Abstract
Asymptomatic infection by Plasmodium falciparum is an important obstacle to eliminating malaria. Asymptomatic carriers do not seek treatment for infection, and therefore they become a reservoir for the parasite. For this reason, these carriers pose a real public health risk. The systematic identification and treatment of asymptomatic infections should reduce the parasite reservoir. A large reduction in this pool will lower the chance of transmission of the disease. In this study, we screened a tribal population of 1,040 individuals in the Purulia district of West Bengal by using a dual-antigen rapid diagnostic kit (RDK), microscopy, and species-specific PCR. All positive individuals were treated with artemisinin-based combination therapy (ACT) (artesunate plus sulfadoxine-pyrimethamine) and followed for 42 days. Polymorphisms in candidate genes were screened by DNA sequencing. A significant proportion (8.4%) of the study population was infected with P. falciparum but showed no clinical manifestations. The PCR method was more sensitive in detecting infection than the RDK or microscopy. The efficacy of the ACT was 97%. In the pfcrt gene, the mutation K76T (the mutated amino acid is indicated by bold type) was found in 100% of the cases. In the pfmdr1 gene, the mutations N86Y and Y184F were noted in 55.5% and 11% of the cases, respectively. Six different haplotypes were identified in the pfdhfr-pfdhps genes. Most importantly, the quintuple mutant A16I51R59N108I164-S436G437E540A581A613 was found in 10% of the isolates, which is potentially important for the development of sulfadoxine-pyrimethamine resistance. A significant proportion of the study population harboring P. falciparum does not seek treatment and therefore serves as a reservoir for the parasite, maintaining the natural cycle. If the National Vector Borne Disease Control Programme (NVBDCP) of India is to eliminate malaria, then this hidden parasite burden needs to be addressed properly. Similar study in other parts of the country could help to determine the magnitude of the problem.
INTRODUCTION
India is one of the 11 countries in the WHO South-East Asia region and has nearly 980 million people at risk of acquiring malaria. According to the WHO's estimates, India has the largest number of malaria cases outside Africa. India's official statistics suggest that Plasmodium falciparum accounts for about 50% of the clinical cases in India. India has a long history of attacking malaria. Organized control programs, which started in the 1940s, used dichlorodiphenyltrichloroethane (DDT) to control mosquitoes. These programs were originally very successful in India and nearly eliminated malaria by 1961. In the subsequent years, however, the disease has reestablished itself in India.
The resurgence of malaria globally and the rising numbers of deaths due to this disease have drawn the attention of the international community. In 1998, the WHO, the World Bank, and several charitable organizations launched the Roll Back Malaria (RBM) Partnership, a global initiative that coordinates actions against malaria. The mission of the RBM Partnership was outlined recently in its global malaria action plan (1). A few of the major goals of the partnership were to reduce the number of global malaria cases from the levels seen in 2000 by 50% by 2010 and by 75% by 2015, to reduce global malaria deaths from the levels seen in 2000 by 50% by 2010 and to near zero by 2015, to eliminate malaria in 8 to 10 countries by 2015, and to achieve eradication of malaria worldwide.
Asymptomatic infection, mainly by P. falciparum, is an important obstacle to reaching those goals. Because asymptomatic carriers do not seek treatment for infection, the life cycle of the infection continues. The systematic identification and treatment of individuals with asymptomatic P. falciparum, as part of a surveillance intervention strategy, are important for reducing the parasite reservoir and helping to decrease transmission of the disease.
In countries in which malaria is endemic, a significant proportion of P. falciparum infections are asymptomatic or subclinical. Microscopy-detected levels of asymptomatic carriage as high as 39% have been reported in other countries (2–6). Similar data from India are not yet available. The present study was designed to determine the prevalence of asymptomatic P. falciparum malaria and the epidemiology of antimalarial drug-resistant molecular markers among the prevailing strains of P. falciparum in the study population.
MATERIALS AND METHODS
Selection of the study population.
This study was conducted in four neighboring villages of the Bundwan block in the Purulia district of West Bengal (22°47′N and 86°30′E), in which malaria is endemic (Fig. 1), and they all have a previous record of malaria transmission. The study villages are situated in a valley surrounded by thick forests and inhabited by a tribal population. The average annual rainfall of Purulia varies between 1,100 and 1,500 mm, and the rainy season is from July to September. Malaria transmission in this area is seasonal with two peaks, the first in July through October and the second in January. P. falciparum is its predominant malarial species.
