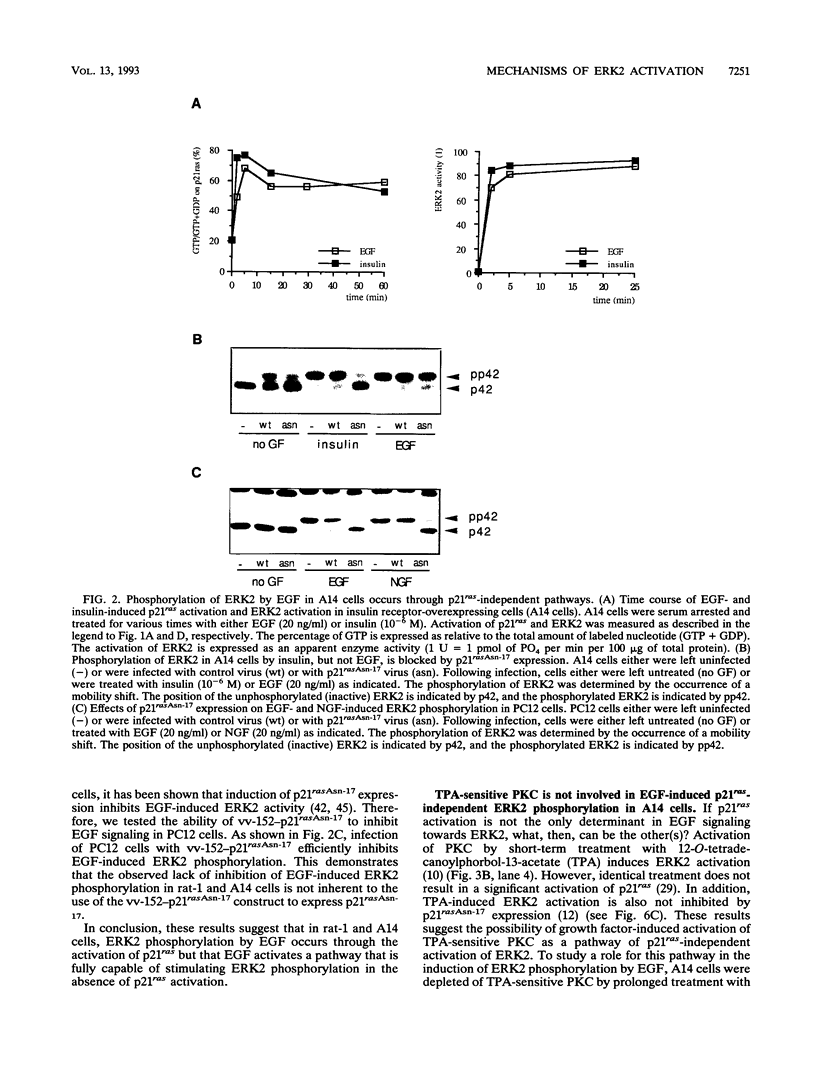
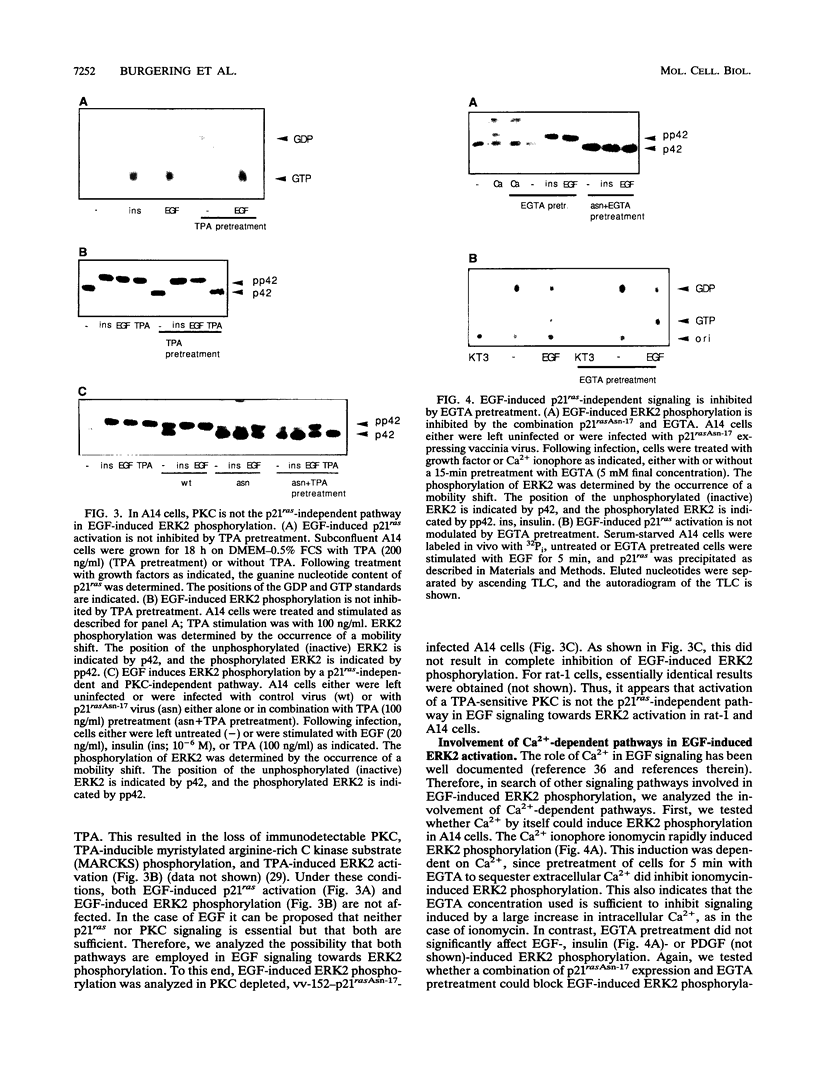
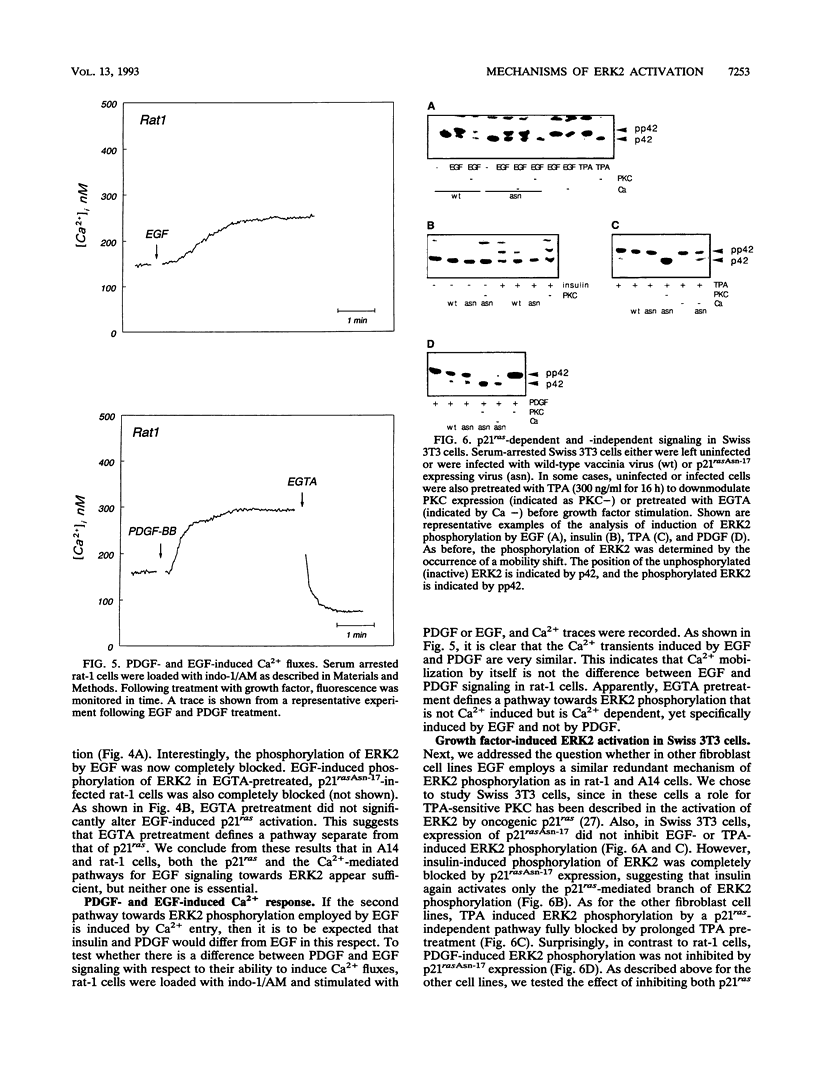
Abstract
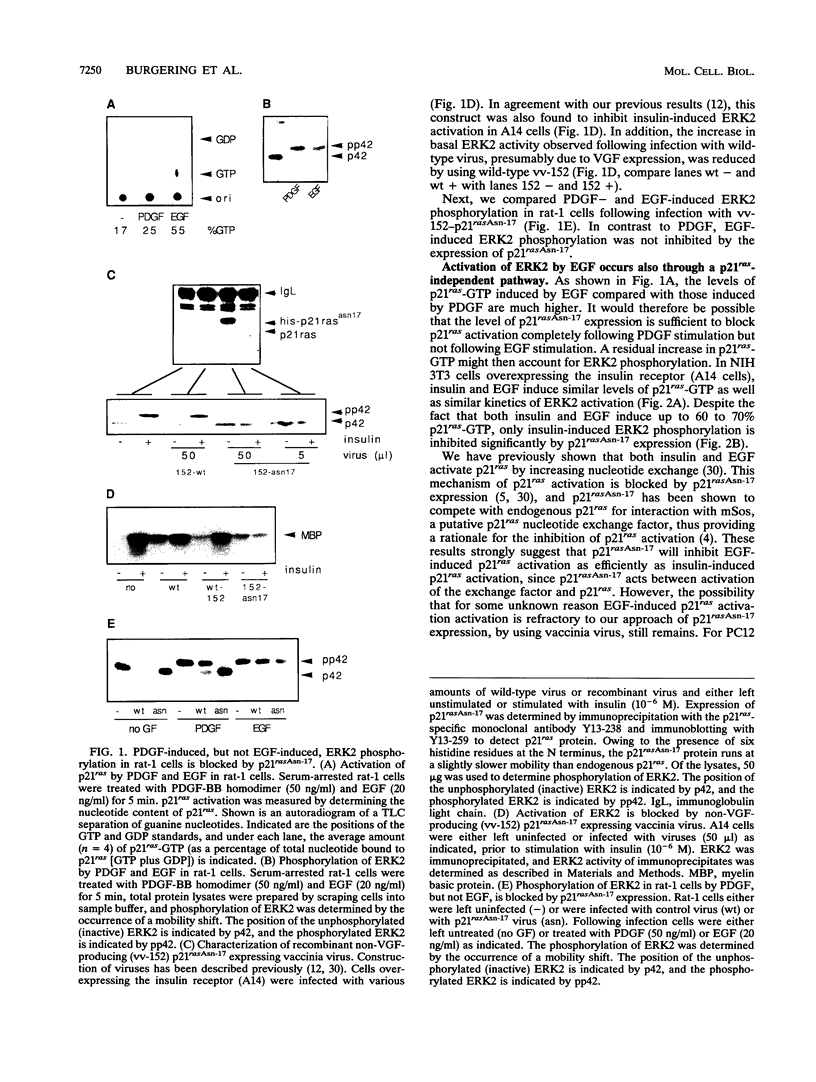
Expression of p21rasAsn-17, a dominant negative mutant of p21ras that blocks p21ras activation by growth factors, inhibits activation of extracellular signal-regulated kinase 2 (ERK2) by insulin and platelet-derived growth factor in rat-1 cells [A. M. M. de Vries-Smits, B. M. T. Burgering, S. J. Leevers, C. J. Marshall, and J. L. Bos, Nature (London) 357:602-604, 1992]. Here we report that expression of p21rasAsn-17 does not abolish epidermal growth factor (EGF)-induced phosphorylation of ERK2 in fibroblasts. Since EGF activates p21ras in these cells, this indicates that EGF induces a p21ras-independent pathway for the phosphorylation of ERK2 as well. We investigated whether activation of protein kinase C (PKC) or increase in intracellular calcium could be involved in p21ras-independent signaling. In rat-1 cells, inhibition of either PKC, by prolonged 12-O-tetradecanoylphorbol-13-acetate (TPA) pretreatment, or calcium influx, by ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA) pretreatment, did not abolish EGF-induced ERK2 phosphorylation. However, a combined inhibition of both p21ras and calcium influx, but not PKC, resulted in a complete inhibition of EGF-induced ERK2 phosphorylation. In contrast, in Swiss 3T3 cells, inhibition of both p21ras activation and TPA-sensitive PKC, but not calcium influx, inhibited EGF-induced ERK2 phosphorylation. These results demonstrate that in fibroblasts, EGF induces alternative pathways of ERK2 phosphorylation in a cell-type-specific manner.