Abstract
In May 2011, a large food-borne outbreak was traced to an unusual O104:H4 enteroaggregative Escherichia coli (EAEC) strain that produced Shiga toxin (Stx) type 2 (Stx2). We developed a mouse model to study the pathogenesis and treatment for this strain and examined the virulence of the isolate for Dutch belted rabbits. O104:H4 strain C227-11 was gavaged into C57BL/6 mice at 109 to 1011 CFU/animal. The infected animals were then given water with ampicillin (Amp; 5 g/liter) ad libitum. The C227-11-infected, Amp-treated C57BL/6 mice exhibited both morbidity and mortality. Kidneys from mice infected with C227-11 showed acute tubular necrosis, a finding seen in mice infected with typical Stx-producing E. coli. We provided anti-Stx2 antibody after infection and found that all of the antibody-treated mice gained more weight than untreated mice and, in another study, that all of the antibody-treated animals lived, whereas 3/8 phosphate-buffered saline-treated mice died. We further compared the pathogenesis of C227-11 with that of an Stx-negative (Stx−) O104:H4 isolate, C734-09, and an Stx2− phage-cured derivative of C227-11. Whereas C227-11-infected animals lost weight or gained less weight over the course of infection and died, mice infected with either of the Stx− isolates did not lose weight and only one mouse died. When the Stx-positive (Stx+) and Stx2− O104:H4 strains were compared in rabbits, greater morbidity and mortality were observed in rabbits infected with the Stx2+ isolates than the Stx2− isolates. In conclusion, we describe two animal models for EAEC pathogenesis, and these studies show that Stx2 is responsible for most of the virulence observed in C227-11-infected mice and rabbits.
INTRODUCTION
May 2011 marked the beginning of a large outbreak in northern Germany of a food-borne illness characterized by bloody diarrhea and the hemolytic-uremic syndrome (HUS). In that outbreak and a related outbreak in France (1–3), nearly 4,000 individuals were affected, including over 900 cases of HUS and 54 deaths (4–6). This epidemic represented the largest outbreak of HUS worldwide (7). Recommendations were made to abstain from eating raw tomatoes, cucumbers, and leafy salads (7), until sprouts were confirmed to be the vehicle of infection by case-control, trace-back, and trace-forward studies (3, 8, 9).
The outbreak isolate produced Shiga toxin (Stx) type 2 (Stx2), a virulence factor typically associated with the development of HUS after presentation of bloody diarrhea. The causative agent was further typed as an O104:H4 enteroaggregative Escherichia coli (EAEC) strain because it encoded EAEC markers (aatA, aggA, aggR, and aap) and carried the EAEC pAA virulence plasmid (4, 7, 10–13). The Stx2-positive (Stx2+) EAEC O104:H4 outbreak strain also displayed resistance to several antibiotics, such as ampicillin (Amp) and streptomycin (Str), due to a large plasmid that encoded extended-spectrum beta-lactamases (ESBLs) and a multidrug resistance gene cluster on the chromosome (4, 7, 12, 14), respectively.
The O104:H4 outbreak strain represents a rare combination of EAEC characteristics and Stx2 expression; only sporadic cases of disease associated with Stx-producing EAEC have previously been described (in Germany [15, 16], France [17, 18], the United Kingdom [19], the Republic of Georgia [20], and Japan [21]). EAEC without Stx causes diarrhea in persons who reside in developed countries, such as the United Kingdom (22, 23), Switzerland (24), and Japan (25), although EAEC is more commonly associated with acute and persistent (>14 days) diarrhea of infants, children (26–31), HIV-positive persons (32–34), and travelers to developing countries (35–39). EAEC isolates are a highly heterogeneous group of bacteria (28, 40, 41), and each isolate carries a particular subset of EAEC-associated virulence genes; no single virulence factor is consistently associated with EAEC pathogenesis. The addition of stx2 to the EAEC virulence gene repertoire, however, has led to a pathogen that has the capacity to cause disease on a large scale with a potentially deadly outcome due to the possibility of the development of HUS.
In this study, we sought to develop and characterize a mouse model of Stx2+ EAEC disease. Previous small-animal models used to study infection with non-Stx-producing EAEC showed intestinal colonization by EAEC to be intermittent and variable (42–44). Consistent colonization by Stx-negative (Stx−) EAEC can be achieved when mice are treated with streptomycin, although morbidity and mortality are not observed in those mice (44–47). Recently, Roche et al. demonstrated growth impairment, persistent stool shedding, and colonization of intestinal tissue in the absence of antibiotic treatment in a neonatal and weanling C57BL/6 mouse model of EAEC disease (48). Very recently, two other groups reported infection of mice with the German Stx2+ EAEC O104:H4 strain, but in neither case was mortality attributed to infection reported (47, 49). Here we show that the Stx2+ O104:H4 outbreak strain is virulent (causes death and consistent weight loss) in an Amp-treated mouse model and a previously described rabbit model of Shiga toxin-producing E. coli (STEC) pathogenesis (50–52). The virulence of the outbreak strain was correlated with the presence of stx2.
(A portion of this work was presented at the 8th International Symposium on Shiga Toxin [Verocytotoxin] Producing Escherichia coli Infections, Amsterdam, The Netherlands, 6 to 9 May 2012 [53].)
MATERIALS AND METHODS
Bacterial strains.
Enteroaggregative E. coli (EAEC) O104:H4 strains were kindly provided by Flemming Scheutz (Statens Serum Institut, Copenhagen, Denmark). The Stx2+ O104:H4 strain C227-11 was isolated on 24 May 2011 from a German patient at Hvidovre University Hospital in Denmark during the O104:H4 outbreak (10). (Note that in this report, the use of Stx2 and stx2 refers to the prototypic Stx2a and stx2a, respectively.) Stx2− E. coli O104:H4 strain C734-09 was isolated from a child in Africa who presented with watery diarrhea. The stx2-bearing phage-cured derivative of C227-11 was isolated as described below. All strains used in the studies were resistant to Amp. For each animal infection study, the challenge strain was obtained directly from a freezer stock and inoculated onto an LB plate supplemented with Amp (100 μg/ml). The plate was then incubated overnight at 37°C, and colonies were picked from the plate and inoculated into LB broth without or with Amp (100 μg/ml). These broth cultures were grown overnight at 37°C with aeration, and bacteria were subsequently harvested from them by centrifugation. The bacterial pellets were resuspended in phosphate-buffered saline (PBS) or 20% glucose-PBS to a 50× to 100× final concentration.
Intact commensal flora (ICF) and Str-treated or Amp-treated mouse models.
These studies were conducted in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals (54). All animal studies were approved by the Institutional Animal Care and Use Committee of the Uniformed Services University of the Health Sciences. All procedures complied with the guidelines in Biosafety in Microbiological and Biomedical Laboratories (55). Male Crl:CD-1(ICR) (CD-1), female BALB/cAnNCrl (BALB/c), or female C57BL/6NCrl (C57BL/6) mice (weight, 10 to 12 g; age, 3 to 5 weeks) from Charles River Labs (Wilmington, MA) were used for all mouse experiments. Mice were fasted for ∼16 h and deprived of water 1 to 2 h prior to bacterial inoculation. For all models, mice were orally infected with 109 to 1011 CFU in 100 μl by intragastric gavage. For the Str-treated model, mice were given drinking water with Str (5 g/liter) 18 h before infection. For the Amp-treated model, mice were given water with Amp (5 or 10 g/liter) at 4 h postinfection (p.i.) unless otherwise indicated, and new Amp water was prepared every 48 h. Food was returned at 4 h p.i. We chose to return food and water to the mice at 4 h p.i. to preclude potential dilution of the inoculum in the gut by these substances. Str or Amp treatment continued throughout the course of the experiment. For the low-protein-diet (LPD) model, 15 BALB/c mice and 15 C57BL/6 mice were given a 6% protein chow (Harlan Laboratories, Indianapolis, IN) for 5 days prior to infection; 5 control animals were given the typical chow (2018 Tecklad Global 18% protein rodent diet; Harlan Laboratories, Indianapolis, IN). Mice were monitored for survival and weight change for up to 28 days p.i. Weight change was determined as the weight p.i. compared to the weight of the mice before infection. In these studies, we define “morbidity” as exhibiting a symptom(s) of disease (see below) and “mortality” as death. Infected mice were euthanized if they met two of the following morbidity symptoms: weight loss, lethargy, ruffled fur, difficulty breathing, and/or difficulty moving.