Fig 1.
Map of study site.
Study procedures.
At the commencement of the study, 10 field staff members from the study area were trained on the purpose and procedures of the project. The study group was divided into five teams with three members each, headed by a member from the Calcutta School of Tropical Medicine. Each team visited households door-to-door to clinically examine all available individuals and to collect blood samples for the laboratory diagnosis of malarial parasites. Rapid diagnostic testing (RDT) was performed at the field site, and microscopy was performed on the same day at the Bundwan Block Primary Health Centre. All positive individuals were treated with an artemisinin-based combination therapy (ACT), a combination of artesunate (AS) plus sulfadoxine-pyrimethamine (SP), per the National Vector Borne Disease Control Programme (NVBDCP) guidelines. Artesunate at 4 mg/kg body weight was administered once daily for 3 days, a single dose of SP (25/1.25 mg base/kg body weight) was administered on day 0, and a single dose of primaquine (PQ) (0.75 mg/kg) was administered on day 1. Study investigators directly observed and documented the administration of each dose of medication. In the event of vomiting within 30 min of administration, a full dose was repeated.
The PCR-based diagnosis was done at the Calcutta School of Tropical Medicine. The individuals who tested positive by this method but tested negative by microscopy and RDT were also treated as described above. All microscopy-positive patients were revisited on posttreatment days 1, 2, 3, 7, 14, 21, 28, 35, and 42 for therapeutic efficacy evaluations.
Laboratory evaluations. (i) Microscopy.
Both thick and thin blood films were prepared from venous blood, stained with Giemsa stain, and examined by two microscopists for detection of malarial parasites and parasite species.
Parasite counts, performed by an experienced microscopist using a light microscope, were calculated by counting the number of parasites per 200 white blood cells (WBCs). Assuming a healthy WBC count to be 8,000/μl of blood, parasitemia was calculated and expressed as the number of parasites per μl of blood.
(ii) RDT-based diagnosis.
RDT was performed with a kit containing a monoclonal anti-P. falciparum histidine-rich protein II (HRP-II)-specific antibody and an anti-Plasmodium vivax p-LDH-specific antibody (Tulip Group, Goa, India) to detect any malarial parasite infection.
(iii) PCR-based diagnosis.
Genomic DNA of the parasite was isolated from the EDTA blood samples using a QIAamp DNA blood minikit (Qiagen, Valencia, CA) according to the manufacturer's instructions.
The Plasmodium genus- and species-specific primers were used separately in a two-step procedure for the detection of malarial infection. Three sets of oligonucleotide primers were used in this study. The primer set containing primers L1 (5′-TTAAAATTGTTGCAGTTAAAACG-3′) and L2 (5′-CCTGTTGTTGCCTTAAACTTC-3′) is genus specific and was used to detect the presence of the Plasmodium parasite. The species-specific primers for P. falciparum, Pf1 (5′-TTAAACTGGTTTGGGAAAACCAAATATATT-3′) and Pf2 (5′-ACACAATGAACTCAATCATGACTACCCGTC-3′), and for P. vivax, Pv1 (5′-CGCTTCTAGCTTAATCCACATAACTGATAC-3′) and Pv2 (5′-ACTTCCAAGCCGAAGCAAAGAAAGTCCTTA-3′), were used together in a single multiplex PCR as described previously (7).
The amplified PCR products were detected on a 1.0% agarose gel, which was subsequently stained with an ethidium bromide solution and visualized under UV light.
(iv) msp1 and msp2 genotyping.
Genotyping of P. falciparum parasites was performed with a nested PCR assay based on the amplification of msp1 and msp2 as described in detail elsewhere (8) to differentiate between recrudescence and reinfection.
(v) Polymorphisms in pfcrt, pfmdr1, pfdhfr, pfdhps, and pfATPase6.