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackshear P. J., Witters L. A., Girard P. R., Kuo J. F., Quamo S. N. Growth factor-stimulated protein phosphorylation in 3T3-L1 cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1985 Oct 25;260(24):13304–13315. [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993 May 7;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates the exchange rate of guanine nucleotides on p21ras in fibroblasts. Mol Cell Biol. 1993 Mar;13(3):1903–1910. doi: 10.1128/mcb.13.3.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Chakrabarti S., Moss B., Fredrickson T. Cell proliferative response to vaccinia virus is mediated by VGF. Virology. 1988 May;164(1):182–192. doi: 10.1016/0042-6822(88)90635-6. [DOI] [PubMed] [Google Scholar]
- Burgering B. M., Medema R. H., Maassen J. A., van de Wetering M. L., van der Eb A. J., McCormick F., Bos J. L. Insulin stimulation of gene expression mediated by p21ras activation. EMBO J. 1991 May;10(5):1103–1109. doi: 10.1002/j.1460-2075.1991.tb08050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgering B. M., Snijders A. J., Maassen J. A., van der Eb A. J., Bos J. L. Possible involvement of normal p21 H-ras in the insulin/insulinlike growth factor 1 signal transduction pathway. Mol Cell Biol. 1989 Oct;9(10):4312–4322. doi: 10.1128/mcb.9.10.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao T. S., Byron K. L., Lee K. M., Villereal M., Rosner M. R. Activation of MAP kinases by calcium-dependent and calcium-independent pathways. Stimulation by thapsigargin and epidermal growth factor. J Biol Chem. 1992 Oct 5;267(28):19876–19883. [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews C. M., Alessandrini A., Erikson R. L. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992 Oct 16;258(5081):478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gutierrez L., Magee A. I., Marshall C. J., Hancock J. F. Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. EMBO J. 1989 Apr;8(4):1093–1098. doi: 10.1002/j.1460-2075.1989.