Site of C227-11 colonization in C57BL/6 mice.
Mice were infected by intragastric gavage with 100 μl of 1 × 1010 CFU of C227-11 suspended in 20% glucose-PBS. Control animals received 20% glucose-PBS alone. At 3 h p.i., three infected and two PBS-treated mice were anesthetized with isoflurane and euthanized by cervical dislocation. Half of the infected mice were given water with Amp (5g/liter) at 4 h p.i. At 6, 24, 72, and 192 h p.i., infected Amp-treated (n = 3), ICF (n = 3), and PBS-treated control (n = 2) mice were anesthetized with isoflurane and euthanized by cervical dislocation. Kidneys from at least one animal at each time point in each group were removed and fixed in 10% neutral buffered formalin, pH 6.8 to 7.2 (Fisher Scientific L.L.C., Kalamazoo, MI), for histopathological analyses. The cecum, large intestine, and a span of the terminal section of the small intestine equal in length to the large intestine were removed from each animal; the luminal contents were then separated from each organ by application of gentle pressure to the organ. Both the tissues and contents were weighed, diluted in PBS (1:10, wt/vol), and homogenized. The supernatants were serially diluted, plated on LB-Amp, and incubated overnight at 37°C. Colonies were enumerated to determine the number of CFU/g sample. The limit of detection was 102 CFU.
Curing C227-11 of the Stx2-bearing phage.
To isolate a derivative of O104:H4 cured of the stx2-bearing phage (C227-11ϕcu), C227-11 was grown in LB broth at 37°C with aeration for 4 h before the addition of mitomycin C to a final concentration of 1 μg/ml. The culture was then grown overnight and plated on LB-Amp. Isolated colonies were screened by colony blotting for the loss of stxA2a. Potential phage-cured mutants were further screened by PCR to confirm the loss of the phage and toxin gene. Loss of the stx2-bearing phage junctions was validated as described by Shaikh and Tarr (56). To assess for the loss of stx2, PCR for stx2 was done as per the protocol of Scheutz et al. (57). Loss of stx2 or Stx2 was verified by Southern blotting and Vero cell cytotoxicity assay, respectively.
Colony and Southern blotting.
Colony and Southern blot analyses were done as directed by the Roche Applied Sciences digoxigenin (DIG) system manual (DIG Application Manual for Filter Hybridization [58]). For the Southern blots, total genomic DNA was isolated from each sample and then digested with EcoRI. The resultant restricted fragments were then separated on a 0.7% agarose gel, transferred to a nitrocellulose membrane, fixed by UV, and probed for stxA2a. The stxA2a probe for Southern and colony blot analyses was amplified from pJES120 (59) by PCR according to the manufacturer's directions (PCR DIG probe synthesis kit; Roche Applied Sciences). The primers used to amplify the stxA2a probe were stx2F (5′-GGGTACTGTGCCTGTTACTG-3′) and stx2R (5′-TACCACTGAACTCCATTAAC-3′).
Vero cell cytotoxicity assays.
Vero cell cytotoxicity assays were done as previously described (60, 61). Briefly, overnight cultures were pelleted by centrifugation at 13,000 rpm for 5 min. The supernatants were again spun at 13,000 rpm for 5 min, and 100 μl of these supernatants was added to Vero cells that had been seeded into microtiter plates 24 h previously. The overlaid Vero cell plates were incubated for 40 to 48 h at 37°C in 5% CO2. The residual cells in the wells were then fixed in Formalde-Fresh solution and stained with crystal violet, and the color intensity per well was read at 600 nm on a spectrophotometric plate reader.
Rabbit studies.
These studies were conducted at the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited animal facility in the Program of Comparative Medicine at the University of Maryland School of Medicine. All procedures conformed to the guidelines of the Guide for the Care and Use of Laboratory Animals (54) and the policies of the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine. All procedures complied with the guidelines in the Biosafety in Microbiological and Biomedical Laboratories (55). Rabbit infection studies were done as previously described (51). Rabbit feces were prescreened for the presence of E. coli via bacterial culture assays and plating on sorbitol MacConkey agar. All rabbits used in this study were free of sorbitol nonfermenters and were found to have <103 CFU/g feces sorbitol fermenters. Additionally, the animals were evaluated for clinical signs of disease, such as diarrhea or hemorrhagic colitis, prior to infection. Briefly, Dutch belted rabbits (Covance, Denver, PA) aged 5 to 8 weeks were fasted overnight, and water was removed from the cages 2 h prior to bacterial inoculation. Animals were anesthetized and gavaged with 5 or 10 ml 10% sodium bicarbonate solution and then were inoculated by intragastric gavage through an infant feeding tube with 109 to 1012 CFU of E. coli O104:H4 strain C227-11, C734-09, or C227-11ϕcu suspended in 1 ml PBS. After inoculation, rabbits were monitored daily for colonization and development of clinical signs of disease for 7 days. Colonization was monitored by the levels of O104:H4 organisms shed in the feces. Fecal pellets were collected and weighed, diluted 1:10 by weight into PBS, and homogenized. The supernatants were serially diluted and plated on MacConkey agar supplemented with Amp (100 μg/ml), and plates were incubated overnight at 37°C. Colonies were enumerated to determine the number of CFU/g feces. Rabbits that showed signs of disease (lethargy, fever, hemorrhagic colitis, or weight loss) and did not respond to interventions (analgesic and rehydration therapy) were euthanized at alternative endpoints: when they experienced a weight loss of more than 20% compared with the preinoculation weight, an inability to eat or drink for >12 h, or a lack of movement or at the conclusion of the study (7 days p.i.). The analgesic was buprenorphine given at 0.02 mg/kg of body weight subcutaneously. Rehydration therapy was administered to the rabbits as lactated Ringer's solution at 25 ml/kg daily until signs resolved. Organs (including kidneys, small intestine, cecum, and colon) were collected at necropsy for histopathological analyses.
Baseline blood samples were collected from the ear veins of rabbits before bacterial inoculation. In addition, blood was collected for complete blood count (CBC) and biochemical analysis by cardiocentesis of each animal at euthanasia. Biochemical and hematological assessments were conducted at a reference veterinary laboratory (Antech Diagnostics, Lake Success, NY) using standard operating procedures. CBC analysis was performed using a hematology analyzer (Advia). Serum chemistry values were measured in a clinical chemistry analyzer (AU; Beckman Coulter). Analyzers were normalized for rabbit parameters. Hematological and serum biochemistry values derived from blood collected before inoculation and at euthanasia were compared for each animal.
Hematological and kidney function analyses in mouse studies.
Female C57BL/6 mice were infected and given water with Amp (10 g/liter) as described above. Mice received either C227-11 or C227-11ϕcu; control mice received no bacteria but were provided Amp water. Animal weight was measured as an indicator of morbidity. Blood was collected from C227-11-infected animals that lost 10 to 15% of the highest weight achieved p.i.; blood was also collected from an uninfected control and a C227-11ϕcu-infected mouse on each day that a C227-11-infected mouse was sacrificed. Blood was collected by tail bleed or cardiac puncture and placed either into Microtainer tubes that contained potassium EDTA (BD Diagnostics, Franklin Lakes, NJ) for CBCs and erythrocyte (RBC) morphology or into microcentrifuge tubes to process sera for kidney function analyses. Each sample contained a similar volume of blood that was required to perform analyses (100 to 200 μl). Bleeds were terminal. To obtain serum, blood in each microcentrifuge tube was first allowed to clot at 4°C. Each tube was then subjected to two consecutive 10-min centrifugation steps (8,000 × g and then 10,000 × g) to separate the serum from the clotted red cells. Serum samples were stored at 4°C until they could be analyzed by the Uniformed Services University of the Health Sciences Diagnostic Services & Comparative Medicine Clinical Pathology Laboratory (Bethesda, MD) for levels of blood urea nitrogen (BUN) and creatinine. Whole-blood samples for determination of CBC and RBC morphology were stored at 4°C and evaluated by facilities at BioReliance (Rockville, MD). Serum analyses on mouse blood samples were carried out using automated equipment and reported through a laboratory information management system (LIMS). A clinical chemistry analyzer (Cobas 600 series) was used to measure serum biochemistry results. CBC determination was performed using a hematology analyzer (Advia 120). Analyzers were normalized for mouse parameters (using multispecies software).
