Abstract
We applied real-time genome sequencing to a Staphylococcus epidermidis strain that caused native-aortic-valve endocarditis in a 26-year-old patient. The 2.5-Mb genome from strain CSUR P278 exhibited a unique sequence type among S. epidermidis strains and contained 32 genes previously considered virulence genes in this species.
CASE REPORT
In June 2012, a 26-year-old man presented to his primary care physician for an anal fissure that had lasted for several months. Physical examination revealed an aortic regurgitation. He waited until 8 August 2012 before he consulted a cardiologist. A transthoracic echocardiography identified a grade III aortic insufficiency. On 12 August 2012, he was subsequently referred to the Cardiology Department of the Timone Hospital, Marseille, France, for evaluation of his aortic insufficiency. The patient was not an intravenous drug user but had a history of psoriasis vulgaris that involved his upper limbs and had required oral corticosteroid therapy, which had been discontinued in January 2012. Being on a diet because of an anal fissure, he had lost 13 kg over the previous 4 months but had neither been hospitalized nor had any surgery or intravenous or intramuscular injection in the past year. He reported scraping his psoriasis lesions due to intense pruritus. On admission, the clinical examination was normal except for a fever of 38.8°C and psoriasis vulgaris lesions on both elbows. A transesophageal echography revealed a bicuspid aortic valve and an aortic valve destruction causing a severe aortic insufficiency. Laboratory investigations revealed a white blood cell count of 13,000/ml (normal range, 4,000 to 10,000/ml), with 84.1% polymorphonuclear lymphocytes (20 to 80%), 11.1% lymphocytes (20 to 40%), and 2.2% monocytes (1.5 to 4.0%), a hemoglobin level of 107 g/liter (130 to 160 g/liter), a platelet count of 299,000/ml (150,000 to 400,000/ml), a creatinine level of 93 μmol/liter (70 to 115 μmol/liter), and a C reactive protein level of 68 mg/liter (≤5 mg/liter). An endocarditis diagnostic kit comprising three pairs of blood culture vials, two blood tubes without additive for serology and determination of rheumatoid factor, antinuclear and antiphospholipid antibodies, a heparinized blood tube for cell culture, and an EDTA blood tube for PCR was performed as previously described (1). Subsequently, an intravenous antibiotic combination of amoxicillin (12 g/day) and gentamicin (160 mg/day) was started. All three blood culture vials in the kit, sampled at 1-h intervals, grew an Staphylococcus epidermidis strain that was susceptible to oxacillin, gentamicin, clindamycin, fosfomycin, rifampin, co-trimoxazole, ofloxacin, fusidic acid, teicoplanin, and vancomycin. The results of all other diagnostic tests in the kit were negative. According to the modified Duke criteria (2), the patient was classified as having definite endocarditis. The patient was transferred to the Cardiac Surgery Department, where he underwent aortic valve replacement with a bioprosthesis on 13 August 2012. Microbiological analysis of the resected valve enabled isolation of an S. epidermidis strain that exhibited the same antibiotic susceptibility and matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry profile as the strains grown from blood, and histological analysis confirmed the diagnosis of endocarditis by demonstrating the intratissular presence of altered polymorphonuclear lymphocytes and clumps of cocci. The initial antibiotic therapy was changed to intravenous vancomycin (2 g/day) for 6 weeks and gentamicin (160 mg/day) for 2 weeks when the blood culture results were known. Under this treatment, the patient improved and, to date, has remained asymptomatic. Our patient had previous aortic valve disease but underwent no surgical or intravenous procedure (as a possible source of infection) in the months preceding the endocarditis episode. However, we identified two putative portals of entry, including the scraping lesions on his legs and the chronic anal fissure, neither of which exhibited local inflammation on admission. The patient signed an informed consent form.
