Abstract
The use of matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) for rapid detection and identification of the enzymes responsible for carbapenem resistance in Acinetobacter spp. appears as a promising option, but it will be necessary to have a standardized protocol that facilitates routine use. Based on the results reported herein and comparisons of several previously published reports, we identified the significant peaks for imipenem detection. Optimal bacterial inoculum and incubation time were established, and results obtained with and without dipicolinic acid (DPA) and Zn2+ allowed us to distinguish between metallo-beta-lactamases and oxacillinases.
TEXT
Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) has been routinely used for bacterial identification in many clinical laboratories, and recently several authors have validated this technology to detect antibiotic resistance (1–5). Most of these studies have been focused on the group of beta-lactam antibiotics, mainly carbapenems.
Carbapenems possess broad-spectrum activity and can be considered the last antibiotics reliably active against many multidrug-resistant Gram-negative bacteria, including Acinetobacter spp., with imipenem being the most active agent. However, carbapenem resistance has increasingly been reported (6, 7). Even so, only one previous work focuses on Acinetobacter baumannii (but not on other species in the genus) and imipenem (4).
These published studies were developed using nonuniform conditions both in regard to the solution and incubation times employed. The main peaks of the spectrum in which the analysis focused also varied from one study to another.
In order to develop a standardized method that can be routinely used in any clinical microbiology laboratory, the current study compares several of the previously described conditions and establishes the parameters for easily and quickly detecting and identifying Acinetobacter carbapenemases.
Seventy isolates of Acinetobacter spp. previously identified to species level by rpoB sequencing (8) were included in this study. MICs of imipenem and meropenem were determined according to CLSI guidelines by microdilution methods (9) and confirmed by Etest. Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922 were tested as controls.
PCRs for metallo-beta-lactamase (MBL) genes (blaIMP, blaVIM, blaSIM, and blaNDM), oxacillinase genes (blaOXA-23-like, blaOXA-24-like, blaOXA-51-like, and blaOXA-58), and the insertion sequence (IS) ISAba1 were performed for all isolates as previously described (10, 11).
The MICs of imipenem and meropenem and enzymes present in each isolate appear in Table 1.
Table 1.
Characteristics of Acinetobacter species isolates included in the study
| No. of isolates (control strain) | Acinetobacter species (no. of isolates)b | MIC (mg/ml)a |
Carbapenemase gene(s) detected | |
|---|---|---|---|---|
| Imipenem | Meropenem | |||
| 2 (A93) | A. bereziniae/AG10 | 32 | 4 | blaIMP |
| 10 | A. baumannii | 256–512 | 256 | blaOXA23-like, blaOXA51-like |
| 9 (A26) | A. baumannii | 256–512 | 256 | blaOXA24-like, blaOXA51-like |
| 15 (A31) | A. baumannii | 32–64 | 4 | blaOXA58, blaOXA51-like |
| 8 | A. baumannii | 512 | 128 | blaOXA51-like-ISAba1 |
| 8 | A. baumannii | 32 | 8 | blaOXA51-like-ISAba1 |
| 5 | A. baumannii | 1 | 1 | blaOXA51-like-ISAba1 |
| 5 (A1 and A4) | A. baumannii | 2 | 0.5 | blaOXA51-like |
| 8 | A. bereziniae/AG10 (2), A. calcoaceticus (1), A. beijerinckii (1), A. soli (1), A. junii (1), A. pittii (1), AG14BJ (1) | 0.5–2 | 0.25–2 | None |
The breakpoint for both imipenem and meropenem is 4 mg/ml (CLSI criteria).
AG10, Acinetobacter genospecies 10; AG14BJ Acinetobacter genospecies 14BJ.
MALDI-TOF MS analysis was performed by analyzing all peaks in the range of 100 to 1,000 m/z in a Microflex mass spectrometer (Bruker Daltonik, Bremen, Germany). Data were processed with FlexControl, version 3.3, software. A total of 480 shots were acquired in automatic mode by random measurements. All experiments were repeated at least three times. Calibration and measurements were performed as previously described (5).
