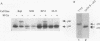Abstract
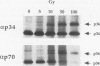
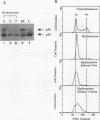
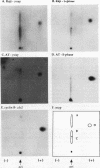
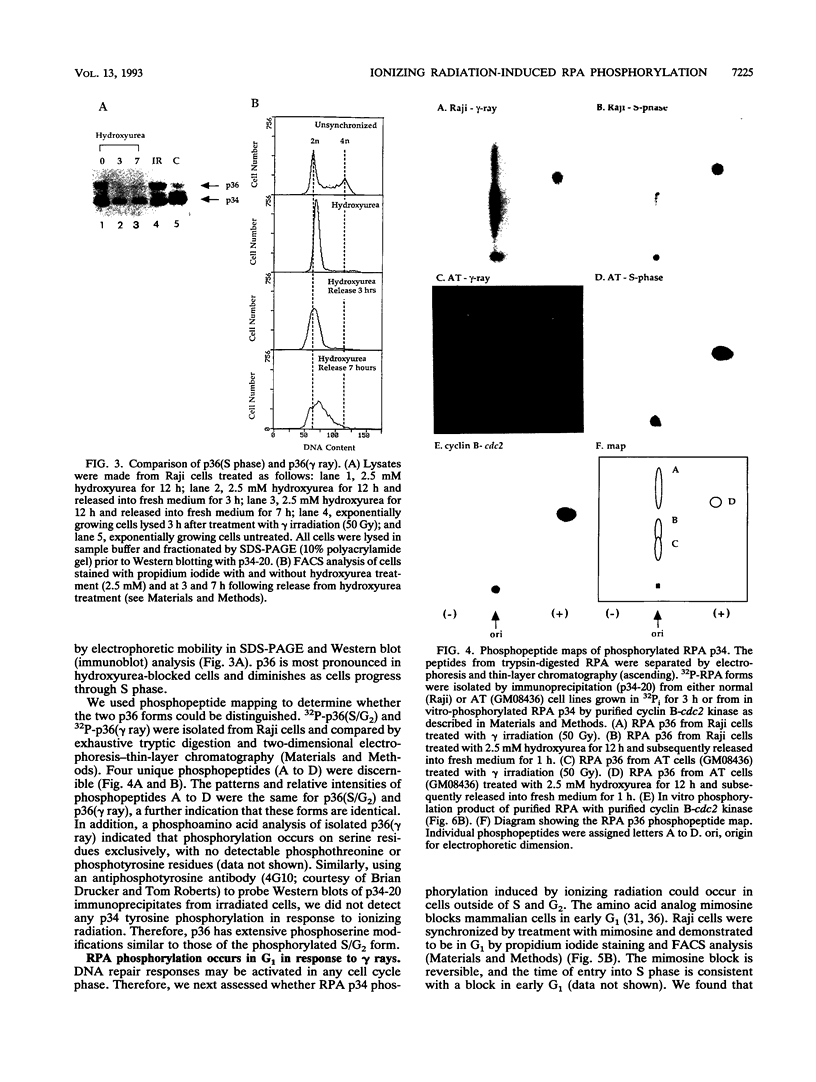
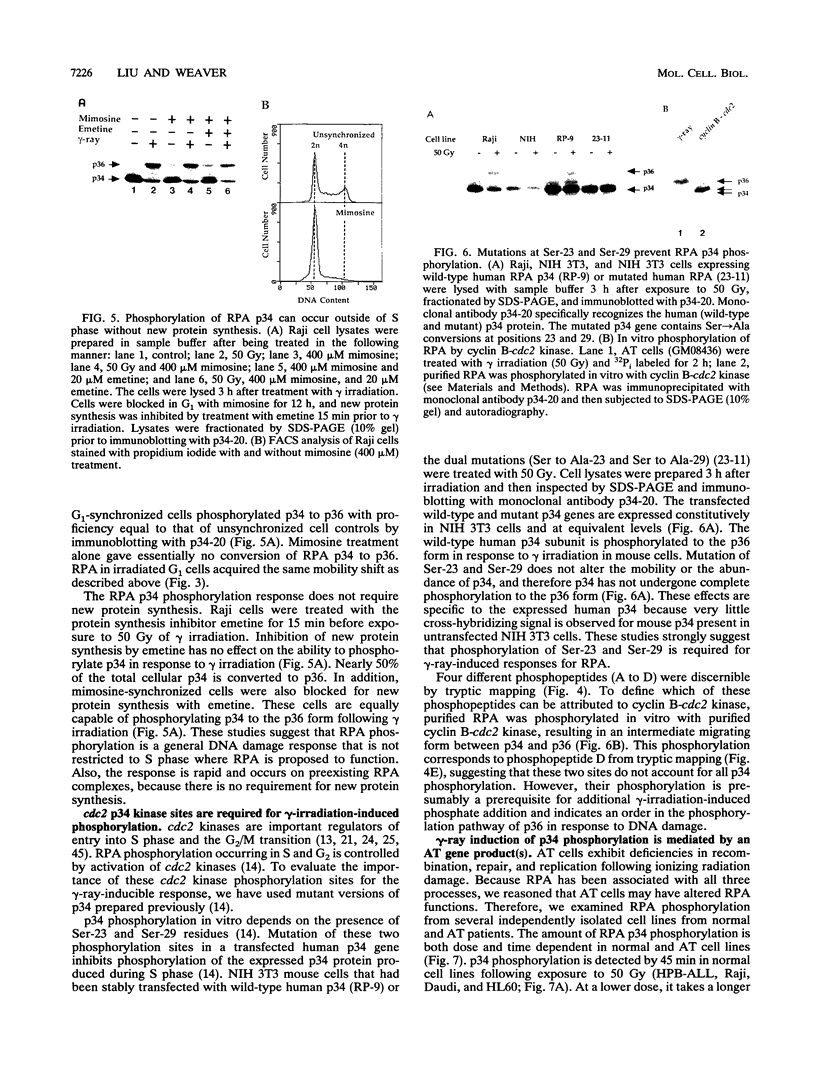
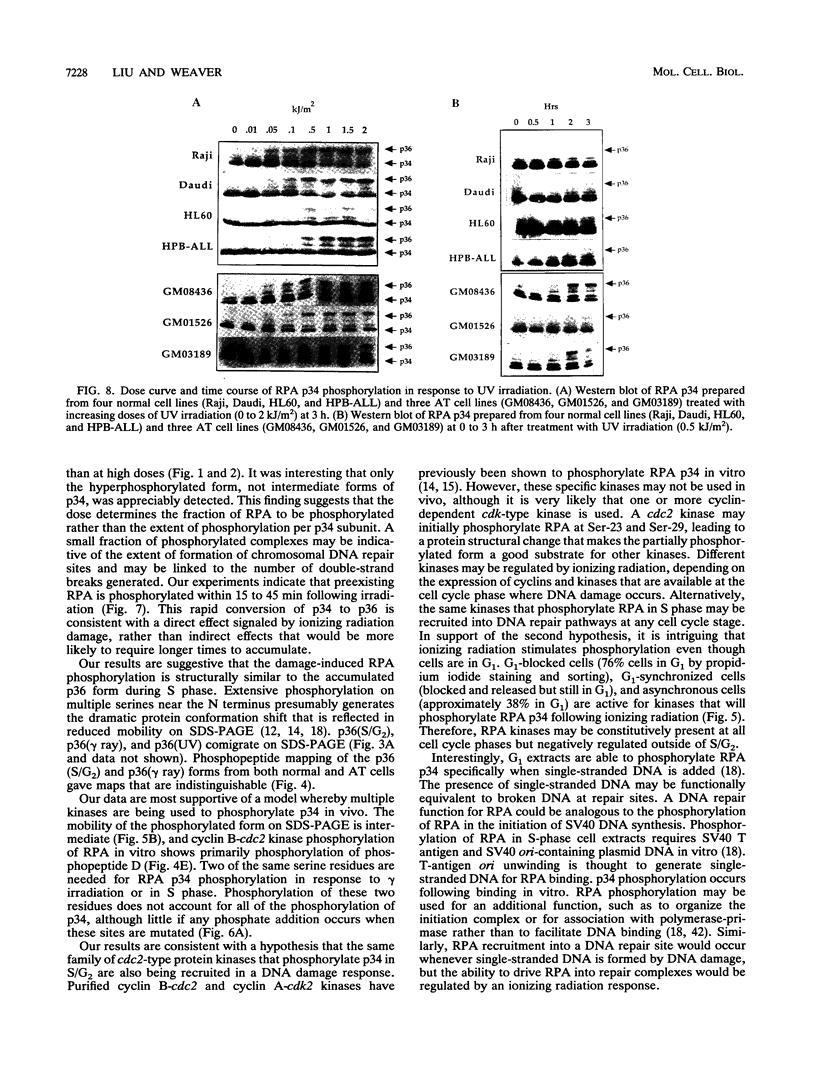
Replication protein A (RPA), the trimeric single-stranded DNA-binding protein complex of eukaryotic cells, is important to DNA replication and repair. Phosphorylation of the p34 subunit of RPA is modulated by the cell cycle, occurring during S and G2 but not during G1. The function of phosphorylated p34 remains unknown. We show that RPA p34 phosphorylation is significantly induced by ionizing radiation. The phosphorylated form, p36, is similar if not identical to the phosphorylated S/G2 form. gamma-Irradiation-induced phosphorylation occurs without new protein synthesis and in cells in G1. Mutation of cdc2-type protein kinase phosphorylation sites in p34 eliminates the ionizing radiation response. The gamma-irradiation-induced phosphorylation of RPA p34 is delayed in cells from ataxia telangiectasia, a human inherited disease conferring DNA repair defects and early-onset tumorigenesis. UV-induced phosphorylation of RPA p34 occurs less rapidly than gamma-irradiation-induced phosphorylation but is kinetically similar between ataxia telangiectasia and normal cells. This is the first time that modification of a repair protein, RPA, has been linked with a DNA damage response and suggests that phosphorylation may play a role in regulating DNA repair pathways.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedermann K. A., Sun J. R., Giaccia A. J., Tosto L. M., Brown J. M. scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1394–1397. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brach M. A., Hass R., Sherman M. L., Gunji H., Weichselbaum R., Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J Clin Invest. 1991 Aug;88(2):691–695. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill S. J., Stillman B. Replication factor-A from Saccharomyces cerevisiae is encoded by three essential genes coordinately expressed at S phase. Genes Dev. 1991 Sep;5(9):1589–1600. doi: 10.1101/gad.5.9.1589. [DOI] [PubMed] [Google Scholar]
- Brill S. J., Stillman B. Yeast replication factor-A functions in the unwinding of the SV40 origin of DNA replication. Nature. 1989 Nov 2;342(6245):92–95. doi: 10.1038/342092a0. [DOI] [PubMed] [Google Scholar]