Preparation of tissue sections for histopathology and immunostaining.
Fixed organs were embedded in paraffin and then sectioned. Tissue sections were then placed on charged glass slides and stained with hematoxylin-eosin (H&E) at HistoServ (Germantown, MD). The H&E-stained sections were evaluated microscopically by a veterinary pathologist who was blinded to the treatment groups. To locate E. coli O104:H4 bacteria bound to or near the surface of intestinal tissue, slides with intestinal sections were immunostained with rabbit anti-O104 serum (Statens Serum Institute, Copenhagen Denmark). Briefly, slides were deparaffinized in the Histoclear agent (National Diagnostics, Atlanta, GA) and rehydrated in a graded ethanol series. To increase antibody recognition of the antigen in tissue sections, slides were treated with antigen retrieval buffer (5× AntigenPlus buffer, pH 10 [EMD Biosciences, San Diego, CA], diluted to 1×), heated in a microwave oven for 15 min at 50% power (or, alternatively, heated in 1× antigen retrieval buffer at 95°C for 8 min), cooled, and rinsed with water. Slides were then incubated overnight in 3% bovine serum albumin diluted in PBS (blocking buffer). Next, the anti-O104 serum was incubated with the tissue sections at a dilution of 1:50 in PBS for 1 h. The antiserum-exposed tissue sections were then washed, and secondary donkey or goat anti-rabbit antibody conjugated to Alexa Fluor 488 was added for 1 h at a dilution of 1:200 in PBS. The slides were rinsed in PBS, Slowfade reagent was added, and a coverslip was applied. Immunofluorescence of stained tissue sections was visualized with an Olympus BX60 microscope with a BX-FLA fluorescent attachment. Digital images of the fluorescent images were obtained with a SPOT RT charge-coupled-device digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI).
Protection studies with anti-Stx2 monoclonal antibody 11E10.
Female C57BL/6 mice (10 per group) were infected with 5.5 × 109 CFU of C227-11 by gavage and given either PBS or 2 μg anti-Stx2 monoclonal antibody 11E10 (CRL-1907; ATTC, Manassas, VA) in PBS by the intraperitoneal route on day −1, +3, +5, or + 7 relative to the time of infection. The intraperitoneal route was used in preference to the intravenous route due to the difficulty in locating the tail vein in C57BL/6 mice. At 4 h p.i., the mice were given Amp water, and Amp treatment was maintained throughout the study. The mice were monitored for survival and weight change for 28 days p.i. The mice that died in this study succumbed to infection and were not euthanized; therefore, tissue was not collected for histopathological analysis.
Statistical analyses.
All statistical analyses were done through application of the GraphPad Prism v5.04 for Windows software (GraphPad Software, San Diego, CA). The repeated-measures (RM) analysis of variance (ANOVA) was applied to assess statistical differences in weight and colonization of infected animals. When infected animals died, the last observation was carried forward and used in all subsequent calculations. Statistical differences in the mean weight between mice infected with the E. coli O104:H4 strains were determined by RM two-way ANOVA. When a few intermediate values were missing, the ANOVA was estimated by a mixed-models analysis. The RM ANOVA was also used to assess statistical significance among the numbers of E. coli O104:H4 strain C227-11 organisms in various segments of the intestine over time. After checking for significance by RM ANOVA, we conducted a separate one-way ANOVA to compare bacterial counts at the same time point among the organs. In the diet studies, comparison of survival curves was done by the log-rank (Mantel-Cox) test. Statistical differences in the geometric mean level of C227-11 colonization in the diet studies did not meet the requirements for ANOVA and were therefore analyzed by t test. For CBC analyses and serum chemistries, differences between infected and control mice were compared by independent Student t test. Kaplan-Meier analysis was done to ascertain differences in survival of infected rabbits. Statistical differences in the mean weight between rabbits infected with the E. coli O104:H4 strains were determined by RM two-way ANOVA. The RM ANOVA was also used to assess statistical significance among the numbers of E. coli O104:H4 C227-11 or C734-09 organisms shed in the feces of infected rabbits.
RESULTS
Development of a mouse model to study the pathogenesis of the Stx2+ O104:H4 outbreak strain.
To investigate the virulence of the Stx2+ EAEC O104:H4 strain in an animal model, we began by testing the pathogenicity of the isolate in established STEC mouse models. We infected Str-treated CD-1 mice (62) or BALB/c mice (63) with an intact commensal flora (ICF; i.e., the mice were not treated with antibiotics) with 109 to 1010 CFU of E. coli O104:H4 strain C227-11 and assessed colonization and mortality (Table 1). Although Str-treated CD-1 mice were highly colonized by O104:H4, the mice gained weight, remained healthy, and were apparently unaffected by the bacteria. Similarly, although some of the O104:H4-infected ICF BALB/c mice lost weight over the course of the study (starting at about day 4 and continuing to day 8), the animals recovered. When we repeated the study, the ICF BALB/c mice again did not become highly colonized (<105 CFU/g feces) with C227-11, so we gave half of those infected animals water with Amp at 2 days p.i. to increase the levels of the outbreak strain in the intestine. Two of the Amp-treated, O104:H4-infected mice died (Table 1).
Table 1.
Development of a mouse model for Stx2+ O104:H4 strain C227-11
| Mouse strain | Treatment | No. of mice with the indicated condition/total no. of mice in treatment group |
|
|---|---|---|---|
| Weight lossa | Mortality | ||
| CD-1 | Str | 0/10 | 0/10 |
| BALB/c | None | 9/25 | 0/25 |
| BALB/c | Amp at 2 days p.i. | 4/5b | 2/5 |
| BALB/c | Amp at 4 h p.i. | 4/10 | 0/10 |
| BALB/c | LPDc | 0/5 | 0/5 |
| BALB/c | LPD + Amp at 4 h p.i. | 1/10 | 0/10 |
| C57BL/6 | LPDg,k,l | 0/5 | 1/5 |
| C57BL/6 | Amp at 4 h p.i.h,j,l | 3/5 | 3/5d |
| C57BL/6 | LPD + Amp at 4 h p.i.i,j,k | 2/10 | 0/10d |
| BALB/c | Amp at 18 h before infection | 3/5 | 0/5 |
| C57BL/6 | None | 0/5 | 0/5e |
| C57BL/6 | Amp at 18 h before infection | 3/5 | 2/5f |
| C57BL/6 | Amp at 4 h p.i. | 4/5 | 5/5e,f |
An animal was considered to have lost weight if more than 5% weight was lost from the highest to the lowest weight after day 1 (the day after feeding).
The mouse that did not lose weight was one of the animals that died; however, no weight data were taken on the 4 days prior to death.
LPD, low-protein diet.
P < 0.005 for survival of C57BL/6 mice treated with LPD plus Amp versus Amp-treated mice on a normal diet.
P < 0.005 for survival of C57BL/6 mice treated with Amp at 4 h p.i. versus mice receiving no Amp treatment.
P < 0.005 for survival of C57BL/6 mice treated with Amp at 4 h p.i. versus mice treated with Amp at 18 h before infection.
Geometric mean numbers of CFU/g feces: 2.53 × 107 on day 1 and 1.91 × 104 on day 4.
Geometric mean numbers of CFU/g feces: 4.13 × 109 on day 1 and 3.83 × 109 on day 4.
Geometric mean numbers of CFU/g feces: 3.83 × 107 on day 1 and 7.63 × 108 on day 4.
P < 0.005 for day 1 and P < 0.001 for day 4 (colonization levels, C57BL/6 mice treated with Amp at 4 h p.i. versus mice treated with LPD plus Amp).
Data are not significant for day 1 and P was <0.005 for day 4 (colonization levels, C57BL/6 mice treated with LPD versus mice treated with LPD plus Amp).
P < 0.05 for day 1 and P < 0.0001 for day 4 (colonization levels, C57BL/6 mice treated with LPD versus treated with Amp at 4 h p.i.).