Commercially available imipenem that contains cilastatin (500 mg) (Tienam; MSD) was used for the experiments. Four different imipenem concentrations (0.25, 0.5, 1, and 5 mg/ml) were diluted in three testing solutions (0.46% NaCl, 20 mM Tris-HCl [pH 6.8], and 10 mM ammonium hydrogen citrate [pH 7.0]), all of them with and without 0.01% SDS. This compound has been used by others to enhance the visualization of degradation products (2). Incubation was carried out at 35°C under constant shaking (500 rpm).
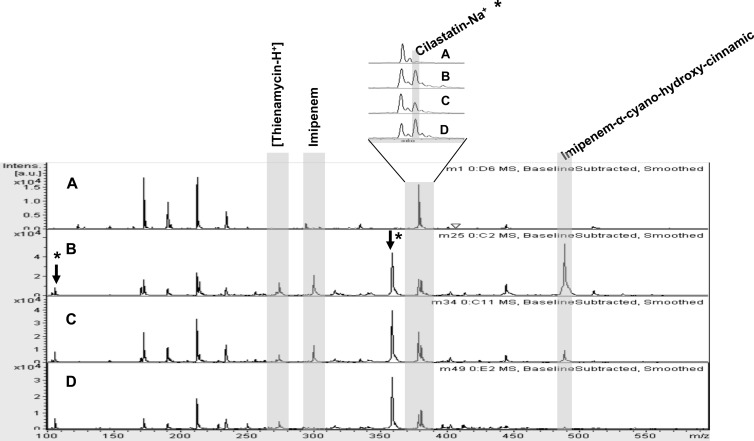
Initially, the characteristic mass spectrum of imipenem was established. Spectrum comparison of all solutions studied with and without imipenem-cilastatin allowed us to select eight peaks theoretically related to the antibiotic used. Only peaks common to all solutions and conditions were selected. Incubation with the IMP-8-producing Acinetobacter strain A93 caused a significant decrease in the intensity of four of those peaks: 274 m/z ([thienamycin-H+]), 300 m/z (imipenem), 489 m/z (imipenem–α-cyano-4-hydroxy-cinnamic acid), 658 m/z (imipenem-cilastatin) (Fig. 1). Peaks that were due to imipenem addition but were nonresponsive to carbapenem-resistant Acinetobacter incubation were assumed to be related to cilastatin present in the imipenem-cilastatin compound. The cilastatin peak at 381 Da (cilastatin-Na+) was identified and used for standardization. After data processing was performed, peaks at 300 and 489 m/z were selected to assess carbapenemase detection in further assays. The absolute signal intensity for 489 m/z was the strongest signal in all the assays regardless of the conditions used. The remaining peaks observed were detected with weak signals. Alpha-cyano-4-hydroxycinnamic acid as a matrix facilitates the ionization of compounds in MALDI-TOF MS, but it is important to considerer that the matrix itself also ionizes, and so do adducts. However, matrix adducts are not usually considered.
Fig 1.
MALDI-TOF MS spectra showing the most representative imipenem peaks. Spectra were determined with the incubation solution alone (0.46% NaCl) (A), imipenem-cilastatin solution (B), an OXA-24-like-producing Acinetobacter strain (C), and an IMP-8-producing Acinetobacter strain (D). Peaks represent [thienamycin-H+] at m/z 274, imipenem at m/z 300, cilastatin-Na+ at m/z 381, and imipenem–α-cyano-hydroxy-cinnamic acid at m/z 489. Arrows and asterisks show peaks due to imipenem-cilastatin addition that were nonresponsive to resistant Acinetobacter incubation. au, arbitrary units.
A decrease in the intensity of selected peaks could readily be detected at three different sample amounts (1 × 109, 2.5 × 109, and 2.5 × 1010 CFU/ml). An inverse relationship between incubation time and peak intensity was observed.
The same spectra were obtained with all antibiotic concentrations and all bacterial inocula tested. The main difference observed was related to the time required for the complete disappearance of antibiotic, but no significant differences were found in the number and complexity of the spectrum peaks.
To corroborate these results, the same experiments were conducted with two other carbapenem-resistant (strain A26, blaOXA-24-like; strain A31, blaOXA-58) and two carbapenem-susceptible (strains A1 and A4, both blaOXA-51-like) well-characterized Acinetobacter species strains. Samples prepared without imipenem and without microorganisms were included in all the assays as controls.