- Bryant P. E. Enzymatic restriction of mammalian cell DNA: evidence for double-strand breaks as potentially lethal lesions. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Jul;48(1):55–60. doi: 10.1080/09553008514551061. [DOI] [PubMed] [Google Scholar]
- Bryant P. E. Use of restriction endonucleases to study relationships between DNA double-strand breaks, chromosomal aberrations and other end-points in mammalian cells. Int J Radiat Biol. 1988 Dec;54(6):869–890. doi: 10.1080/09553008814552291. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Cornforth M. N., Bedford J. S. On the nature of a defect in cells from individuals with ataxia-telangiectasia. Science. 1985 Mar 29;227(4694):1589–1591. doi: 10.1126/science.3975628. [DOI] [PubMed] [Google Scholar]
- Coverley D., Kenny M. K., Munn M., Rupp W. D., Lane D. P., Wood R. D. Requirement for the replication protein SSB in human DNA excision repair. Nature. 1991 Feb 7;349(6309):538–541. doi: 10.1038/349538a0. [DOI] [PubMed] [Google Scholar]
- Datta R., Hass R., Gunji H., Weichselbaum R., Kufe D. Down-regulation of cell cycle control genes by ionizing radiation. Cell Growth Differ. 1992 Sep;3(9):637–644. [PubMed] [Google Scholar]
- Denekamp J. Cell kinetics and radiation biology. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Feb;49(2):357–380. doi: 10.1080/09553008514552591. [DOI] [PubMed] [Google Scholar]
- Din S., Brill S. J., Fairman M. P., Stillman B. Cell-cycle-regulated phosphorylation of DNA replication factor A from human and yeast cells. Genes Dev. 1990 Jun;4(6):968–977. doi: 10.1101/gad.4.6.968. [DOI] [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Dutta A., Stillman B. cdc2 family kinases phosphorylate a human cell DNA replication factor, RPA, and activate DNA replication. EMBO J. 1992 Jun;11(6):2189–2199. doi: 10.1002/j.1460-2075.1992.tb05278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S. J., Richman R., Hall F. L., Williams R. T., Lodgson N., Harper J. W. CDK2 encodes a 33-kDa cyclin A-associated protein kinase and is expressed before CDC2 in the cell cycle. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2907–2911. doi: 10.1073/pnas.89.7.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdile L. F., Heyer W. D., Kolodner R., Kelly T. J. Characterization of a cDNA encoding the 70-kDa single-stranded DNA-binding subunit of human replication protein A and the role of the protein in DNA replication. J Biol Chem. 1991 Jun 25;266(18):12090–12098. [PubMed] [Google Scholar]
