Abstract
A protocol was optimized for the isolation of Mycobacterium avium subsp. paratuberculosis (MAP) from milk and colostrum, with parameters including chemical decontamination, antibiotics, and different culture media. This study demonstrates that the efficiency of MAP recovery from milk is highly dependent upon the culturing protocol, and such protocols should be optimized to ensure that low concentrations of MAP in milk can be detected.
TEXT
In the later stages of infection, Mycobacterium avium subsp. paratuberculosis (MAP) can disseminate throughout the body and has been isolated from milk, supramammary lymph nodes, and lymph fluid from the udder of cattle (1, 2). Calves that are fed maternal colostrum are more likely to become infected with MAP than those that are fed colostrum replacer or pasteurized colostrum, indicating that raw colostrum and milk may be the initial source of exposure for neonates (3, 4). The culture of MAP from milk presents many difficulties because of the low growth rate of MAP compared with that of other microorganisms present in milk, the fastidious nature of MAP, and the low numbers of MAP (<2 to 8 CFU/50 ml to 10 to 560 CFU/ml) naturally present in milk from infected cows (1, 5). The basic protocol for the isolation of MAP from milk includes centrifugation to concentrate bacterial cells, chemical decontamination (often using hexadecylpyridinium chloride [HPC]) to decrease contaminating microorganisms, and culture in either liquid or solid medium optimized for the recovery of MAP. Protocols for the isolation of MAP from milk vary significantly, and a comprehensive study to identify the most efficacious method for the isolation of MAP from the complex milk matrix is necessary (1, 6–11). Media most commonly used for the culture of MAP from milk include Bactec 12B, para-JEM, and Herrold's egg yolk (HEY) (10, 12–14). These media vary greatly in composition, including the presence of growth supplements and antibiotics, as well as growth detection methods, and therefore vary in their ability to support MAP growth (15, 16).
In the present study, triplicate samples of milk obtained from a known Johne's disease-free cow were spiked with MAP (clinical isolate 167; National Animal Disease Center, Ames, IA) to final concentrations of 102, 104, 106, and 108 CFU/ml. For each experiment, a positive control consisting of 106 CFU of MAP/ml in phosphate-buffered saline (PBS), a negative control consisting of milk with no bacteria, and a no-treatment control consisting of 106 CFU of MAP/ml of milk were included. Within each protocol, the samples (20 ml) were centrifuged at 1,865 × g for 30 min at 4°C to partition the milk unless otherwise specified. The whey layer was discarded, and the cream and pellet layers were retained for the experimental procedure. Effects of temperature (room temperature [RT], 22 to 25°C, and 39°C), centrifugation speed (1,865, 3,500, and 5,600 × g), concentration of HPC (Sigma Chemical Co., St. Louis, MO) decontaminant (0.75, 1.00, 1.25, and 1.50%), time of decontamination (5, 24, and 48 h), and antibiotics (100 μg/ml vancomycin, 50 μg/ml amphotericin B, and 100 μg/ml nalidixic acid; Sigma) on the recovery of viable MAP from milk were investigated. Two liquid culture media, Bactec 12B and para-JEM, and one solid medium, HEY, were compared for the ability to recover MAP from spiked milk samples. The Bactec 12B medium (Becton, Dickinson, Franklin, NJ) was supplemented with 0.5 ml of sterile unmodified raw egg yolk (in-house), 0.2 ml PANTA Plus (BD), and 0.1 ml of 50 μg/ml mycobactin J and then inoculated with 200 μl of decontaminated milk sample and placed in a 37°C incubator for 84 days. The para-JEM medium (Thermo Fisher Scientific [TREK Diagnostic Systems, Inc.], Cleveland, OH) was supplemented according to the manufacturer's instructions, inoculated with 500 μl of decontaminated milk sample, and placed into the ESP Culture System II machine for up to 65 days. All bottles were subjected to MAP growth confirmation procedures of Ziehl-Neelsen acid fast stain for MAP colonies and PCR of the IS900 gene target as previously described (17). HEY medium (BD) was inoculated with 100 μl of decontaminated milk sample and incubated at 39°C for 12 weeks, and colonies were counted. Statistical analysis was performed with the GLIMMIX procedure of the Statistical Analysis System (SAS Institute, Cary, NC), and a Tukey-Kramer post hoc test was performed if results were significant (P < 0.05).