For genome sequencing, the patient's S. epidermidis strain, isolated from a valvular biopsy specimen (deposited into the CSUR collection under reference number CSUR P278), was grown on 5% sheep blood-enriched Columbia agar (bioMérieux, Marcy l'Etoile, France). Four petri dishes were spread with the culture, and the dishes were resuspended in 1.0 ml of 4× Tris-EDTA (TE) buffer. Two samples of 100 and 200 μl of this suspension were individually processed with a QIAamp tissue kit (Qiagen, Hilden, Germany). DNA was eluted (50 μl per sample), and then samples were pooled. The yield and concentration were measured with the Quant-iT PicoGreen kit (Invitrogen, Saint-Aubin, France) on a GENios (Tecan) fluorometer at 16.7 ng/μl.
The genomic sequence was generated using an Ion Torrent PGM sequencer (Life Technologies, Saint-Aubin, France) from 1 μg of DNA. The fragment library was constructed using enzymatic DNA fragmentation and adaptor ligation with the Ion Xpress Plus fragment library kit (Life Technologies). Fragment size selection was performed by electrophoresis using agarose gel electrophoresis. The target fragment size distribution was 200 to 250 bp. This enrichment was purified using the Agencourt AMPure XP system (Beckman, Roissy, France). The size distribution of DNA fragments was analyzed on a Bioanalyzer using a high-sensitivity kit (Agilent, Santa Clara, CA). After dilution of the library at 11.62 pM, template preparation, and emulsion PCR, Ion Sphere particle (ISP) enrichment results were obtained using an Ion OneTouch 200 template kit (v.2). The quality of the resultant ISPs was assessed using the Qubit 2.0 fluorometer (Life Technologies). ISPs were loaded and sequenced on an Ion 316 chip (Life Technologies). A total of 1,915,420 reads were obtained, with an average length of 127 bp, which generated 244 Mb of sequences. No prior quality filtering was actually used for the de novo assembly, performed using Newbler version 2.3 software (Roche), with 90% identity and 40 bp as the overlap. All 139 contigs larger than 50 bp were included in the assembly. A genome size of 2.54 Mb was obtained. Although not closed, this genome size was in the range of previously sequenced S. epidermidis genomes. Open reading frames (ORFs) were predicted using Prodigal (http://prodigal.ornl.gov), with default parameters, but the predicted ORFs were excluded if they spanned a sequencing gap region. The predicted bacterial protein sequences were searched against the GenBank database (3) and the Clusters of Orthologous Groups (COGs) database using BLASTP. tRNA and rRNA genes were detected using tRNAscan-SE v.1.21 (4) and RNAmmer 1.2 (5), respectively. SignalP (6) and TMHMM (7) were used to identify signal peptides and transmembrane helices, respectively. The absence of plasmids was verified both by searching the gene annotation for any plasmid-related gene and by mapping all contigs against previously published S. epidermidis plasmid sequences.
S. epidermidis is a common commensal of the human skin. As such, it is often considered a culture contaminant. However, this bacterium has emerged as a major pathogen in hospital-acquired infections and is currently the most common agent of infections of intravenous catheters and indwelling prosthetic devices (8). In addition to being a cause of hospital-acquired infections, S. epidermidis may cause endocarditis in patients with native valves (9). Although its causative role in native endocarditis was initially underestimated, several studies on native-valve endocarditis (NVE) have demonstrated that S. epidermidis may account for up to 5% of cases (10, 11). However, because the number of prosthetic-valve infections caused by this bacterium exceed by far the number of cases of NVE, the specific genomic attributes of NVE-causing S. epidermidis have been poorly studied.
Thanks to the development of rapid benchtop sequencers, genome sequencing is becoming faster, less expensive, and more throughput efficient. This makes it possible to obtain the annotated sequence of a bacterial genome in 3 to 4 days, propelling real-time genomics as a strategy that may be used in the workflow of a routine microbiology laboratory, notably for epidemic strains or strains of unusual virulence or antibiotic resistance. In the present study, we sequenced the genome of a Staphylococcus epidermidis strain responsible for acute native-valve aortic endocarditis in order to study its virulence markers.
