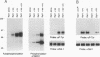Abstract
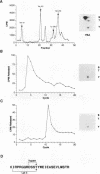
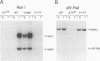
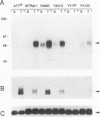
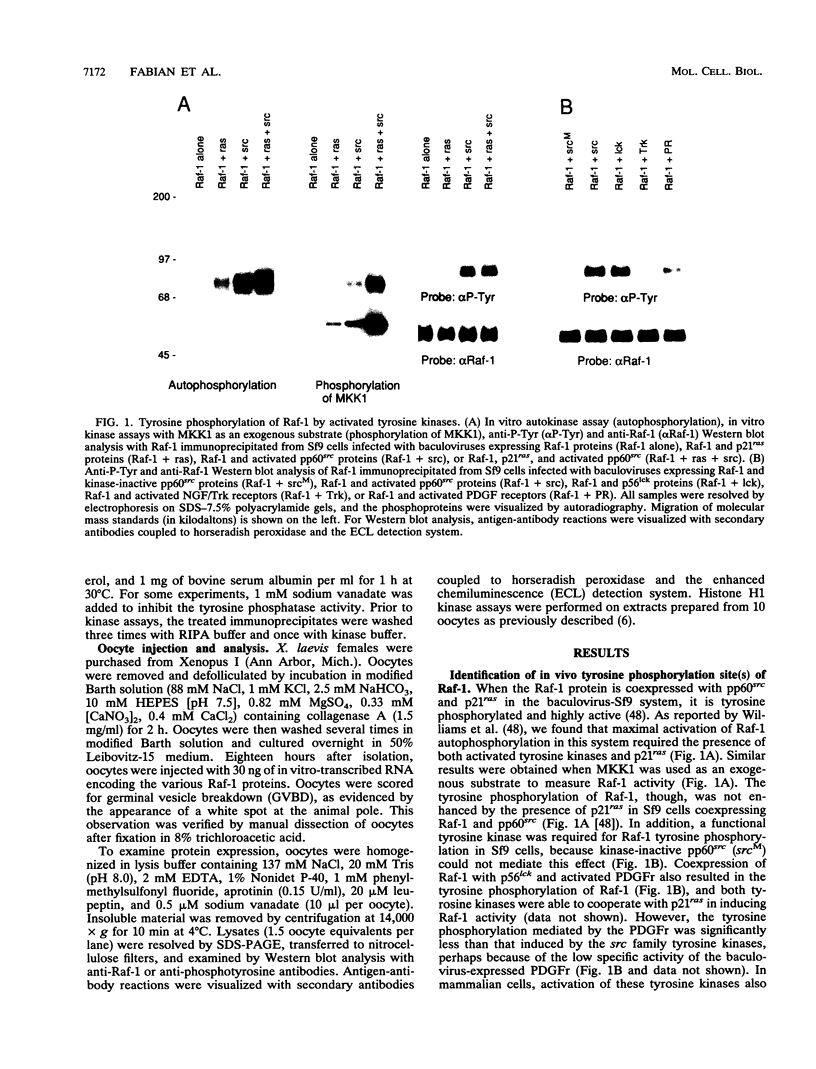
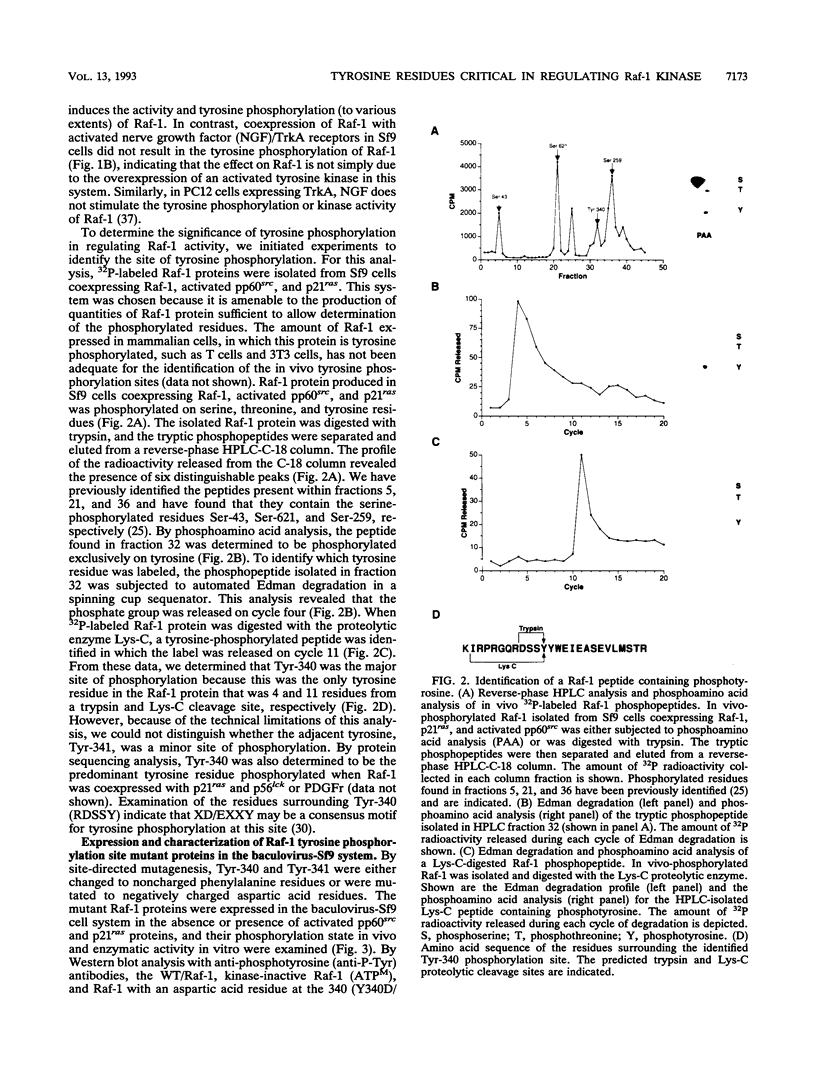
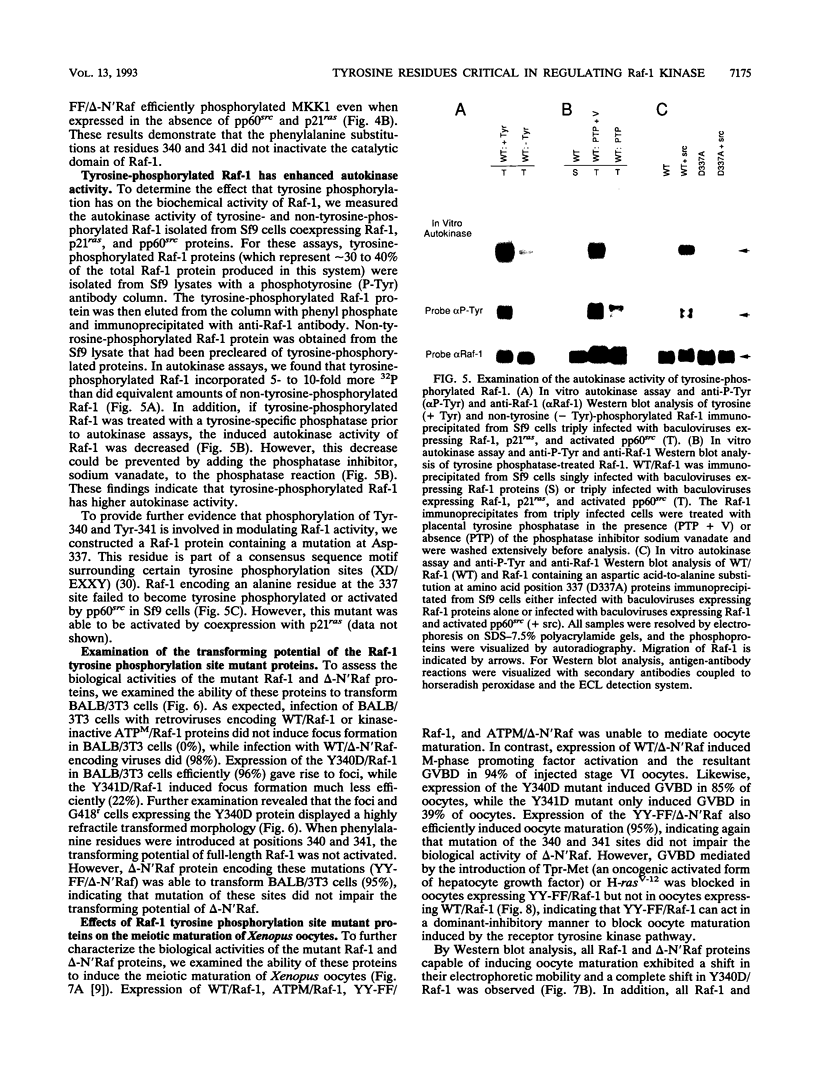
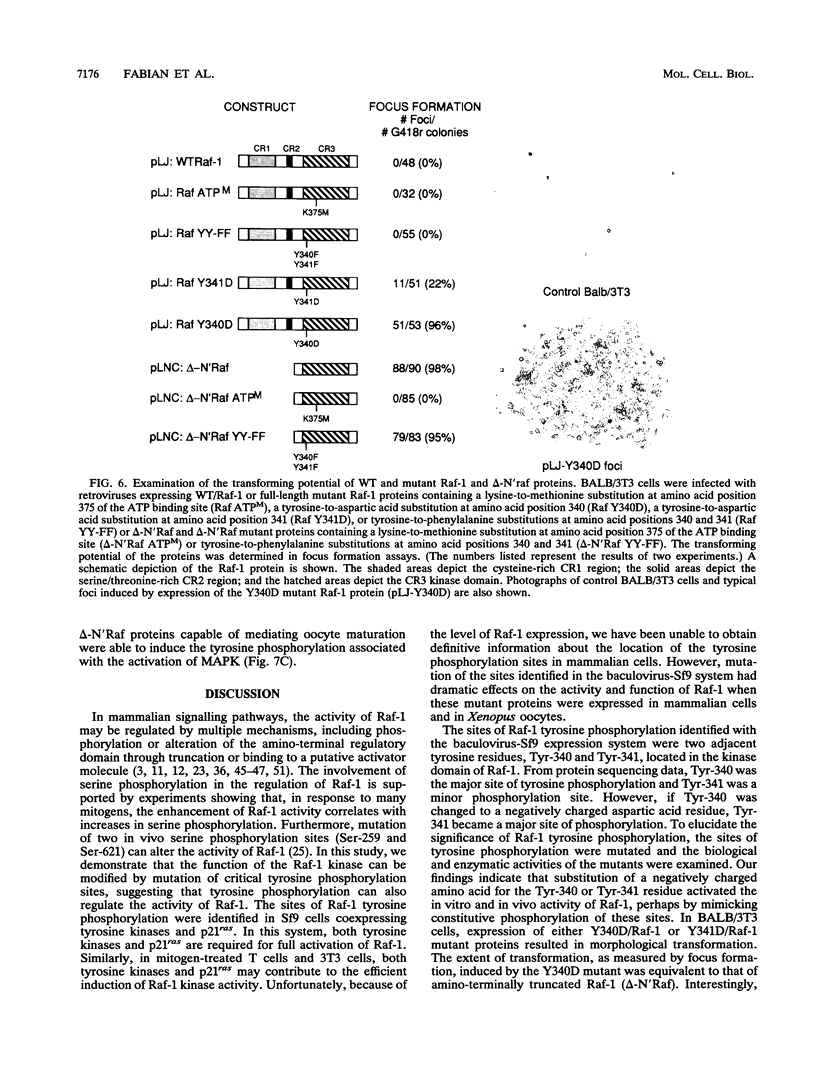
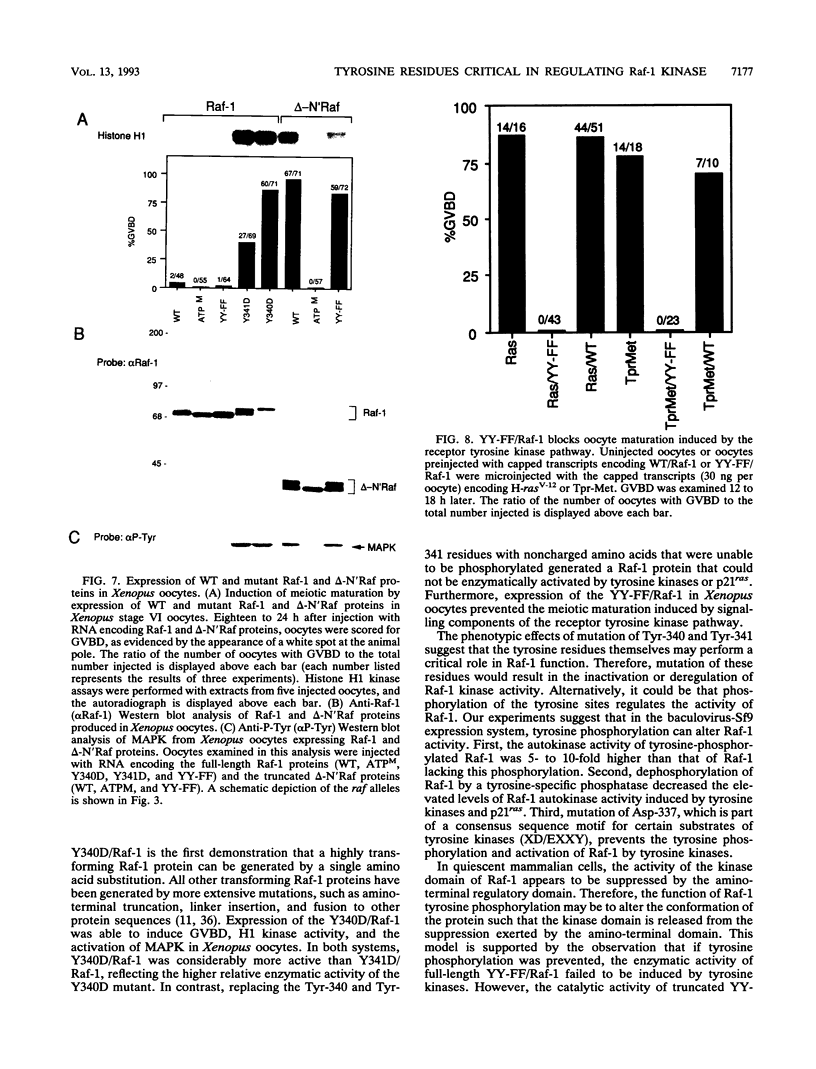
The serine/threonine kinase activity of the Raf-1 proto-oncogene product is stimulated by the activation of many tyrosine kinases, including growth factor receptors and pp60v-src. Recent studies of growth factor signal transduction pathways demonstrate that Raf-1 functions downstream of activated tyrosine kinases and p21ras and upstream of mitogen-activated protein kinase. However, coexpression of both activated tyrosine kinases and p21ras is required for maximal activation of Raf-1 in the baculovirus-Sf9 expression system. In this study, we investigated the role of tyrosine kinases and tyrosine phosphorylation in the regulation of Raf-1 activity. Using the baculovirus-Sf9 expression system, we identified Tyr-340 and Tyr-341 as the major tyrosine phosphorylation sites of Raf-1 when coexpressed with activated tyrosine kinases. Introduction of a negatively charged residue that may mimic the effect of phosphorylation at these sites activated the catalytic activity of Raf-1 and generated proteins that could transform BALB/3T3 cells and induce the meiotic maturation of Xenopus oocytes. In contrast, substitution of noncharged residues that were unable to be phosphorylated produced a protein that could not be enzymatically activated by tyrosine kinases and that could block the meiotic maturation of oocytes induced by components of the receptor tyrosine kinase pathway. These findings demonstrate that maturation of the tyrosine phosphorylation sites can dramatically alter the function of Raf-1. In addition, this is the first report that a transforming Raf-1 protein can be generated by a single amino acid substitution.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck T. W., Huleihel M., Gunnell M., Bonner T. I., Rapp U. R. The complete coding sequence of the human A-raf-1 oncogene and transforming activity of a human A-raf carrying retrovirus. Nucleic Acids Res. 1987 Jan 26;15(2):595–609. doi: 10.1093/nar/15.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Bruder J. T., Heidecker G., Rapp U. R. Serum-, TPA-, and Ras-induced expression from Ap-1/Ets-driven promoters requires Raf-1 kinase. Genes Dev. 1992 Apr;6(4):545–556. doi: 10.1101/gad.6.4.545. [DOI] [PubMed] [Google Scholar]
- Crews C. M., Alessandrini A., Erikson R. L. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992 Oct 16;258(5081):478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- Daar I., Yew N., Vande Woude G. F. Inhibition of mos-induced oocyte maturation by protein kinase A. J Cell Biol. 1993 Mar;120(5):1197–1202. doi: 10.1083/jcb.120.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P., Haser W., Haystead T. A., Vincent L. A., Roberts T. M., Sturgill T. W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992 Sep 4;257(5075):1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- Dickson B., Sprenger F., Morrison D., Hafen E. Raf functions downstream of Ras1 in the Sevenless signal transduction pathway. Nature. 1992 Dec 10;360(6404):600–603. doi: 10.1038/360600a0. [DOI] [PubMed] [Google Scholar]
- Fabian J. R., Morrison D. K., Daar I. O. Requirement for Raf and MAP kinase function during the meiotic maturation of Xenopus oocytes. J Cell Biol. 1993 Aug;122(3):645–652. doi: 10.1083/jcb.122.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Golden A., Han Y., Sternberg P. W. C. elegans lin-45 raf gene participates in let-60 ras-stimulated vulval differentiation. Nature. 1993 May 13;363(6425):133–140. doi: 10.1038/363133a0. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Huleihel M., Cleveland J. L., Kolch W., Beck T. W., Lloyd P., Pawson T., Rapp U. R. Mutational activation of c-raf-1 and definition of the minimal transforming sequence. Mol Cell Biol. 1990 Jun;10(6):2503–2512. doi: 10.1128/mcb.10.