Abstract
Quantitative PCR on nasopharyngeal aspirate (NPA) can achieve high sensitivity and specificity in diagnosing Pneumocystis pneumonia (PCP) compared to microscopic examination of bronchoscopic specimens in a population with low HIV prevalence. Since NPA is a minimally invasive procedure, it is ideal as a screening test for PCP.
TEXT
Currently, microbiological confirmation of Pneumocystis pneumonia (PCP) requires the identification of Pneumocystis cysts or trophozoites using microscopy. Bronchoscopic specimens are preferred because Pneumocystis resides mainly in the alveolar space (1). However, both transbronchoscopic bronchoalveolar lavage and sputum induction pose significant risk to the patient (2–4). Upper respiratory tract specimens are not recommended because of low sensitivity when traditional microscopic examination is used (5). PCR has higher sensitivity than microscopic examination in the detection of Pneumocystis (6) and may overcome the problem of low sensitivity associated with microscopic examination of upper respiratory tract specimens. In this study, we sought to determine whether nasopharyngeal aspirate (NPA) specimens could be used for the diagnosis of PCP.
Patients of the Hong Kong West Cluster Hospitals with bronchoscopic specimens (bronchoalveolar lavage fluid [BALF] or bronchial aspirate) submitted to our laboratory for Pneumocystis examination from 1 January 2010 to 31 March 2012 were identified using the laboratory information system. In our laboratory, Pneumocystis cysts were visualized with methenamine silver stain by using standard procedures (7). With this method, Pneumocystis trophozoites or sporozoites could not be detected. Archived NPA specimens from these patients collected during the same hospitalization were retrieved. Only the earliest NPA specimen was tested if multiple specimens were available from the same patient. NPA specimens were collected as described previously (8). Clinical and laboratory data were obtained using the clinical management system. Patients without archived NPA specimens were excluded. A patient was considered to have definite PCP if Pneumocystis was identified in the bronchoscopic specimen by microscopic examination (9). This study has been approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster.
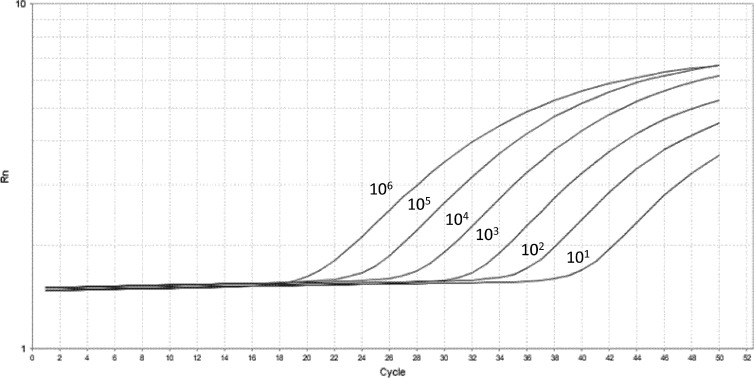
DNA was extracted from 200 μl of NPA specimens and from other fungi using the QIAamp DNA kit (Qiagen, Hilden, Germany) and DNeasy plant minikit (Qiagen, Hilden, Germany), respectively, according to the manufacturer's instructions (10). Real-time quantitative PCR (qPCR) targeting a 124-bp fragment of the mitochondrial large subunit rRNA (mt LSU rRNA) gene of Pneumocystis jirovecii was performed as described previously (11). Plasmid suspensions were used as standards for quantification and positive controls (Fig. 1).
Fig 1.
Standard curve for quantitative analysis of Pneumocystis jirovecii mt LSU rRNA.
