Abstract
Time to detection (TTD) in automated blood culture systems is delayed for sensitive microorganisms in the presence of antimicrobial substances and has been associated with worse outcomes for sepsis patients on inadequate empirical therapy. While resin addition removes antimicrobial substances to various degrees from blood culture media, media formulations and the blend of resins may influence performance. The BacT/Alert 3D system (bioMérieux) was investigated using the new resin-containing medium types FA Plus (aerobic) and FN Plus (anaerobic). TTD was compared between control and test bottles containing relevant bacteria or Candida albicans, with and without defined concentrations of antimicrobials. Failure of neutralization was defined as a negative blood culture on day 3. In general, growth delay was nonlinear, concentration dependent, bottle type specific, and reciprocally associated with MICs. Substance-specific serum drug concentrations corresponding to a predefined, clinically relevant 3-h delay of TTD were calculated. Where appropriate, a time interval allowing for drug elimination below this critical level was obtained by pharmacokinetic modeling. Clarithromycin, clindamycin, gentamicin, linezolid, tigecycline, vancomycin, and fluconazole were neutralized. For ciprofloxacin and piperacillin-tazobactam, which were only incompletely neutralized in combination with the most sensitive test strains, a maximum waiting time for blood draw of 1 h was determined based on pharmacokinetics. One or more test strains did not grow in bottles containing either amoxicillin-clavulanate, cefepime, cefotaxime, meropenem, or metronidazole, and we thus recommend particular caution in timing of blood draws if patients have been pretreated with these agents.
INTRODUCTION
Pathogen detection in blood culture (BC) is significantly lower when cultures are taken during antimicrobial therapy (1). Nevertheless, blood samples are obtained (1, 2) on unavoidable occasions, under conditions such as emergence of signs of infection in neutropenic patients (3) or in perioperative patients on prophylaxis (4).
In contrast to clinical studies, simulated BC studies comparing culture bottles differing only in the presence or absence of antimicrobial substances (5–9) have been able to demonstrate that the increased recovery rate from BC systems designed for neutralization of antibiotic agents is truly through the latter effect (10). Nevertheless, recovery rates are unevenly distributed with respect to the class of antimicrobial substances used, as well as the type of test microorganisms, and differ also between the systems under investigation (5–7, 11). For instance, BacT/Alert FAN BC bottles (bioMérieux, Marcy l'Etoile, France) contain Ecosorb, a proprietary substance composed, in part, of Fuller's earth and activated charcoal particles, while products from others contain resin-like materials (10) or rely only on an optimal 1:9 blood-broth dilution (7). Resin-containing media have been found to be most effective in neutralization of antimicrobials (5–7). Due to this fact, and since use of blood culture aliquots from charcoal-based media is not readily compatible with rapid contemporary identification methods, such as molecular assays (12), peptide nucleic acid probe-fluorescent in situ hybridization (AdvanDx, Inc., Woburn, MA) (13), or matrix-assisted laser desorption ionization–time of flight mass spectrometry directly from positive BC bottles (14), and also because it may interfere with Gram stain reading, the charcoal-containing bioMérieux system had to be changed.
Shortening of time to detection (TTD) has a clinical impact, for instance, in septic shock, where each hour's delay in achieving administration of effective antibiotics is associated with a measurable increase in mortality (15) or earlier deescalation from empirical broad-spectrum therapy (16). Furthermore, random delay of TTD by antimicrobial activity in BC is undesirable, since TTD may be used to provide clinically relevant information; for example, TTD-dependent approaches have been proposed for estimation of prognosis (17–19), duration of empirical therapy (20), and distinction of bacteremia from contamination (21). Finally, a gradual difference in TTD can translate into a categorical one, if positive flagging of an automated BC exceeds the working hours of a microbiology laboratory (22).
In the present seeded study, we investigated antimicrobial neutralization in the new BacT/Alert FA and FN Plus resin-containing BC bottles (bioMérieux).
MATERIALS AND METHODS
BC media and instrument.
BacT/Alert FA Plus (aerobic) and FN plus (anaerobic) media differ in their compositions as well as their relative contents of two different resins. The anaerobic formulation includes a complex amino acid component, not contained in the aerobic formulation, destined for neutralization of additional substances. Multiple lots were used in combination with the automated BacT/Alert 3D BC system (bioMérieux).
Antimicrobial substances and concentrations.
Antibiotics frequently used in severely ill patients were chosen (see Table 1). Peak serum drug concentrations (Cmax) achieved after standard adult dosing (23) (see Table 2) were used to inoculate BC bottles. Because the bottle types contained different volumes of growth medium (aerobic, 30 ml; anaerobic, 40 ml), the final concentrations differed. In addition, decreasing concentrations of 1/2 and 1/4 the Cmax were tested for all microorganisms. If antimicrobial substances could not be neutralized, 1/8 and 1/16 concentrations were also tested (see Fig. 1).