The region of the pfmdr1 gene containing codons 86 and 184 was amplified by two pairs of primers as described elsewhere (9). A part of the pfcrt gene covering the single nucleotide polymorphisms (SNPs) at codons 72 to 76 was amplified as described previously (10). The region of the pfATPase6 gene spanning codon 769 was amplified as described previously (9). A portion of the pfdhfr gene spanning codons 16, 51, 59, 108, and 164 and a portion of the pfdhps gene spanning codons 436, 437, 540, 581, and 613 were amplified by two rounds of PCR using the primers described earlier (11, 12).
Direct sequencing of the PCR products was done on an ABI 3130xl genetic analyzer (Applied Biosystems, Foster City, CA). The sequences were analyzed using BioEdit software.
Statistical analysis.
The free statistical software program R (version 2.13.1) was used to calculate the Z-test value for the comparison of the sensitivity of the three diagnostic tests used.
Ethical approval.
The study protocol was approved by the institutional ethics committee of the Calcutta School of Tropical Medicine.
RESULTS
Prevalence of asymptomatic malaria in the study area.
The field study was conducted in June 2012 in four adjoining villages of the Bundwan block of the Purulia district. The total population of the study area was 1,040 (487 male and 553 female). A total of 963 individuals were available in the villages and examined for malarial parasite infection by the three methods. At the field level, 68 people were found to be positive for the P. falciparum antigen, and 4 people were positive for P. vivax. Out of 68 P. falciparum-positive individuals, as determined by RDT, a malarial parasite was detected by microscopy in 58 cases. In addition, the P. falciparum parasite was detected in another 8 individuals who were negative according to the dual-antigen rapid diagnostic kit (RDK). The P. falciparum parasite was detected in 66 individuals, and of them, 29 were positive for both the ring form and gametocytes, 26 were positive for the ring form only, and 11 were positive for gametocytes only. The average parasite load of the asexual form of P. falciparum was 1,718, ranging between 120 and 12,800. The P. vivax parasite was detected in all 4 RDT-positive individuals. By PCR, P. falciparum infection was demonstrated in 78 individuals, and of them, 73 were positive by either RDT, microscopy, or both. In 5 cases, P. falciparum infection was detected by PCR only. It is interesting to note that 3 individuals were positive by RDT or microscopy but were negative by PCR.
A total of 81 individuals were found to be infected with P. falciparum (overall prevalence, 8.4%). As microscopy remains the gold standard for diagnostic purposes, 66 parasitologically confirmed individuals were proven asymptomatic carriers (prevalence, 6.8%). The remaining 15 individuals were suspected carriers who were positive by either RDT, PCR, or both (prevalence, 1.5%). Four cases were detected as P. vivax monoinfection by both RDT and microscopy.
Among the three diagnostic methods used, PCR was more sensitive (96.3%, 78/81) than RDT (83.9%, 68/81) and microscopy (81.9%, 66/81) (Table 1). The sensitivity of PCR was significantly higher than that of RDT (Z = 2.684, P = 0.0036) and that of microscopy (Z = 2.681, P = 0.0036). No significant difference between the sensitivities of RDT and microscopy (Z = 0.401, P = 0.344) was detected.
Table 1.
Prevalence of asymptomatic P. falciparum malaria and the sensitivity of three diagnostic methods
| Village | Population (n) | Individuals examined (n) | Total positive (n [%]) | No. of cases with the following test results: |
|||||
|---|---|---|---|---|---|---|---|---|---|
| RDT+ PBS+a PCR+ | RDT+ PBS− PCR+ | RDT+ PBS+ PCR− | RDT− PBS+ PCR+ | RDT− PBS+ PCR− | RDT− PBS− PCR+ | ||||
| Tharkadaha | 250 | 224 | 30 | 20 | 4 | 1 | 2 | 2 | 1 |
| Burijhore | 305 | 275 | 24 | 14 | 4 | 0 | 2 | 0 | 4 |
| Asampani | 236 | 225 | 25 | 21 | 2 | 0 | 2 | 0 | 0 |
| Karu | 249 | 239 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| Total | 1,040 | 963 | 81 (8.4) | 57 | 10 | 1 | 6 | 2 | 5 |
PBS, peripheral blood smear.
Efficacy of artesunate plus sulfadoxine-pyrimethamine in asymptomatic P. falciparum malaria.