Because administration of a low-protein diet (LPD) to mice resulted in a failure of just-weaned C57BL/6 mice to gain weight after infection with typical EAEC and EAEC-infected adult C57BL/6 mice were shown to gain less weight than control animals (48), we included C57BL/6 mice along with BALB/c mice in our next set of studies. First, we infected either BALB/c or C57BL/6 mice given an LPD with C227-11 and then gave them water with or without Amp. We found that although 1/5 of the O104:H4-infected C57BL/6 mice on an LPD died, the animals that were on Amp and an LPD survived, while 3/5 of the Amp-treated infected mice on a normal diet died (Table 1). None of the BALB/c animals died in this study. The reason for the reduced pathogenicity in the animals on the LPD compared to that for the Amp-only-treated animals is most likely due to reduced colonization by C227-11 in the LPD groups (Table 1).
Next we wanted to assess the effect of providing Amp treatment either before or after infection. We infected C57BL/6 mice with 1010 CFU of E. coli O104:H4 C227-11, either did not treat the animals or gave them Amp water at 18 h before or 4 h p.i., and monitored them for colonization and mortality. For comparison, we also infected and treated ICF BALB/c mice in the same manner. All five of the C227-11-infected C57BL/6 mice given Amp after infection lost weight (not shown) and died, and 2/5 of the animals given Amp before infection died, while none of the infected ICF C57BL/6 mice died (Table 1); none of the ICF BALB/c mice died.
Overall in these initial studies, the model that most consistently induced morbidity (weight loss) and mortality due to Stx2+ O104:H4 was the C57BL/6 mouse given Amp water at 4 h p.i. (Note that the growth of C227-11 in the presence of Amp did not induce the expression of stx2, as measured by Vero cell assay [data not shown].) Of note, when we screened mouse-passaged C227-11 colonies by PCR for the aggR gene, we found that a large proportion of the colonies were negative for aggR, a result that suggests loss of the pAA plasmid. That some mouse-passaged C227-11 colonies appeared to have lost the pAA plasmid was consistent with findings in humans; i.e., O104:H4 isolates from a percentage of patients with long-term carriage of the outbreak strain were found to have lost the pAA plasmid (Helge Karch, unpublished observation).
Colonization of C227-11 in the gi tracts of C57BL/6 mice.
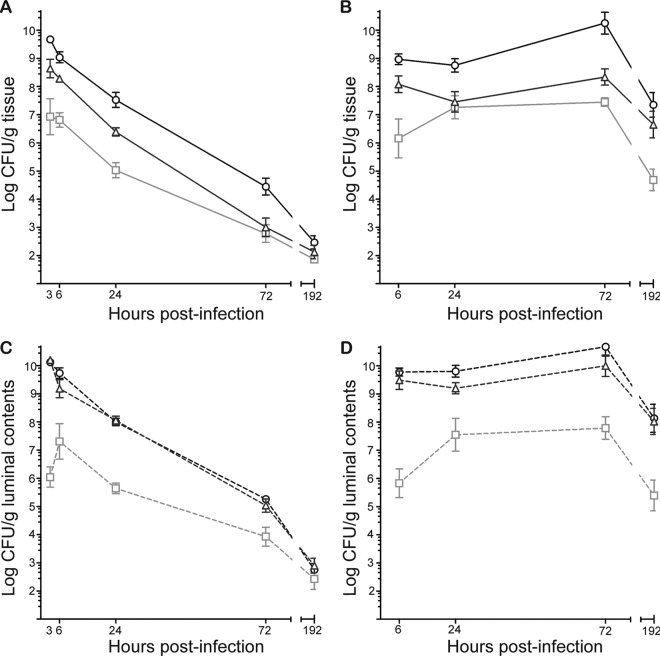
To characterize the site of C227-11 colonization within the gastrointestinal (gi) tract, we orally inoculated ICF C57BL/6 mice or Amp-treated C57BL/6 mice with 1010 CFU by intragastric gavage. At 3, 6, 24, 72, and 192 h after infection, we enumerated the O104:H4 bacteria associated with tissue or luminal contents of the cecum, small intestine, or large intestine. In both animal models, the number of O104:H4 bacteria was the highest overall in the cecum, followed by the large intestinal tissue (Fig. 1A and B). Also, in both animal models, the O104:H4 bacterial load of the cecal tissues was significantly higher than that of the small intestine, at least until 192 h, for the ICF model (Fig. 1A), whereas in the Amp-treated model, the O104:H4 counts of the cecal tissue remained significantly higher than those of the small intestinal tissue through the end of the study (Fig. 1B). At the final time point, the numbers of C227-11 bacteria recovered from all tissue sites of the intestine of ICF mice were similar.
Fig 1.
E. coli O104:H4 colonization over time at sites within the gi tract of C57BL/6 mice. ICF or Amp-treated mice were infected with 1010 CFU of C227-11. The geometric mean numbers of CFU/g tissue (A and B) and luminal contents (C and D) are shown for the small intestine (□), cecum (○), and large intestine (△). Each point represents the geometric mean numbers of CFU/g feces of 3 mice. Bars represent ±1 standard error of the mean. (A) The numbers of C227-11 organisms recovered from the small intestinal tissue of ICF mice were significantly less than those recovered from the cecum (P < 0.0001 at 3, 6, and 24 h p.i.; P < 0.01 at 72 h p.i.). C227-11 counts of large intestinal tissue were greater than those of the small intestinal tissue at 3 h, 6 h (P < 0.01), and 24 h (P < 0.05) p.i. (B) E. coli O104:H4 counts from the cecal tissues of Amp-treated mice were significantly higher than those from the small intestinal tissue (P < 0.001, except for no difference at 24 h p.i.). The numbers of C227-11 recovered from the small intestinal and large intestinal tissue were not significantly different (except at 6 and 192 h p.i., P < 0.05). (C) The numbers of E. coli O104:H4 bacteria recovered from the contents of ceca and large intestine of ICF mice were greater than the numbers recovered from the small intestine: cecal and small intestine contents were significantly different (P < 0.0001) at 3 h, 6 h, 24 h (P < 0.05), and 72 h p.i.; small intestinal contents and large intestinal contents were significantly different (P < 0.0001) at 3 h, 24 h, (P < 0.001), and 6 h p.i. (D) In Amp-treated mice, significantly greater numbers of C227-11 bacteria were recovered from the contents of the ceca and large intestine than the small intestinal contents: cecal contents and small intestinal contents were significantly different (P < 0.01 at 24 h p.i.; P < 0.0001 at all other time points); small intestinal contents and large intestinal contents were significantly different (P < 0.0001 at 6 h p.i., P < 0.05 at 24 h p.i., P < 0.01 at 72 h p.i., and P < 0.001 at 192 h p.i.).
When we compared the counts of C227-11 bacteria recovered from the luminal contents of the cecum, small intestine, or large intestine of ICF and Amp-treated mice, we again observed that the numbers of O104:H4 bacteria were the highest in the cecum and large intestine, but in this case, the counts from both sites were similar in each animal model (Fig. 1C and D). Furthermore, the general patterns of cecal luminal O104:H4 counts versus those of the small intestinal luminal counts mirrored those of the tissue counts from each model. Specifically, in both Amp-treated and ICF mice, significantly greater numbers of C227-11 bacteria were recovered from the contents of ceca than the contents of the small intestine throughout the whole study (Fig. 1C and D), until the bacterial counts approached and fell below the limit of detection at final time points in the ICF mice (Fig. 1C).
As expected, we found that the number of bacteria recovered from the gi tract of ICF mice decreased over the course of the experiment (Fig. 1A and C), while Amp-treated mice maintained a level of C227-11 colonization of between 105 and 1010 CFU for the duration of the study (Fig. 1B and D). The high bacterial counts recovered from the ceca of ICF and Amp-treated mice indicate that the cecum is the main site of O104:H4 colonization in both models (Fig. 1A and B).