We verified the positive results from the mitochondrial (mt) large-subunit (LSU) rRNA qPCR (LSU-qPCR) by a conventional PCR targeting the mitochondrial small-subunit rRNA (mt SSU rRNA) gene of P. jirovecii. A 220-bp fragment of the mt SSU rRNA of P. jirovecii was amplified using 1 μM forward primer (5′-ACCCACGATAAATCTTACCACTTC-3′) and reverse primer (5′-AGCACGTCTGTAGCCCACTT-3′). The PCR mixture (25 μl) contained DNA, PCR buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 3 mM MgCl2), 0.4 mM each deoxynucleoside triphosphates (dNTPs), and 0.625U Taq polymerase (Applied Biosystems, Branchburg, NJ). The mixtures were amplified at 95°C for 10 min; 45 cycles of 94°C for 1 min, 63°C for 1 min, and 72°C for 1 min; and a final extension at 72°C for 10 min in an automated thermal cycler (Applied Biosystems, Foster City, CA). PCR products were purified and sequenced as described previously (12).
All statistical analyses were performed using PASW Statistics 18 and VassarStats (http://vassarstats.net/). Sensitivity, specificity, positive predictive value, and negative predictive value were determined using the standard formula (13, 14). A P value of <0.05 was considered to represent statistical significance.
During the study period, a total of 416 bronchoscopic specimens (382 BALF and 34 bronchial aspirate specimens) from 317 patients were submitted to our laboratory for Pneumocystis examination, among which Pneumocystis was detected in 27 patients by microscopy. Archived NPA specimens were available for 117 patients, and only these patients were included in subsequent analysis (Table 1). NPA specimens were collected from 7 days before to 2 days after the collection of the first bronchoscopic specimen. Pneumocystis was detected in bronchoscopic specimens by microscopic examination in 15 patients (14 from BALF, 1 from bronchial aspirate). Among these patients, the median duration from hospital admission to diagnosis was 2 days (range, 1 to 10 days). Eight (53%) out of the 15 patients with Pneumocystis detected by microscopic examination of bronchoscopic specimens were non-HIV-immunocompromised patients.
Table 1.
Comparison of demographics and clinical characteristics between patients with and without PCP as determined by microscopic examination of bronchoscopic specimens
| Characteristic | Value |
P valueb | |
|---|---|---|---|
| PCP (n = 15) | Non-PCPa (n = 102) | ||
| Median age (yr) (interquartile range) | 46 (41–56) | 59 (47–71) | 0.012 |
| No. (%) of females | 6 (40) | 48 (47) | 0.783 |
| No. (%) of patients with underlying disease | |||
| HIV | 7 (47) | 3 (3) | <0.001 |
| Chronic obstructive pulmonary disease | 0 (0) | 8 (8) | 0.594 |
| Bronchiectasis | 0 (0) | 5 (5) | 1.000 |
| Interstitial lung disease | 1 (7) | 10 (10) | 1.000 |
| Lung malignancy | 0 (0) | 3 (3) | 1.000 |
| Active tuberculosis | 1 (7) | 5 (5) | 0.569 |
| Diabetes mellitus | 1 (7) | 16 (16) | 0.694 |
| Solid organ tumor | 0 (0) | 9 (9) | 0.602 |
| Hematological malignancy | 0 (0) | 22 (22) | 0.071 |
| Transplant recipient | 7 (47) | 26 (26) | 0.123 |
| Connective tissue disease | 1 (7) | 17 (17) | 0.461 |
| Immunosuppressive drugs | 8 (53) | 49 (48) | 0.786 |
| Cardiovascular disease | 1 (7) | 23 (23) | 0.301 |
| Liver disease | 3 (20) | 12 (12) | 0.407 |
| Renal disease | 5 (33) | 16 (16) | 0.142 |
| Neurological disease | 1 (7) | 12 (12) | 1.000 |
| Median lymphocyte count, × 109 cells/liter (interquartile range) | 0.7 (0.4–1.1) | 0.6 (0.4–1.1) | 0.545 |
Pneumonia with no causative organism identified (26 patients), bacterial pneumonia (26 patients), viral pneumonia (12 patients), culture-confirmed tuberculosis or empirical antituberculosis treatment (10 patients), culture-confirmed fungal pneumonia or empirical antifungal treatment (6 patients), empirical treatment for PCP (3 patients), bronchiolitis obliterans organizing pneumonia (7 patients), interstitial lung disease (3 patients), organizing pneumonia (3 patients), graft versus host disease (2 patients), gold-induced pneumonitis (1 patient), idiopathic pneumonia syndrome (1 patient), immune reconstitution inflammatory syndrome (1 patient), and flare-up of Churg-Strauss disease (1 patient).