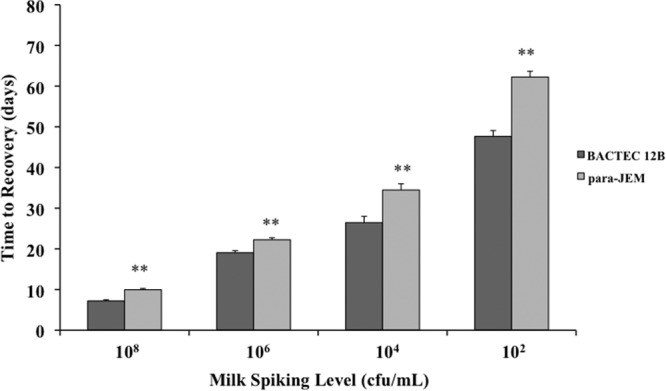
The three media tested, Bactec 12B, para-JEM, and HEY, differed considerably in the recovery of MAP from decontaminated milk samples. Results demonstrated that HEY medium was inferior to the liquid media in recovery of MAP from milk, with limits of detection estimated to be 104 CFU/ml (data not shown). These findings were comparable to a previous study using HPC decontamination prior to culture on HEY medium (6). Therefore, no further analyses with HEY medium were conducted. Bactec 12B medium consistently recovered MAP at lower concentrations and earlier in incubation (P < 0.01) than did the para-JEM medium (Fig. 1), even though the volume of inoculum added to bottles was 2.5-fold greater for para-JEM than for Bactec 12B. Over all experiments, Bactec 12B medium failed to recover only 3% of the milk samples spiked with 102 CFU/ml of MAP, whereas para-JEM medium failed to recover 71% of samples at this concentration.
Fig 1.
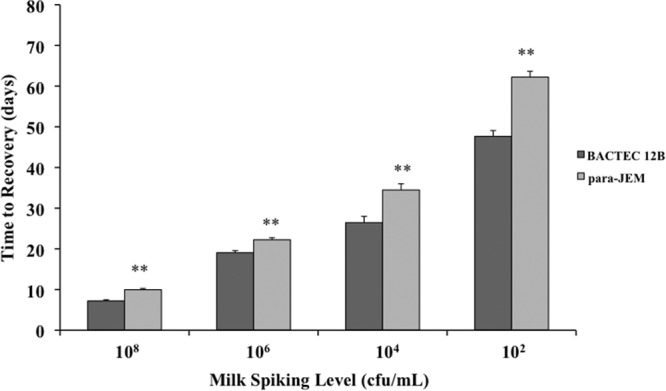
Comparison of the Bactec 12B and para-JEM media for time to recovery and detection sensitivity of Mycobacterium avium subsp. paratuberculosis in raw milk spiked with 102 to108 CFU/ml. Data are expressed as means ± standard errors of the means (SEM). Significant differences between the two media are represented by asterisks (**, P < 0.01).
The temperature of decontamination with HPC did not have a significant effect on MAP recovery (P = 0.63, data not shown), regardless of culture medium. However, increasing the temperature from RT to 39°C during decontamination of milk samples increased the contamination rates (15.6 to 31.1%) in Bactec 12B and (33.3 to 42.2%) para-JEM media. Additionally, increasing centrifugation force from 1,865 to 5,600 × g did not enhance the recovery of MAP from milk samples (P = 0.45, data not shown). Increasing concentration of HPC from 0.75 to 1.50% and time of exposure to HPC from 5 to 48 h resulted in significant reductions (P < 0.05) in the recovery of viable MAP, as seen for samples spiked with 102 CFU/ml of MAP and incubated in Bactec 12B medium (Fig. 2). Therefore, the recommended protocol for chemical decontamination of milk to maximize the recovery of MAP in culture was determined to be 0.75% HPC for 5 h at RT.
Fig 2.
Time to recovery of Mycobacterium avium subsp. paratuberculosis in raw milk spiked with 102 CFU/ml after decontamination with 0.75, 1.00, 1.25, and 1.50% hexadecylpyridinium chloride (HPC) for 5, 24, and 48 h in Bactec 12B medium. Data are expressed as means ± SEM. Significant differences between times of decontamination within an HPC concentration are represented by asterisks (* and **, P < 0.05).
Overnight incubation of the milk samples with the antibiotic cocktail VAN (consisting of 100 μg/ml vancomycin [V], 50 μg/ml amphotericin B [A], and 100 μg/ml nalidixic acid [N]), following decontamination with 0.75% HPC, resulted in detrimental effects on the recovery of viable MAP (Fig. 3). There was an increase (P < 0.01) in the time to recovery after secondary treatment with VAN compared with HPC alone, regardless of liquid medium used or concentration of the MAP inoculum. Treatment with VAN doubled the time to recovery from 33 to 70 days for milk samples spiked with 102 CFU/ml of MAP and incubated in Bactec 12B medium. Exposure to any singular antibiotic (V, A, or N) caused a consistent decrease (P < 0.05) in viability in both culture media as well (data not shown).