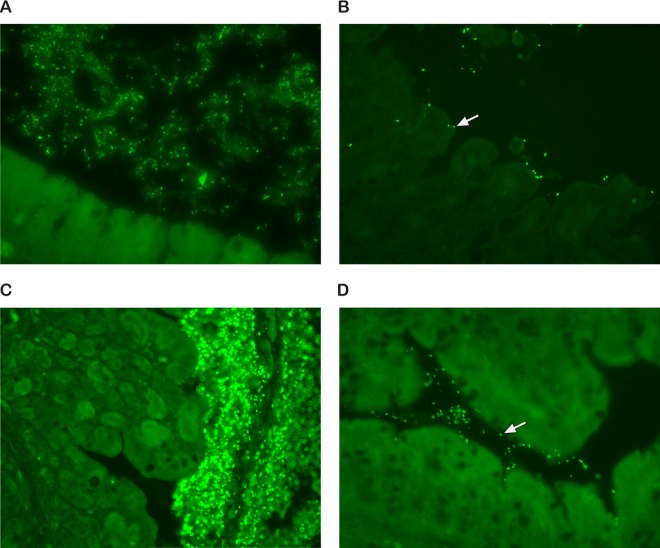
EAEC prototype strain 17-2 has been shown to aggregate in association with a thick mucus layer overlying the intestinal epithelium of infected animals (64) and on the surface of intestinal mucosa samples cultured with EAEC (65). To visualize how C227-11 colonizes the Amp-treated mouse intestine, we collected intestinal tissue from infected mice and stained it with rabbit anti-O104 serum. We detected O104:H4-positive bacteria in aggregates within the lumen of the cecum (Fig. 2A) and in close association with cecal tissue (Fig. 2B, arrow). We also detected massive aggregates of O104 bacteria in the colon (Fig. 2C) and in close association with colonic tissue (Fig. 2D, arrow). Additionally, we noted that the ceca of infected mice were enlarged compared to those of the controls but found upon histological examination that the intestines of most of the infected mice were normal. However, one C227-11-infected mouse showed extensive inflammation in the cecum and colon (not shown).
Fig 2.
Localization of C227-11 in the cecum and colon of Amp-treated mice. Cecal (A and B) or colonic (C and D) tissue was stained with an anti-O104 serum followed by Alexa Fluor-labeled secondary antibody. (A) Note aggregates of O104 bacteria in the lumen of the cecum; (B) close association of O104 bacterium with cecal tissue (white arrow); (C) massive aggregates of O104 bacteria in the lumen of the colon; (D) note the close association of an O104 bacterium with colonic tissue (white arrow). Magnifications, ×400.
Systemic effects of C227-11 infection.
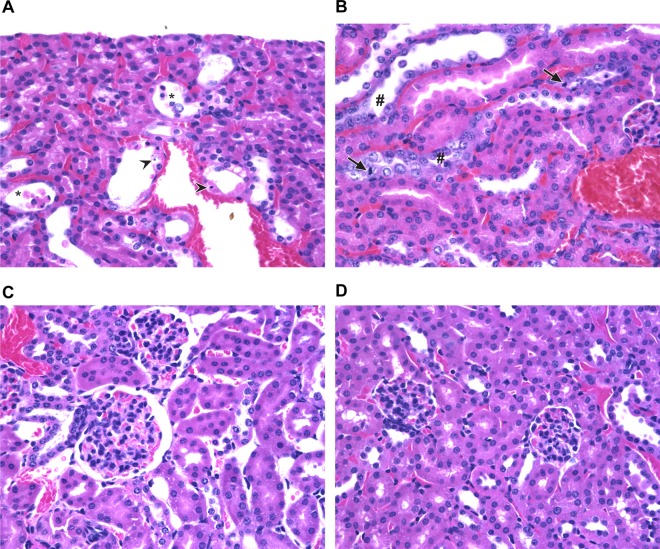
We previously reported that the death of STEC-infected Str-treated mice is due to Stx2-mediated acute renal tubular necrosis (66). To assess kidney damage in the Amp-treated Stx2+ O104:H4 C57BL/6 mouse model, we collected kidneys for histopathology from C227-11-infected mice that met the criteria for euthanasia. We found acute tubular necrosis in all kidney sections examined from C227-11-infected mice and none of the control mice; additionally, we detected areas of tubule regeneration, a finding that indicates recent kidney damage (Fig. 3C and D). We concluded that Stx2 is almost certainly responsible for kidney damage and death in C227-11-infected Amp-treated mice.
Fig 3.
Tubular damage and regeneration in renal sections from C227-11-infected mice. (A) Hematoxylin-eosin-stained kidney section that shows the renal cortices from a mouse that succumbed to infection. There is acute tubular necrosis in the renal cortex characterized by tubules lined by necrotic epithelial cells (arrowhead) and tubular lumina containing sloughed necrotic cells (*). (B) Similar to panel A, but note tubules (#) with features characteristic of regeneration: cells have large vesiculate nuclei, increased cytoplasmic basophilia, and mitotic figures (arrows). (C and D) Hematoxylin-eosin-stained kidney section of uninfected mouse. Magnifications, ×400.
We further assessed the systemic effects of Stx2+ O104:H4 infection in the Amp-treated mouse model by analyzing blood hematological parameters and chemistries. We collected blood from C227-11-infected mice once they lost 10 to 15% of their body weight compared to their maximum weight achieved p.i., an endpoint at which mice were not so moribund as to impede blood collection. Whole-blood samples were analyzed for complete blood counts (CBCs), and sera were evaluated for markers of kidney function, specifically, blood urea nitrogen (BUN) and creatinine levels. E. coli Stx2+ O104:H4-infected mice had significantly higher BUN and creatinine values than uninfected mice (Table 2), a finding indicative of kidney damage in the infected animals. Significant neutrophilia was observed in the blood samples of the C227-11-infected group compared to the control mice (Table 2). None of the primary hematological features of HUS were detected in the infected animals; there were no differences in platelets, hemoglobin, or hematocrit values between infected and control mice.
Table 2.
Serum chemistry and CBC analyses of uninfected or infected (C227-11 or C227-11ϕcu) Amp-treated C57BL/6 mice
| Strain | BUN concn (mg/dl) | Creatinine concn (mg/dl) | % neutrophils | % lymphocytes | Hemoglobin concn (g/dl) | % hematocrit | No. of platelets/μl (103) |
|---|---|---|---|---|---|---|---|
| Control | 17.5 | 0.32 | 4.7 | 90.2 | 14.1 | 52.6 | 893 |
| C227-11 | 56.22a,b | 0.53a,c | 16.77a | 69.6 | 16.23b | 49.7 | 1,195 |
| C227-11ϕcud | 24.88 | 0.33 | 6.5 | 81.45 | 13.4 | 41.5 | 898.5 |
P ≤ 0.05, C227-11 versus control.
P = 0.01, C227-11 versus C227-11ϕcu.
P = 0.06, C227-11 versus C227-11ϕcu.
No significant difference in C227-11ϕcu values compared to the control.
Contribution of Stx2 to virulence of the O104:H4 outbreak strain. (i) Generation of an E. coli O104:H4 derivative cured of the Stx2-encoding phage.
To study the contribution of Stx2 to the pathogenesis of O104:H4, we constructed a mutant of C227-11 cured of the phage that encodes Stx2. Loss of the phage genome and stx2 was confirmed by PCR and Southern blot analysis (Fig. 4). Additionally, PCR to amplify the junctions of the stx2-encoding phage, inserted into wrbA on the bacterial chromosome, confirmed the loss of the phage. Culture supernatants of C227-11ϕcu were not cytotoxic to Vero cells, a finding that further substantiates the loss of the Stx2-encoding phage, as the 50% cytotoxic dose for the wild type was 105 per ml culture supernatant.
Fig 4.
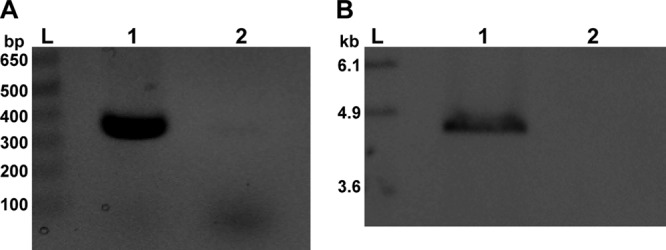
PCR and Southern blot analysis of C227-11ϕcu. (A) PCR amplification of a portion of stx2 in wild-type strain C227-11 (lane 1) which was absent in C227-11ϕcu (lane 2). An amplicon at 349 bp indicates the presence of stx2. (B) Southern blot of C227-11 (lane 1) and C227-11ϕcu (lane 2) genomic DNA probed for stxA2 (found on a 4.8-kb fragment). Lanes L, molecular size ladders. The size of the ladder bands is indicated in bp (A) or kbp (B). The brightness and contrast of the images were adjusted linearly in Adobe Photoshop CS5 (version 12.0, ×32) software.