Once the data were processed, it was clear that the two selected peaks had a significant reduction after 1 h of incubation time with both resistant microorganisms under all the assay conditions tested. This demonstrates that the two selected peaks are good markers to detect carbapenemase activity.
When the effects of different assay solutions are compared, it can be seen that 0.46% NaCl and 20 mM Tris-HCl, pH 6.8, led to a higher stability of compounds throughout the incubation time than 10 mM ammonium hydrogen citrate, pH 7.0. For this reason the ammonium hydrogen citrate was not used in subsequent assays. SDS supplementation produced no change in the antibiotic spectrum in any instances (data not shown). A possible explanation suggested for this fact could be that the hydrolyzed products were very labile and therefore should be analyzed immediately after sample preparation (2). In addition, the difficulty of ionizing degradation products might result in their not being detected in the cellular supernatant (5).
On the basis of all results, a standard protocol was designed. The chosen imipenem concentration was 1 mg/ml, and the inocula were adjusted to achieve a final concentration of 2.5 × 1010 CFU/ml in each tube. The incubation time was reduced to 1 h at 35°C under constant shaking (500 rpm). No SDS (0.01%) was added to the buffer solutions.
Once the procedure had been successfully assayed in control strains, MALDI-TOF MS was performed on 65 selected strains based on the type of enzyme produced (Table 1).
All strains were analyzed in 0.46% NaCl and 20 mM Tris-HCl, pH 6.8, buffers. The spectra obtained with the two solutions were compared between themselves and with those obtained for the corresponding controls.
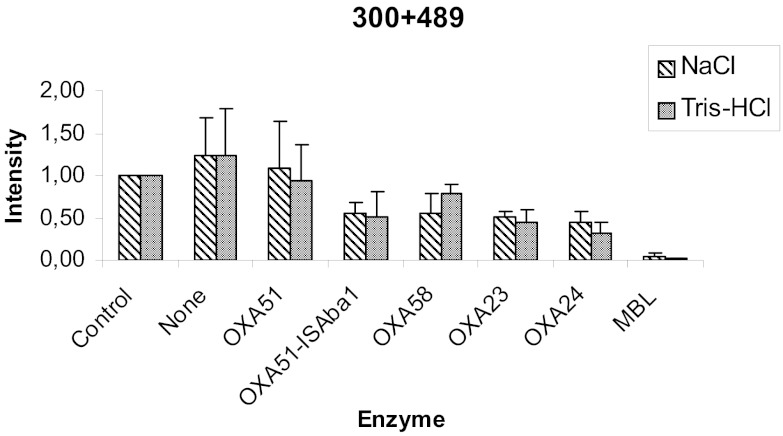
Results showed that after 1 h of incubation time, carbapenemase activity was observed for all the carbapenem-resistant strains tested with a significant reduction in the 300 and 489 m/z peaks. As other investigators have noted (1), imipenem hydrolysis was greater with the microorganisms that produced metallo-beta-lactamases than with those producing carbapenem-hydrolyzing class D (OXA-type) beta-lactamases. Moreover, regardless of the incubation solution used, significant differences were detected among different tested oxacillinases (Fig. 2).
Fig 2.
Representative comparison of the average intensities of the most representative peaks of imipenem (m/z 300 and 489) obtained after a 1-h incubation with the different carbapenemases included in the study. As controls, 1 mg/ml imipenem was used in both solutions with no microorganism.
The capacity of NaCl for oxacillinase inhibition has been mentioned previously, but no major effects were detected in our study.
Acinetobacter carbapenemase activity was then determined under Zn-rich and -chelated conditions to confirm the presence of metallo-beta-lactamases by MALDI-TOF MS. Samples were incubated with agitation (500 rpm) for 1 h at 35°C in 0.46% NaCl-imipenem unsupplemented or supplemented with 2 mM ZnSO4, 2 mM dipicolinic acid (DPA), or 2 mM DPA plus 2 mM ZnSO4. The procedure was repeated five times using fresh samples each time.