The draft genome sequencing of S. epidermidis strain CSUR P278 consisted of 139 contigs (approximately 96-fold coverage) arranged into a single chromosomic scaffold. No plasmid was detected. The chromosome size, G+C content, and number of genes were 2,563,459 bases, 31.92%, and 2,581 genes, respectively. Among these genes, 2,522 were protein-coding genes and 60 were RNAs (1 rRNA operon, an additional 5S rRNA, 55 tRNAs, and 1 transfer-messenger RNA [tmRNA]). A total of 1,997 genes were assigned to COGs (79.1%). Of these, 1,887 genes were assigned a putative function (74.9%). Twenty-four genes (0.9%) were ORFs with no detectable homology to other ORFs in the database (ORFans), and 402 (15.9%) were annotated as hypothetical proteins. As many as 202 genes had peptide signals, and 616 had transmembrane helices.
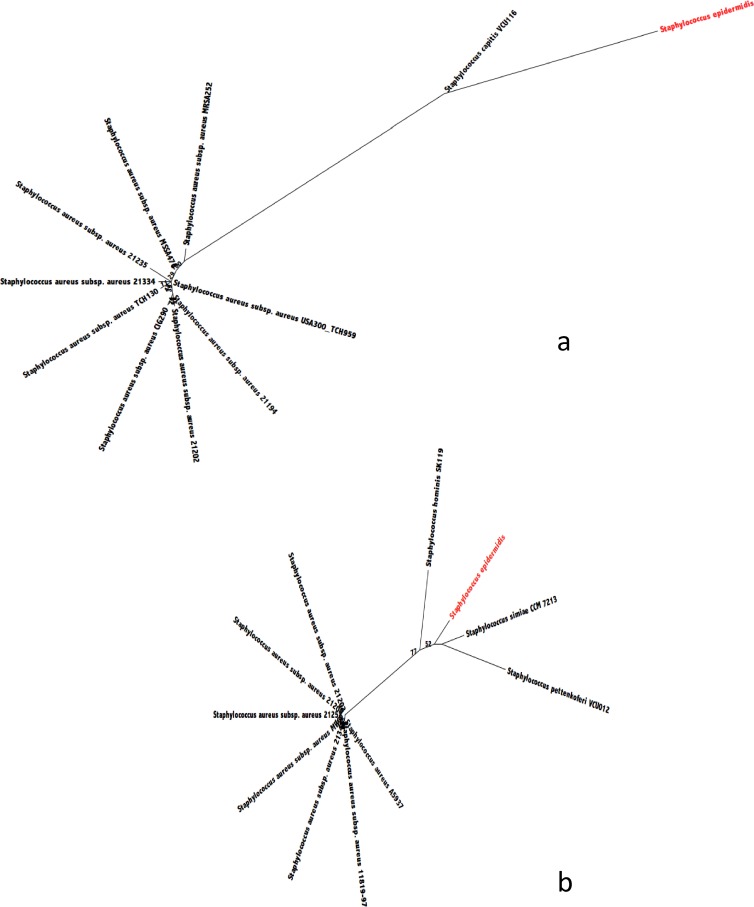
Compared to the multilocus sequence type (MLST) database (www.mlst.net), strain CSUR P278 exhibited a unique sequence type (arc allele 8, aroE24, gtr-12, mutS23, new alleles for pyr, tpi, and ygiL). By comparison with the genomes from the noninfectious S. epidermidis strain ATCC 12228 and the pathogenic strain RP62a (12, 13), strain CSUR P278 exhibited an almost perfect synteny. Two gene clusters present in both pathogenic S. epidermidis strains were absent from strain ATCC 12228. A first cluster of 42 kb comprised of 64 genes was identified by BLASTP as a complete S. epidermidis bacteriophage previously described as vB_SepiS-phiIPLA7 (14). As in other bacterial species, bacteriophages are known in S. epidermidis to play a role in lateral gene transfer (15). A second cluster of 34 kb was identified as arginine catabolic mobile element II (ACME II) (16). This genetic element was detected mostly in methicillin-resistant S. epidermidis strains (17–19), although Granslo et al. found it mostly in methicillin-susceptible isolates (16), as is the case for strain CSUR P278. In contrast, strain CSUR P278 had a toxin-antitoxin (TA) module (Table 1) absent from the other two S. epidermidis genomes. Compared to sequences in GenBank, this TA module was most closely related to Staphylococcus simiae for the toxin and to Staphylococcus capitis for the antitoxin, but no ortholog was found in S. epidermidis, making it possible that its acquisition resulted from a lateral gene transfer from another coagulase-negative staphylococcus (Fig. 1) at a recombination hot spot. TA modules, known as addiction molecules, have been hypothesized to play a role in virulence in human bacterial and epidemic pathogens (20). We assume that the unique presence of this TA module is linked to the aggressive behavior of this S. epidermidis isolate, causing native-valve endocarditis. In addition, 32 genes previously described in S. epidermidis as associated with virulence, classified mostly as adhesins, exoenzymes, or exotoxins, were detected in our strain (Table 1). However, the exact role of some of these genes in pathogenicity is uncertain, as many are also present in the non-biofilm-forming, non-infection-associated S. epidermidis strain ATCC 12228 (13). In