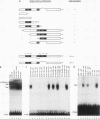
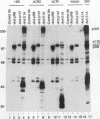
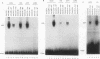
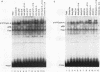
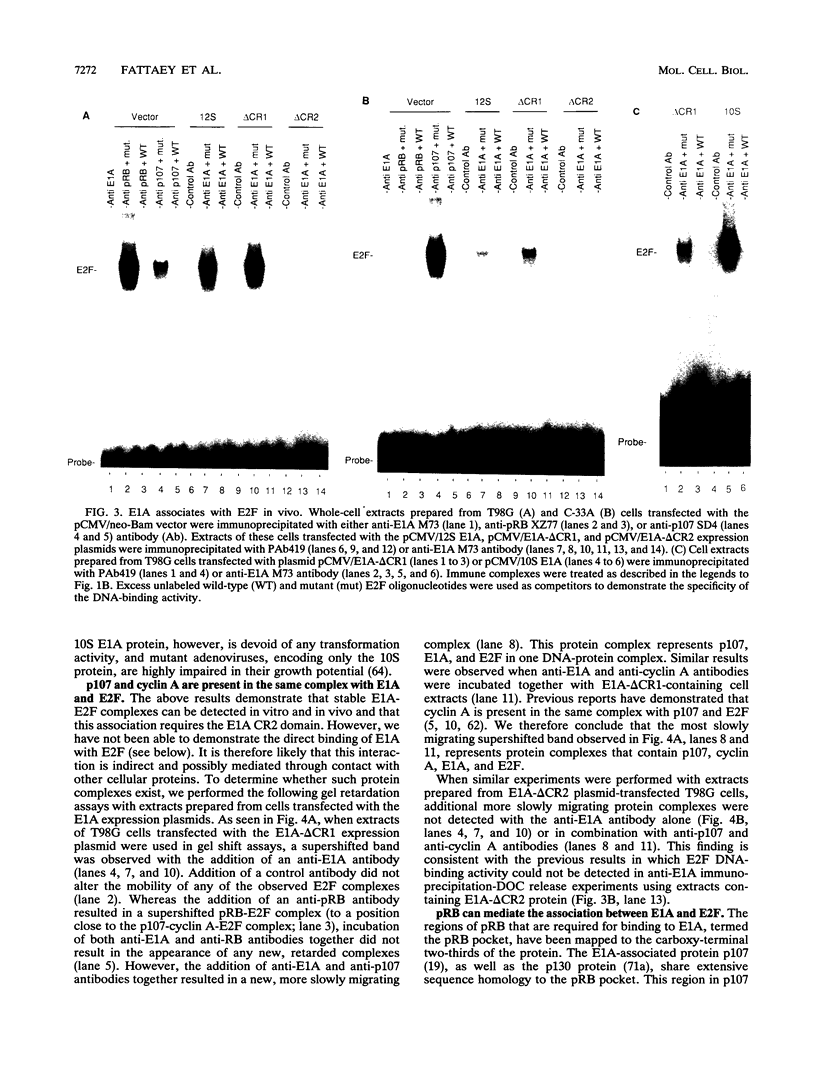
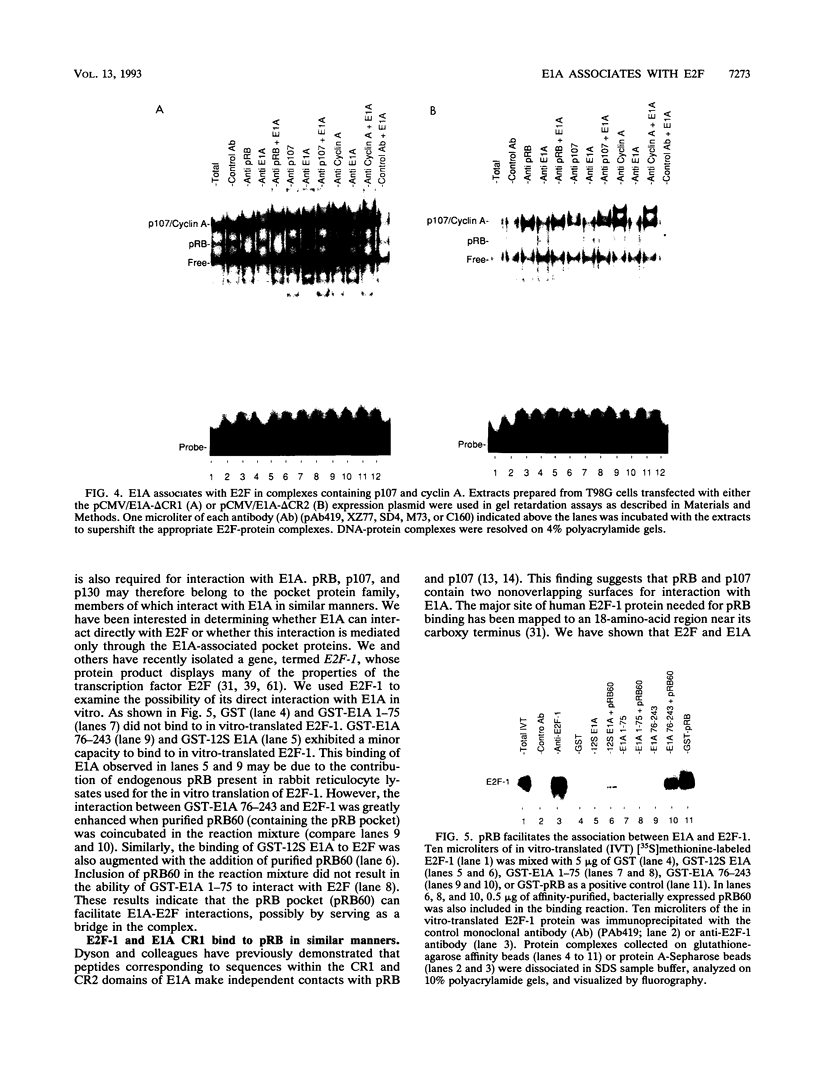
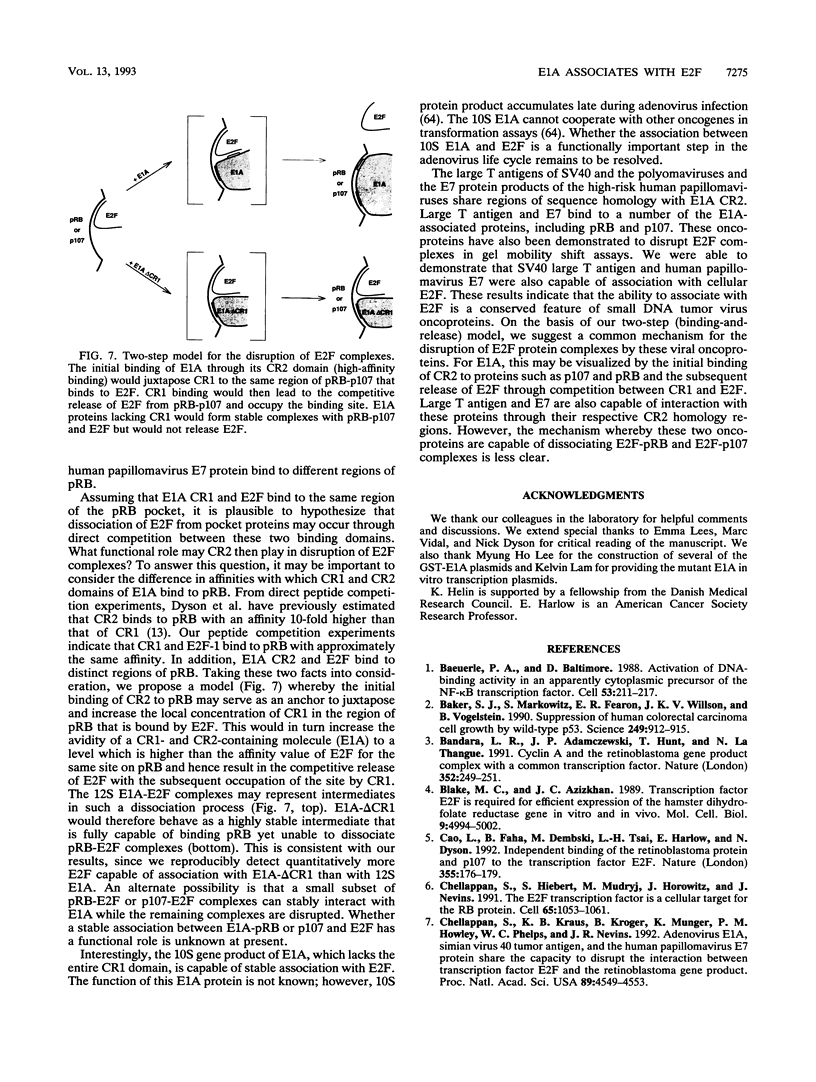
Abstract
The transcription factor E2F is present in independent complexes with the product of the retinoblastoma susceptibility gene, pRB, and a related gene product, p107, in association with the cyclin A-cdk2 or the cyclin E-cdk2 kinase complex. pRB and p107 can negatively regulate E2F activity, since overexpression of pRB or p107 in cells lacking a functional pRB leads to the repression of E2F activity. The products of the adenovirus E1A gene can disrupt E2F complexes and result in free and presumably active E2F transcription factor. The regions of E1A required for this function are also essential for binding to a number of cellular proteins, including pRB and p107. Through the use of a number of glutathione S-transferase fusion proteins representing different regions of E1A, as well as in vivo expression of E1A proteins containing deletions of either conserved region 1 (CR1) or CR2, we find that CR2 of E1A can form stable complexes with E2F. E1A proteins containing both CR1 and CR2 also associate with E2F, although the presence of these proteins results in the release of free E2F from its complexes. In vitro reconstitution experiments indicate that E1A-E2F interactions are not direct and that pRB can serve to facilitate these interactions. Complexes containing E1A, p107, cyclin A, and E2F were identified in vivo, which indicates that E1A may associate with E2F through either p107 or pRB. Peptide competition experiments demonstrate that the pRB-binding domain of the human E2F-1 protein can compete with the CR1 but not CR2 domain of E1A for binding to pRB. These results indicate that E1A CR1 and E2F-1 may bind to the same or overlapping sites on pRB and that E1A CR2 binds to an independent region. On the basis of our results, we propose a two-step model for the release of E2F from pRB and p107 cellular proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Bandara L. R., Adamczewski J. P., Hunt T., La Thangue N. B. Cyclin A and the retinoblastoma gene product complex with a common transcription factor. Nature. 1991 Jul 18;352(6332):249–251. doi: 10.1038/352249a0. [DOI] [PubMed] [Google Scholar]
- Blake M. C., Azizkhan J. C. Transcription factor E2F is required for efficient expression of the hamster dihydrofolate reductase gene in vitro and in vivo. Mol Cell Biol. 1989 Nov;9(11):4994–5002. doi: 10.1128/mcb.9.11.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Faha B., Dembski M., Tsai L. H., Harlow E., Dyson N. Independent binding of the retinoblastoma protein and p107 to the transcription factor E2F. Nature. 1992 Jan 9;355(6356):176–179. doi: 10.1038/355176a0. [DOI] [PubMed] [Google Scholar]
- Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991 Jun 14;65(6):1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Chellappan S., Kraus V. B., Kroger B., Munger K., Howley P. M., Phelps W. C., Nevins J. R. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton S. Cell cycle regulation of the human cdc2 gene. EMBO J. 1992 May;11(5):1797–1804. doi: 10.1002/j.1460-2075.1992.tb05231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Devoto S. H., Mudryj M., Pines J., Hunter T., Nevins J. R. A cyclin A-protein kinase complex possesses sequence-specific DNA binding activity: p33cdk2 is a component of the E2F-cyclin A complex. Cell. 1992 Jan 10;68(1):167–176. doi: 10.1016/0092-8674(92)90215-x. [DOI] [PubMed] [Google Scholar]
- Dyson N., Bernards R., Friend S. H., Gooding L. R., Hassell J. A., Major E. O., Pipas J. M., Vandyke T., Harlow E. Large T antigens of many polyomaviruses are able to form complexes with the retinoblastoma protein. J Virol. 1990 Mar;64(3):1353–1356. doi: 10.1128/jvi.64.3.1353-1356.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Dembski M., Fattaey A., Ngwu C., Ewen M., Helin K. Analysis of p107-associated proteins: p107 associates with a form of E2F that differs from pRB-associated E2F-1. J Virol. 1993 Dec;67(12):7641–7647. doi: 10.1128/jvi.67.12.7641-7647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Guida P., McCall C., Harlow E. Adenovirus E1A makes two distinct contacts with the retinoblastoma protein. J Virol. 1992 Jul;66(7):4606–4611. doi: 10.1128/jvi.66.7.4606-4611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Guida P., Münger K., Harlow E. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J Virol. 1992 Dec;66(12):6893–6902. doi: 10.1128/jvi.66.12.6893-6902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Harlow E. Adenovirus E1A targets key regulators of cell proliferation. Cancer Surv. 1992;12:161–195. [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Faha B., Harlow E., Livingston D. M. Interaction of p107 with cyclin A independent of complex formation with viral oncoproteins. Science. 1992 Jan 3;255(5040):85–87. doi: 10.1126/science.1532457. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Ludlow J. W., Marsilio E., DeCaprio J. A., Millikan R. C., Cheng S. H., Paucha E., Livingston D. M. An N-terminal transformation-governing sequence of SV40 large T antigen contributes to the binding of both p110Rb and a second cellular protein, p120. Cell. 1989 Jul 28;58(2):257–267. doi: 10.1016/0092-8674(89)90840-4. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Xing Y. G., Lawrence J. B., Livingston D. M. Molecular cloning, chromosomal mapping, and expression of the cDNA for p107, a retinoblastoma gene product-related protein. Cell. 1991 Sep 20;66(6):1155–1164. doi: 10.1016/0092-8674(91)90038-z. [DOI] [PubMed] [Google Scholar]
- Faha B., Ewen M. E., Tsai L. H., Livingston D. M., Harlow E. Interaction between human cyclin A and adenovirus E1A-associated p107 protein. Science. 1992 Jan 3;255(5040):87–90. doi: 10.1126/science.1532458. [DOI] [PubMed] [Google Scholar]
- Faha B., Harlow E., Lees E. The adenovirus E1A-associated kinase consists of cyclin E-p33cdk2 and cyclin A-p33cdk2. J Virol. 1993 May;67(5):2456–2465. doi: 10.1128/jvi.67.5.2456-2465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A., McCall C., Whyte P., Franza B. R., Jr Human cyclin A and the retinoblastoma protein interact with similar but distinguishable sequences in the adenovirus E1A gene product. Oncogene. 1991 Mar;6(3):481–485. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J., Heijneker H. L. Size and location of the transforming region in human adenovirus type 5 DNA. Nature. 1974 Oct 25;251(5477):687–691. doi: 10.1038/251687a0. [DOI] [PubMed] [Google Scholar]
- Haley K. P., Overhauser J., Babiss L. E., Ginsberg H. S., Jones N. C. Transformation properties of type 5 adenovirus mutants that differentially express the E1A gene products. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5734–5738. doi: 10.1073/pnas.81.18.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel P. A., Gill R. M., Phillips R. A., Gallie B. L. Transcriptional repression of the E2-containing promoters EIIaE, c-myc, and RB1 by the product of the RB1 gene. Mol Cell Biol. 1992 Aug;12(8):3431–3438. doi: 10.1128/mcb.12.8.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Franza B. R., Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helin K., Harlow E., Fattaey A. Inhibition of E2F-1 transactivation by direct binding of the retinoblastoma protein. Mol Cell Biol. 1993 Oct;13(10):6501–6508. doi: 10.1128/mcb.13.10.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helin K., Lees J. A., Vidal M., Dyson N., Harlow E., Fattaey A. A cDNA encoding a pRB-binding protein with properties of the transcription factor E2F. Cell. 1992 Jul 24;70(2):337–350. doi: 10.1016/0092-8674(92)90107-n. [DOI] [PubMed] [Google Scholar]