Fisher's exact test was used to compare categorical variables, whereas Mann-Whitney U test was used for continuous variables.
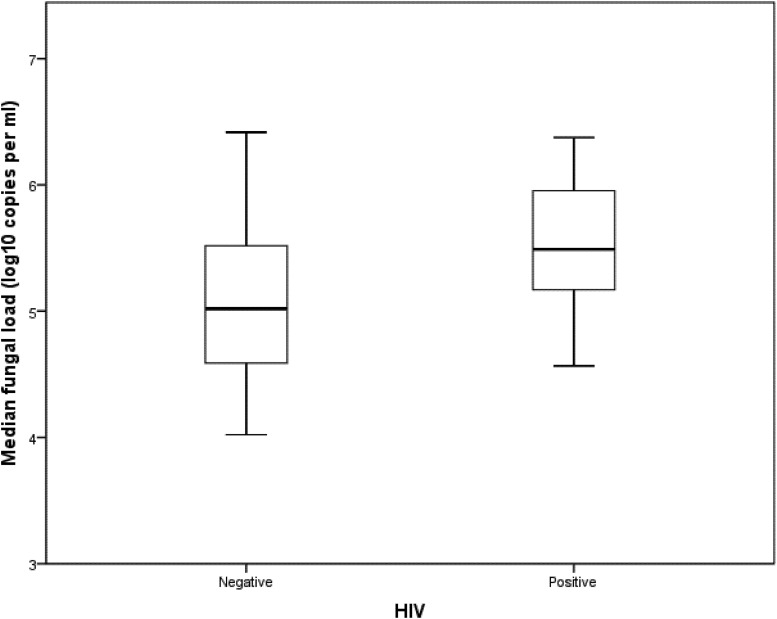
The detection limit for LSU-qPCR was 10 copies per reaction of extracted DNA, corresponding to a cycle threshold value of 41. LSU-qPCR was negative on the DNA extracted from Candida albicans, Cryptococcus neoformans, Saccharomyces cerevisiae, and Aspergillus fumigatus. Compared to microscopic examination of the bronchoscopic specimens, LSU-qPCR of NPA specimens had a sensitivity, specificity, positive predictive value, and negative predictive value of 100% (95% confidence interval [CI], 75% to 100%), 96.1% (95% CI, 90% to 99%), 78.9% (95% CI, 54% to 93%), and 100% (95% CI, 95% to 100%), respectively (Table 2). All NPA specimens positive for Pneumocystis using LSU-qPCR were further confirmed to be positive using mt SSU rRNA conventional PCR (SSU-PCR), while 8.2% (8/98) of NPA specimens negative for LSU-qPCR were positive by SSU-PCR. In true positive cases where bronchoscopic specimens were positive by microscopy and NPA specimens were positive by LSU-qPCR and SSU-PCR, the median Pneumocystis load in NPA specimens was lower in non-HIV than in HIV patients, though not reaching statistical significance (P = 0.203; Fig. 2).
Table 2.
Performance of LSU-qPCR in NPA specimens for the diagnosis of PCP, with microscopic examination of bronchoscopic specimens as the gold standard
| LSU-qPCR in NPA specimens | No. of specimens (microscopic examination of bronchoscopic specimens using methenamine silver staining) |
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 15 | 4 | 19 |
| Negative | 0 | 98 | 98 |
| Total | 15 | 102 | 117 |
Fig 2.
Comparison of Pneumocystis load between HIV-positive and -negative patients. Mann-Whitney U test was used for statistical analysis. P = 0.203.
Four patients were negative for Pneumocystis by microscopy of the BALF specimen but positive by LSU-qPCR in the NPA specimens (Table 3). For all four NPA specimens, SSU-PCR was positive, and sequencing of PCR products showed 99 to 100% nucleotide identity with the P. jirovecii mt SSU rRNA gene (GenBank accession no. HQ228547.1). Notably, two of these patients were treated with cotrimoxazole due to treatment failure with broad-spectrum antibacterial coverage and a high clinical suspicion of PCP despite negative BALF microscopy result. Both patients improved clinically after empirical treatment with cotrimoxazole. Moreover, these two patients had higher Pneumocystis loads than the other two patients who did not receive specific treatment against Pneumocystis.