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Hoshi M., Nishida E., Sakai H. Characterization of a mitogen-activated, Ca2+-sensitive microtubule-associated protein-2 kinase. Eur J Biochem. 1989 Sep 15;184(2):477–486. doi: 10.1111/j.1432-1033.1989.tb15040.x. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Izquierdo M., Downward J., Graves J. D., Cantrell D. A. Role of protein kinase C in T-cell antigen receptor regulation of p21ras: evidence that two p21ras regulatory pathways coexist in T cells. Mol Cell Biol. 1992 Jul;12(7):3305–3312. doi: 10.1128/mcb.12.7.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan P. L., Ozanne B. Cellular responsiveness to growth factors correlates with a cell's ability to express the transformed phenotype. Cell. 1983 Jul;33(3):931–938. doi: 10.1016/0092-8674(83)90036-3. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Protein kinase C mediates platelet-derived growth factor-induced tyrosine phosphorylation of p42. J Cell Biol. 1988 Apr;106(4):1395–1402. doi: 10.1083/jcb.106.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Kochs G., Hummel R., Vahidi H., Mischak H., Finkenzeller G., Marmé D., Rapp U. R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993 Jul 15;364(6434):249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Lloyd P., Rapp U. R. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991 Jan 31;349(6308):426–428. doi: 10.1038/349426a0. [DOI] [PubMed] [Google Scholar]
- Kosako H., Nishida E., Gotoh Y. cDNA cloning of MAP kinase kinase reveals kinase cascade pathways in yeasts to vertebrates. EMBO J. 1993 Feb;12(2):787–794. doi: 10.1002/j.1460-2075.1993.tb05713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Sturgill T. W., Weber M. J. Defective regulation of mitogen-activated protein kinase activity in a 3T3 cell variant mitogenically nonresponsive to tetradecanoyl phorbol acetate. Mol Cell Biol. 1991 Feb;11(2):1002–1008. doi: 10.1128/mcb.11.2.