contrast, two complete operons absent from the noninfectious strain but found in virulent strains are present in strain CSUR P278. The ica operon, comprised of 4 genes, is known to be involved in biofilm formation and biofilm-associated infection in animal models (8). The capABCD operon is involved in resistance to neutrophil phagocytosis (8). Finally, in a pan-genomic study of S. epidermidis, it was suggested that isolates having a formate dehydrogenase-encoding gene (fdh) were characterized by a reduced virulence and that this gene was lost in virulent strains (21). This is consistent with our study, as strain CSUR P278 lacked the fdh gene. However, in contrast with other pathogenic S. epidermidis isolates, strain CSUR P278 lacked the IS256 insertion sequence (22).
Table 1.
Putative virulence-associated genes detected in S. epidermidis strain CSUR P278a
| Gene category and function | Gene name | Contig no.-ORF | Function(s) |
|---|---|---|---|
| Biofilm formation | |||
| Primary attachment | atlE | 34-2 | Autolysin |
| sdrG (fbe) | 10-1 | Fibrinogen-binding protein | |
| sdrF | 15-49 | Ser-Asp-rich fibrinogen-binding protein, surface adhesin | |
| sdrH | 17-31 | Ser-Asp-rich fibrinogen-binding protein, surface adhesin | |
| sdrG | 31-1 | Ser-Asp-rich fibrinogen-binding protein, surface adhesin | |
| ebp | 1-48 | Elastin-binding protein | |
| ebh | 1-88 | Cell wall-associated fibronectin-binding protein | |
| ebha | 1-90 | EbhA protein | |
| Intercellular aggregation and protective exopolymers | icaA | 3-100 | Poly N-acetylglucosamine, protects from IgG, AMPs, phagocytosis, and complement |
| icaB | 3-98 | Poly N-acetylglucosamine, protects from IgG, AMPs, phagocytosis, and complement | |
| icaC | 3-97 | Poly N-acetylglucosamine, protects from IgG, AMPs, phagocytosis, and complement | |
| icaD | 3-99 | Poly N-acetylglucosamine, protects from IgG, AMPs, phagocytosis and complement | |
| icaR | 3-101 | Poly N-acetylglucosamine, protects from IgG, AMPs, phagocytosis, and complement | |
| capA | 4-89 | Poly-γ-glutamyl acid, protects from AMPs and phagocytosis | |
| capB | 6-6 | Poly-γ-glutamyl acid, protects from AMPs and phagocytosis | |
| capC | 6-7 | Poly-γ-glutamyl acid, protects from AMPs and phagocytosis | |
| capD | 6-10 | Poly-γ-glutamyl acid, protects from AMPs and phagocytosis | |
| sepA | 26-3 | Protease, AMP degradation | |
| Resistance to AMPs | dltA | 18-30 | Dealanylation of teichoic acids |
| dltB | 18-31 | Dealanylation of teichoic acids | |
| dltC | 18-32 | Dealanylation of teichoic acids | |
| dltD | 18-33 | Dealanylation of teichoic acids | |
| mprF | 1-172 | Lysylation of phospholipids | |
| vraF | 5-71 | AMP exporter | |
| vraG | 5-72 | AMP exporter | |
| aprR | 5-69 | AMP sensor | |
| aprS | 5-70 | AMP sensor | |
| Exoenzymes | |||
| sspA | 51-4 | Serine protease, V8 protease | |
| sspB | 3-4 | Serine protease precursor | |
| geh | 15-40, 3-5, 3-6 | Glycerol ester hydrolase, lipase | |
| lip | 3-96 | Lipase A | |
| Toxins | |||
| psmβ | 2-39 | Beta-hemolysin | |
| ficD | 7-68 | Toxin | |
| Antitoxin | 7-67 | Antitoxin |
AMPs, antimicrobial peptides.