(ii) Role of Stx2 in the mouse model.
We infected Amp-treated C57BL/6 mice with C227-11 (Stx2+), C734-09 (Stx−), or C227-11ϕcu (Stx2−). The lethality of the O104:H4 outbreak isolate was variable among experiments in this mouse model: the mortality of C227-11-infected mice ranged from 0 to 100% (12/50 mice died in four experiments). However, only 1 of 10 C734-09-infected mice succumbed to infection and none of the C227-11ϕcu-infected mice died. Additionally, C227-11-infected animals lost weight or gained less weight over the course of infection, whereas mice infected with either of the Stx2− O104:H4 strains did not lose weight (Fig. 5). Histopathological examination of kidneys from mice that succumbed to C227-11 infection revealed acute tubular necrosis, a result not seen in Stx2− EAEC-infected mice. Further, serum chemistry analyses from C227-11-infected mice revealed significantly elevated BUN and creatinine levels compared to the values from C227-11ϕcu-infected mice (Table 2). Taken together, we conclude that the virulence, as assessed by consistent weight loss, kidney structural and functional damage, and occasional marked lethality, seen from infection with C227-11 is due to the presence and expression of stx2.
Fig 5.
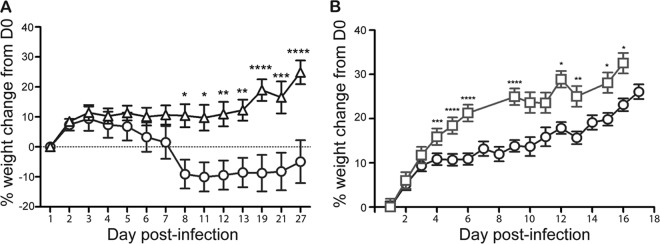
Percent weight change of Amp-treated C57BL/6 mice orally infected with E. coli O104:H4 strains. Mice were infected with O104:H4 strain C227-11 (○) or C734-09 (△) (A) and O104:H4 strain C227-11 (○) or C227-11ϕcu (□) (B). Points on the graph represent the mean weight of 10 animals per group (A) or 40 animals per group (B). Bars represent 1 standard error of the mean; asterisks indicate days that demonstrated a significant difference between mean weights (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). D0, day of infection.
(iii) Protection of and enhanced weight gain in C227-11-infected mice treated with anti-Stx2 monoclonal antibody 11E10.
We did a preliminary study in the Amp-treated mouse model to determine whether anti-Stx2 monoclonal antibody 11E10 could protect O104:H4-infected mice. The infected mice were given 2 μg antibody on day 8 p.i. None of the animals that received 11E10 died (n = 8), and 3 of 8 PBS-treated animals succumbed to infection. We next expanded the study to include administration of antibody on different days relative to the time of infection. Amp-treated C227-11-infected mice received nothing, 11E10, or PBS either before infection (−1 day) or after infection (3, 5, or 7 days p.i.). We assessed weight change and mortality for 14 days p.i. Only 1/10 mice treated with 11E10 on day −1 died, whereas 4/10 of the PBS-treated (day −1) and 4/9 untreated (but infected) animals died, findings that indicate that 11E10 was partially protective. The 11E10-treated animal that succumbed to infection was found dead, so organs were not collected for histopathological analysis. In contrast, the mortality rates for the animals given either PBS or antibody on day 3 or 5 p.i. were similar (20 to 30%). Furthermore, treated (PBS or 11E10) mice (from all groups) generally died later, with a mean time to death of 15.3 days compared to a mean time to death of 12.3 days for untreated, infected mice. However, the 11E10-treated animals from groups treated on days −1, +3 (Fig. 6), and +7 of infection gained significantly more weight than untreated mice. All but one of the mice given 11E10 on day 7 p.i. survived, whereas 7/10 mice given the PBS control survived. The single 11E10-treated animal that succumbed did so on day 8, a result that suggests that the antibody was given too late to rescue that mouse, which had already lost more than 20% of its body weight at the time of treatment.
Fig 6.
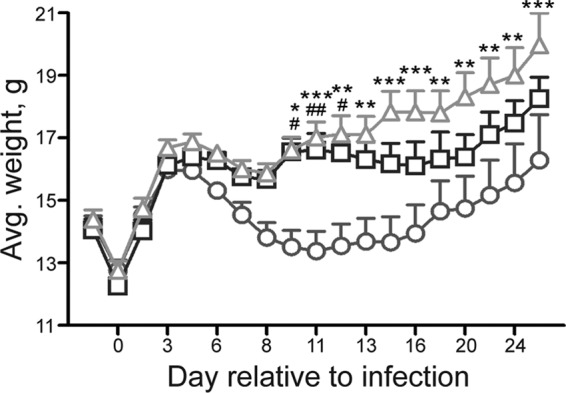
Average weight of C227-11-infected, Amp-treated C57BL/6 mice either untreated or receiving PBS or 11E10. Mice were infected with C227-11 and on day 3 p.i. received nothing (○), PBS (□), or anti-Stx2 monoclonal antibody 11E10 (△) by intraperitoneal injection. Bars indicate ±1 standard error of the mean. Pound signs indicate days that demonstrated a significant difference between mean weights for no treatment compared to PBS treatment (#, P < 0.05; ##, P < 0.01). Asterisks indicate days that demonstrated a significant difference between mean weights for no treatment compared to 11E10 treatment (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
(iv) Role of Stx2 in the rabbit model.
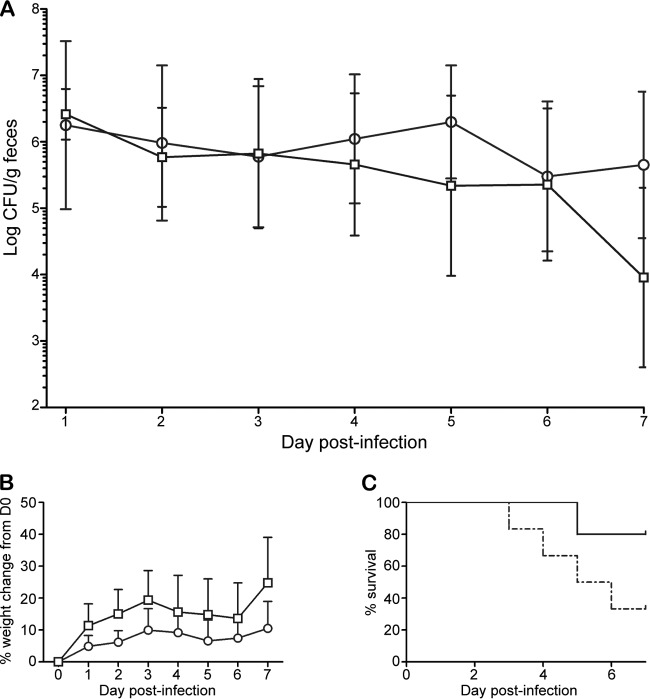
Garcia and colleagues observed that Dutch belted rabbits naturally or experimentally infected with enterohemorrhagic E. coli display systemic disease similar to the HUS seen in humans (50, 52). We also found that Dutch belted rabbits exhibit weight loss, diarrhea, and occasional kidney damage in response to infection with EHEC O157:H7 (51). Here we sought to determine whether (i) Dutch belted rabbits were susceptible to infection by EAEC and (ii) the Stx2-expressing EAEC German outbreak strain was virulent in the Dutch belted rabbit model. Rabbits given a large dose (1012 CFU) of C227-11 or C734-09 became infected and remained colonized throughout the experiment (Fig. 7A). The Stx2+ isolate was more virulent than C734-09, in that nearly all C227-11-infected animals displayed signs of disease beginning on day 2 p.i., and 4/6 died, whereas only 1 death occurred among 5 animals in the C734-09-fed group, with fewer animals displaying signs of morbidity, such as weight loss (Fig. 7B and C; Table 3). In this study, 4/6 C227-11-infected rabbits met the criteria and received analgesics and rehydration therapy, and 2/5 C734-09-infected animals were provided those therapies. Histopathological evaluation of tissues from a few C227-11-infected rabbits revealed mild erosion and necrosis of the mucosal epithelium in the large and small intestines and the cecum. Additionally, we noted hemorrhage in the cecum of a rabbit infected with C227-11. Conversely, C734-09-infected rabbits did not display significant intestinal pathology. We assessed kidneys from rabbits infected with C227-11 and C734-09; we did not observe any significant abnormalities in the kidneys of these rabbits. We collected blood and assessed hematological changes in rabbits that met criteria for euthanasia. Of the two C227-11-infected rabbits that became ill and met criteria for euthanasia, the levels of both BUN (63 and 77 mg/dl, respectively) and creatinine (2.6 and 0.5 mg/dl, respectively) were elevated compared to baseline values (BUN, 17 and 24 mg/dl, respectively; creatinine, 0.4 and 0.3 mg/dl, respectively). In contrast, the BUN (26 mg/dl) and creatinine (0.5 mg/dl) values of the C734-09-infected rabbit that met criteria for euthanasia were not different from the values obtained preinfection (BUN, 25 mg/dl; creatinine, 0.4 mg/dl).