All 81 P. falciparum-positive individuals were treated by AS plus SP as per the government of India's national guidelines for the treatment of malaria. Sixty-six microscopically positive true asymptomatic individuals were followed for 42 days posttreatment. The demographic parameters of these cases are listed in Table 2. Splenic enlargement was noted in 5 (7.6%) cases, and hepatic enlargement was seen in 2 (3.03%) cases. Out of these 66 cases, recurrent parasitemia was recorded in 2 cases which were classified as late parasitological failure (LPF) by msp1 and msp2 genotyping (efficacy rate, 97% [64/66]).
Table 2.
Baseline characteristics of parasite-positive patients
| Characteristic (n = 66) | Result |
|---|---|
| Sex (no. [%]) | |
| Male | 40 (49.4) |
| Female | 41 (50.6) |
| Age category (no. [%]) | |
| 0–4 yr | 10 (12.3) |
| 5–8 yr | 13 (16.1) |
| 9–14 yr | 10 (12.3) |
| Adult | 48 (59.3) |
| Liver size (cm) | |
| Mean | 0.189 |
| Range | 0–5 |
| SD | 0.876 |
| 95% CIa | 0–0.482 |
| Spleen size (cm) | |
| Mean | 0.365 |
| Range | 0–5 |
| SD | 0.976 |
| 95% CI | 0.039–0.691 |
| Parasite count (no./μl) | |
| Mean | 1,718 |
| Range | 120–12,800 |
| SD | 2,733.1 |
| 95% CI | 1,045.5–2,390 |
CI, confidence interval.
Prevalence of polymorphisms in marker genes associated with antimalarial drug resistance.
We attempted to study the polymorphisms of the pfcrt, pfdhfr, pfdhps, pfmdr1, and pfATPase6 genes in 30 randomly selected isolates. The mutant South American haplotype CVIET was found in the pfcrt gene of all isolates. The pfmdr1 gene was sequenced successfully in 27 isolates. The mutation N86Y was observed in 15 isolates (55.5%), and the Y184F mutation was demonstrated in 3 (11%) isolates only (the mutated amino acids are indicated by bold type). No S769N mutation in the pfATPase6 gene was observed in the prevailing parasite population. The pfdhfr gene was sequenced successfully in 24 isolates that covered codons 16, 51, 59, 108, and 164. An N51I mutation at codon 51 was found in 12.5% of the isolates, and C59R and S108N mutations at codons 59 and 108, respectively, were found in 100% of the isolates. Three different haplotypes were recorded, among which the double mutant A16N51R59N108I164 was the most prevalent (77%). A triple mutant, A16I51R59N108I164, was recorded in 12.5% of the isolates. Sequencing of the pfdhps gene was successful in 26 isolates. Mutations at codons 437 (A437G) and 540 (A540E) were noted in 92.3% and 15.3% of the isolates, respectively. Among the three different haplotypes, S436G437K540A581A613 was the most prevalent (80%).
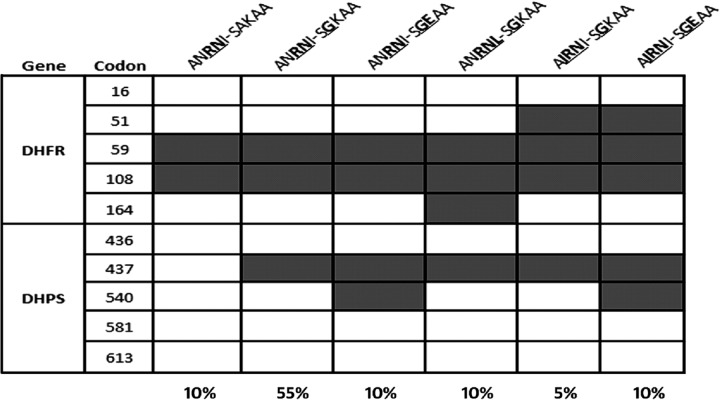
Both the pfdhfr and the pfdhps genes were sequenced in 20 isolates. The triple mutation haplotype A16N51R59N108I164-S436G437K540A581A613 was found in 55% of the isolates. A quintuple mutation, A16I51R59N108I164-S436G437E540A581A613, with triple pfdhfr and double pfdhps was recorded in 10% of the cases (Fig. 2). This particular mutant haplotype has been found to be associated with SP resistance.