Table 1.
MICs of test strains
| Druga | MIC (mg/liter) for test strain |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. albicans | E. faecium | S. epidermidis |
S. aureus |
S. pneumoniae |
P. aeruginosa | E. coli |
K. pneumoniae |
B. fragilis | ||||
| MSSA | MRSA | SP1 | SP2 | KP1 | KP2 | |||||||
| AMC | —b | — | 0.25 | 0.19 | 24 | 0.032 | 0.016 | — | 1.5 | 12 | 0.5 | — |
| FEB | — | — | — | — | — | 0.032 | 0.047 | 1 | 0.016 | 0.032 | 0.016 | — |
| CTX | — | — | — | — | — | 0.016 | 0.016 | — | 0.016 | 1 | 0.16 | — |
| CIP | — | — | 0.094 | 0.19 | 32 | 0.38 | 1 | 0.094 | 0.004 | 0.19 | 0.002 | — |
| CLR | — | — | 0.5 | 0.38 | 256 | 0.064 | 0.19 | — | — | — | — | — |
| CLI | — | — | 0.094 | 0.032 | 256 | — | — | — | — | — | — | — |
| FLC | 1 | — | — | — | — | — | — | — | — | — | — | — |
| GEN | — | — | 0.094 | 0.38 | 128 | — | — | 2 | 0.125 | 2 | 0.094 | — |
| LZD | — | 0.5 | 1.5 | 1 | 0.75 | — | — | — | — | — | — | — |
| MEM | — | — | 0.064 | 0.64 | 32 | — | — | 0.094 | 0.008 | 0.047 | 0.012 | 0.094 |
| MZ | — | — | — | — | — | — | — | — | — | — | — | 0.19 |
| TZP | — | — | 0.5 | 0.75 | 256 | — | — | 3 | — | — | — | 0.094 |
| TGC | — | 0.032 | 0.5 | 0.125 | 0.094 | 0.023 | 0.016 | — | 0.064 | 0.5 | 0.047 | 0.19 |
| VAN | — | 0.125 | 1.5 | 1.5 | 1 | — | — | — | — | — | — | — |
AMC, amoxicillin-clavulanate; FEB, cefepime; CTX, cefotaxime; CIP, ciprofloxacin; CLR, clarithromycin; CLI, clindamycin; FLC, fluconazole; GEN, gentamicin; LZD, linezolid; MEM, meropenem; MZ, metronidazole; TZP, piperacillin-tazobactam; TGC, tigecycline; VAN, vancomycin.
—, not determined.
Table 2.
Estimated serum drug concentrations that led to TTD delays of ≥3 ha
| Drugb | Peak serum drug concn (mg/liter) | Microorganism/bottle type | Serum drug concn (mg/liter) causing ≥3-h delay | 95% CI (mg/liter) |
|---|---|---|---|---|
| AMC | 11.6 | MSSA/FA | 1 | 1–2 |
| MSSA/FN | 6 | 3–42 | ||
| MRSA/FA | 21 | 12–98 | ||
| MRSA/FN | 55 | 46–70 | ||
| S. epidermidis/FA | 0.4 | 0.39–0.41 | ||
| S. epidermidis/FN | 2 | 1–5 | ||
| K. pneumoniae KP2/FA | 15 | 11–21 | ||
| K. pneumoniae KP2/FN | 15 | 22–158 | ||
| CTX | 214.1 | K. pneumoniae KP1/FA | 204 | 182–233 |
| K. pneumoniae KP1/FN | 89 | 61–167 | ||
| CIP | 4.6 | MSSA/FA | 191 | 96–380 |
| S. pneumoniae SP2/FN | 58 | 35–178 | ||
| P. aeruginosa/FA | 39 | 29–61 | ||
| P. aeruginosa/FN | 22 | 18–27 | ||
| E. coli/FA | 6 | 6–7 | ||
| E. coli/FN | 4 | 3–5 | ||
| K. pneumoniae KP2/FA | 3 | 3–3 | ||
| K. pneumoniae KP2/FN | 2 | 1–5 | ||
| FLC | 6.72 | C. albicans/FN | 249 | 151–704 |
| GEN | 7.6 | S. epidermidis/FA | 43 | 34–57 |
| S. epidermidis/FN | 27 | 21–38 | ||
| E. coli/FA | 251 | 126–500 | ||
| E. coli/FN | 146 | 73–292 | ||
| K. pneumoniae KP2/FN | 265 | 132–532 | ||