Fig 7.
Pathogenesis of O104:H4 in Dutch belted rabbits orally infected with 1011 to 1012 CFU C227-11 or C734-09. Rabbits were infected with O104:H4 strain C227-11 (○) or C734-09 (□). (A) Colonization over time. Each point represents the geometric mean of the number of E. coli O104:H4 bacteria shed into the feces from infected animals (6 per group); bars indicate ±1 standard error of the mean. The limit of detection was 102 CFU/g feces. (B) Percent weight change of infected rabbits. Points on the graph indicate the mean weight of 6 animals per group; bars indicate ±1 standard error of the mean. D0, day of infection. (C) Percent survival of rabbits infected with C227-11 (dashed line) or C734-09 (solid line) on each day p.i.
Table 3.
Morbidity and mortality of Dutch belted rabbits infected with 1011 to 1012 CFU of C227-11 or C734-09
| Characteristic | No. of rabbits with characteristic/total no. |
|
|---|---|---|
| C227-11 | C734-09 | |
| Loose stools | 5/6 | 2/5 |
| Weight loss | 3/6 | 1/5 |
| Lethargy | 5/6 | 2/5 |
| Death | 4/6a | 1/5b |
Two rabbits were euthanized on days 3 and 5 p.i., and two succumbed to disease on days 3 and 4 p.i.
One rabbit was euthanized on day 4 p.i.
When we infected rabbits with 109 to 1011 CFU of C227-11 or C227-11ϕcu, only low-level colonization was achieved in each group, and none of the rabbits died or showed signs of morbidity (not shown). These data taken in aggregate led us to conclude that Dutch belted rabbits are susceptible to infection by some EAEC strains, although a large dose is required to cause morbidity and mortality, and that the Stx2+ O104:H4 strain is more virulent in this model than Stx2− O104:H4 strains. Further, the levels of colonization with stx2-positive (C227-11) and stx2-negative (C734-09 and C227-11ϕcu) EAEC bacteria were similar (Fig. 7A and data not shown), a finding that indicates that Stx2 does not play a role in colonization in this model.
DISCUSSION
In this study, we developed a small-animal model to study the pathogenesis of the Stx2+ O104:H4 E. coli strain associated with a large outbreak of bloody diarrhea and HUS (3–5, 7). We found that C57BL/6 mice given water containing Amp at 4 h p.i. were susceptible to infection with E. coli O104:H4 strain C227-11 and demonstrated signs of disease that included consistent weight loss and, in some cases, mortality. Moreover, this is the first report of a small-animal model in which abundant biofilm aggregates of EAEC were demonstrated in the colon of infected animals. C227-11 was also virulent in a Dutch belted rabbit model of STEC pathogenesis (50–52), although a high dose (>1011 CFU) was required to achieve the colonization level necessary to cause morbidity and mortality. We further showed that Stx2 is a major virulence factor for C227-11, since a mutant cured of the Stx2 phage was not virulent in Amp-treated mice or Dutch belted rabbits and because treatment of infected mice with anti-Stx2 antibody (11E10) reduced morbidity and mortality compared to those that occurred after administration of PBS alone or no treatment. While there were no significant differences in the weight of mice receiving PBS or 11E10, there was a trend toward greater weight gain in the mice treated with anti-Stx2 antibody; mice fared best if given 11E10, as this group had fewer deaths overall.
We found that Amp treatment was necessary to observe mortality of adult C57BL/6 mice infected with Stx2+ O104:H4 and that death occurred at as early as 5 days and as late as 27 days p.i. In contrast, although Str treatment of infected CD-1 or BALB/c mice resulted in high levels of gi colonization by C227-11, the infected animals did not become ill. Our results with Str are consistent with the findings of others who demonstrated that Str treatment of mice promotes persistent and prolonged gi colonization but not death due to Stx− EAEC infection (44–47, 67, 68). We hypothesize that Amp treatment increases gi colonization by EAEC compared to that after administration of Str. In our model, Amp treatment resulted in a more prolonged high level of colonization with C227-11 than was reported for Str-treated mice (O104:H4 colonized the cecum of Str-treated CD-1 mice at 2.3 × 103 ± 9.7 × 102 CFU on day 7 p.i [47]), while we found that O104:H4 levels in the ceca of Amp-treated mice remained above 107 CFU at 8 days p.i. The reason for the higher level of C227-11 colonization in the Amp-treated model may be that Amp is more effective against anaerobes than Str in the gi tract (69, 70), and it has been shown that anaerobes play an essential role in resistance to microbial colonization of the mouse gut (71, 72).
We further identified the main site of C227-11 colonization to be the cecum in both Amp-treated and ICF models, a finding that is in agreement with the findings obtained with other O104:H4 infection models (47, 49, 68). However, unlike Torres et al. (47), we found that the O104:H4 outbreak strain clearly also colonized the small and large intestines. That EAEC colonizes both the small and large intestines in an animal model was also shown by Harrington et al. (44) and Maura et al. (68). A correlation between the small intestinal colonization and virulence was demonstrated by Wadolkowski et al. (66) in the Str-treated mouse model of STEC pathogenesis. In that study, a mouse-passaged, plasmid-cured derivative of an Stx1+, Stx2+ O157:H7 strain was found in high numbers in the small intestine. Those authors hypothesized that the increased virulence of that strain (over that of the wild-type O157:H7 parent strain) was attributable to colonization of the small intestine and increased absorption of Stx2 from that site (anti-Stx2 but not anti-Stx1 was protective).
That Stx2 contributed to the pathogenesis of C227-11 is predicted from the large number of bloody diarrhea and HUS cases in the German outbreak (6, 11, 12). Here we demonstrated the role of Stx2 in E. coli O104:H4 pathogenesis in two animal models. Amp-treated mice infected with C227-11 showed less weight gain and greater mortality than mice infected with C227-11ϕcu (which is cured of the stx2-encoding phage) or C734-09 (an unrelated stx2− O104:H4 EAEC isolate); additionally, greater morbidity and mortality were observed in rabbits infected with the Stx2+ isolate. Further corroborating the role of Stx2 in pathogenesis of the O104:H4 outbreak strain, treatment of C227-11-infected mice with the anti-Stx2 monoclonal antibody 11E10 resulted in protection and enhanced weight gain. Treatment with PBS alone was somewhat protective initially but ultimately did not protect infected mice from morbidity as effectively as anti-Stx2 monoclonal antibody 11E10. That isotonic fluids alone can be partially protective from renal injury has also been observed in humans (73). In that study, the authors attributed attenuated renal injury failure to early parenteral volume expansion during E. coli O157:H7 infection.
Acute tubular necrosis in mice is a hallmark of STEC-mediated disease and was determined to be the cause of death in Str-treated CD-1 mice infected with Stx2+ E. coli O157:H7 (66). We found acute tubular necrosis in the kidneys of Amp-treated C57BL/6 mice that succumbed to infection with C227-11 as early as 5 days p.i. Our observation of renal tubular necrosis at 5 days p.i. is earlier than the time that it had been detected in a germfree mouse model, in which similar kidney damage was not noted in O104:H4-infected mice at 7 days p.i., though acute tubular necrosis was identified in mice euthanized on days 13 to 15 p.i. in that study (49). There have been a few publications describing acute tubular necrosis in the kidneys of STEC-infected individuals. Specifically, Karpman et al. identified tubular damage in renal biopsy specimens of HUS patients (74). Furthermore, Kaneko et al. reported cell death of renal tubules in Stx-mediated HUS in a 7-year-old boy (75). The editorial by Meyers and Kaplan additionally cited other studies in which renal tubular injury in Stx-mediated HUS was shown (76). To the best of our knowledge, reports describing acute tubular necrosis in individuals infected in the O104:H4 E. coli outbreak have not yet been published; however, we would expect that those patients could have developed acute tubular necrosis, as this strain produces Stx2.