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G., Kölch W., Morrison D. K., Rapp U. R. The role of Raf-1 phosphorylation in signal transduction. Adv Cancer Res. 1992;58:53–73. doi: 10.1016/s0065-230x(08)60290-0. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein kinase classification. Methods Enzymol. 1991;200:3–37. doi: 10.1016/0076-6879(91)00125-g. [DOI] [PubMed] [Google Scholar]
- Ikawa S., Fukui M., Ueyama Y., Tamaoki N., Yamamoto T., Toyoshima K. B-raf, a new member of the raf family, is activated by DNA rearrangement. Mol Cell Biol. 1988 Jun;8(6):2651–2654. doi: 10.1128/mcb.8.6.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakura Y., Druker B., Wood K. W., Mamon H. J., Okuda K., Roberts T. M., Griffin J. D. Granulocyte-macrophage colony-stimulating factor and interleukin-3 induce rapid phosphorylation and activation of the proto-oncogene Raf-1 in a human factor-dependent myeloid cell line. Blood. 1991 Jan 15;77(2):243–248. [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Lloyd P., Rapp U. R. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991 Jan 31;349(6308):426–428. doi: 10.1038/349426a0. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., Force T. L., Rapp U. R., Bonventre J. V., Avruch J. Mitogen regulation of c-Raf-1 protein kinase activity toward mitogen-activated protein kinase-kinase. J Biol Chem. 1993 Jul 25;268(21):16009–16019. [PubMed] [Google Scholar]
- Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Le Guellec R., Couturier A., Le Guellec K., Paris J., Le Fur N., Philippe M. Xenopus c-raf proto-oncogene: cloning and expression during oogenesis and early development. Biol Cell. 1991;72(1-2):39–45. doi: 10.1016/0248-4900(91)90076-y. [DOI] [PubMed] [Google Scholar]
- Li P., Wood K., Mamon H., Haser W., Roberts T. Raf-1: a kinase currently without a cause but not lacking in effects. Cell. 1991 Feb 8;64(3):479–482. doi: 10.1016/0092-8674(91)90228-q. [DOI] [PubMed] [Google Scholar]