- Hiebert S. W., Blake M., Azizkhan J., Nevins J. R. Role of E2F transcription factor in E1A-mediated trans activation of cellular genes. J Virol. 1991 Jul;65(7):3547–3552. doi: 10.1128/jvi.65.7.3547-3552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert S. W., Chellappan S. P., Horowitz J. M., Nevins J. R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992 Feb;6(2):177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- Hiebert S. W., Lipp M., Nevins J. R. E1A-dependent trans-activation of the human MYC promoter is mediated by the E2F factor. Proc Natl Acad Sci U S A. 1989 May;86(10):3594–3598. doi: 10.1073/pnas.86.10.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Hu Q. J., Bautista C., Edwards G. M., Defeo-Jones D., Jones R. E., Harlow E. Antibodies specific for the human retinoblastoma protein identify a family of related polypeptides. Mol Cell Biol. 1991 Nov;11(11):5792–5799. doi: 10.1128/mcb.11.11.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q. J., Dyson N., Harlow E. The regions of the retinoblastoma protein needed for binding to adenovirus E1A or SV40 large T antigen are common sites for mutations. EMBO J. 1990 Apr;9(4):1147–1155. doi: 10.1002/j.1460-2075.1990.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P. S., Patrick D. R., Edwards G., Goodhart P. J., Huber H. E., Miles L., Garsky V. M., Oliff A., Heimbrook D. C. Protein domains governing interactions between E2F, the retinoblastoma gene product, and human papillomavirus type 16 E7 protein. Mol Cell Biol. 1993 Feb;13(2):953–960. doi: 10.1128/mcb.13.2.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Krek W., Sellers W. R., DeCaprio J. A., Ajchenbaum F., Fuchs C. S., Chittenden T., Li Y., Farnham P. J., Blanar M. A. Expression cloning of a cDNA encoding a retinoblastoma-binding protein with E2F-like properties. Cell. 1992 Jul 24;70(2):351–364. doi: 10.1016/0092-8674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- Kim Y. K., Lee A. S. Identification of a 70-base-pair cell cycle regulatory unit within the promoter of the human thymidine kinase gene and its interaction with cellular factors. Mol Cell Biol. 1991 Apr;11(4):2296–2302. doi: 10.1128/mcb.11.4.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986 Apr 25;45(2):219–228. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- Lees E., Faha B., Dulic V., Reed S. I., Harlow E. Cyclin E/cdk2 and cyclin A/cdk2 kinases associate with p107 and E2F in a temporally distinct manner. Genes Dev. 1992 Oct;6(10):1874–1885. doi: 10.1101/gad.6.10.1874. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Mathews M. B. Control of adenovirus early gene expression: a class of immediate early products. Cell. 1980 Aug;21(1):303–313. doi: 10.1016/0092-8674(80)90138-5. [DOI] [PubMed] [Google Scholar]