We found similar numbers of C227-11 and C734-09 bacteria shed in the feces of infected Dutch belted rabbits, and we therefore conclude that Stx2 does not play a role in colonization in this model of EAEC infection. This is in contrast to a model of STEC O157:H7 pathogenesis in ICF BALB/c mice (77) in which the Stx2+ O157:H7 E. coli 86-24 strain was found to colonize animals better than the Stx2− isogenic mutant. Those authors proposed a model of bacterial attachment to intestinal epithelial cells whereby Stx2 induces expression of nucleolin on the surface of intoxicated eukaryotic cells; nucleolin binds to the bacterial adhesin intimin (78). We hypothesize that Stx2 does not play a role in colonization of the O104:H4 isolate, as this EAEC strain does not express intimin.
Despite previous reports that adult ICF mice challenged with EAEC show variable colonization and intermittently shed the bacteria into feces (43, 44, 48), in our hands, C227-11 colonization persisted in all adult C57BL/6 ICF mice through 72 h p.i., a result that suggests that ICF C57BL/6 mice are an acceptable model for short-term EAEC colonization and infection studies. However, Dutch belted rabbits may represent a useful ICF animal model to study prolonged colonization by and pathogenesis of EAEC: we demonstrated fecal shedding of all EAEC strains (C227-11, C227-11ϕcu, and C734-09) from infected animals on all 7 days of the experiment, in contrast to previous EAEC rabbit studies, in which colonization was variable (42). Furthermore, when given at a high dose, C227-11 caused disease (weight loss, lethargy, dehydration) and death in infected rabbits. Interestingly, a few C734-09-infected rabbits demonstrated disease, including weight loss, in contrast to PBS control animals, a finding that highlights the possible utility of this model to study Stx2− EAEC strains.
In conclusion, we developed and characterized an Amp-treated mouse model that is useful to study the pathogenesis of both Stx2+ and Stx2− EAEC strains. We determined that the high-level virulence seen in both mice and rabbits with O104:H4 outbreak isolate C227-11 is due to the presence and expression of stx2. Our data support the hypothesis that the combination of Stx2 and EAEC virulence factors was responsible for the increased pathogenesis of the 2011 O104:H4 outbreak strain.
ACKNOWLEDGMENTS
We thank the entire A. D. O'Brien lab, particularly Farhang Alem, Stephen Darnell, James Vergis, and Alyssa Flora, at the Uniformed Services University of the Health Sciences, for expert technical assistance. We thank Cara Olsen for her invaluable help with the statistical analyses. At the University of Maryland, we thank Annabelle Crusan for her clinical veterinary support; Michael M. Lipsky, Theresa Alexander, Michelle Izuka, Elisa Luna, Dawn McKenna, Dawn Engel, and Rebecca Yerkey for veterinary technical support; and Eileen Barry for expert guidance. We also thank Flemming Scheutz from the Statens Serum Institut, Copenhagen, Denmark, for providing C227-11 and C734-09 and James B. Kaper, University of Maryland, School of Medicine, for use of laboratory facilities.
The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the U.S. Department of Defense, the Uniformed Services University of the Health Sciences, or the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This work was funded by grants R37 AI020148 and R37 AI02014827S1 (ARRA Supplement) to A.D.O. and R37 AI033096 to J.P.N.
Footnotes
Published ahead of print 25 February 2013
REFERENCES
- 1. Gault G, Weill FX, Mariani-Kurkdjian P, Jourdan-da Silva N, King L, Aldabe B, Charron M, Ong N, Castor C, Mace M, Bingen E, Noel H, Vaillant V, Bone A, Vendrely B, Delmas Y, Combe C, Bercion R, d'Andigne E, Desjardin M, de Valk H, Rolland P. 2011. Outbreak of haemolytic uraemic syndrome and bloody diarrhoea due to Escherichia coli O104:H4, south-west France, June 2011. Euro Surveill. 16(26):pii=19905. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19905 [DOI] [PubMed] [Google Scholar]
- 2. Mariani-Kurkdjian P, Bingen E, Gault G, Jourdan-Da Silva N, Weill FX. 2011. Escherichia coli O104:H4 south-west France, June 2011. Lancet Infect. Dis. 11:732–733 [DOI] [PubMed] [Google Scholar]
- 3. King LA, Nogareda F, Weill FX, Mariani-Kurkdjian P, Loukiadis E, Gault G, Jourdan-DaSilva N, Bingen E, Mace M, Thevenot D, Ong N, Castor C, Noel H, Van Cauteren D, Charron M, Vaillant V, Aldabe B, Goulet V, Delmas G, Couturier E, Le Strat Y, Combe C, Delmas Y, Terrier F, Vendrely B, Rolland P, de Valk H. 2012. Outbreak of Shiga toxin-producing Escherichia coli O104:H4 associated with organic fenugreek sprouts, France, June 2011. Clin. Infect. Dis. 54:1588–1594 [DOI] [PubMed] [Google Scholar]
- 4. Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, Bernard H, Fruth A, Prager R, Spode A, Wadl M, Zoufaly A, Jordan S, Kemper MJ, Follin P, Muller L, King LA, Rosner B, Buchholz U, Stark K, Krause G, HUS Investigation Team 2011. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N. Engl. J. Med. 365:1771–1780 [DOI] [PubMed] [Google Scholar]
- 5. Robert Koch Institute September 2011. Final presentation and evaluation of epidemiological findings in the EHEC O104:H4 outbreak, Germany 2011. Robert Koch Institute, Berlin, Germany [Google Scholar]
- 6. World Health Organization 2011. Outbreaks of E. coli O104:H4 infection: update 30. World Health Organization, Geneva, Switzerland [Google Scholar]
- 7. Frank C, Faber MS, Askar M, Bernard H, Fruth A, Gilsdorf A, Hohle M, Karch H, Krause G, Prager R, Spode A, Stark K, Werber D, HUS Investigation Team 2011. Large and ongoing outbreak of haemolytic uraemic syndrome, Germany, May 2011. Euro Surveill. 16(21):pii=19878. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19878 [PubMed] [Google Scholar]
- 8. European Food Safety Authority 2011. Tracing seeds, in particular fenugreek (Trigonella foenum-graecum) seeds, in relation to the Shiga toxin-producing E. coli (STEC) O104:H4 2011 outbreaks in Germany and France. European Food Safety Authority, Parma, Italy [Google Scholar]
- 9. Buchholz U, Bernard H, Werber D, Bohmer MM, Remschmidt C, Wilking H, Delere Y, an der Heiden M, Adlhoch C, Dreesman J, Ehlers J, Ethelberg S, Faber M, Frank C, Fricke G, Greiner M, Hohle M, Ivarsson S, Jark U, Kirchner M, Koch J, Krause G, Luber P, Rosner B, Stark K, Kuhne M. 2011. German outbreak of Escherichia coli O104:H4 associated with sprouts. N. Engl. J. Med. 365:1763–1770 [DOI] [PubMed] [Google Scholar]
- 10. Scheutz F, Nielsen EM, Frimodt-Moller J, Boisen N, Morabito S, Tozzoli R, Nataro JP, Caprioli A. 2011. Characteristics of the enteroaggregative Shiga toxin/verotoxin-producing Escherichia coli O104:H4 strain causing the outbreak of haemolytic uraemic syndrome in Germany, May to June 2011. Euro Surveill. 16(24):pii=19889. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19889 [DOI] [PubMed] [Google Scholar]
- 11. Rasko DA, Webster DR, Sahl JW, Bashir A, Boisen N, Scheutz F, Paxinos EE, Sebra R, Chin CS, Iliopoulos D, Klammer A, Peluso P, Lee L, Kislyuk AO, Bullard J, Kasarskis A, Wang S, