Abstract
In the title compound, (C5H6N)[Fe(NCS)4(C5H5N)2]·4C5H3N3·C5H5N, the FeIII ion is located on an inversion centre and is six-coordinated by four N atoms of the thiocyanate ligands and two pyridine N atoms in a trans arrangement, forming a slightly distorted octahedral geometry. A half-occupied H atom attached to a pyridinium cation forms an N—H⋯N hydrogen bond with a centrosymmetrically-related pyridine unit. Four pyrazine-2-carbonitrile molecules crystallize per complex anion. In the crystal, π–π stacking interactions are present [centroid–centroid distances = 3.6220 (9), 3.6930 (9), 3.5532 (9), 3.5803 (9) and 3.5458 (8) Å].
Related literature
For the use of molecular assemblies comprising cationic and anionic modules, see: Fritsky et al. (1998 ▶, 2004 ▶); Kanderal et al. (2005 ▶). For FeII–thiocyanate complexes with aromatic N-donor ligands indicating spin crossover, see: Gamez et al. (2009 ▶); Niel et al. (2001 ▶). For related structures, see: Moroz et al. (2010 ▶); Penkova et al. (2010 ▶); Petrusenko et al. (1997 ▶); Real et al. (1991 ▶).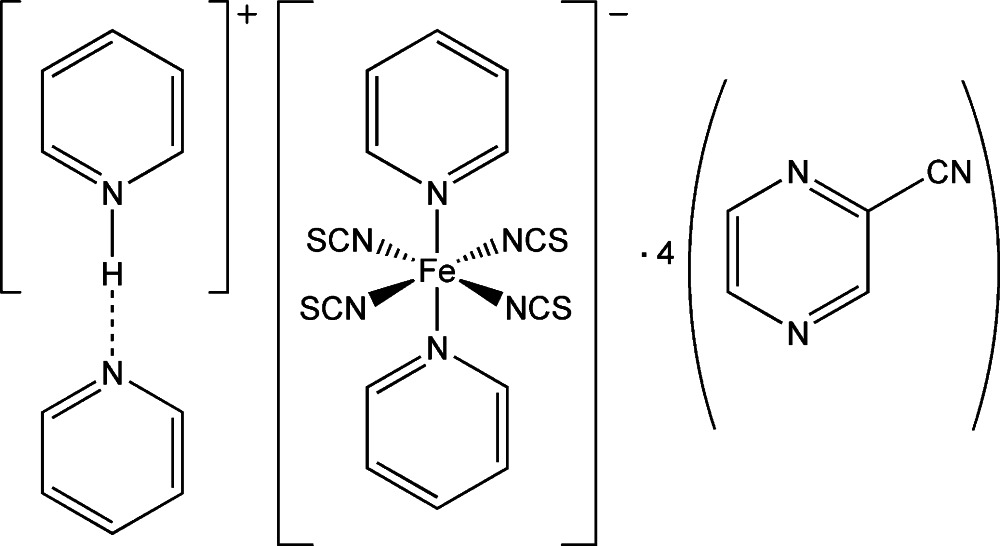
Experimental
Crystal data
(C5H6N)[Fe(NCS)4(C5H5N)2]·4C5H3N3·C5H5N
M r = 1025.99
Triclinic,

a = 8.1766 (2) Å
b = 11.9362 (3) Å
c = 12.7519 (3) Å
α = 102.982 (1)°
β = 97.799 (1)°
γ = 97.684 (1)°
V = 1184.02 (5) Å3
Z = 1
Mo Kα radiation
μ = 0.55 mm−1
T = 120 K
0.38 × 0.19 × 0.17 mm
Data collection
Bruker Kappa APEXII DUO CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.818, T max = 0.910
18588 measured reflections
5482 independent reflections
4470 reflections with I > 2σ(I)
R int = 0.024
Refinement
R[F 2 > 2σ(F 2)] = 0.031
wR(F 2) = 0.076
S = 1.01
5482 reflections
313 parameters
H-atom parameters constrained
Δρmax = 0.33 e Å−3
Δρmin = −0.37 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Brandenburg, 1997 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813010362/hy2622sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813010362/hy2622Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813010362/hy2622Isup3.cdx
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N4—H4N⋯N4i | 0.88 | 1.80 | 2.677 (3) | 179 |
Symmetry code: (i)  .