- Means A. L., Slansky J. E., McMahon S. L., Knuth M. W., Farnham P. J. The HIP1 binding site is required for growth regulation of the dihydrofolate reductase gene promoter. Mol Cell Biol. 1992 Mar;12(3):1054–1063. doi: 10.1128/mcb.12.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Resolving the functions of overlapping viral genes by site-specific mutagenesis at a mRNA splice site. Nature. 1982 Feb 4;295(5848):380–384. doi: 10.1038/295380a0. [DOI] [PubMed] [Google Scholar]
- Moran B., Zerler B. Interactions between cell growth-regulating domains in the products of the adenovirus E1A oncogene. Mol Cell Biol. 1988 Apr;8(4):1756–1764. doi: 10.1128/mcb.8.4.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E., Zerler B., Harrison T. M., Mathews M. B. Identification of separate domains in the adenovirus E1A gene for immortalization activity and the activation of virus early genes. Mol Cell Biol. 1986 Oct;6(10):3470–3480. doi: 10.1128/mcb.6.10.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudryj M., Devoto S. H., Hiebert S. W., Hunter T., Pines J., Nevins J. R. Cell cycle regulation of the E2F transcription factor involves an interaction with cyclin A. Cell. 1991 Jun 28;65(7):1243–1253. doi: 10.1016/0092-8674(91)90019-u. [DOI] [PubMed] [Google Scholar]
- Münger K., Werness B. A., Dyson N., Phelps W. C., Harlow E., Howley P. M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989 Dec 20;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992 Oct 16;258(5081):424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Transcriptional activation by viral regulatory proteins. Trends Biochem Sci. 1991 Nov;16(11):435–439. doi: 10.1016/0968-0004(91)90171-q. [DOI] [PubMed] [Google Scholar]
- Pagano M., Draetta G., Jansen-Dürr P. Association of cdk2 kinase with the transcription factor E2F during S phase. Science. 1992 Feb 28;255(5048):1144–1147. doi: 10.1126/science.1312258. [DOI] [PubMed] [Google Scholar]
- Pearson B. E., Nasheuer H. P., Wang T. S. Human DNA polymerase alpha gene: sequences controlling expression in cycling and serum-stimulated cells. Mol Cell Biol. 1991 Apr;11(4):2081–2095. doi: 10.1128/mcb.11.4.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricaudet M., Akusjärvi G., Virtanen A., Pettersson U. Structure of two spliced mRNAs from the transforming region of human subgroup C adenoviruses. Nature. 1979 Oct 25;281(5733):694–696. doi: 10.1038/281694a0. [DOI] [PubMed] [Google Scholar]