Table 3.
Clinical characteristics of four patients with negative BAL staining and positive NPA PCR for Pneumocystis
| Patient no. | Sexh/age (yr) | Underlying disease | Pulmonary radiological appearance | Lymphocyte count (× 109 cells/liter) | Anti-Pneumocystis treatment | Pneumocystis load (copies/ml) |
|---|---|---|---|---|---|---|
| 1 | F/52 | Systemic lupus erythematosus/scleroderma | Patchy ground glass densities and tiny lung nodules at bilateral upper lobesa | 0.53 | Nild | 4.68 × 104 |
| 2 | M/63 | Immunoblastic T cell lymphoma, receiving chemotherapy | Bilateral symmetrical airspace opacities, particularly at the lower zonesb | 0.83 | Given cotrimoxazole with clinical improvemente | 8.88 × 104 |
| 3 | F/13 | Juvenile dermatomyositis | Bilateral symmetrical airspace and reticular opacities, predominantly involving perihilar and lower zonesb | 0.88 | Given cotrimoxazole with clinical improvementf | 1.08 × 105 |
| 4 | F/54 | Lung adenocarcinoma with metastasis to brain, hypertension | Multifocal bilateral patchy consolidation with upper zone predominancec | 0.60 | Nilg | 3.59 × 104 |
Positron emission tomography/computed tomography.
Chest radiograph.
Computed tomography.
Clinically improved after receiving empirical tuberculosis treatment for suspected pulmonary tuberculosis.
Received imipenem-cilastatin and ticarcillin-clavulanate before cotrimoxazole therapy.
Received amoxicillin-clavulanate and azithromycin before cotrimoxazole therapy.
Clinically improved after receiving piperacillin-tazobactam and doxycycline for the treatment of community-acquired pneumonia.
F, female; M, male.
Our study shows that LSU-qPCR in NPA specimens has high sensitivity, specificity, positive predictive value, and negative predictive value for the diagnosis of PCP compared with microscopic examination of bronchoscopic specimens. NPA specimens have several advantages over bronchoscopic specimens. First, NPA is a technically simple procedure and therefore feasible even in busy clinical settings (15, 16). Second, since NPA specimens are often collected upon admission in patients with upper or lower respiratory tract infections, the use of NPA specimens for diagnosis of PCP can potentially minimize the delay compared to when bronchoscopic specimens are used. Third, obtaining NPA specimens is less invasive than obtaining bronchoscopic specimens and therefore is associated with less patient discomfort and a lower risk of complications (17).
In this study, 8.2% of NPA specimens were LSU-qPCR negative but SSU-PCR positive, suggesting that the SSU-PCR could be oversensitive in the diagnosis of PCP. Four patients have false-positive NPA LSU-qPCR and SSU-PCR results compared with microscopic examination of BALF specimens. The patients who were given cotrimoxazole due to high clinical suspicion of PCP had higher Pneumocystis loads than those without specific treatment for Pneumocystis. The result suggests that patients with higher Pneumocystis load in NPA specimens have a higher likelihood of true PCP rather than mere colonization.
The use of upper respiratory tract specimens in the diagnosis of Pneumocystis infection has been evaluated in previous studies (18–24). Our study differs from these studies in several aspects. First, our study is the first to assess a population consisting of mainly adult non-HIV patients, with less than 10% of HIV-positive patients in the studied population. Second, some of the previous studies used PCR in the BALF as the gold standard for diagnosis, which may be oversensitive due to frequent colonization of Pneumocystis in immunocompromised patients.
This is the first study to evaluate the performance of qPCR in NPA specimens for the microbiological confirmation of PCP encompassing mainly non-HIV patients. NPA specimens are superior to bronchoscopic specimens in their ease of collection and low associated risks, making them preferable to bronchoscopic specimens in routine clinical settings. Bronchoscopic procedures should be reserved for patients in whom NPA specimens cannot settle the diagnosis.