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Leevers S. J., Marshall C. J. Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J. 1992 Feb;11(2):569–574. doi: 10.1002/j.1460-2075.1992.tb05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F. Signal transduction. How receptors turn Ras on. Nature. 1993 May 6;363(6424):15–16. doi: 10.1038/363015a0. [DOI] [PubMed] [Google Scholar]
- Medema R. H., Burgering B. M., Bos J. L. Insulin-induced p21ras activation does not require protein kinase C, but a protein sensitive to phenylarsine oxide. J Biol Chem. 1991 Nov 5;266(31):21186–21189. [PubMed] [Google Scholar]
- Medema R. H., de Vries-Smits A. M., van der Zon G. C., Maassen J. A., Bos J. L. Ras activation by insulin and epidermal growth factor through enhanced exchange of guanine nucleotides on p21ras. Mol Cell Biol. 1993 Jan;13(1):155–162. doi: 10.1128/mcb.13.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodie S. A., Willumsen B. M., Weber M. J., Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993 Jun 11;260(5114):1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- Muldoon L. L., Enslen H., Rodland K. D., Magun B. E. Stimulation of Ca2+ influx by endothelin-1 is subject to negative feedback by elevated intracellular Ca2+. Am J Physiol. 1991 Jun;260(6 Pt 1):C1273–C1281. doi: 10.1152/ajpcell.1991.260.6.C1273. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. F., Weber M. J., Sturgill T. W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991 Apr;10(4):885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppelenbosch M. P., Tertoolen L. G., den Hertog J., de Laat S. W. Epidermal growth factor activates calcium channels by phospholipase A2/5-lipoxygenase-mediated leukotriene C4 production. Cell. 1992 Apr 17;69(2):295–303. doi: 10.1016/0092-8674(92)90410-e. [DOI] [PubMed] [Google Scholar]