- Fairman M. P., Stillman B. Cellular factors required for multiple stages of SV40 DNA replication in vitro. EMBO J. 1988 Apr;7(4):1211–1218. doi: 10.1002/j.1460-2075.1988.tb02933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotedar R., Roberts J. M. Cell cycle regulated phosphorylation of RPA-32 occurs within the replication initiation complex. EMBO J. 1992 Jun;11(6):2177–2187. doi: 10.1002/j.1460-2075.1992.tb05277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenberg D., Frankenberg-Schwager M., Blöcher D., Harbich R. Evidence for DNA double-strand breaks as the critical lesions in yeast cells irradiated with sparsely or densely ionizing radiation under oxic or anoxic conditions. Radiat Res. 1981 Dec;88(3):524–532. [PubMed] [Google Scholar]
- Fulop G. M., Phillips R. A. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990 Oct 4;347(6292):479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., Piwnica-Worms H., Ernst T. J., Kanakura Y., Griffin J. D. cdc2 gene expression at the G1 to S transition in human T lymphocytes. Science. 1990 Nov 9;250(4982):805–808. doi: 10.1126/science.2237430. [DOI] [PubMed] [Google Scholar]
- Gatti R. A., Boder E., Vinters H. V., Sparkes R. S., Norman A., Lange K. Ataxia-telangiectasia: an interdisciplinary approach to pathogenesis. Medicine (Baltimore) 1991 Mar;70(2):99–117. [PubMed] [Google Scholar]
- Giordano A., Whyte P., Harlow E., Franza B. R., Jr, Beach D., Draetta G. A 60 kd cdc2-associated polypeptide complexes with the E1A proteins in adenovirus-infected cells. Cell. 1989 Sep 8;58(5):981–990. doi: 10.1016/0092-8674(89)90949-5. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Hallahan D. E., Sukhatme V. P., Sherman M. L., Virudachalam S., Kufe D., Weichselbaum R. R. Protein kinase C mediates x-ray inducibility of nuclear signal transducers EGR1 and JUN. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2156–2160. doi: 10.1073/pnas.88.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989 Nov 3;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Hendrickson E. A., Qin X. Q., Bump E. A., Schatz D. G., Oettinger M., Weaver D. T. A link between double-strand break-related repair and V(D)J recombination: the scid mutation. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4061–4065. doi: 10.1073/pnas.88.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer W. D., Kolodner R. D. Purification and characterization of a protein from Saccharomyces cerevisiae that binds tightly to single-stranded DNA and stimulates a cognate strand exchange protein. Biochemistry. 1989 Apr 4;28(7):2856–2862. doi: 10.1021/bi00433a017. [DOI] [PubMed] [Google Scholar]