- Phelps W. C., Bagchi S., Barnes J. A., Raychaudhuri P., Kraus V., Münger K., Howley P. M., Nevins J. R. Analysis of trans activation by human papillomavirus type 16 E7 and adenovirus 12S E1A suggests a common mechanism. J Virol. 1991 Dec;65(12):6922–6930. doi: 10.1128/jvi.65.12.6922-6930.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri P., Bagchi S., Devoto S. H., Kraus V. B., Moran E., Nevins J. R. Domains of the adenovirus E1A protein required for oncogenic activity are also required for dissociation of E2F transcription factor complexes. Genes Dev. 1991 Jul;5(7):1200–1211. doi: 10.1101/gad.5.7.1200. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Schneider J. F., Fisher F., Goding C. R., Jones N. C. Mutational analysis of the adenovirus E1a gene: the role of transcriptional regulation in transformation. EMBO J. 1987 Jul;6(7):2053–2060. doi: 10.1002/j.1460-2075.1987.tb02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. K., Devoto S. H., Smith E. J., Chellappan S. P., Jakoi L., Nevins J. R. Interactions of the p107 and Rb proteins with E2F during the cell proliferation response. EMBO J. 1993 Mar;12(3):1013–1020. doi: 10.1002/j.1460-2075.1993.tb05742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan B., Zhu X., Chen P. L., Durfee T., Yang Y., Sharp D., Lee W. H. Molecular cloning of cellular genes encoding retinoblastoma-associated proteins: identification of a gene with properties of the transcription factor E2F. Mol Cell Biol. 1992 Dec;12(12):5620–5631. doi: 10.1128/mcb.12.12.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirodkar S., Ewen M., DeCaprio J. A., Morgan J., Livingston D. M., Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle-regulated manner. Cell. 1992 Jan 10;68(1):157–166. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- Slansky J. E., Li Y., Kaelin W. G., Farnham P. J. A protein synthesis-dependent increase in E2F1 mRNA correlates with growth regulation of the dihydrofolate reductase promoter. Mol Cell Biol. 1993 Mar;13(3):1610–1618. doi: 10.1128/mcb.13.3.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens C., Harlow E. Differential splicing yields novel adenovirus 5 E1A mRNAs that encode 30 kd and 35 kd proteins. EMBO J. 1987 Jul;6(7):2027–2035. doi: 10.1002/j.1460-2075.1987.tb02467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian T., Kuppuswamy M., Nasr R. J., Chinnadurai G. An N-terminal region of adenovirus E1a essential for cell transformation and induction of an epithelial cell growth factor. Oncogene. 1988 Feb;2(2):105–112. [PubMed] [Google Scholar]
- Thalmeier K., Synovzik H., Mertz R., Winnacker E. L., Lipp M. Nuclear factor E2F mediates basic transcription and trans-activation by E1a of the human MYC promoter. Genes Dev. 1989 Apr;3(4):527–536. doi: 10.1101/gad.3.4.527. [DOI] [PubMed] [Google Scholar]
- Tsai L. H., Harlow E., Meyerson M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature. 1991 Sep 12;353(6340):174–177. doi: 10.1038/353174a0. [DOI] [PubMed] [Google Scholar]