.
supplementary crystallographic information
Comment
Molecular assemblies comprising from cationic and anionic modules are of special interest for crystal engineering and molecular magnetism (Fritsky et al., 2004). Formation of such compounds often can be mediated by different types of intermolecular interactions, such as co-ordination and hydrogen bonds, ionic and van der Waals interactions (Fritsky et al., 1998; Kanderal et al., 2005). Such assemblies may possess interesting functional properties and, in particular, indicate spin crossover behavior. In this regard, FeII thiocyanate complexes with aromatic N-donor ligands attract much attention considering the possible metal ion spin state modulation by variation of a ligand (Gamez et al., 2009) accompanied by coordination polymer formation. Particularly, as one of the simplest bridging N-donor ligands to design coordination polymers, pyrazine (pz) is known for the construction of spin crossover Hofmann-like clathrates with general formula [FeIIMII(pz)(CN)4]∞ (M = Ni, Pd or Pt) (Niel et al., 2001). A combination of pz and thiocyanate ligands leads to the formation of two-dimensional coordination polymer [Fe(NCS)2(pz)2] with an antiferromagnetic exchange between metal centres (Real et al., 1991). In this context, we attempted to synthesize FeII thiocyanate complex with pyrazine-2-carbonitrile (cnpz). However, the reaction of [FeII(NCS)2(py)4] (py = pyridine) and cnpz in an organic media in air led to oxidation of FeII and to the formation of the title compound.
The compound consists of one complex anion [Fe(NCS)4(py)2]-, one pyridinium cation, one pyridine and four pyrazine-2-carbonitrile molecules (Fig. 1). The FeIII ion is located on an inversion centre and is sixfold coordinated by four N atoms of four thiocyanate anions and two N atoms of two pyridine ligands in a trans arrangement, forming a slightly distorted octahedral coordination geometry. The thiocyanate ligands are bound through N atoms and are quasi-linear [S1—C1—N2 = 179.41 (14), S2—C9—N3 = 179.33 (15)°], while the Fe—NCS linkages are bent [Fe1—N2—C1 = 163.20 (12), Fe1—N3—C9 = 167.56 (12)°]. These structural features are typical for the complexes where the NCS group is N-bound (Petrusenko et al., 1997). The distances between FeIII ion and N atoms of the thiocyanate anions [Fe1—N2 = 2.0424 (13), Fe1—N3 = 2.0370 (13) Å] are considerably shorter than those between FeIII and N atoms of the pyridine ligands [Fe1—N1 = 2.1320 (12)Å], that could be related to the higher affinity of the metal ion to negatively charged thiocyanate comparing with the neutral organic ligand. The C—N and C—C bond lenths in the coordinated pyridine ligands are normal and close to the values observed in the related structures (Moroz et al., 2010; Penkova et al., 2010).
In the title compound there are four solvent molecules of pyrazine-2-carbonitrile per each FeIII ion that interact with one another through π–π stacking, with distances between the centroids of 3.5532 (9), 3.5803 (9) and 3.5458 (8) Å (Fig. 2). One of the uncoordinated pyridines is protonated and the N-bound H atom is disodered between two equally populated positions, forming N—H···N hydrogen bonds (Table 1). Coordinated and solvent pyridine molecules also interact with one another viaπ–π contacts, with distances between the centroids of 3.6220 (9) and 3.6930 (9) Å (Fig. 3).
Experimental
Crystals of the title compound were obtained by adding pyrazine-2-carbonitrile (52.5 mg, 0.5 mmol) to tetrakis(pyridine)bis(isothiocyanato)iron(II), [Fe(NCS)2(py)4], (48.8 mg, 0.1 mmol) in acetone (5 ml). The solution was left to evaporate in air. In one day this yielded red crystals that were collected, washed with water and dried in air (yield: 21 mg, 20%).
Refinement
H atoms were positioned geometrically and refined as riding atoms, with C—H = 0.95 and N—H = 0.88 Å and with Uiso(H) = 1.2Ueq(C,N). N-bound H atom of the uncoordinated pyridine is half-occupied due to the requirement of symmetry.
Figures
Fig. 1.
Molecular structure of the title compound with displacement ellipsoids drawn at the 50% probability level. [Symmetry code: (i) -x+1, -y+1, -z+1.]
Fig. 2.
Crystal structure of the title compound, showing π–π contacts between the cnpz molecules as dashed lines (carmine: Fe, yellow: S, blue: N, grey: C, light-grey: H).
Fig. 3.