- Heyer W. D., Rao M. R., Erdile L. F., Kelly T. J., Kolodner R. D. An essential Saccharomyces cerevisiae single-stranded DNA binding protein is homologous to the large subunit of human RP-A. EMBO J. 1990 Jul;9(7):2321–2329. doi: 10.1002/j.1460-2075.1990.tb07404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B. D., Hanauske-Abel H. M., Flint A., Lalande M. A new class of reversible cell cycle inhibitors. Cytometry. 1991;12(1):26–32. doi: 10.1002/cyto.990120105. [DOI] [PubMed] [Google Scholar]
- Jaspers N. G., Gatti R. A., Baan C., Linssen P. C., Bootsma D. Genetic complementation analysis of ataxia telangiectasia and Nijmegen breakage syndrome: a survey of 50 patients. Cytogenet Cell Genet. 1988;49(4):259–263. doi: 10.1159/000132673. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kenny M. K., Schlegel U., Furneaux H., Hurwitz J. The role of human single-stranded DNA binding protein and its individual subunits in simian virus 40 DNA replication. J Biol Chem. 1990 May 5;265(13):7693–7700. [PubMed] [Google Scholar]
- Lalande M. A reversible arrest point in the late G1 phase of the mammalian cell cycle. Exp Cell Res. 1990 Feb;186(2):332–339. doi: 10.1016/0014-4827(90)90313-y. [DOI] [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Lock R. B., Ross W. E. Inhibition of p34cdc2 kinase activity by etoposide or irradiation as a mechanism of G2 arrest in Chinese hamster ovary cells. Cancer Res. 1990 Jun 15;50(12):3761–3766. [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Eki T., Hurwitz J. Studies on the initiation and elongation reactions in the simian virus 40 DNA replication system. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9712–9716. doi: 10.1073/pnas.87.24.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon P. J. Ataxia-telangiectasia: an inherited disorder of ionizing-radiation sensitivity in man. Progress in the elucidation of the underlying biochemical defect. Hum Genet. 1987 Mar;75(3):197–208. doi: 10.1007/BF00281059. [DOI] [PubMed] [Google Scholar]
- Melendy T., Stillman B. An interaction between replication protein A and SV40 T antigen appears essential for primosome assembly during SV40 DNA replication. J Biol Chem. 1993 Feb 15;268(5):3389–3395. [PubMed] [Google Scholar]