Nucleotide sequence accession numbers.
The nucleotide sequences obtained in this study have been deposited within the GenBank sequence database (accession numbers JX567346, JX567347, JX567348, and JX567349).
ACKNOWLEDGMENTS
This work is partly supported by donations from Hui Hoy and Chow Sin Lan Charity Fund; the Hong Kong Special Administrative Region Research Fund for the Control of Infectious Diseases of the Food and Health Bureau; the Providence Foundation Limited, in memory of the late Lui Hac Minh; and National Science and Technology Major Project of China (grant 2012ZX10004-213-002).
We thank Siddharth Sridhar for comments on the manuscript.
The authors declare no conflicts of interests.
Footnotes
Published ahead of print 13 February 2013
REFERENCES
- 1. del Rio C, Barragan M, Franco-Paredes C. 2004. Pneumocystis pneumonia. N. Engl. J. Med. 351:1262–1263 [DOI] [PubMed] [Google Scholar]
- 2. Sakamoto K, Taniguchi H, Kondoh Y, Wakai K, Kimura T, Kataoka K, Hashimoto N, Nishiyama O, Hasegawa Y. 2012. Acute exacerbation of IPF following diagnostic bronchoalveolar lavage procedures. Respir. Med. 106:436–442 [DOI] [PubMed] [Google Scholar]
- 3. Ramirez P, Valencia M, Torres A. 2007. Bronchoalveolar lavage to diagnose respiratory infections. Semin. Respir. Crit. Care Med. 28:525–533 [DOI] [PubMed] [Google Scholar]
- 4. Bell D, Leckie V, McKendrick M. 2004. Use of the induced sputum procedure in the investigation of smear-negative pulmonary tuberculosis. Clin. Infect. Dis. 38:1509–1510 [DOI] [PubMed] [Google Scholar]
- 5. Ruffini DD, Madhi SA. 2002. The high burden of Pneumocystis carinii pneumonia in African HIV-1-infected children hospitalized for severe pneumonia. AIDS 16:105–112 [DOI] [PubMed] [Google Scholar]
- 6. Rabodonirina M, Raffenot D, Cotte L, Boibieux A, Mayencon M, Bayle G, Persat F, Rabatel F, Trepo C, Peyramond D, Piens MA. 1997. Rapid detection of Pneumocystis carinii in bronchoalveolar lavage specimens from human immunodeficiency virus-infected patients: use of a simple DNA extraction procedure and nested PCR. J. Clin. Microbiol. 35:2748–2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Versalovic J, Funke G, Landry ML, Warnock DW. 2011. Manual of clinical microbiology, 11 ed ASM Press, Washington, DC [Google Scholar]
- 8. Hung IF, Cheng VC, Wu AK, Tang BS, Chan KH, Chu CM, Wong MM, Hui WT, Poon LL, Tse DM, Chan KS, Woo PC, Lau SK, Peiris JS, Yuen KY. 2004. Viral loads in clinical specimens and SARS manifestations. Emerg. Infect. Dis. 10:1550–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Azoulay E, Bergeron A, Chevret S, Bele N, Schlemmer B, Menotti J. 2009. Polymerase chain reaction for diagnosing Pneumocystis pneumonia in non-HIV immunocompromised patients with pulmonary infiltrates. Chest 135:655–661 [DOI] [PubMed] [Google Scholar]
- 10. Chan KH, Yam WC, Pang CM, Chan KM, Lam SY, Lo KF, Poon LL, Peiris JS. 2008. Comparison of the NucliSens easyMAG and Qiagen BioRobot 9604 nucleic acid extraction systems for detection of RNA and DNA respiratory viruses in nasopharyngeal aspirate samples. J. Clin. Microbiol. 46:2195–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. To KK, Hung IF, Xu T, Poon RW, Ip WC, Li PT, Li CP, Lau SK, Yam WC, Chan KH, Yuen KY. 2013. Clinical significance of Pneumocystis jirovecii in patients with active tuberculosis. Diagn. Microbiol. Infect. Dis. 75:260–265 [DOI] [PubMed] [Google Scholar]