Crystal structure of the title compound, showing hydrogen bonds and π–π contacts between the pyridine molecules as dashed lines (carmine: Fe, yellow: S, blue: N, grey: C, light-grey: H).
Crystal data
| (C5H6N)[Fe(NCS)4(C5H5N)2]·4C5H3N3·C5H5N | Z = 1 |
| Mr = 1025.99 | F(000) = 527 |
| Triclinic, P1 | Dx = 1.439 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.1766 (2) Å | Cell parameters from 7810 reflections |
| b = 11.9362 (3) Å | θ = 2.6–27.6° |
| c = 12.7519 (3) Å | µ = 0.55 mm−1 |
| α = 102.982 (1)° | T = 120 K |
| β = 97.799 (1)° | Block, red |
| γ = 97.684 (1)° | 0.38 × 0.19 × 0.17 mm |
| V = 1184.02 (5) Å3 |
Data collection
| Bruker Kappa APEXII DUO CCD diffractometer | 5482 independent reflections |
| Radiation source: fine-focus sealed tube | 4470 reflections with I > 2σ(I) |
| Curved graphite crystal monochromator | Rint = 0.024 |
| Detector resolution: 16 pixels mm-1 | θmax = 27.7°, θmin = 1.7° |
| φ and ω scans with κ offset | h = −10→10 |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | k = −15→15 |
| Tmin = 0.818, Tmax = 0.910 | l = −16→16 |
| 18588 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.031 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.076 | H-atom parameters constrained |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.0363P)2 + 0.3715P] where P = (Fo2 + 2Fc2)/3 |
| 5482 reflections | (Δ/σ)max = 0.001 |
| 313 parameters | Δρmax = 0.33 e Å−3 |
| 0 restraints | Δρmin = −0.37 e Å−3 |
Special details
| Experimental. Hydrogen atoms were positioned geometrically and constrained to ride on their parent atoms, with C—H = 0.95 Å, N—H = 0.88 Å, and Uiso = 1.2 Ueq(parent atom). The highest peak is located 0.70 Å from atom C18 and the deepest hole is located 0.48 Å from atom Fe1. |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Fe1 | 0.5000 | 0.5000 | 0.5000 | 0.01474 (8) | |
| S1 | 0.18557 (5) | 0.25687 (4) | 0.16391 (3) | 0.02717 (11) | |
| S2 | 0.31797 (5) | 0.74869 (4) | 0.27623 (4) | 0.02695 (11) | |
| N1 | 0.72282 (15) | 0.50545 (11) | 0.43036 (10) | 0.0163 (3) | |
| N2 | 0.38008 (16) | 0.37328 (11) | 0.36553 (11) | 0.0200 (3) | |
| N3 | 0.42809 (15) | 0.62208 (11) | 0.42297 (11) | 0.0193 (3) | |
| N4 | 0.86353 (18) | 0.50989 (12) | 0.04639 (11) | 0.0279 (3) | |
| H4N | 0.9528 | 0.5042 | 0.0153 | 0.033* | 0.50 |
| N5 | 0.40216 (18) | 0.10177 (12) | 0.42369 (12) | 0.0278 (3) | |
| N6 | 0.09833 (17) | −0.12537 (12) | 0.62053 (11) | 0.0271 (3) | |
| N7 | 0.90967 (18) | 0.11755 (13) | −0.07429 (12) | 0.0302 (3) | |
| N8 | 0.59044 (17) | −0.09046 (12) | 0.13024 (11) | 0.0237 (3) | |