The comparative analysis of MBL- and OXA-type-producing Acinetobacter strains under the described conditions resulted in the inhibition of metallo-beta-lactamases by DPA. No effect was observed in OXA-type producers. The concentration of DPA used was not enough to completely inhibit the enzymatic activity. The 1-h DPA incubation assay reduced the activity to 70% relative to the activity observed for nontreated samples. Zinc addition to the incubation solution completely reversed the chelating agent action. Although these results have been obtained with Acinetobacter spp., DPA should have the same effect with other MBL producers.
This study resulted in the identification of the most representative peaks associated with imipenem, particularly those involved in carbapenemase activity, and could be useful to establish a standardized protocol that can be used in routine assays.
ACKNOWLEDGMENTS
We are very grateful to Faustino Huertas for chemistry advice. We thank Alvaro Gómez for technical assistance during this project.
The study was developed with resources of the Hospital Clínico San Carlos.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 27 February 2013
REFERENCES
- 1. Burckhardt I, Zimmermann S. 2011. Using matrix-assisted laser desorption ionization-time of flight mass spectrometry to detect carbapenem resistance within 1 to 2.5 hours. J. Clin. Microbiol. 49:3321–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hrabak J, Studentova V, Walkova R, Zemlickova H, Jakubu V, Chudackova E, Gniadkowski M, Pfeifer Y, Perry JD, Wilkinson K, Bergerova T. 2012. Detection of NDM-1, VIM-1, KPC, OXA-48, and OXA-162 carbapenemases by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 50:2441–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hrabak J, Walkova R, Studentova V, Chudackova E, Bergerova T. 2011. Carbapenemase activity detection by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 49:3222–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kempf M, Bakour S, Flaudrops C, Berrazeg M, Brunel JM, Drissi M, Mesli E, Touati A, Rolain JM. 2012. Rapid detection of carbapenem resistance in Acinetobacter baumannii using matrix-assisted laser desorption ionization-time of flight mass spectrometry. PLoS One 7:e31676 doi:10.1371/journal.pone.0031676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sparbier K, Schubert S, Weller U, Boogen C, Kostrzewa M. 2012. Matrix-assisted laser desorption ionization-time of flight mass spectrometry-based functional assay for rapid detection of resistance against beta-lactam antibiotics. J. Clin. Microbiol. 50:927–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu YM, Chen YS, Toh HS, Huang CC, Lee YL, Ho CM, Lu PL, Ko WC, Chen YH, Wang JH, Tang HJ, Yu KW, Liu YC, Chuang YC, Xu Y, Ni Y, Liu CE, Hsueh PR. 2012. In vitro susceptibilities of non-Enterobacteriaceae isolates from patients with intra-abdominal infections in the Asia-Pacific region from 2003 to 2010: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int. J. Antimicrob. Agents 40(Suppl):S11–S17 [DOI] [PubMed] [Google Scholar]
- 7. Kempf M, Rolain JM. 2012. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int. J. Antimicrob. Agents 39:105–114 [DOI] [PubMed] [Google Scholar]
- 8. Alvarez-Buylla A, Culebras E, Picazo JJ. 2012. Identification of Acinetobacter species: is Bruker biotyper MALDI-TOF mass spectrometry a good alternative to molecular techniques? Infect. Genet. Evol. 12:345–349 [DOI] [PubMed] [Google Scholar]
- 9. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supple-ment. Document M100-MS120. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10. Culebras E, Gonzalez-Romo F, Head J, Gomez M, Morales G, Picazo JJ. 2010. Outbreak of Acinetobacter baumannii producing OXA-66 in a Spanish hospital: epidemiology and study of patient movements. Microb. Drug Resist. 16:309–315 [DOI] [PubMed] [Google Scholar]
- 11. Villalon P, Valdezate S, Medina-Pascual MJ, Carrasco G, Vindel A, Saez-Nieto JA. 9 November 2012, posting date Epidemiology of the Acinetobacter-derived cephalosporinase, carbapenem-hydrolysing oxacillinase and metallo-beta-lactamase genes, and of common insertion sequences, in epidemic clones of Acinetobacter baumannii from Spain. J. Antimicrob. Chemother. doi:10.1093/jac/dks448 [DOI] [PubMed] [Google Scholar]