Abstract
We selected 180 clinical isolates of the Mycobacterium tuberculosis complex (MTBC) from patients in China and performed comparative sequence analysis of the mpt64 gene after amplification. From the results, we found that polymorphisms of the mpt64 gene in the MTBC may be the reason for changes in the antigen produced, which may in turn cause alterations of related functions, thereby allowing immune evasion.
TEXT
Tuberculosis (TB) is a major infectious disease with a worldwide prevalence. About one-third of the world's population is infected with Mycobacterium tuberculosis. Among those infected, 9.3 million people develop active TB, and 1.8 million people die of TB each year (1). Current efforts to reduce the global problem of TB are focused on improving diagnosis and effective vaccines. Biochemical, immunological, and molecular biological characterization of M. tuberculosis has led to the identification of several antigens that may be useful in the development of improved diagnostic methods and vaccines (2).
MPT64 (Rv1980c), a 24-kDa protein of M. tuberculosis, is an important protein secreted by this pathogen (3, 4). It is hypothesized that actively secreted proteins of M. tuberculosis are the first to interact with the host immune system, and therefore that such proteins are important for activating the immune response in individuals infected with M. tuberculosis. Furthermore, this protein is recognized by human Th1 cells, and thus it could be useful for TB diagnosis or as part of a novel candidate vaccine against TB (5). A large amount of variability in the diagnostic accuracy of MPT64 has been reported, depending on the recombinant antigen used in assays (6, 7). Previous studies showed that the MPT64 antigen is highly conserved, but these studies were based on small samples (8). In this study, we selected 180 clinical isolates of the M. tuberculosis complex (MTBC) from patients in China, amplified the gene encoding the MPT64 antigen, and compared the sequences.
The 180 clinical isolates were selected from 2,346 MTBC strains that were previously isolated in China and genotyped by spoligotyping (9). All major and rare genotypes in China were included (Table 1; Fig. 1). Considering the predominance of Beijing family strains in China, we chose about half Beijing family strains (92 strains) and half non-Beijing family strains (88 strains). We randomly selected the 92 Beijing family strains from 1,738 Beijing strains among 2,346 MTBC strains. The remaining 88 strains were selected from 608 non-Beijing family isolates. Furthermore, we attempted to purposely include strains representing different spoligotypes that were isolated from different places. Table 2 shows the numbers of strains used in this study that were obtained from different provinces and regions in China. The strains were cultured using standard Löwenstein-Jensen medium, heat inactivated, and then subjected to PCR. The nucleotide sequences of the primers (5′ to 3′) were designed by DNAstar software according to the H37Rv genome sequence and were as follows: GCTTGTGGATCGCATATCCT and GACGACGTTTTGCCCTACAT.
Table 1.
Spoligotype patterns of strains used in this study
| Spoligotype | No. of strains |
|---|---|
| Beijing | 92 |
| T | 13 |
| U | 28 |
| MANU | 11 |
| Haarlem | 5 |
| EAI | 1 |
| LAM | 2 |
| H37Rv family | 1 |
| BCG | 2 |
| S | 1 |
| CAS | 4 |
| New | 20 |
Fig 1.
Spoligotypes of 180 strains of the MTBC.
Table 2.
Distribution of strains in different areas of China
| Area | No. of isolates |
|---|---|
| Anhui Province | 12 |
| Shanxi Province | 17 |
| Beijing Municipality | 11 |
| Fujian Province | 29 |
| Gansu Province | 12 |
| Guangxi Zhuang Autonomous Region | 29 |
| Sichuan Province | 1 |
| Henan Province | 12 |
| Hunan Province | 7 |
| Xizang (Tibet) Autonomous Region | 11 |
| Xinjiang Uygur Autonomous Region | 13 |
| Jilin Province | 14 |
| Zhejiang Province | 12 |
PCR was performed in a total volume of 20 μl. The PCR mix contained 10 μl of PCR buffer, 100 nM (each) primers, a 200 μM concentration of each deoxynucleoside triphosphate (dNTP), and 0.5 U of DNA Taq polymerase (TaKaRa). An initial denaturation of 5 min at 94°C was followed by 35 cycles of denaturation at 94°C for 45 s, annealing at 62°C for 45 s, and extension at 72°C for 1 min, followed by a final extension at 72°C for 10 min. Negative controls (reagents only, no DNA) were included with each PCR cycle. The positive control was 500 pg of DNA from M. tuberculosis H37Rv. The presence and size of each PCR product were determined by electrophoresis on a 2% agarose gel in Tris-boric acid-EDTA buffer followed by staining with ethidium bromide. All PCR tests were performed at least twice to validate the reproducibility. The sequences of the PCR products were determined by use of an ABI 3730xl DNA analyzer and then compared and sliced using Bioedit software. The protein structures were predicted by PyMol software (http://www.pymol.org/). Spoligotyping was performed according to a previously described method (10), and the spoligotyped families were assigned according to the international SpolDB4 database (11).