Fig 2.
Prevalence of pfdhfr-pfdhps haplotypes in the study population (n = 20).
DISCUSSION
Jalpaiguri and Purulia are the two districts in West Bengal which have recorded the largest numbers of P. falciparum malaria cases during the last few years. A focus of chloroquine (CQ)-resistant (resistance level III [R-III] type) P. falciparum malaria was first reported from Purulia (13). Malaria transmission in this part of the state is seasonal. The prevalence of symptomatic malaria during the dry season is relatively low or even zero. The present study was done during the month of June, with temperatures ranging as high as 47°C to 49°C. Malaria transmission during that period was very low due to reduced vector population owing to the high atmospheric temperature. Under the existing conditions, an overall prevalence of 8.4% P. falciparum infection was detected without any signs or symptoms of malaria, of which 6.8% were parasitologically confirmed to be true carriers and 1.6% were suspected carriers. It has been observed that P. falciparum infection can persist asymptomatically in semi-immune individuals for more than 18 months when the possibility of reinfection is excluded (14). Similar to the observation of our present study, the persistence of asymptomatic P. falciparum infection interseasonally, in areas with seasonal transmission (15), has been reported in African countries (12, 16, 17). Such findings have driven presumptive intermittent antimalarial treatment strategies for asymptomatic carriers, regardless of their infection status, to reduce the disease burden in African countries.
Age is one of the most important factors that correlate with protective immunity in areas in which malaria is endemic. Infection among nonimmune individuals invariably results in clinical manifestations and often leads to death, particularly in young children if left untreated, as they have not yet acquired immunity (18). Adults and older children have a lower prevalence of infection and a lower incidence of clinical symptoms (19), as these individuals acquire immunity from several episodes of malaria during the early part of their lives (20). As a result, an adolescent or adult is more likely to develop uncomplicated or asymptomatic malaria (21). In our present study, we observed that out of 81 P. falciparum-positive cases, only 10 cases were within the <4-year age group. Similarly, the prevalence of asymptomatic carriage has been reported to be the greatest in adolescents and children. In a study in the Gambia, the prevalence of asexual parasitemia (61%) was highest in individuals aged 5 to 15 years (5).
Identification and management of asymptomatic carriers have become new and important challenges for malaria control programs. One aspect of these challenges, in particular, is the systematic diagnosis of asymptomatic cases. A PCR-based method can detect low-level parasitemia which is below the microscopic potency level. In the present study, 5 cases were diagnosed by PCR only, but in the field and for mass screening, it is impossible to perform PCR for all cases.
An artemisinin-based combination therapy (ACT), which is advocated as the first line of antimalarial treatment, has been reported to be effective in reducing even submicroscopic levels of gametocytes (22–24). In the present study, we used an ACT, a combination of AS and SP, to treat all positive cases, and we observed that this combination was highly effective in the study population against the P. falciparum strain (efficacy, 97%). As a higher level of resistance to this combination has been reported from the Jalpaiguri district of the state (25), the efficacy of this combination should be monitored periodically.
The K76T mutation in the pfcrt gene and the N86Y mutation in the pfmdr1 gene were widely distributed, indicating a high potential for resistance against chloroquine in the prevailing parasite population. A mutant quadruple or quintuple haplotype with mutations at codons 51, 59, and 108 in the pfdhfr gene and codon 437, with or without 540 point mutations, in the pfdhps gene were detected in 10% of the isolates. This figure indicates the enhancing resistance power of P. falciparum strains to SP in the study area.
A significant proportion of our study population was harboring P. falciparum infection without any signs or symptoms of malaria. Naturally, this population does not tend to visit a diagnostic facility or seek antimalarial treatment. Thus, these individuals become reservoirs within the population for parasites, which enables further transmission of the disease. The parasite strains of the study area are resistant to CQ, as evidenced by the prevailing mutant pfcrt and pfmdr1 molecular markers. However, these strains are sensitive to AS plus SP, an ACT recommended by the National Drug Policy of India, as evidenced by the in vivo efficacy study of the combination as well as the prevailing pfdhfr, pfdhps, and pfATPase6 molecular markers. If malaria control programs are going to be successful in eliminating malaria, this hidden parasite burden needs to be addressed properly. This can be achieved by either active mass screening of the entire population or mass drug administration. Similar studies in other parts of the country might be helpful to determine the magnitude of the problem.