| LZD | 12.7 | MSSA/FN | 1,292 | 841–2,787 |
| MEM | 62 | MSSA/FN | 15 | 8–95 |
| MRSA/FA | 6 | 4–30 | ||
| MRSA/FN | 123 | 94–179 | ||
| S. epidermidis/FN | 6 | 4–15 | ||
| P. aeruginosa/FN | 36 | 23–83 | ||
| K. pneumoniae KP1/FN | 36 | 21–125 | ||
| K. pneumoniae KP2/FN | 38 | 26–71 | ||
| MZ | 26 | B. fragilis/FN | 2 | 1–2 |
| FEB | 163.9 | P. aeruginosa/FA | 4,141 | 2,619–9,886 |
| P. aeruginosa/FN | 28 | 26–31 | ||
| TZP | 224 | MSSA/FA | 288 | 231–384 |
| MSSA/FN | 222 | 199–251 | ||
| MRSA/FA | 634 | 478–945 | ||
| MRSA/FN | 590 | 542–647 | ||
| S. epidermidis/FA | 448 | 390–526 | ||
| S. epidermidis/FN | 428 | 383–485 | ||
| P. aeruginosa/FN | 4,692 | 3,465–7,263 | ||
| TGC | 1.45 | S. pneumoniae SP2/FA | 14 | 8–114 |
| S. pneumoniae SP2/FN | 32 | 17–226 | ||
| K. pneumoniae KP2/FA | 32 | 19–115 | ||
| K. pneumoniae KP2/FN | 32 | 20–72 | ||
| VAN | 25 | E. faecium/FA | 221 | 156–380 |
| E. faecium/FN | 120 | 88–190 | ||
| S. epidermidis/FA | 961 | 541–1,707 |
Only microorganism-substance combinations for which the TTD was significantly different from control (control data not shown) were used for calculations. Concentrations are rounded to whole numbers.
AMC, amoxicillin-clavulanate; CTX, cefotaxime; CIP, ciprofloxacin; FLC, fluconazole; GEN, gentamicin; LZD, linezolid; MEM, meropenem; MZ, metronidazole; FEB, cefepime; TZP, piperacillin-tazobactam; TGC, tigecycline; VAN, vancomycin.
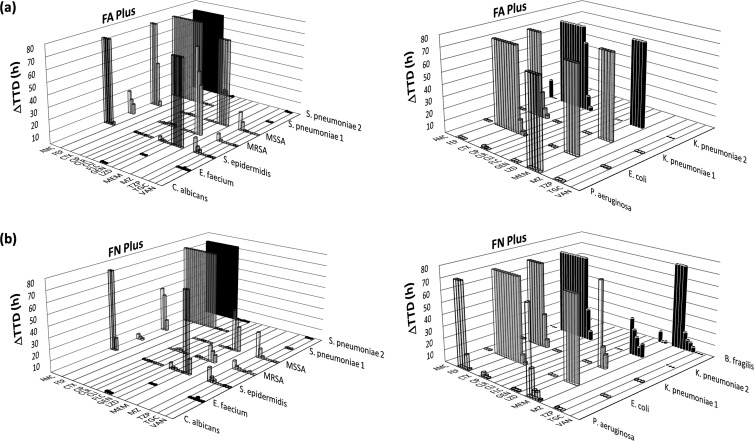
Fig 1.
Differences in the TTDs with and without antimicrobial substances: (a) BacT/Alert FA Plus (aerobic) bottle; (b) BacT/Alert FN Plus (anaerobic) bottle. Arithmetic means of triplicates were used for the graphic illustration. Each column illustrates the difference in the TTD between bottles containing antimicrobial substances and controls. Consecutive columns along the x axis show the change in TTD (ΔTTD) related to decreasing concentrations of the antimicrobial substances. The ΔTTD for bottles that remained negative due to insufficient antimicrobial neutralization after 3 days was set as 72 h.
Microorganisms.
Microbial species were chosen according to their frequency of isolation from positive BCs. The following ATCC and clinical strains were used: Staphylococcus epidermidis 12228, methicillin-sensitive Staphylococcus aureus (MSSA) 29213, methicillin-resistant S. aureus (MRSA) BAA-1026, Enterococcus faecium 27270, Streptococcus pneumoniae 10015 (referred to here as strain SP1), Streptococcus pneumoniae (bloodstream isolate, referred to as strain SP2 [see below]), Escherichia coli 10536, Klebsiella pneumoniae BAA-1144 (referred to as strain KP1), Klebsiella pneumoniae 10031 (referred to as strain KP2), Pseudomonas aeruginosa 15442, Bacteroides fragilis 25585, and Candida albicans 25585.