| N9 | 0.67103 (17) | 0.14539 (11) | 0.12764 (11) | 0.0220 (3) | |
| N10 | 0.15882 (17) | 0.11284 (12) | 0.62270 (11) | 0.0224 (3) | |
| C1 | 0.29957 (18) | 0.32456 (13) | 0.28113 (12) | 0.0173 (3) | |
| C2 | 0.80428 (18) | 0.60594 (13) | 0.41827 (12) | 0.0193 (3) | |
| H2 | 0.7656 | 0.6766 | 0.4459 | 0.023* | |
| C3 | 0.94253 (19) | 0.60979 (14) | 0.36697 (13) | 0.0213 (3) | |
| H3 | 0.9981 | 0.6821 | 0.3597 | 0.026* | |
| C4 | 0.99889 (19) | 0.50744 (14) | 0.32645 (13) | 0.0214 (3) | |
| H4 | 1.0925 | 0.5080 | 0.2897 | 0.026* | |
| C5 | 0.9167 (2) | 0.40386 (14) | 0.34019 (13) | 0.0225 (3) | |
| H5 | 0.9541 | 0.3323 | 0.3142 | 0.027* | |
| C6 | 0.77991 (19) | 0.40658 (13) | 0.39215 (12) | 0.0197 (3) | |
| H6 | 0.7235 | 0.3354 | 0.4014 | 0.024* | |
| C7 | 0.6803 (2) | 0.62600 (15) | 0.12971 (14) | 0.0297 (4) | |
| H7 | 0.6508 | 0.7007 | 0.1539 | 0.036* | |
| C8 | 0.5837 (2) | 0.52690 (15) | 0.14229 (13) | 0.0271 (4) | |
| H8 | 0.4869 | 0.5328 | 0.1754 | 0.032* | |
| C9 | 0.38283 (18) | 0.67553 (13) | 0.36185 (12) | 0.0176 (3) | |
| C10 | 0.8197 (2) | 0.61399 (15) | 0.08152 (14) | 0.0283 (4) | |
| H10 | 0.8867 | 0.6817 | 0.0730 | 0.034* | |
| C11 | 0.32510 (19) | 0.07281 (13) | 0.48410 (13) | 0.0204 (3) | |
| C12 | 0.22339 (18) | 0.03216 (13) | 0.55739 (12) | 0.0183 (3) | |
| C13 | 0.1944 (2) | −0.08487 (14) | 0.55571 (13) | 0.0238 (3) | |
| H13 | 0.2437 | −0.1378 | 0.5074 | 0.029* | |
| C14 | 0.0340 (2) | −0.04577 (15) | 0.68615 (13) | 0.0255 (4) | |
| H14 | −0.0350 | −0.0704 | 0.7338 | 0.031* | |
| C15 | 0.0642 (2) | 0.07162 (15) | 0.68746 (13) | 0.0252 (4) | |
| H15 | 0.0157 | 0.1246 | 0.7363 | 0.030* | |
| C16 | 0.7703 (2) | 0.41443 (15) | 0.05894 (15) | 0.0309 (4) | |
| H16 | 0.8024 | 0.3407 | 0.0344 | 0.037* | |
| C17 | 0.6293 (2) | 0.42005 (15) | 0.10637 (14) | 0.0292 (4) | |
| H17 | 0.5645 | 0.3511 | 0.1142 | 0.035* | |
| C18 | 0.72593 (18) | 0.05927 (13) | 0.06193 (12) | 0.0182 (3) | |
| C19 | 0.53568 (19) | −0.00576 (14) | 0.19602 (12) | 0.0209 (3) | |
| H19 | 0.4667 | −0.0256 | 0.2456 | 0.025* | |
| C20 | 0.5757 (2) | 0.11054 (14) | 0.19485 (12) | 0.0221 (3) | |
| H20 | 0.5334 | 0.1675 | 0.2439 | 0.026* | |
| C21 | 0.68690 (19) | −0.05677 (14) | 0.06260 (12) | 0.0211 (3) | |
| H21 | 0.7296 | −0.1138 | 0.0139 | 0.025* | |
| C22 | 0.82945 (19) | 0.09294 (14) | −0.01369 (13) | 0.0219 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Fe1 | 0.01377 (15) | 0.01447 (15) | 0.01549 (15) | 0.00130 (11) | 0.00307 (11) | 0.00305 (12) |
| S1 | 0.0231 (2) | 0.0345 (2) | 0.0178 (2) | 0.00335 (17) | 0.00294 (16) | −0.00485 (17) |