ACKNOWLEDGMENTS
We thank all the residents of Bundwan, Purulia, who participated in this study.
We thank the government of West Bengal, Department of Health and Family Welfare, for funding this project.
We have no conflicts of interest concerning the work reported in this article.
Footnotes
Published ahead of print 20 February 2013
REFERENCES
- 1.Roll Back Malaria Partnership. Global malaria action plan. 2008. http://www.rollbackmalaria.org/gmap/gmap.pdf.
- 2. Mabunda S, Aponte JJ, Tiago A, Alonso PA. 2009. A country-wide malaria survey in Mozambique. II. Malaria attributable proportion of fever and establishment of malaria case definition in children across different epidemiological settings. Malar. J. 8:74 doi:10.1186/1475-2875-8-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vafa M, Troye-Blomberg M, Anchang J, Garcia A, Migot-Nabias F. 2008. Multiplicity of Plasmodium falciparum infection in asymptomatic children in Senegal: relation to transmission, age and erythrocyte variants. Malar. J. 7:17 doi:10.1186/1475-2875-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dal-Bianco MP, Koster KB, Kombila UD, Kun JF, Grobusch MP, Ngoma GM, Matsiequi PB, Supan C, Salazar CL, Missinou MA, Issifou S, Lell B, Kremsner P. 2007. High prevalence of asymptomatic Plasmodium falciparum infection in Gabonese adults. Am. J. Trop. Med. Hyg. 77:939–942 [PubMed] [Google Scholar]
- 5. Dunyo S, Miligan P, Edwards T, Sutherland C, Targett G, Pinder M. 2006. Gametocytaemia after drug treatment of asymptomatic Plasmodium falciparum. PLoS Clin. Trials 1:e20 doi:10.1371/journal.pctr.0010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baliraine FN, Afrane YA, Amenya DA, Bonizzoni M, Menge DM, Zhou G, Zhong D, Vardo-Zalik AM, Githeko A, Yan G. 2009. High prevalence of asymptomatic Plasmodium falciparum infections in a highland area of western Kenya: a cohort study. J. Infect. Dis. 200:66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boonma P, Christensen PR, Suwanarusk R, Price RN, Russell B, Lek-Uthai U. 2007. Comparison of molecular methods for the detection and speciation of Plasmodium vivax and Plasmodium falciparum. Malar. J. 6:124 doi:10.1186/1475-2875-6-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Snounou G. 2002. Genotyping of Plasmodium spp. nested PCR. Methods Mol. Med. 72:103–116 [DOI] [PubMed] [Google Scholar]
- 9. Imwong M, Dondorp AM, Nosten F, Yi P, Mungthin M, Hanchana S, Das D, Phyo AP, Lwin KM, Pukrittayakamee S, Lee SJ, Saisung S, Koecharoen K, Nguon C, Day NP, Socheat D, White NJ. 2010. Exploring the contribution of candidate genes to artemisinin resistance in Plasmodium falciparum. Antimicrob. Agents Chemother. 54:2886–2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liljander A, Wiklund L, Falk N, Kweku M, Mårtensson A, Felger I, Färnert A. 2009. Optimization and validation of multi-coloured capillary electrophoresis for genotyping of Plasmodium falciparum merozoite surface proteins (msp1 and 2). Malar. J. 8:78 doi:10.1186/1475-2875-8-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahmed A, Bararia D, Vinayak S, Yameen M, Biswas S, Dev Kumar VA, Ansari MA, Sharma YD. 2004. Plasmodium falciparum isolates in India exhibit a progressive increase in mutations associated with sulfadoxine-pyrimethamine resistance. Antimicrob. Agents Chemother. 48:879–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andriantsoanirina V, Ratsimbasoa A, Bouchier C, Jahevitra M, Rabearimanana S, Radrianjafy R, Andrianaranjaka V, Randriantsoa T, Rason MA, Tichit M, Rabarijaona LP, Mercereau-Puijalon O, Durand R, Ménard D. 2009. Plasmodium falciparum drug resistance in Madagascar: facing the spread of unusual pfdhfr and pfmdr-1 haplotypes and the decrease of dihydroartemisinin susceptibility. Antimicrob. Agents Chemother. 53:4588–4597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pandya AP, Barkakaty BN, Narasimham MV. 1991. Changing response of Plasmodium falciparum to chloroquine in West Bengal during 1980-1988. J. Commun. Dis. 23:103–108 [PubMed] [Google Scholar]