In addition, 3 ATCC pneumococcus strains (ATCC 10015, 49619, and 6301), as well as routinely cryopreserved BC isolates (n = 10, recovered previously in FAN BC bottles [bioMérieux]) and isolates from nasopharyngeal colonization (n = 10) from the years 2009 to 2011 were inoculated under the same conditions as for neutralization testing. This was done in order to assess strain-specific differences in TTD and recovery rates on Columbia agar with 5% sheep blood (Becton, Dickinson GmbH, Heidelberg, Germany), since pneumococci may proliferate or even survive poorly in blood culture media, which might interfere with the generalizability of results. Five categories of semiquantitative growth (0 to 4) were used for evaluation of subcultivability.
Strain- and substance-specific MICs obtained by Etest (bioMérieux) following the manufacturer's instructions are compiled in Table 1, below.
All microorganisms were passaged at least once after reconstitution or thawing on Columbia agar with 5% sheep blood or CHROMagar Candida (Becton, Dickinson). Pneumococcus strains were subcultured three times before inoculation into BC bottles.
Bottle inoculation.
For each organism-antimicrobial substance combination (see Table 1 and Fig. 1) inoculated at once (workup unit), controls without added antimicrobials were included. If additional antimicrobial substance concentrations were tested, new controls were included. All incubations (including controls) were performed in triplicate. To simulate clinical usage, bottles were inoculated with 10 ml banked blood (Austrian Red Cross; kindly provided by the Department of Blood Group Serology and Transfusion Medicine, Medical University Vienna). Blood was stored at 4°C and used within 7 days after the end of the expirationdate. To compensate for antimicrobial substances possibly present in banked blood, the same batch was used to inoculate bottles containing antimicrobial test substances and corresponding control bottles. All microorganisms were suspended to a McFarland density standard 1 in sterile NaCl and inoculated to a final concentration of 10 to 100 microorganisms per bottle (6, 7). Required predilutions were determined by colony count plating in triplicate. Antimicrobial substances were diluted in banked blood and added to the BC bottles at first. Directly thereafter, microorganisms were inoculated and bottles loaded into the BactT/Alert instrument. Assuming that a growth delay of more than 3 days would not allow for timely correction of inappropriate empirical therapy (24), BCs that were not flagged positive within 3 days were considered negative.
Processing of positive-flagged BC bottles.
Subcultures were performed on the same growth media as used prior to inoculation of BC bottles. If one bottle of a workup unit was flagged as positive but the inoculated microorganism was not recovered or contaminants appeared in subculture, testing of the whole workup unit was repeated.
Data analysis and statistics.
To calculate the relationship between the concentration of the antibiotic in the medium and TTD in the first step, the censoring at 72 h had to be considered. If one or two measurements of three replicates were censored at 72 h, the values were adjusted based on the assumption of a log-normal distribution of TTD values. The values for the censored TTD were imputed such that the geometric mean of the triplet was equal to that of a log-normal distribution, with 33% or 67% of the observation at >72 h. The log ratio of the TTD value obtained at different concentrations to that of the reference without antibiotics was the dependent variable in a linear regression on the concentration of the antibiotic. Considering varying and naturally occurring lags in translation of laboratory results into corrections for empirical therapies, a time interval of delay in the TTD of ≥3 h was deliberately defined as clinically significant. For each analysis conducted for all strains and antibiotics and for both media, the concentration resulting in a 3-h delay was estimated together with its 95% confidence interval based on the regression coefficients.
Time after dosing at which the 3-h delay concentration was no longer exceeded (the waiting time) was calculated based on the following equation: C = C0 × e−kel × t, where C is the concentration to be reached, C0 is the concentration of the antibiotic at a certain time after dosing as reported in the literature, e is the Euler constant, kel is the elimination constant, obtained from the literature, and t is the time difference between the times for C and C0. Where the critical concentration was close to Cmax (period of α-elimination), the waiting time was read from elimination curves (see Table 3).
Table 3.
Blood draw waiting time required to avoid a TTD delay of ≥3 hb
| Druga | Blood draw waiting time (h) |
Result | Reference or source | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. albicans | E. faecium | S. epidermidis | MSSA | S. pneumoniae SP2 | P. aeruginosa | E. coli | K. pneumoniae KP2 | B. fragilis | |||
| AMC | — | — | 1.7 | <1 | NN | — | 0 | 0 | — | NN | 27 |
| FEB | — | — | — | — | NN | 1.6 | NN | NN | — | NN | 28 |
| CTX | — | — | — | — | NN | — | NN | <1 | — | NN | 29 |
| CIP | — | — | 0 | 0 | 0 | 0 | 0 | <1 | — | <1 | 30 |
| CLR | — | — | 0 | 0 | 0 | — | — | — | — | 0 | NA |
| CLI | — | — | 0 | 0 | — | — | — | — | — | 0 | NA |
| FLC | 0 | — | — | — | — | — | — | — | — | 0 | NA |
| GEN | — | — | 0 | 0 | — | 0 | 0 | 0 | — | 0 | NA |
| LZD | — | 0 | 0 | 0 | — | — | — | — | — | 0 | NA |
| MEM | — | — | 5 | 2.1 | — | <1 | NN | <1 | NN | NN | 31 |
| MZ | — | — | — | — | — | — | — | — | NN | NN | NA |
| TZP | — | — | 0 | <1 | — | 0 | — | — | 0 | <1 | 32 |
| TGC | — | 0 | 0 | 0 | 0 | — | 0 | 0 | 0 | 0 | NA |
| VAN | — | 0 | 0 | 0 | — | — | — | — | — | 0 | NA |
AMC, amoxicillin-clavulanate; FEB, cefepime; CTX, cefotaxime; CIP, ciprofloxacin; CLR, clarithromycin; CLI, clindamycin; FLC, fluconazole; GEN, gentamicin; LZD, linezolid; MEM, meropenem; MZ, metronidazole; TZP, piperacillin-tazobactam; TGC, tigecycline; VAN, vancomycin.