| S2 | 0.0290 (2) | 0.0289 (2) | 0.0305 (2) | 0.00821 (18) | 0.00977 (18) | 0.01836 (18) |
| N1 | 0.0150 (6) | 0.0167 (6) | 0.0168 (6) | 0.0016 (5) | 0.0026 (5) | 0.0042 (5) |
| N2 | 0.0187 (6) | 0.0182 (7) | 0.0213 (7) | 0.0017 (5) | 0.0032 (5) | 0.0018 (5) |
| N3 | 0.0177 (6) | 0.0178 (7) | 0.0224 (7) | 0.0020 (5) | 0.0045 (5) | 0.0050 (5) |
| N4 | 0.0315 (8) | 0.0282 (8) | 0.0275 (8) | 0.0064 (6) | 0.0127 (6) | 0.0086 (6) |
| N5 | 0.0284 (8) | 0.0291 (8) | 0.0268 (7) | 0.0039 (6) | 0.0103 (6) | 0.0062 (6) |
| N6 | 0.0268 (8) | 0.0264 (8) | 0.0279 (8) | −0.0001 (6) | 0.0022 (6) | 0.0108 (6) |
| N7 | 0.0279 (8) | 0.0364 (9) | 0.0255 (7) | −0.0008 (6) | 0.0082 (6) | 0.0076 (6) |
| N8 | 0.0275 (7) | 0.0216 (7) | 0.0223 (7) | 0.0020 (6) | 0.0076 (6) | 0.0054 (6) |
| N9 | 0.0244 (7) | 0.0212 (7) | 0.0212 (7) | 0.0048 (5) | 0.0052 (6) | 0.0054 (6) |
| N10 | 0.0244 (7) | 0.0238 (7) | 0.0204 (7) | 0.0061 (6) | 0.0061 (5) | 0.0059 (6) |
| C1 | 0.0160 (7) | 0.0161 (7) | 0.0215 (8) | 0.0043 (6) | 0.0085 (6) | 0.0041 (6) |
| C2 | 0.0181 (8) | 0.0165 (7) | 0.0228 (8) | 0.0018 (6) | 0.0025 (6) | 0.0048 (6) |
| C3 | 0.0174 (8) | 0.0209 (8) | 0.0259 (8) | −0.0004 (6) | 0.0037 (6) | 0.0083 (7) |
| C4 | 0.0145 (7) | 0.0285 (9) | 0.0223 (8) | 0.0026 (6) | 0.0059 (6) | 0.0076 (7) |
| C5 | 0.0210 (8) | 0.0217 (8) | 0.0257 (8) | 0.0070 (6) | 0.0064 (7) | 0.0045 (7) |
| C6 | 0.0206 (8) | 0.0166 (8) | 0.0219 (8) | 0.0018 (6) | 0.0045 (6) | 0.0054 (6) |
| C7 | 0.0383 (10) | 0.0239 (9) | 0.0268 (9) | 0.0102 (8) | 0.0048 (8) | 0.0038 (7) |
| C8 | 0.0264 (9) | 0.0330 (10) | 0.0236 (8) | 0.0089 (7) | 0.0064 (7) | 0.0073 (7) |
| C9 | 0.0144 (7) | 0.0164 (7) | 0.0219 (8) | 0.0011 (6) | 0.0081 (6) | 0.0023 (6) |
| C10 | 0.0363 (10) | 0.0226 (9) | 0.0265 (9) | 0.0023 (7) | 0.0065 (7) | 0.0081 (7) |
| C11 | 0.0201 (8) | 0.0194 (8) | 0.0204 (8) | 0.0036 (6) | 0.0017 (6) | 0.0029 (6) |
| C12 | 0.0158 (7) | 0.0227 (8) | 0.0158 (7) | 0.0024 (6) | 0.0007 (6) | 0.0054 (6) |
| C13 | 0.0231 (8) | 0.0232 (8) | 0.0238 (8) | 0.0032 (7) | 0.0029 (7) | 0.0042 (7) |
| C14 | 0.0191 (8) | 0.0380 (10) | 0.0202 (8) | 0.0004 (7) | 0.0017 (6) | 0.0124 (7) |
| C15 | 0.0238 (8) | 0.0337 (10) | 0.0202 (8) | 0.0080 (7) | 0.0072 (7) | 0.0073 (7) |
| C16 | 0.0364 (10) | 0.0219 (9) | 0.0371 (10) | 0.0087 (7) | 0.0108 (8) | 0.0081 (8) |
| C17 | 0.0307 (9) | 0.0255 (9) | 0.0337 (10) | 0.0032 (7) | 0.0079 (8) | 0.0119 (8) |
| C18 | 0.0153 (7) | 0.0238 (8) | 0.0149 (7) | 0.0019 (6) | 0.0016 (6) | 0.0049 (6) |
| C19 | 0.0179 (8) | 0.0277 (9) | 0.0168 (7) | 0.0021 (6) | 0.0038 (6) | 0.0055 (6) |
| C20 | 0.0228 (8) | 0.0263 (9) | 0.0178 (8) | 0.0085 (7) | 0.0056 (6) | 0.0028 (6) |