- Ulfendahl P. J., Linder S., Kreivi J. P., Nordqvist K., Sevensson C., Hultberg H., Akusjärvi G. A novel adenovirus-2 E1A mRNA encoding a protein with transcription activation properties. EMBO J. 1987 Jul;6(7):2037–2044. doi: 10.1002/j.1460-2075.1987.tb02468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Elsen P., Houweling A., Van der Eb A. Expression of region E1b of human adenoviruses in the absence of region E1a is not sufficient for complete transformation. Virology. 1983 Jul 30;128(2):377–390. doi: 10.1016/0042-6822(83)90264-7. [DOI] [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a ras cooperation activity is separate from its positive and negative transcription regulatory functions. Mol Cell Biol. 1988 May;8(5):2177–2183. doi: 10.1128/mcb.8.5.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Ruley H. E., Harlow E. Two regions of the adenovirus early region 1A proteins are required for transformation. J Virol. 1988 Jan;62(1):257–265. doi: 10.1128/jvi.62.1.257-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Wu E. W., Clemens K. E., Heck D. V., Münger K. The human papillomavirus E7 oncoprotein and the cellular transcription factor E2F bind to separate sites on the retinoblastoma tumor suppressor protein. J Virol. 1993 Apr;67(4):2402–2407. doi: 10.1128/jvi.67.4.2402-2407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee A. S., Raychaudhuri P., Jakoi L., Nevins J. R. The adenovirus-inducible factor E2F stimulates transcription after specific DNA binding. Mol Cell Biol. 1989 Feb;9(2):578–585. doi: 10.1128/mcb.9.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee A. S., Reichel R., Kovesdi I., Nevins J. R. Promoter interaction of the E1A-inducible factor E2F and its potential role in the formation of a multi-component complex. EMBO J. 1987 Jul;6(7):2061–2068. doi: 10.1002/j.1460-2075.1987.tb02471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian M., La Thangue N. B. Adenovirus E1a prevents the retinoblastoma gene product from repressing the activity of a cellular transcription factor. EMBO J. 1992 Jul;11(7):2603–2610. doi: 10.1002/j.1460-2075.1992.tb05325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerler B., Moran B., Maruyama K., Moomaw J., Grodzicker T., Ruley H. E. Adenovirus E1A coding sequences that enable ras and pmt oncogenes to transform cultured primary cells. Mol Cell Biol. 1986 Mar;6(3):887–899. doi: 10.1128/mcb.6.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., van den Heuvel S., Helin K., Fattaey A., Ewen M., Livingston D., Dyson N., Harlow E. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 1993 Jul;7(7A):1111–1125. doi: 10.1101/gad.7.7a.1111. [DOI] [PubMed] [Google Scholar]