Calculation of waiting times was based on the mean peak serum drug concentration (Cmax) and the more effectively neutralizing bottle within a blood culture pair. The cumulative substance-specific waiting time is shown: —, not tested; NN, not neutralized, i.e., maximum waiting time was reached; 0, complete neutralization, i.e., critical concentration was higher than the Cmax and there was no latency in the indicated setting; NA, not applicable. Values other than 0 indicate the waiting time that allowed for a <3-h delay in the TTD.
Mann-Whitney tests were applied to analyze differences in TTD and subculture results between invasive and noninvasive types of S. pneumoniae and between aerobic and anaerobic media. For all statistical tests, a significance level of 5% was chosen.
RESULTS
Antimicrobial neutralization.
As expected, growth delay depended not only on neutralization capacity of the media formulations but also on MICs of the test strains (Table 1 and Fig. 1). If antimicrobial substances were neutralized to some degree, a concentration-dependent nonlinear course of growth delay was observed (Fig. 1). In these cases and if the TTD of test bottles differed significantly from controls (data not shown), the strain- and substance-specific serum drug concentrations were calculated at which a delay in TTD of 3 h (considered clinically significant) would occur (Table 2). If those concentrations ranged below or near the Cmax, a time interval between drug delivery and blood draw was calculated or determined from the elimination kinetics graphs, resulting in the elimination of the drug below this critical level (Table 3).
Figure 1 shows the differences in TTD between antimicrobial substance-containing BC bottles versus controls. Among the group of β-lactam antibiotics, there were differences in neutralization that were dependent on the chemical nature of the substance under investigation. While piperacillin-tazobactam could be neutralized well, for the other substances tested, a significant growth delay of up to 3 days (or more) occurred in relation to strain-specific MICs. In Gram-positive test strains exposed to the combination amoxicillin-clavulanate, growth was detected earlier in the anaerobic bottle. Similarly, meropenem was more effectively neutralized in the anaerobic than in the aerobic medium, while the opposite was observed for cefotaxime and cefepime. Neither of these agents was sufficiently inactivated in the low-MIC test strains.
Pneumococci did not grow in any β-lactam-containing BC bottle. However, the majority of the other antimicrobial substances (clarithromycin, clindamycin, gentamicin, linezolid, tigecycline, vancomycin, and fluconazole) were neutralized so effectively that no, or nearly no, growth delay resulted. Ciprofloxacin was generally inactivated well, particularly in the aerobic medium, but showed a MIC-dependent growth-inhibitory effect in the most sensitive test strains. Metronidazole neutralization was hardly possible, as only 1/16 of the Cmax could be tolerated by the B. fragilis strain.
Streptococcus pneumoniae recovery.
Since growth characteristics of pneumococcus might interfere with generalization of neutralization results, several invasive isolates as well as colonizing isolates were investigated. All inoculated bottles were flagged positive, while 7 of 24 strains (29%) did not grow in subculture of the aerobic bottle. In the anaerobic bottles, the TTD was highly significant longer (P = 0.009), but for 21 of 23 (91%) strains, growth in subculture was at least in semiquantitative category 3, in comparison to only 10 of 23 (44%) strains previously inoculated in the aerobic bottles (P = 0.002). No significant differences in the TTD and semiquantitative growth in subculture were observed between invasive and colonizing strains, neither by evaluation of aerobic and anaerobic bottle types together (P = 0.152 and 0.135, respectively) nor by separate analysis of aerobic BC (P = 0.136 and 0.238, respectively) and anaerobic BC (P = 0.619 and 0.204, respectively).
DISCUSSION
Our discussion is limited to resin-containing media, because the superior neutralizing capacity of resin-based media over the bioMérieux charcoal medium has been well documented (5–7) and the new medium type might replace the predecessor in the near future. We were able to compare results of six previously published seeded studies on resin-containing BC bottles (Becton, Dickinson) with the results of the present study on new the BacT/Alert resin-containing BC bottles (5–7, 9, 25, 26).