- Moodie S. A., Willumsen B. M., Weber M. J., Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993 Jun 11;260(5114):1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Heidecker G., Rapp U. R., Copeland T. D. Identification of the major phosphorylation sites of the Raf-1 kinase. J Biol Chem. 1993 Aug 15;268(23):17309–17316. [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rapp U., Roberts T. M. Signal transduction from membrane to cytoplasm: growth factors and membrane-bound oncogene products increase Raf-1 phosphorylation and associated protein kinase activity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8855–8859. doi: 10.1073/pnas.85.23.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K. The Raf-1 kinase as a transducer of mitogenic signals. Cancer Cells. 1990 Dec;2(12):377–382. [PubMed] [Google Scholar]
- Nishida E., Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem Sci. 1993 Apr;18(4):128–131. doi: 10.1016/0968-0004(93)90019-j. [DOI] [PubMed] [Google Scholar]
- Nishida Y., Hata M., Ayaki T., Ryo H., Yamagata M., Shimizu K., Nishizuka Y. Proliferation of both somatic and germ cells is affected in the Drosophila mutants of raf proto-oncogene. EMBO J. 1988 Mar;7(3):775–781. doi: 10.1002/j.1460-2075.1988.tb02875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Goldsborough M. D., Mark G. E., Bonner T. I., Groffen J., Reynolds F. H., Jr, Stephenson J. R. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4218–4222. doi: 10.1073/pnas.80.14.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Heidecker G., Huleihel M., Cleveland J. L., Choi W. C., Pawson T., Ihle J. N., Anderson W. B. raf family serine/threonine protein kinases in mitogen signal transduction. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):173–184. doi: 10.1101/sqb.1988.053.01.023. [DOI] [PubMed] [Google Scholar]
- Rapp U. R. Role of Raf-1 serine/threonine protein kinase in growth factor signal transduction. Oncogene. 1991 Apr;6(4):495–500. [PubMed] [Google Scholar]