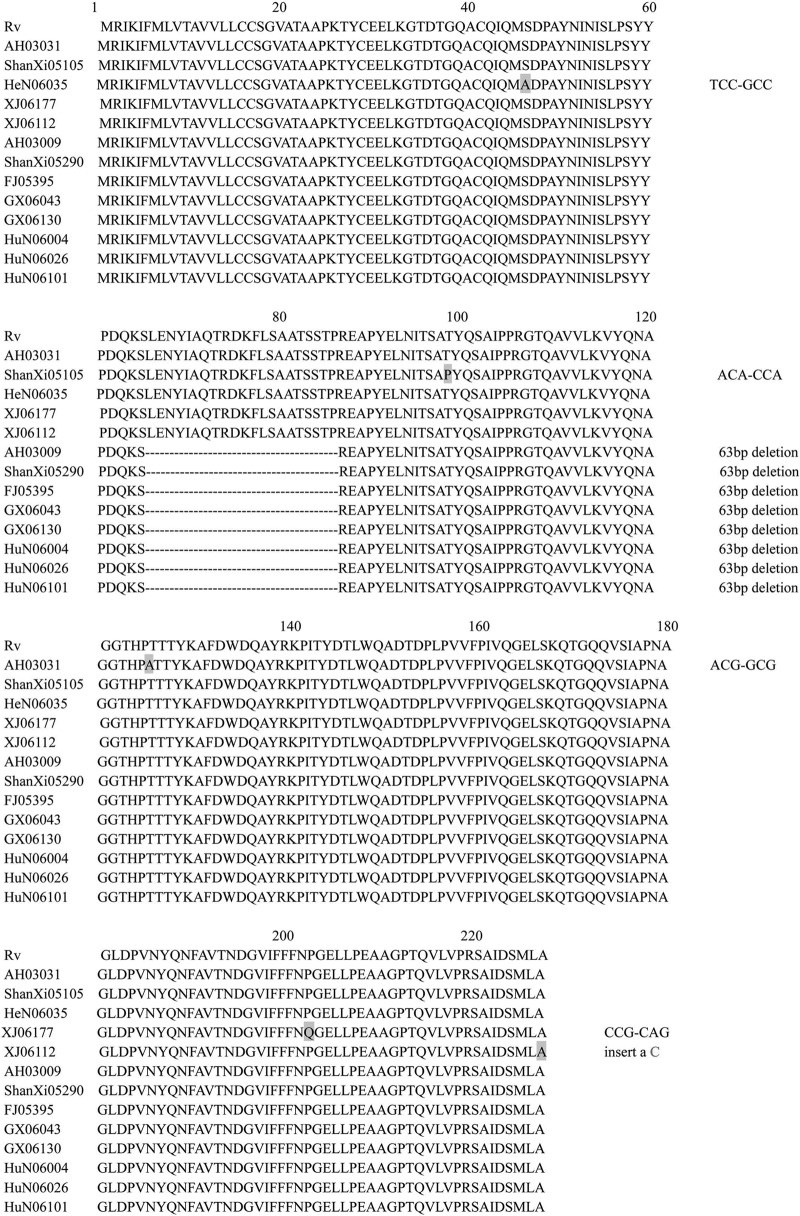
In this study, we selected 180 clinical isolates of the MTBC in China, which originated from a very large geographical area and contained different spoligotyping patterns; hence, the data from these strains are representative of the genetic diversity that might be present within China, at least to some extent. With the exception of 2 M. bovis bacillus Calmette-Guérin (BCG) strains, all other strains yielded PCR products in the MPT64 reaction. Fifteen strains (including the 2 BCG strains) presented polymorphisms in the mpt64 gene, accounting for 8.33% of the strains. Eight strains had a 63-bp deletion, whereas 4 strains showed a single-base mutation (Fig. 2 and 3); all of these mutations were nonsynonymous. Strain XJ06112 had a single-base insertion, but this did not result in an amino acid change, as the C insertion occurred in the codon for the last amino acid (Ala) at the carboxyl terminus, in the second base of the “GCC.” Strains AH03031, ShanXi05105, HeN06035, and XJ06177 had one nonsynonymous nucleotide mutation each, which resulted in rare changes in protein structure and function. The 63-bp deletion in the 8 strains led to the loss of amino acids 66 to 86 from MPT64; these residues were within the second alpha helix of MPT64. Figure 4 shows the tertiary structure of the MPT64 protein with or without the 63-bp deletion. Because of the deficiency of the alpha helix, the curvatures of adjacent beta-sheets were greatly increased. This resulted in a drastic change in the overall structural topology and surface exposure, which likely had a major impact on the biological function of this