Differences in the types and compositions of resins and culture media may account for the observed differences. MICs of test strains were not provided in some of the above-mentioned studies. As we chose a 3-day incubation time while other studies used different protocols (e.g., 5-day incubation), very weak neutralization effects may have remained undetected and thus limit comparability. Nevertheless, we believe that differences in the TTD essentially beyond those detected in the present study are unlikely to significantly impact clinical outcomes.
Spaargaren et al. tested several gentamicin concentrations on Escherichia coli in Bactec Plus Aerobic/F BC bottles (Becton, Dickinson) (9). At 10 mg/liter, a delay in the TTD of 3.8 h was observed, corresponding closely to a calculated concentration in BacT/Alert FA bottles of 10.75 mg/liter (calculated serum concentration, 43 mg/liter [Table 2]), translating to a delay in the TTD of 3 h in the present study.
Nzeako and Al-Qasabi (25) found neutralization of vancomycin and gentamicin when the tested Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa isolates in Bactec Plus medium up to a concentration of 100 mg/liter. This was in accordance with our results, although we did not test beyond the Cmax of 25 mg/liter (23).
Viganò et al. reported concentration-dependent neutralization in Bactec Plus BC bottles containing cefotaxime, even for low-MIC Escherichia coli and pneumococci strains. In strains with a comparable MIC and at comparable drug concentrations, cefotaxime could not be inactivated in the present study. While gentamicin and ciprofloxacin inactivation was similarly effective, vancomycin delayed the TTD on the order of several hours, in contrast to complete neutralization in BacT/Alert Plus bottles. This difference could be clinically important in the case of continuous vancomycin infusion.
Great differences exist when we compare our results to those of Flayhart et al. (6) in the testing of cefepime with Enterobacteriaceae and Pseudomonas aeruginosa. At all tested concentrations (ranging from 10 to 164 mg/liter), complete recovery was achieved (the TTD was not provided). At comparable concentrations and even below (lowest bottle concentration, 2.56 mg/liter), no growth was detected in the present study for enterobacteria, while Pseudomonas aeruginosa was recovered without delay from the aerobic bottle type although only at 5.12 mg/liter and below from the anaerobic bottles. Of note, the MIC of the Pseudomonas aeruginosa isolate tested in the present study was 30- to 60-fold lower than those of the Enterobacteriaceae tested. Flayhart et al. did not provide information on the MICs of their test strains. Neutralization of gentamicin, vancomycin, and piperacillin-tazobactam was consistent.
Miller et al. (7) showed more effective neutralization of ampicillin in the Bactec Plus Aerobic/F bottle than in the anaerobic counterpart, while in the present study the opposite was observed with amoxicillin-clavulanate. Moreover, the ampicillin effect on Streptococcus pneumoniae could be neutralized completely at comparable lowest test concentrations, while no growth was detected in our setting. Pseudomonas aeruginosa was only incompletely recovered from comparable cefepime- or piperacillin-tazobactam-containing test bottles, in contrast to complete neutralization even at the Cmax in the present study. For the combination of vancomycin and MSSA, a similar observation was made.
Complete neutralization of fluconazole in Bactec Plus Aerobic/F bottles was demonstrated in a recent seeded study involving Candida spp., which was similar to our findings using the new BacT/Alert Plus bottles (26).
In summary, gentamicin, ciprofloxacin, and piperacillin-tazobactam were comparably well neutralized in the Becton, Dickinson and bioMérieux systems. Cefepime and possibly vancomycin may be more effectively neutralized in the bioMérieux system. However, even results for bottles from the same manufacturer varied between studies, which has been previously noticed (7). Most likely, these discrepancies would be resolved if the MICs of the test strains were always available.
The present study design proved appropriate for pneumococci, since growth of all strains was detected in aerobic and anaerobic new BacT/Alert BC media and there was no significant difference in survival between strains. The fact that the TTD was longer in the anaerobic medium type while growth in subculture was increased is remarkable. Media composition in the aerobic medium possibly does not support sustained survival, while initial proliferation seems sufficient for growth detection by the BacT/Alert 3D instrument. Microbial use of different metabolic pathways with different capacities for acid generation from carbohydrates may provide an explanation for the staggered growth detection. Alternatively, an initial active metabolism in the unfavorable aerobic bottle type may contribute to an earlier disintegration of cellular structures under conditions of suboptimal growth requirements.