- Roberts T. M. Cell biology. A signal chain of events. Nature. 1992 Dec 10;360(6404):534–535. doi: 10.1038/360534a0. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Copeland T. D., Mark G. E., Rapp U. R., Oroszlan S. Detection of the myristylated gag-raf transforming protein with raf-specific antipeptide sera. Virology. 1985 Oct 15;146(1):78–89. doi: 10.1016/0042-6822(85)90054-6. [DOI] [PubMed] [Google Scholar]
- Stanton V. P., Jr, Nichols D. W., Laudano A. P., Cooper G. M. Definition of the human raf amino-terminal regulatory region by deletion mutagenesis. Mol Cell Biol. 1989 Feb;9(2):639–647. doi: 10.1128/mcb.9.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. M., Sithanandam G., Copeland T. D., Kaplan D. R., Rapp U. R., Morrison D. K. 95-kilodalton B-Raf serine/threonine kinase: identification of the protein and its major autophosphorylation site. Mol Cell Biol. 1992 Sep;12(9):3733–3742. doi: 10.1128/mcb.12.9.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm S. M., Cleveland J. L., Rapp U. R. Expression of raf family proto-oncogenes in normal mouse tissues. Oncogene. 1990 Mar;5(3):345–351. [PubMed] [Google Scholar]
- Thompson P. A., Ledbetter J. A., Rapp U. R., Bolen J. B. The Raf-1 serine-threonine kinase is a substrate for the p56lck protein tyrosine kinase in human T-cells. Cell Growth Differ. 1991 Dec;2(12):609–617. [PubMed] [Google Scholar]
- Troppmair J., Bruder J. T., App H., Cai H., Liptak L., Szeberényi J., Cooper G. M., Rapp U. R. Ras controls coupling of growth factor receptors and protein kinase C in the membrane to Raf-1 and B-Raf protein serine kinases in the cytosol. Oncogene. 1992 Sep;7(9):1867–1873. [PubMed] [Google Scholar]
- Tsuda L., Inoue Y. H., Yoo M. A., Mizuno M., Hata M., Lim Y. M., Adachi-Yamada T., Ryo H., Masamune Y., Nishida Y. A protein kinase similar to MAP kinase activator acts downstream of the raf kinase in Drosophila. Cell. 1993 Feb 12;72(3):407–414. doi: 10.1016/0092-8674(93)90117-9. [DOI] [PubMed] [Google Scholar]