Fig 1.
Phylogenetic positions of the toxin (a) and antitoxin (b) modules from S. epidermidis strain CSUR P278. Phylogenetic relationships were inferred from amino acid sequences using the maximum likelihood method.
In conclusion, although it did not directly impact patient management, our real-time genomics strategy enabled, with a short delay, the identification of the unusual gene content of our patient's strain among pathogenic S. epidermidis strains, as well as the analysis of its virulence repertoire. This study also highlighted the need for sequencing large collections of both pathogenic and nonpathogenic S. epidermidis strains to improve our understanding of the genetic background of each phenotype.
Nucleotide sequence accession number.
The genome sequence was deposited in GenBank under accession number AMSJ00000000.
ACKNOWLEDGMENTS
We thank Julien Paganini at Xegen Company (www.xegen.fr) for automating the genomic annotation process.
This study was funded by the Méditerranée Infection Foundation.
Footnotes
Published ahead of print 30 January 2013
REFERENCES
- 1. Raoult D, Casalta JP, Richet H, Khan M, Bernit E, Rovery C, Branger S, Gouriet F, Imbert G, Bothello E, Collart F, Habib G. 2005. Contribution of systematic serological testing in diagnosis of infective endocarditis. J. Clin. Microbiol. 43:5238–5242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VGJ, Ryan T, Bashore T, Corey GR. 2000. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 30:633–638 [DOI] [PubMed] [Google Scholar]
- 3. Benson DA, Karsch-Mizrachi I, Clark K, Lipman DJ, Ostell J, Sayers EW. 2012. GenBank. Nucleic Acids Res. 40:D48–D53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lowe TM, Eddy SR. 1997. tRNAscan-SE: a program for improved detection of transfer RNA gene in genomic sequence. Nucleic Acids Res. 25:955–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. 2007. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 35:3100–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8:785–786 [DOI] [PubMed] [Google Scholar]
- 7. Krogh A, Larsson B, von Heijne G, Sonnhammer EL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567–580 [DOI] [PubMed] [Google Scholar]
- 8. Otto M. 2009. Staphylococcus epidermidis—the ‘accidental’ pathogen. Nat. Rev. Microbiol. 7:555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arber N, Militianu A, Ben-Yehuda A, Krivoy N, Pinkhas J, Sidi Y. 1991. Native valve Staphylococcus epidermidis endocarditis: report of seven cases and review of the literature. Am. J. Med. 90:758–762 [PubMed] [Google Scholar]
- 10. Etienne J, Eykyn SJ. 1990. Increase in native valve endocarditis caused by coagulase negative staphylococci: an Anglo-French clinical and microbiological study. Br. Heart J. 64:381–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whitener C, Caputo GM, Weitekamp MR, Karchmer AW. 1993. Endocarditis due to coagulase-negative staphylococci. Microbiologic, epidemiologic, and clinical considerations. Infect. Dis. Clin. North Am. 7:81–96 [PubMed] [Google Scholar]