Due to a study design that included a combination of microorganisms frequently isolated in bacteremia or sepsis with commonly used antimicrobial agents, the neutralization data obtained in our study may characterize the performance of the novel BacT/Alert BC bottles if they are used in patients already receiving antimicrobial agents. As the antimicrobial substance in use is normally known but the potential bloodstream pathogen and exact drug serum concentrations are not, the time intervals and overall results given in Table 3 can only give a rough estimate when a compromise must be achieved between rapid diagnosis and microbial cultivability. Nevertheless, our observations of situations in which neutralization of the antimicrobial agent was impossible or, in contrast, when total neutralization occurred, should be generalizable to the clinical setting. However, it should be remembered that the complete neutralization observed in the present setting might be insufficient in the case of exceptionally sensitive microorganisms, as residual activity of antimicrobial substances after incubation in resin-containing bottles has been demonstrated with biochemical methods (9).
ACKNOWLEDGMENTS
Blood culture bottles, strains, and antimicrobial drugs, as well as the BacT/Alert 3D incubator for the duration of the study, were provided by bioMérieux.
We thank Stefan Winkler, Clinical Division of Infection and Tropical Medicine, Department of Medicine I, Medical University of Vienna, for valuable discussions.
Footnotes
Published ahead of print 13 March 2013
REFERENCES
- 1. Darby JM, Linden P, Pasculle W, Saul M. 1997. Utilization and diagnostic yield of blood cultures in a surgical intensive care unit. Crit. Care Med. 25:989–994 [DOI] [PubMed] [Google Scholar]
- 2. Weinstein MP. 1996. Current blood culture methods and systems: clinical concepts, technology, and interpretation of results. Clin. Infect. Dis. 23:40–46 [DOI] [PubMed] [Google Scholar]
- 3. Peters RPH, van Agtmael MA, Danner SA, Savelkoul PHM, Vandenbroucke-Grauls CMJE. 2004. New developments in the diagnosis of bloodstream infections. Lancet Infect. Dis. 4:751–760 [DOI] [PubMed] [Google Scholar]
- 4. Appelbaum PC, Beckwith DG, Dipersio JR, Dyke JW, Salventi JF, Stone LL. 1983. Enhanced detection of bacteremia with a new Bactec resin blood culture medium. J. Clin. Microbiol. 17:48–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vigano EF, Vasconi E, Agrappi C, Clerici P, Melloni P. 2004. Use of simulated blood cultures for antibiotic effect on time to detection of the two blood culture systems BacT/Alert and Bactec 9240. N. Microbiol. 27:235–248 [PubMed] [Google Scholar]
- 6. Flayhart D, Borek AP, Wakefield T, Dick J, Carroll KC. 2007. Comparison of Bactec Plus blood culture media to BacT/Alert FA blood culture media for detection of bacterial pathogens in samples containing therapeutic levels of antibiotics. J. Clin. Microbiol. 45:816–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller NS, Rogan D, Orr BL, Whitney D. 2011. Comparison of BD Bactec Plus blood culture media to VersaTREK Redox blood culture media for detection of bacterial pathogens in simulated adult blood cultures containing therapeutic concentrations of antibiotics. J. Clin. Microbiol. 49:1624–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chern IF, Steer JA, Wilson APR. 1999. Effect of teicoplanin on isolation of Staphylococcus aureus from blood culture media. J. Antimicrob. Chemother. 43:589–591 [DOI] [PubMed] [Google Scholar]
- 9. Spaargaren J, van Boven CPA, Voorn GP. 1998. Effectiveness of resins in neutralizing antibiotic activities in Bactec Plus Aerobic/F culture medium. J. Clin. Microbiol. 36:3731–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reimer LG, Wilson ML, Weinstein MP. 1997. Update on detection of bacteremia and fungemia. Clin. Microbiol. Rev. 10:444–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Passerini R, Riggio D, Radice D, Bava L, Cassatella C, Salvatici M, Zorzino L, Sandri M. 2009. Interference of antibiotic therapy on blood cultures time-to-positivity: analysis of a 5-year experience in an oncological hospital. Eur. J. Clin. Microbiol. Infect. Dis. 28:95–98 [DOI] [PubMed] [Google Scholar]
- 12. Hogg GM, McKenna JP, Ong G. 2008. Rapid detection of methicillin-susceptible and methicillin-resistant Staphylococcus aureus directly from positive BacT/Alert blood culture bottles using real-time polymerase chain reaction: evaluation and comparison of 4 DNA extraction methods. Diagn. Microbiol. Infect. Dis. 61:446–452 [DOI] [PubMed] [Google Scholar]