antigen. Although the function of MPT64 is currently unclear, we propose that the 8 strains harboring the deletion interact differently with human T cells and may represent a special type of MTBC strain that merits further investigation. In addition, variability in the accuracy of diagnosis using MPT64 may be due partly to the polymorphisms in the gene.
Fig 2.
Sequence alignment of antigen MPT64 sequences of 180 Mycobacterium tuberculosis complex strains. For 2 BCG strains, no PCR product was obtained; 13 strains exhibited changes at the gene level; and the remaining 165 strains were the same as strain H37Rv.
Fig 3.
Spoligotypes of 13 variant strains among the MTBC isolates.
Fig 4.
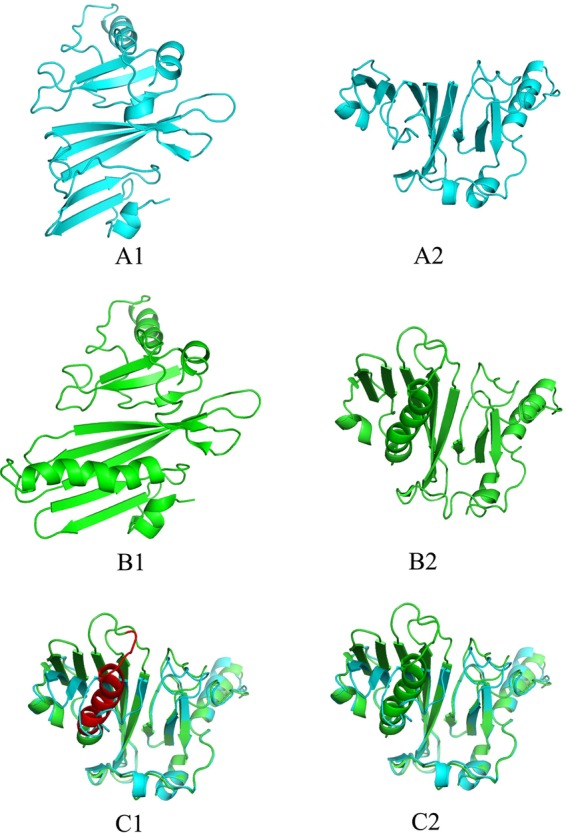
Tertiary structure of the MPT64 protein as predicted by PyMol software. Structures A1 and A2 were produced with the sequence with a 63-bp deletion, structures B1 and B2 were produced using the intact MPT64 sequence, and structures C1 and C2 were produced by overlapping the A and B structures.
There are 23 T-cell epitopes in the MPT64 antigen (Table 3), according to the Immune Epitopes Database (IEDB) (12). More than half (52.17%) of these epitopes have amino acid changes that are because of nucleotide alterations. The 63-bp deletion affected 5 of 23 T-cell epitopes in the antigen (IEDB ID numbers 61834, 53370, 33402, 28594, and 75172). This suggests that the gene may undergo antigenic variation in response to host immune pressure and that it may be involved in diversifying selection to evade host immunity. Additionally, the large number of amino acid substitutions in these epitopes may reflect ongoing immune evasion.