| C21 | 0.0231 (8) | 0.0211 (8) | 0.0187 (8) | 0.0045 (6) | 0.0056 (6) | 0.0021 (6) |
| C22 | 0.0207 (8) | 0.0235 (8) | 0.0197 (8) | 0.0014 (6) | 0.0025 (6) | 0.0039 (6) |
Geometric parameters (Å, º)
| Fe1—N3 | 2.0370 (13) | C6—H6 | 0.9500 |
| Fe1—N2 | 2.0424 (13) | C7—C10 | 1.375 (2) |
| Fe1—N1 | 2.1320 (12) | C7—C8 | 1.385 (2) |
| S1—C1 | 1.6229 (16) | C7—H7 | 0.9500 |
| S2—C9 | 1.6220 (16) | C8—C17 | 1.374 (2) |
| N1—C6 | 1.3412 (19) | C8—H8 | 0.9500 |
| N1—C2 | 1.3430 (19) | C10—H10 | 0.9500 |
| N2—C1 | 1.164 (2) | C11—C12 | 1.452 (2) |
| N3—C9 | 1.166 (2) | C12—N10 | 1.340 (2) |
| N4—C10 | 1.336 (2) | C12—C13 | 1.380 (2) |
| N4—C16 | 1.337 (2) | C13—H13 | 0.9500 |
| N4—H4N | 0.8800 | C14—C15 | 1.386 (2) |
| N5—C11 | 1.142 (2) | C14—H14 | 0.9500 |
| N6—C14 | 1.332 (2) | C15—N10 | 1.333 (2) |
| N6—C13 | 1.337 (2) | C15—H15 | 0.9500 |
| N7—C22 | 1.141 (2) | C16—C17 | 1.375 (2) |
| N8—C19 | 1.330 (2) | C16—H16 | 0.9500 |
| N8—C21 | 1.3355 (19) | C17—H17 | 0.9500 |
| C2—C3 | 1.381 (2) | C18—N9 | 1.3411 (19) |
| C2—H2 | 0.9500 | C19—C20 | 1.387 (2) |
| C3—C4 | 1.380 (2) | C19—H19 | 0.9500 |
| C3—H3 | 0.9500 | C20—N9 | 1.331 (2) |
| C4—C5 | 1.385 (2) | C20—H20 | 0.9500 |
| C4—H4 | 0.9500 | C21—C18 | 1.381 (2) |
| C5—C6 | 1.376 (2) | C21—H21 | 0.9500 |
| C5—H5 | 0.9500 | C22—C18 | 1.453 (2) |
| N3i—Fe1—N3 | 179.999 (1) | N1—C6—H6 | 118.6 |
| N3i—Fe1—N2i | 88.77 (5) | C5—C6—H6 | 118.6 |
| N3—Fe1—N2i | 91.23 (5) | C10—C7—C8 | 118.63 (16) |
| N3i—Fe1—N2 | 91.23 (5) | C10—C7—H7 | 120.7 |
| N3—Fe1—N2 | 88.77 (5) | C8—C7—H7 | 120.7 |
| N2i—Fe1—N2 | 180.0 | C17—C8—C7 | 119.32 (16) |
| N3i—Fe1—N1 | 90.30 (5) | C17—C8—H8 | 120.3 |
| N3—Fe1—N1 | 89.70 (5) | C7—C8—H8 | 120.3 |
| N2i—Fe1—N1 | 90.58 (5) | N3—C9—S2 | 179.33 (15) |
| N2—Fe1—N1 | 89.42 (5) | N4—C10—C7 | 121.91 (16) |
| N3i—Fe1—N1i | 89.70 (5) | N4—C10—H10 | 119.0 |
| N3—Fe1—N1i | 90.30 (5) | C7—C10—H10 | 119.0 |
| N2i—Fe1—N1i | 89.42 (5) | N5—C11—C12 | 177.71 (17) |
| N2—Fe1—N1i | 90.58 (5) | N10—C12—C13 | 123.29 (14) |
| N1—Fe1—N1i | 180.0 | N10—C12—C11 | 116.65 (14) |
| C6—N1—C2 | 118.34 (13) | C13—C12—C11 | 120.04 (14) |
| C6—N1—Fe1 | 120.20 (10) | N6—C13—C12 | 121.47 (15) |
| C2—N1—Fe1 | 121.37 (10) | N6—C13—H13 | 119.3 |
| C1—N2—Fe1 | 163.20 (12) | C12—C13—H13 | 119.3 |
| C9—N3—Fe1 | 167.56 (12) | N6—C14—C15 | 122.38 (15) |
| C10—N4—C16 | 119.32 (15) | N6—C14—H14 | 118.8 |
| C10—N4—H4N | 120.3 | C15—C14—H14 | 118.8 |
| C16—N4—H4N | 120.3 | N10—C15—C14 | 122.39 (15) |
| C14—N6—C13 | 115.74 (14) | N10—C15—H15 | 118.8 |
| C19—N8—C21 | 115.86 (14) | C14—C15—H15 | 118.8 |