Fig 3.
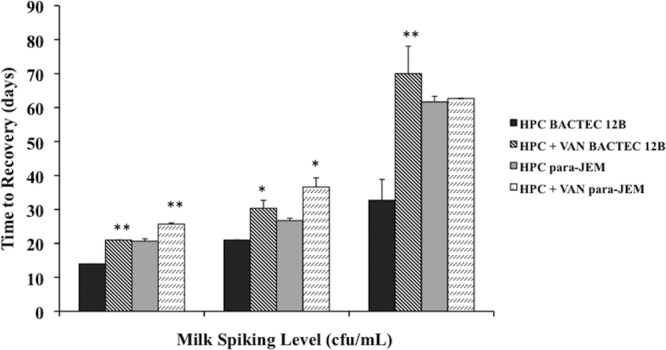
Effects on the time to recovery of Mycobacterium avium subsp. paratuberculosis in milk cultured in Bactec 12B and para-JEM media after treatment with the antibiotic cocktail (VAN) consisting of 100 μg/ml vancomycin (V), 50 μg/ml amphotericin B (A), and 100 μg/ml nalidixic acid (N) compared with treatment alone with hexadecylpyridinium chloride (HPC). Data are expressed as means ± SEM. Significant differences between HPC alone or HPC plus VAN within each medium are represented by asterisks (**, P < 0.01; *, P < 0.05).
The three culture media demonstrated different abilities to recover viable MAP from milk. HEY medium proved to be far inferior to the liquid media, with a sensitivity of detection estimated at 104 CFU/ml of MAP. Bactec 12B medium consistently detected inoculation concentrations of 102 CFU/ml, had faster detection times, and had fewer incidences of machine-indicated false-positive and -negative samples compared to para-JEM medium. Differences in medium formulation, such as the source of egg yolk and composition of supplemental antibiotics, could have affected the recovery of viable MAP. The manufacturer of Bactec 12B medium recommends the use of PANTA PLUS, which consists of polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin. The para-JEM medium contains the same antibiotics as the VAN cocktail used in our decontamination protocol. Vancomycin is known to be detrimental to the viability of MAP as demonstrated by a 28- to 49-day increase in the time to detection when vancomycin (86 μg/ml) was added to the Bactec 12B medium (18). The para-JEM medium also contains a reagent called para-JEM BLUE which has been shown to retard the growth of MAP by increasing times to detection by up to 1 week (19). The lethality of VAN on the viability of MAP may be compounded, because treatment followed exposure to HPC (6, 20). HPC can damage the MAP bacterium and may cause MAP to become more vulnerable to the antibiotics to which they are normally resistant. Further studies should investigate whether different antibiotics would have similar effects on MAP or whether decreasing the concentration of each antibiotic in the cocktail would decrease the detrimental effects observed for VAN on MAP in this study.
The optimization of a culture method allows the accurate assessment of shedding of MAP into milk of naturally infected cattle, providing critical information to producers about the risk of transmitting MAP to calves via consumption of milk from infected cows.
ACKNOWLEDGMENTS
We thank Philip Dykema, Robin Swanson, and Hannah Wilson for their training, advice, and assistance. We also thank Erica Hellmich and Margaret Walker for their technical assistance in the laboratory.
Footnotes
Published ahead of print 20 February 2013
REFERENCES
- 1. Sweeney R, Whitlock R, Rosenberger A. 1992. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J. Clin. Microbiol. 30:166–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khol J, Pinedo P, Buergelt C, Neumann L, Baumgartner W, Rae D. 2012. The collection of lymphatic fluid from the bovine udder and its use for the detection of Mycobacterium avium subsp. paratuberculosis in the cow. J. Vet. Diagn. Invest. 24:23–31 [DOI] [PubMed] [Google Scholar]
- 3. Stabel J. 2008. Pasteurization of colostrum reduces the incidence of paratuberculosis in neonatal dairy calves. J. Dairy Sci. 91:3600–3606 [DOI] [PubMed] [Google Scholar]
- 4. Pithua P, Godden S, Wells S, Oakes M. 2009. Efficacy of feeding plasma-derived commercial colostrum replacer for the prevention of transmission of Mycobacterium avium subsp. paratuberculosis in Holstein calves. J. Vet. Med. Assoc. 234:1167–1176 [DOI] [PubMed] [Google Scholar]
- 5. Slana I, Kralik P, Kralova A, Pavlik I. 2008. On-farm spread of Mycobacterium avium subsp. paratuberculosis in raw milk studied by IS900 and F57 competitive real-time quantitative PCR and culture examination. Int. J. Food Microbiol. 128:250–257 [DOI] [PubMed] [Google Scholar]