- Moore S. P., Erdile L., Kelly T., Fishel R. The human homologous pairing protein HPP-1 is specifically stimulated by the cognate single-stranded binding protein hRP-A. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9067–9071. doi: 10.1073/pnas.88.20.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel R. J., Zhang H. B., Iliakis G., McKenna W. G. Cyclin B expression in HeLa cells during the G2 block induced by ionizing radiation. Cancer Res. 1991 Oct 1;51(19):5113–5117. [PubMed] [Google Scholar]
- Nurse P., Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981 Aug 6;292(5823):558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- Painter R. B., Young B. R. Radiosensitivity in ataxia-telangiectasia: a new explanation. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7315–7317. doi: 10.1073/pnas.77.12.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandita T. K., Hittelman W. N. Initial chromosome damage but not DNA damage is greater in ataxia telangiectasia cells. Radiat Res. 1992 Apr;130(1):94–103. [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Sargentini N. J., Smith K. C. Quantitation of the involvement of the recA, recB, recC, recF, recJ, recN, lexA, radA, radB, uvrD, and umuC genes in the repair of X-ray-induced DNA double-strand breaks in Escherichia coli. Radiat Res. 1986 Jul;107(1):58–72. [PubMed] [Google Scholar]
- Sherman M. L., Datta R., Hallahan D. E., Weichselbaum R. R., Kufe D. W. Ionizing radiation regulates expression of the c-jun protooncogene. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5663–5666. doi: 10.1073/pnas.87.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccioli G. E., Rathbun G., Oltz E., Stamato T., Jeggo P. A., Alt F. W. Impairment of V(D)J recombination in double-strand break repair mutants. Science. 1993 Apr 9;260(5105):207–210. doi: 10.1126/science.8469973. [DOI] [PubMed] [Google Scholar]
- Taylor Y. C., Duncan P. G., Zhang X., Wright W. D. Differences in the DNA supercoiling response of irradiated cell lines from ataxia-telangiectasia versus unaffected individuals. Int J Radiat Biol. 1991 Feb;59(2):359–371. doi: 10.1080/09553009114550331. [DOI] [PubMed] [Google Scholar]
- Tolmach L. J., Jones R. W., Busse P. M. The action of caffeine on X-irradiated HeLa cells. I. Delayed inhibition of DNA synthesis. Radiat Res. 1977 Sep;71(3):653–665. [PubMed] [Google Scholar]