- 13. Oliveira K, Procop GW, Wilson D, Coull J, Stender H. 2002. Rapid identification of Staphylococcus aureus directly from blood cultures by fluorescence in situ hybridization with peptide nucleic acid probes. J. Clin. Microbiol. 40:247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmidt V, Jarosch A, März P, Sander C, Vacata V, Kalka-Moll W. 2012. Rapid identification of bacteria in positive blood culture by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Eur. J. Clin. Microbiol. Infect. Dis. 31:311–317 [DOI] [PubMed] [Google Scholar]
- 15. Kumar A, Haery C, Paladugu B, Symeoneides S, Taiberg L, Osman J, Trenholme G, Opal SM, Goldfarb R, Parrillo JE. 2006. The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J. Infect. Dis. 193:251–258 [DOI] [PubMed] [Google Scholar]
- 16. Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. 2008. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 36:296–327 [DOI] [PubMed] [Google Scholar]
- 17. Guerti K, Devos H, Ieven MM, Mahieu LM. 2011. Time to positivity of neonatal blood cultures: fast and furious? J. Med. Microbiol. 60:446–453 [DOI] [PubMed] [Google Scholar]
- 18. Kim J, Gregson DB, Ross T, Laupland KB. 2010. Time to blood culture positivity in Staphylococcus aureus bacteremia: association with 30-day mortality. J. Infect. 61:197–204 [DOI] [PubMed] [Google Scholar]
- 19. Martinez JA, Soto S, Fabrega A, Almela M, Mensa J, Soriano A, Marco F, Jimenez de Anta MT, Vila J. 2006. Relationship of phylogenetic background, biofilm production, and time to detection of growth in blood culture vials with clinical variables and prognosis associated with Escherichia coli bacteremia. J. Clin. Microbiol. 44:1468–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaiser JR, Cassat JE, Lewno MJ. 2002. Should antibiotics be discontinued at 48 hours for negative late-onset sepsis evaluations in the neonatal intensive care unit? J. Perinatol. 22:445–447 [DOI] [PubMed] [Google Scholar]
- 21. Kassis C, Rangaraj G, Jiang Y, Hachem RY, Raad I. 2009. Differentiating culture samples representing coagulase-negative staphylococcal bacteremia from those representing contamination by use of time-to-positivity and quantitative blood culture methods J. Clin. Microbiol. 47:3255–3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savinelli T, Parenteau S, Mermel LA. 2004. What happens when automated blood culture instrument detect growth but there are no technologists in the microbiology laboratory? Diagn. Microbiol. Infect. Dis. 48:173–174 [DOI] [PubMed] [Google Scholar]
- 23. Amsden GW. 2010. Tables of antimicrobial agent pharmacology, p 705–761 In Mandell GL, Bennett JE, Dolin R. (ed), Principles and practice of infectious diseases, 7th ed, vol 1 Churchill Livingstone Elsevier, Philadelphia, PA [Google Scholar]
- 24. Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G, Reller LB. 1997. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin. Infect. Dis. 24:584–602 [DOI] [PubMed] [Google Scholar]
- 25. Nzeako BC, Al-Qasabi SS. 2004. Evaluation of the neutralising capacity of Bactec medium for some antibiotics. Br. J. Biomed. Sci. 61:171–174 [DOI] [PubMed] [Google Scholar]
- 26. Jekarl DW, Lee SY, Lee S, Park YJ, Lee J, Baek SM, An YJ, Ock SM, Lee MK. 2012. Comparison of the Bactec Fx Plus, Mycosis IC/F, Mycosis/F Lytic blood culture media and the BacT/Alert 3D FA media for detection of Candida species in seeded blood culture specimens containing therapeutic peak levels of fluconazole. J. Clin. Lab. Anal. 26:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hampel B, Lode H, Bruckner G, Koeppe P. 1988. Comparative pharmacokinetics of sulbactam/ampicillin and clavulanic acid/amoxycillin in human volunteers. Drugs 35(Suppl 7):29–33 [DOI] [PubMed] [Google Scholar]
- 28. Dan M, Asherov J, Poch F. 1999. Comparison of ex-vivo serum bactericidal activity of cefepime, ceftazidime and cloxacillin against Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 33:39–42 [DOI] [PubMed] [Google Scholar]
- 29. Sandoz 2007. Summary of product characteristics: cefotaxime. Electronic Medicines Compendium, Wrexham, United Kingdom: http://www.medicines.org.uk/emc/medicine/12154/SPC/ [Google Scholar]
- 30. Brunner M, Stabeta H, Moller JG, Schrolnberger C, Erovic B, Hollenstein U, Zeitlinger M, Eichler HG, Muller M. 2002. Target site concentrations of ciprofloxacin after single intravenous and oral doses. Antimicrob. Agents Chemother. 46:3724–3730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tomaselli F, Maier A, Matzi V, Smolle-Juttner FM, Dittrich P. 2004. Penetration of meropenem into pneumonic human lung tissue as measured by in vivo microdialysis. Antimicrob. Agents Chemother. 48:2228–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kinzig M, Sorgel F, Brismar B, Nord CE. 1992. Pharmacokinetics and tissue penetration of tazobactam and piperacillin in patients undergoing colorectal surgery. Antimicrob. Agents Chemother. 36:1997–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]