- 6. Gao A, Odumeru J, Raymond M, Mutharia L. 2005. Development of improved method for isolation of Mycobacterium avium subsp. paratuberculosis from bulk tank milk: effect of age of milk, centrifugation, and decontamination. Can. J. Vet. Res. 69:81–87 [PMC free article] [PubMed] [Google Scholar]
- 7. Dundee L, Grant I, Ball H, Rowe M. 2001. Comparative evaluation of four decontamination protocols for the isolation of Mycobacterium avium subsp. paratuberculosis from milk. Lett. Appl. Microbiol. 33:173–177 [DOI] [PubMed] [Google Scholar]
- 8. Streeter R, Hoffisis F, Bech-Nielsen S, Shulaw W, Rings D. 1995. Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. Am. J. Vet. Res. 56:1322–1324 [PubMed] [Google Scholar]
- 9. Stabel J, Wells S, Wagner B. 2002. Relationships between fecal culture, ELISA, and bulk tank milk test results for Johne's disease in US dairy herds. J. Dairy Sci. 85:525–531 [DOI] [PubMed] [Google Scholar]
- 10. Gao A, Odumeru J, Raymond M, Hendrick S, Duffield T, Mutharia L. 2009. Comparison of milk culture, direct and nested polymerase chain reaction (PCR) with fecal culture based on samples from dairy herds infected with Mycobacterium avium subsp. paratuberculosis. Can. J. Vet. Res. 73:58–64 [PMC free article] [PubMed] [Google Scholar]
- 11. Reddacliff L, Vadali A, Whittington R. 2003. The effect of decontamination protocols on the numbers of sheep strain Mycobacterium avium subsp. paratuberculosis isolated from tissues and faeces. Vet. Microbiol. 95:271–282 [DOI] [PubMed] [Google Scholar]
- 12. Grant I, Ball H, Rowe M. 2002. Incidence of Mycobacterium paratuberculosis in bulk raw and commercially pasteurized cows' milk from approved dairy processing establishments in the United Kingdom. Appl. Environ. Microbiol. 68:2428–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slana I, Liapi M, Moravkova M, Kralova A, Pavlik I. 2009. Mycobacterium avium subsp. paratuberculosis in cow bulk tank milk in Cyprus detected by culture and quantitative IS900 and F57 real-time PCR. Prev. Vet. Med. 89:223–226 [DOI] [PubMed] [Google Scholar]
- 14. Ellingson J, Anderson J, Koziczkowski J, Radcliff R, Sloan S, Allen S, Sullivan N. 2005. Detection of viable Mycobacterium avium subsp. paratuberculosis in retail pasteurized whole milk by two culture methods and PCR. J. Food Prot. 68:966–972 [DOI] [PubMed] [Google Scholar]
- 15. Whittington R. 2009. Factors affecting isolation and identification of Mycobacterium avium subsp. paratuberculosis from fecal and tissue samples in a liquid culture system. J. Clin. Microbiol. 47:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tortoli E, Cichero P, Chirillo M, Gismondo M, Bono L, Gesu G, Simonetti M, Volpe G, Nardi G, Marone P. 1998. Multicenter comparison of ESP Culture System II with BACTEC 460TB and with Lowenstein-Jensen medium for recovery of mycobacteria from different clinical specimens, including blood. J. Clin. Microbiol. 36:1378–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leite F, Stokes K, Robbe-Austerman S, Stabel J. 2013. Comparison of fecal DNA extraction kits for the detection of Mycobacterium avium subsp. paratuberculosis by polymerase chain reaction. J. Vet. Diagn. Invest. 25:27–34 [DOI] [PubMed] [Google Scholar]
- 18. Gumber S, Whittington R. 2007. Comparison of BACTEC 460 and MGIT 960 systems for the culture of Mycobacterium avium subsp. paratuberculosis S strain and observations on the effect of inclusion of ampicillin in culture media to reduce contamination. Vet. Microbiol. 119:42–52 [DOI] [PubMed] [Google Scholar]
- 19. Okwumabua O, Moua T, Danz T, Quinn J, O'Connor M, Gibbons-Burgener S. 2010. Growth rate retardation and inhibitory effect of para-JEM BLUE of Mycobacterium avium subspecies paratuberculosis. J. Vet. Diagn. Invest. 22:734–737 [DOI] [PubMed] [Google Scholar]
- 20. Pribylova R, Kubicova L, Babak V, Pavlik I, Kralik P. 2012. Effect of short- and long-term antibiotic exposure on the viability of Mycobacterium avium subsp. paratuberculosis as measured by propidium monoazide F57 real-time quantitative PCR and culture. Vet. J. 194:354–360 [DOI] [PubMed] [Google Scholar]