- 12. Gill SR, Fouts DE, Archer GL, Mongodin EF, DeBoy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, Dodson RJ, Daugherty SC, Madupu R, Angiuoli SV, Durkin AS, Haft DH, Vamathevan J, Khouri H, Utterback T, Lee C, Dimitrov G, Jiang L, Qin H, Weidman J, Tran K, Kang K, Hance IR, Nelson KE, Fraser CM. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 187:2426–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang YQ, Ren SX, Li HL, Wang YX, Fu G, Yang J, Qin ZQ, Miao YG, Wang WY, Chen RS, Shen Y, Chen Z, Yuan ZH, Zhao GP, Qu D, Danchin A, Wen YM. 2003. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol. Microbiol. 49:1577–1593 [DOI] [PubMed] [Google Scholar]
- 14. Gutierrez D, Martinez B, Rodriguez A, Garcia P. 2010. Isolation and characterization of bacteriophages infecting Staphylococcus epidermidis. Curr. Microbiol. 61:601–608 [DOI] [PubMed] [Google Scholar]
- 15. Daniel A, Bonne PE, Fischetti VA. 2007. First complete genome sequence of two Staphylococcus epidermidis bacteriophages. J. Bacteriol. 189:2086–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Granslo HN, Klingenberg C, Fredheim EG, Ronnestad A, Mollnes TE, Flaegstad T. 2010. Arginine catabolic mobile element is associated with low antibiotic resistance and low pathogenicity in Staphylococcus epidermidis from neonates. Pediatr. Res. 68:237–241 [DOI] [PubMed] [Google Scholar]
- 17. Barbier F, Lebeaux D, Hernandez D, Delannoy AS, Caro V, Francois P, Schrenzel J, Ruppe E, Gaillard K, Wolff M, Brisse S, Andremont A, Ruimy R. 2011. High prevalence of the arginine catabolic mobile element in carriage isolates of methicillin-resistant Staphylococcus epidermidis. J. Antimicrob. Chemother. 66:29–36 [DOI] [PubMed] [Google Scholar]
- 18. Miragaia M, de Lencastre H, Perdreau-Remington F, Chambers HF, Higashi J, Sullam PM, Lin J, Wong KI, King KA, Otto M, Sensabaugh GF, Diep BA. 2009. Genetic diversity of arginine catabolic mobile element in Staphylococcus epidermidis. PLoS One 4:e7722 doi:10.1371/journal.pone.0007722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Svensson K, Hellmark B, Soderquist B. 2011. Characterization of SCCmec elements in methicillin-resistant Staphylococcus epidermidis isolated from blood cultures from neonates during three decades. APMIS 119:885–893 [DOI] [PubMed] [Google Scholar]
- 20. Georgiades K, Raoult D. 2011. Genomes of the most dangerous epidemic bacteria have a virulence repertoire characterized by fewer genes but more toxin-antitoxin modules. PLoS One 6:e17962 doi:10.1371/journal.pone.0017962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Conlan S, Mijares LA, NISC Comparative Sequencing Program, Becker J, Blakesley RW, Bouffard GG, Brooks S, Coleman H, Gupta J, Gurson N, Park M, Schmidt B, Thomas PJ, Otto M, Kong HH, Murray PR, Segre JA. 2012. Staphylococcus epidermidis pan-genome sequence analysis reveals diversity of skin commensal and hospital infection-associated isolates. Genome Biol. 13:R64 doi:gb-2012-13-7-r64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gu J, Li H, Li M, Vuong C, Otto M, Wen Y, Gao Q. 2005. Bacterial insertion sequence IS256 as a potential molecular marker to discriminate invasive strains from commensal strains of Staphylococcus epidermidis. J. Hosp. Infect. 61:342–348 [DOI] [PubMed] [Google Scholar]