on microorganisms that can affect the balance within the dynamic interplay between host and microbe (35). For instance, amyloid fibrils formed by prostatic acid phosphatase augment HIV infection and thus tip the balance toward HIV survival and replication (36). Amyloids are present in human native hemostasis components that represent crucial regulatory elements in blood coagulation and clot clearance (hemostatic system). This hemostatic system can be exploited with the help of amyloids produced by microorganisms such as E. coli (a commensal bacterium) and Salmonella spp. (pathogenic bacteria), thus increasing their chances of colonizing their host (37). Hemostasis, amyloid, and microbial pathogenesis are closely related, and interactions between host and bacterial amyloids play a critical role (37).
There are several additional lines of evidence that Als amyloid interactions shift host-microbe interactions in C. albicans. Als5p expression does not correlate with either acute-stage or convalescent-stage candidiasis, and many clinical C. albicans strains have natural ALS5 deletions (34, 38). In contrast, ALS5 expression is obvious in asymptomatic, nonpregnant women, suggesting that it is transcribed under commensal conditions (39). Also, we have recently shown that surface amyloids are present on the surface of C. albicans in situ in autopsy sections of abscesses from candidiasis victims (40). In humans, these surface amyloids can affect the host response by binding serum amyloid P component (SAP), an innate immune system pattern recognition receptor (PRR) that suppresses the inflammatory response (40, 41).
Clearly, the outcome of the interaction between microbial amyloids and the host can determine the benefit or harm to the host. Our data and those of others support the paradigm that microbial surface amyloids change host-microbe interactions and can promote either infection or commensalism. This study only scratches the surface of the complexity of host-microbe interactions and gives novel insights into the molecular basis for how a successful commensal like C. albicans can inhabit its host.
Supplementary Material
ACKNOWLEDGMENTS
We thank Desmond Jackson for his consideration and comments on the manuscript.
This work was supported by grants R01 GM098616 and SC1 GM083756 to P.N.L.
Footnotes
Published ahead of print 8 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00020-13.
REFERENCES
- 1. Hube B. 2004. From commensal to pathogen: stage- and tissue-specific gene expression of Candida albicans. Curr. Opin. Microbiol. 7:336–341 [DOI] [PubMed] [Google Scholar]
- 2. Richard ML, Plaine A. 2007. Comprehensive analysis of glycosylphosphatidylinositol-anchored proteins in Candida albicans. Eukaryot. Cell 6:119–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang YL. 2003. Virulence factors of Candida species. J. Microbiol. Immunol. Infect. 36:223–228 [PubMed] [Google Scholar]
- 4. Hoyer LL. 2001. The ALS gene family of Candida albicans. Trends Microbiol. 9:176–180 [DOI] [PubMed] [Google Scholar]
- 5. Gaur NK, Klotz SA. 1997. Expression, cloning, and characterization of a Candida albicans gene, ALA1, that confers adherence properties upon Saccharomyces cerevisiae for extracellular matrix proteins. Infect. Immun. 65:5289–5294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sheppard DC, Yeaman MR, Welch WH, Phan QT, Fu Y, Ibrahim AS, Filler SG, Zhang M, Waring AJ, Edwards JE., Jr 2004. Functional and structural diversity in the Als protein family of Candida albicans. J. Biol. Chem. 279:30480–30489 [DOI] [PubMed] [Google Scholar]
- 7. Otoo HN, Lee KG, Qiu W, Lipke PN. 2008. Candida albicans Als adhesins have conserved amyloid-forming sequences. Eukaryot. Cell 7:776–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramsook CB, Tan C, Garcia MC, Fung R, Soybelman G, Henry R, Litewka A, O'Meally S, Otoo HN, Khalaf RA, Dranginis AM, Gaur NK, Klotz SA, Rauceo JM, Jue CK, Lipke PN. 2010. Yeast cell adhesion molecules have functional amyloid-forming sequences. Eukaryot. Cell 9:393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frank AT, Ramsook CB, Otoo HN, Tan C, Soybelman G, Rauceo JM, Gaur NK, Klotz SA, Lipke PN. 2010. Structure and function of glycosylated tandem repeats from Candida albicans Als adhesins. Eukaryot. Cell 9:405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rauceo JM, De Armond R, Otoo H, Kahn PC, Klotz SA, Gaur NK, Lipke PN. 2006. Threonine-rich repeats increase fibronectin binding in the Candida albicans adhesin Als5p. Eukaryot. Cell 5:1664–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia MC, Lee JT, Ramsook CB, Alsteens D, Dufrene YF, Lipke PN. 2011. A role for amyloid in cell aggregation and biofilm formation. PLoS One 6:e17632 doi:10.1371/journal.pone.0017632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alsteens D, Garcia MC, Lipke PN, Dufrene YF. 2010. Force-induced formation and propagation of adhesion nanodomains in living fungal cells. Proc. Natl. Acad. Sci. U. S. A. 107:20744–20749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lipke PN, Garcia MC, Alsteens D, Ramsook CB, Klotz SA, Dufrene YF. 2012. Strengthening relationships: amyloids create adhesion nanodomains in yeasts. Trends Microbiol. 20:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mylonakis E, Casadevall A, Ausubel FM. 2007. Exploiting amoeboid and non-vertebrate animal model systems to study the virulence of human pathogenic fungi. PLoS Pathog. 3:e101 doi:10.1371/journal.ppat.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Means TK. 2010. Fungal pathogen recognition by scavenger receptors in nematodes and mammals. Virulence 1:37–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, Tai MF, Stewart CR, Pukkila-Worley R, Hickman SE, Moore KJ, Calderwood SB, Hacohen N, Luster AD, El Khoury J. 2009. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J. Exp. Med. 206:637–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tenor JL, Aballay A. 2008. A conserved Toll-like receptor is required for Caenorhabditis elegans innate immunity. EMBO Rep. 9:103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Komura T, Yasui C, Miyamoto H, Nishikawa Y. 2010. Caenorhabditis elegans as an alternative model host for Legionella pneumophila, and protective effects of Bifidobacterium infantis. Appl. Environ. Microbiol. 76:4105–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anyanful A, Easley KA, Benian GM, Kalman D. 2009. Conditioning protects C. elegans from lethal effects of enteropathogenic E. coli by activating genes that regulate lifespan and innate immunity. Cell Host Microbe 5:450–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matricardi PM. 2010. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: controversial aspects of the ‘hygiene hypothesis’. Clin. Exp. Immunol. 160:98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pujol N, Zugasti O, Wong D, Couillault C, Kurz CL, Schulenburg H, Ewbank JJ. 2008. Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog. 4:e1000105 doi:10.1371/journal.ppat.1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, Kim DH. 2006. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2:e183 doi:10.1371/journal.pgen.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jain C, Yun M, Politz SM, Rao RP. 2009. A pathogenesis assay using Saccharomyces cerevisiae and Caenorhabditis elegans reveals novel roles for yeast AP-1, Yap1, and host dual oxidase BLI-3 in fungal pathogenesis. Eukaryot. Cell 8:1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sifri CD, Begun J, Ausubel FM. 2005. The worm has turned—microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 13:119–127 [DOI] [PubMed] [Google Scholar]
- 25. Keppler-Ross S, Noffz C, Dean N. 2008. A new purple fluorescent color marker for genetic studies in Saccharomyces cerevisiae and Candida albicans. Genetics 179:705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stiernagle T. 11 February 2006. Maintenance of C. elegans. WormBook 2006:1–11 doi:10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nicholas HR, Hodgkin J. 2004. Responses to infection and possible recognition strategies in the innate immune system of Caenorhabditis elegans. Mol. Immunol. 41:479–493 [DOI] [PubMed] [Google Scholar]
- 28. Rae R, Witte H, Rodelsperger C, Sommer RJ. 2012. The importance of being regular: Caenorhabditis elegans and Pristionchus pacificus defecation mutants are hypersusceptible to bacterial pathogens. Int. J. Parasitol. 42:747–753 [DOI] [PubMed] [Google Scholar]
- 29. Twumasi-Boateng K, Shapira M. 2012. Dissociation of immune responses from pathogen colonization supports pattern recognition in C. elegans. PLoS One 7:e35400 doi:10.1371/journal.pone.0035400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGhee JD. 27 March 2007. The C. elegans intestine. WormBook 2007:1–36 doi:10.1895/wormbook.1.133.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seidel HS, Kimble J. 2011. The oogenic germline starvation response in C. elegans. PLoS One 6:e28074 doi:10.1371/journal.pone.0028074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lorenzo A, Yankner BA. 1994. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by Congo red. Proc. Natl. Acad. Sci. U. S. A. 91:12243–12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. 2004. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118:229–241 [DOI] [PubMed] [Google Scholar]
- 34. Cottier F, Pavelka N. 2012. Complexity and dynamics of host-fungal interactions. Immunol. Res. 53:127–135 [DOI] [PubMed] [Google Scholar]
- 35. Oli MW, Otoo HN, Crowley PJ, Heim KP, Nascimento MM, Ramsook CB, Lipke PN, Brady LJ. 18 October 2012. Functional amyloid formation by Streptococcus mutans. Microbiology doi:10.1099/mic.0.060855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Munch J, Rucker E, Standker L, Adermann K, Goffinet C, Schindler M, Wildum S, Chinnadurai R, Rajan D, Specht A, Gimenez-Gallego G, Sanchez PC, Fowler DM, Koulov A, Kelly JW, Mothes W, Grivel JC, Margolis L, Keppler OT, Forssmann WG, Kirchhoff F. 2007. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 131:1059–1071 [DOI] [PubMed] [Google Scholar]
- 37. Fowler DM, Koulov AV, Balch WE, Kelly JW. 2007. Functional amyloid—from bacteria to humans. Trends Biochem. Sci. 32:217–224 [DOI] [PubMed] [Google Scholar]
- 38. Mochon AB, Jin Y, Kayala MA, Wingard JR, Clancy CJ, Nguyen MH, Felgner P, Baldi P, Liu H. 2010. Serological profiling of a Candida albicans protein microarray reveals permanent host-pathogen interplay and stage-specific responses during candidemia. PLoS Pathog. 6:e1000827 doi:10.1371/journal.ppat.1000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheng G, Wozniak K, Wallig MA, Fidel PL, Jr, Trupin SR, Hoyer LL. 2005. Comparison between Candida albicans agglutinin-like sequence gene expression patterns in human clinical specimens and models of vaginal candidiasis. Infect. Immun. 73:1656–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gilchrist KB, Garcia MC, Sobonya R, Lipke PN, Klotz SA. 2012. New features of invasive candidiasis in humans: amyloid formation by fungi and deposition of serum amyloid P component by the host. J. Infect. Dis. 206:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pepys MB. 2012. Invasive candidiasis: new insights presaging new therapeutic approaches? J. Infect. Dis. 206:1339–1341 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.