| N2—C1—S1 | 179.41 (14) | C15—N10—C12 | 114.73 (14) |
| N7—C22—C18 | 178.82 (18) | N4—C16—C17 | 121.84 (16) |
| N8—C21—C18 | 121.37 (14) | N4—C16—H16 | 119.1 |
| N8—C21—H21 | 119.3 | C17—C16—H16 | 119.1 |
| C18—C21—H21 | 119.3 | C8—C17—C16 | 118.98 (16) |
| N1—C2—C3 | 122.04 (14) | C8—C17—H17 | 120.5 |
| N1—C2—H2 | 119.0 | C16—C17—H17 | 120.5 |
| C3—C2—H2 | 119.0 | N9—C18—C21 | 123.30 (14) |
| C4—C3—C2 | 119.23 (14) | N9—C18—C22 | 116.67 (14) |
| C4—C3—H3 | 120.4 | C21—C18—C22 | 120.03 (14) |
| C2—C3—H3 | 120.4 | N8—C19—C20 | 122.36 (14) |
| C3—C4—C5 | 118.89 (14) | N8—C19—H19 | 118.8 |
| C3—C4—H4 | 120.6 | C20—C19—H19 | 118.8 |
| C5—C4—H4 | 120.6 | N9—C20—C19 | 122.46 (14) |
| C6—C5—C4 | 118.72 (15) | N9—C20—H20 | 118.8 |
| C6—C5—H5 | 120.6 | C19—C20—H20 | 118.8 |
| C4—C5—H5 | 120.6 | C20—N9—C18 | 114.64 (13) |
| N1—C6—C5 | 122.77 (14) |
Symmetry code: (i) −x+1, −y+1, −z+1.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N4—H4N···N4ii | 0.88 | 1.80 | 2.677 (3) | 179 |
Symmetry code: (ii) −x+2, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HY2622).
References
- Brandenburg, K. (1997). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Fritsky, I. O., Kozłowski, H., Sadler, P. J., Yefetova, O. P., Świątek-Kozłowska, J., Kalibabchuk, V. A. & Głowiak, T. (1998). J. Chem. Soc. Dalton Trans. pp. 3269–3274.
- Fritsky, I. O., Świątek-Kozłowska, J., Dobosz, A., Sliva, T. Y. & Dudarenko, N. M. (2004). Inorg. Chim. Acta, 357, 3746–3752.
- Gamez, P., Costa, J. S., Quesada, M. & Aromí, G. (2009). Dalton Trans. pp. 7845–7853. [DOI] [PubMed]
- Kanderal, O. M., Kozłowski, H., Dobosz, A., Świątek-Kozłowska, J., Meyer, F. & Fritsky, I. O. (2005). Dalton Trans. pp. 1428–1437. [DOI] [PubMed]
- Moroz, Y. S., Szyrweil, L., Demeshko, S., Kozłowski, H., Meyer, F. & Fritsky, I. O. (2010). Inorg. Chem. 49, 4750–4752. [DOI] [PubMed]
- Niel, V., Martinez-Agudo, J. M., Muñoz, M. C., Gaspar, A. B. & Real, J. A. (2001). Inorg. Chem. 40, 3838–3839. [DOI] [PubMed]
- Penkova, L., Demeshko, S., Pavlenko, V. A., Dechert, S., Meyer, F. & Fritsky, I. O. (2010). Inorg. Chim. Acta, 363, 3036–3040.
- Petrusenko, S. R., Kokozay, V. N. & Fritsky, I. O. (1997). Polyhedron, 16, 267–274.
- Real, J. A., Munno, G., Muñoz, M. C. & Julve, M. (1991). Inorg. Chem. 30, 2701–2704.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813010362/hy2622sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813010362/hy2622Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813010362/hy2622Isup3.cdx
Additional supplementary materials: crystallographic information; 3D view; checkCIF report