Table 3.
Amino acid changes in epitopes included in antigen MPT64a
| IEDB ID no. | Peptide sequence | Antigen | aa change |
|---|---|---|---|
| 65965 | TQVLVPRSAIDSMLA | Rv1980c | No change |
| 19348 | GELLPEAAGPTQVLVPRSAI | Rv1980c | No change |
| 43447 | NDGVIFFFNPGELLPEAAGP | Rv1980c | P-Q |
| 49925 | PVNYQNFAVTNDGVIFFFNP | Rv1980c | P-Q |
| 70980 | VSIAPNAGLDPVNYQNFAVT | Rv1980c | No change |
| 19359 | GELSKQTGQQVSIAPNAGLD | Rv1980c | No change |
| 48458 | PLPVVFPIVQGELSKQTGQQ | Rv1980c | No change |
| 73593 | YDTLWQADTDPLPVVFPIVQ | Rv1980c | No change |
| 72323 | WDQAYRKPITYDTLWQADTD | Rv1980c | No change |
| 24522 | HPTTTYKAFDWDQAYRKPIT | Rv1980c | No change |
| 37059 | LKVYQNAGGTHPTTTYKAFD | Rv1980c | T-A |
| 27981 | IPPRGTQAVVLKVYQNAGGT | Rv1980c | T-A |
| 2048 | AIPPRGTQAVVLKVYQNAGG | Rv1980c | No change |
| 44381 | NITSATYQSAIPPRGTQAVV | Rv1980c | T-P |
| 61834 | STPREAPYELNITSATYQSA | Rv1980c | 63-bp deletion |
| 53370 | RDKFLSAATSSTPREAPYEL | Rv1980c | 63-bp deletion |
| 33402 | KSLENYIAQTRDKFLSAATS | Rv1980c | 63-bp deletion |
| 28594 | ISLPSYYPDQKSLENYIAQT | Rv1980c | 63-bp deletion |
| 75172 | YNINISLPSYYPDQKSLENY | Rv1980c | 63-bp deletion |
| 51643 | QMSDPAYNINISLPSYYPDQ | Rv1980c | S-A |
| 22633 | GTDTGQACQIQMSDPAYNIN | Rv1980c | S-A |
| 3629 | APKTYCEELKGTDTGQACQI | Rv1980c | No change |
| 26805 | IKIFMLVTAVVLLCCSGVAT | Rv1980c | No change |
Shading indicates locations of amino acid changes.
Among the 180 isolates in this study, 2 BCG strains, one (FJ07111) isolated from the Fujian Province and one (JL06005) from the Jilin Province, yielded no PCR products in the MPT64 reaction, which is consistent with the results shown in previous studies (13–15). Thirteen variant strains showed different spoligotyping patterns (Fig. 3); in particular, 8 strains among these 13 showed a 63-bp deletion. This implies that the genotypes of MTBC strains may not be related to the immune reaction between the pathogen and host cell. This assumption is reasonable, because spoligotyping is based on the detection of various nonrepetitive spacer sequences located between small repetitive units (direct repeats [DRs]) in the DR locus of MTBC strains (10), which is located in the noncoding area of the genome. This is in contrast to T-cell epitopes, which are in the antigen coding areas; thus, genetic variation in such regions may obviously translate into functional differences.
Our finding that the MPT64 antigen harbors a large number of amino acid changes suggests that strain diversity should be considered during further development of new vaccines containing MPT64.
ACKNOWLEDGMENTS
We thank the staffs of the 13 provinces for their excellent contributions to this study.
This study was financially supported by the China Mega-Project for Infectious Disease (grant 2011ZX10004-001) and the program for Mycobacterium tuberculosis genome SNP analysis and research on the origin of the Beijing family strains (grant 2011SKLID208) of the State Key Laboratory for Infectious Disease Prevention and Control.