- Turner B. C., Tonks N. K., Rapp U. R., Reed J. C. Interleukin 2 regulates Raf-1 kinase activity through a tyrosine phosphorylation-dependent mechanism in a T-cell line. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5544–5548. doi: 10.1073/pnas.90.12.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B., Rapp U., App H., Greene M., Dobashi K., Reed J. Interleukin 2 induces tyrosine phosphorylation and activation of p72-74 Raf-1 kinase in a T-cell line. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1227–1231. doi: 10.1073/pnas.88.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst L., Barr M., Marcus S., Polverino A., Wigler M. Complex formation between RAS and RAF and other protein kinases. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6213–6217. doi: 10.1073/pnas.90.13.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek A. B., Hollenberg S. M., Cooper J. A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993 Jul 16;74(1):205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Wasylyk B., Heidecker G., Huleihel M., Rapp U. R. Expression of raf oncogenes activates the PEA1 transcription factor motif. Mol Cell Biol. 1989 May;9(5):2247–2250. doi: 10.1128/mcb.9.5.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N. G., Roberts T. M., Li P. Both p21ras and pp60v-src are required, but neither alone is sufficient, to activate the Raf-1 kinase. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2922–2926. doi: 10.1073/pnas.89.7.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. W., Sarnecki C., Roberts T. M., Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992 Mar 20;68(6):1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- Wu J., Harrison J. K., Vincent L. A., Haystead C., Haystead T. A., Michel H., Hunt D. F., Lynch K. R., Sturgill T. W. Molecular structure of a protein-tyrosine/threonine kinase activating p42 mitogen-activated protein (MAP) kinase: MAP kinase kinase. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):173–177. doi: 10.1073/pnas.90.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. F., Settleman J., Kyriakis J. M., Takeuchi-Suzuki E., Elledge S. J., Marshall M. S., Bruder J. T., Rapp U. R., Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993 Jul 22;364(6435):308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]