- 14. Krajden S, Panisko DM, Tobe B, Yang J, Keystone JS. 1991. Prolonged infection with Plasmodium falciparum in a semi-immune patient. Trans. R. Soc. Trop. Med. Hyg. 85:731–732 [DOI] [PubMed] [Google Scholar]
- 15. Babiker HA, Abdel-Muhsin AM, Ranford-Cartwright LC, Satti G, Walliker D. 1998. Characteristics of Plasmodium falciparum parasites that survive the lengthy dry season in eastern Sudan where malaria transmission is markedly seasonal. Am. J. Trop. Med. Hyg. 59:582–590 [DOI] [PubMed] [Google Scholar]
- 16. Males S, Gaye O, Garcia A. 2008. Long-term asymptomatic carriage of Plasmodium falciparum protects from malaria attacks: a prospective study among Senegalese children. Clin. Infect. Dis. 46:516–522 [DOI] [PubMed] [Google Scholar]
- 17. Le Port A, Cot M, Etard JF, Gaye O, Migot-Nabias F, Garcia A. 2008. Relation between Plasmodium falciparum asymptomatic infection and malaria attacks in a cohort of Senegalese children. Malar. J. 7:193 doi:10.1186/1475-2875-7-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Day KP, Marsh K. 1991. Naturally acquired immunity to Plasmodium falciparum. Immunol. Today 12:A68–A71 [DOI] [PubMed] [Google Scholar]
- 19. Djimdé AA, Doumbo OK, Traore O, Guindo AB, Kayentao K, Diourte Y, Niare-Doumbo S, Coulibaly D, Kone AK, Cissoko Y, Tekete M, Fofana B, Dicko A, Diallo DA, Wellems TE, Kwiatkowski D, Plowe CV. 2003. Clearance of drug-resistant parasites as a model for protective immunity in Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 69:558–563 [PubMed] [Google Scholar]
- 20. Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C. 1999. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 5:340–343 [DOI] [PubMed] [Google Scholar]
- 21. Grobusch MP, Kremsner PG. 2005. Uncomplicated malaria. Curr. Top. Microbiol. Immunol. 295:83–104 [PubMed] [Google Scholar]
- 22. Sutherland CJ, Ord R, Dunyo S, Jawara M, Drakeley CJ, Alexander N, Coleman R, Pinder M, Walraven G, Targett GA. 2005. Reduction of malaria transmission to Anophele mosquitoes with a six-dose regimen of co-artemether. PLoS Med. 2:e92 doi:10.1371/journal.pmed.0020092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bousema JT, Schneider P, Gouagna LC, Drakeley CJ, Tostmann A, Houben R, Githure JI, Ord R, Sutherland CJ, Omar SA, Sauerwein RW. 2006. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J. Infect. Dis. 193:1151–1159 [DOI] [PubMed] [Google Scholar]
- 24. Drakeley CJ, Jawara M, Targett GA, Walraven G, Obisike U, Coleman R, Pinder M, Sutherland CJ. 2004. Addition of artesunate to chloroquine for treatment of Plasmodium falciparum malaria in Gambian children causes a significant but short-lived reduction in infectiousness for mosquitoes. Trop. Med. Int. Health 9:53–61 [DOI] [PubMed] [Google Scholar]
- 25. Saha P, Guha SK, Das S, Mullick S, Ganguly S, Biswas A, Bera DK, Chattopadhyay G, Das M, Kundu PK, Ray K, Maji AK. 2012. Comparative efficacy of Artemisinin Combination Therapies (ACTs) in P. falciparum malaria and polymorphism of PfATPase6, Pfcrt, Pfdhfr and Pfdhps genes in tea gardens of Jalpaiguri district, India. Antimicrob. Agents Chemother. 56:2511–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]