Footnotes
Published ahead of print 6 February 2013
REFERENCES
- 1. Donald PR, van Helden PD. 2009. The global burden of tuberculosis—combating drug resistance in difficult times. N. Engl. J. Med. 360:2393–2395 [DOI] [PubMed] [Google Scholar]
- 2. Young DB, Kaufmann SH, Hermans PW, Thole JE. 1992. Mycobacterial protein antigens: a compilation. Mol. Microbiol. 6:133–145 [DOI] [PubMed] [Google Scholar]
- 3. Mustafa AS. 2009. HLA-promiscuous Th1-cell reactivity of MPT64 (Rv1980c), a major secreted antigen of Mycobacterium tuberculosis, in healthy subjects. Med. Princ. Pract. 18:385–392 [DOI] [PubMed] [Google Scholar]
- 4. Mustafa AS, Shaban F. 2010. Mapping of Th1-cell epitope regions of Mycobacterium tuberculosis protein MPT64 (Rv1980c) using synthetic peptides and T-cell lines from M. tuberculosis-infected healthy humans. Med. Princ. Pract. 19:122–128 [DOI] [PubMed] [Google Scholar]
- 5. Mustafa AS. 2010. In silico binding predictions for identification of HLA-DR-promiscuous regions and epitopes of Mycobacterium tuberculosis protein MPT64 (Rv1980c) and their recognition by human Th1 cells. Med. Princ. Pract. 19:367–372 [DOI] [PubMed] [Google Scholar]
- 6. Yang H, Liu ZH, Zhang LT, Wang J, Yang HS, Qin LH, Jin RL, Feng YH, Cui ZL, Zheng RJ, Hu ZY. 2011. Selection and application of peptide mimotopes of MPT64 protein in Mycobacterium tuberculosis. J. Med. Microbiol. 60:69–74 [DOI] [PubMed] [Google Scholar]
- 7. Silva VM, Sardella IG, Luiz RR, Cunha AJ, Cavalcanti AH, Mahavir S, Barreto MM, Rodrigues RS, Carvalho TF, Saad MH. 2008. Immunoreactivity of five antigens of Mycobacterium tuberculosis in patients attending a public health care facility in an area with high endemicity for TB. Microbiol. Immunol. 52:544–550 [DOI] [PubMed] [Google Scholar]
- 8. Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, Ernst JD, Gagneux S. 2010. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat. Genet. 42:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dong H, Liu Z, Lv B, Zhang Y, Liu J, Zhao X, Wan K. 2010. Spoligotypes of Mycobacterium tuberculosis from different provinces of China. J. Clin. Microbiol. 48:4102–4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brudey K, Driscoll JR, Rigouts L, Prodinger WM, Gori A, Al-Hajoj SA, Allix C, Aristimuno L, Arora J, Baumanis V, Binder L, Cafrune P, Cataldi A, Cheong S, Diel R, Ellermeier C, Evans JT, Fauville-Dufaux M, Ferdinand S, Garcia de Viedma D, Garzelli C, Gazzola L, Gomes HM, Guttierez MC, Hawkey PM, van Helden PD, Kadival GV, Kreiswirth BN, Kremer K, Kubin M, Kulkarni SP, Liens B, Lillebaek T, Ho HM, Martin C, Martin C, Mokrousov I, Narvskaïa O, Ngeow YF, Naumann L, Niemann S, Parwati I, Rahim Z, Rasolofo-Razanamparany V, Rasolonavalona T, Rossetti ML, Rüsch-Gerdes S, Sajduda A, Samper S, Shemyakin IG, Singh UB, Somoskovi A, Skuce RA, van Soolingen D, Streicher EM, Suffys PN, Tortoli E, Tracevska T, Vincent V, Victor TC, Warren RM, Yap SF, Zaman K, Portaels F, Rastogi N, Sola C. 2006. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 6:23 doi:10.1186/1471-2180-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ernst JD, Lewinsohn DM, Behar S, Blythe M, Schlesinger LS, Kornfeld H, Sette A. 2008. Meeting report: NIH Workshop on the Tuberculosis Immune Epitope Database. Tuberculosis (Edinb.) 88:366–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li H, Ulstrup JC, Jonassen TO, Melby K, Nagai S, Harboe M. 1993. Evidence for absence of the MPB64 gene in some substrains of Mycobacterium bovis BCG. Infect. Immun. 61:1730–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, Small PM. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520–1523 [DOI] [PubMed] [Google Scholar]
- 15. Oettinger T, Andersen AB. 1994. Cloning and B-cell-epitope mapping of MPT64 from Mycobacterium tuberculosis H37Rv. Infect. Immun. 62:2058–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]