- Tsurimoto T., Stillman B. Multiple replication factors augment DNA synthesis by the two eukaryotic DNA polymerases, alpha and delta. EMBO J. 1989 Dec 1;8(12):3883–3889. doi: 10.1002/j.1460-2075.1989.tb08567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Schieven G. L., Tuel-Ahlgren L. M., Dibirdik I., Myers D. E., Ledbetter J. A., Song C. W. Tyrosine phosphorylation is a mandatory proximal step in radiation-induced activation of the protein kinase C signaling pathway in human B-lymphocyte precursors. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):252–256. doi: 10.1073/pnas.90.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Tuel-Ahlgren L., Song C. W., Waddick K., Myers D. E., Kirihara J., Ledbetter J. A., Schieven G. L. Ionizing radiation stimulates unidentified tyrosine-specific protein kinases in human B-lymphocyte precursors, triggering apoptosis and clonogenic cell death. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9005–9009. doi: 10.1073/pnas.89.19.9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert T. A., Hartwell L. H. Characterization of RAD9 of Saccharomyces cerevisiae and evidence that its function acts posttranslationally in cell cycle arrest after DNA damage. Mol Cell Biol. 1990 Dec;10(12):6554–6564. doi: 10.1128/mcb.10.12.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert T. A., Hartwell L. H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988 Jul 15;241(4863):317–322. doi: 10.1126/science.3291120. [DOI] [PubMed] [Google Scholar]
- Wobbe C. R., Dean F. B., Murakami Y., Weissbach L., Hurwitz J. Simian virus 40 DNA replication in vitro: study of events preceding elongation of chains. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4612–4616. doi: 10.1073/pnas.83.13.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Weinberg D. H., Virshup D. M., Li J. J., Kelly T. J. Identification of cellular proteins required for simian virus 40 DNA replication. J Biol Chem. 1989 Feb 15;264(5):2801–2809. [PubMed] [Google Scholar]
- Young B. R., Painter R. B. Radioresistant DNA synthesis and human genetic diseases. Hum Genet. 1989 May;82(2):113–117. doi: 10.1007/BF00284040. [DOI] [PubMed] [Google Scholar]
- Zampetti-Bosseler F., Scott D. Cell death, chromosome damage and mitotic delay in normal human, ataxia telangiectasia and retinoblastoma fibroblasts after x-irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1981 May;39(5):547–558. doi: 10.1080/09553008114550651. [DOI] [PubMed] [Google Scholar]