- Posada J., Cooper J. A. Requirements for phosphorylation of MAP kinase during meiosis in Xenopus oocytes. Science. 1992 Jan 10;255(5041):212–215. doi: 10.1126/science.1313186. [DOI] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill T. W., Wu J. Recent progress in characterization of protein kinase cascades for phosphorylation of ribosomal protein S6. Biochim Biophys Acta. 1991 May 17;1092(3):350–357. doi: 10.1016/s0167-4889(97)90012-4. [DOI] [PubMed] [Google Scholar]
- Szeberényi J., Cai H., Cooper G. M. Effect of a dominant inhibitory Ha-ras mutation on neuronal differentiation of PC12 cells. Mol Cell Biol. 1990 Oct;10(10):5324–5332. doi: 10.1128/mcb.10.10.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sözeri O., Vollmer K., Liyanage M., Frith D., Kour G., Mark G. E., 3rd, Stabel S. Activation of the c-Raf protein kinase by protein kinase C phosphorylation. Oncogene. 1992 Nov;7(11):2259–2262. [PubMed] [Google Scholar]
- Thomas G. MAP kinase by any other name smells just as sweet. Cell. 1992 Jan 10;68(1):3–6. doi: 10.1016/0092-8674(92)90199-m. [DOI] [PubMed] [Google Scholar]
- Warne P. H., Viciana P. R., Downward J. Direct interaction of Ras and the amino-terminal region of Raf-1 in vitro. Nature. 1993 Jul 22;364(6435):352–355. doi: 10.1038/364352a0. [DOI] [PubMed] [Google Scholar]
- Wood K. W., Sarnecki C., Roberts T. M., Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992 Mar 20;68(6):1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- Zhang X. F., Settleman J., Kyriakis J. M., Takeuchi-Suzuki E., Elledge S. J., Marshall M. S., Bruder J. T., Rapp U. R., Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993 Jul 22;364(6435):308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]
- de Vries-Smits A. M., Burgering B. M., Leevers S. J., Marshall C. J., Bos J. L. Involvement of p21ras in activation of extracellular signal-regulated kinase 2. Nature. 1992 Jun 18;357(6379):602–604. doi: 10.1038/357602a0. [DOI] [PubMed] [Google Scholar]