- 12. To KK, Wong SS, Poon RW, Trendell-Smith NJ, Ngan AH, Lam JW, Tang TH, Ahchong AK, Kan JC, Chan KH, Yuen KY. 2012. A novel Dirofilaria species causing human and canine infections in Hong Kong. J. Clin. Microbiol. 50:3534–3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Altman DG, Bland JM. 1994. Diagnostic tests 2: predictive values. BMJ 309:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Altman DG, Bland JM. 1994. Diagnostic tests. 1: sensitivity and specificity. BMJ 308:1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. To KK, Hung IF, Li IW, Lee KL, Koo CK, Yan WW, Liu R, Ho KY, Chu KH, Watt CL, Luk WK, Lai KY, Chow FL, Mok T, Buckley T, Chan JF, Wong SS, Zheng B, Chen H, Lau CC, Tse H, Cheng VC, Chan KH, Yuen KY. 2010. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin. Infect. Dis. 50:850–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. To KK, Chan KH, Fung YF, Yuen KY, Ho PL. 2010. Azithromycin treatment failure in macrolide-resistant Mycoplasma pneumoniae pneumonia. Eur. Respir. J. 36:969–971 [DOI] [PubMed] [Google Scholar]
- 17. Rano A, Agusti C, Jimenez P, Angrill J, Benito N, Danes C, Gonzalez J, Rovira M, Pumarola T, Moreno A, Torres A. 2001. Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using non-invasive and bronchoscopic procedures. Thorax 56:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Samuel CM, Whitelaw A, Corcoran C, Morrow B, Hsiao NY, Zampoli M, Zar HJ. 2011. Improved detection of Pneumocystis jirovecii in upper and lower respiratory tract specimens from children with suspected Pneumocystis pneumonia using real-time PCR: a prospective study. BMC Infect. Dis. 11:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Graham SM, Mankhambo L, Phiri A, Kaunda S, Chikaonda T, Mukaka M, Molyneux EM, Carrol ED, Molyneux ME. 2011. Impact of human immunodeficiency virus infection on the etiology and outcome of severe pneumonia in Malawian children. Pediatr. Infect. Dis. J. 30:33–38 [DOI] [PubMed] [Google Scholar]
- 20. Helweg-Larsen J, Jensen JS, Benfield T, Svendsen UG, Lundgren JD, Lundgren B. 1998. Diagnostic use of PCR for detection of Pneumocystis carinii in oral wash samples. J. Clin. Microbiol. 36:2068–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Respaldiza N, Montes-Cano MA, Friaza V, Munoz-Lobato F, Medrano FJ, Varela JM, Calderon E, De la Horra C. 2006. Usefulness of oropharyngeal washings for identifying Pneumocystis jirovecii carriers. J. Eukaryot. Microbiol. 53:S100–S101 [DOI] [PubMed] [Google Scholar]
- 22. Matos O, Costa MC, Lundgren B, Caldeira L, Aguiar P, Antunes F. 2001. Effect of oral washes on the diagnosis of Pneumocystis carinii pneumonia with a low parasite burden and on detection of organisms in subclinical infections. Eur. J. Clin. Microbiol. Infect. Dis. 20:573–575 [DOI] [PubMed] [Google Scholar]
- 23. Fischer S, Gill VJ, Kovacs J, Miele P, Keary J, Silcott V, Huang S, Borio L, Stock F, Fahle G, Brown D, Hahn B, Townley E, Lucey D, Masur H. 2001. The use of oral washes to diagnose Pneumocystis carinii pneumonia: a blinded prospective study using a polymerase chain reaction-based detection system. J. Infect. Dis. 184:1485–1488 [DOI] [PubMed] [Google Scholar]
- 24. Larsen HH, Huang L, Kovacs JA, Crothers K, Silcott VA, Morris A, Turner JR, Beard CB, Masur H, Fischer SH. 2004. A prospective, blinded study of quantitative touch-down polymerase chain reaction using oral-wash samples for diagnosis of Pneumocystis pneumonia in HIV-infected patients. J. Infect. Dis. 189:1679–1683 [DOI] [PubMed] [Google Scholar]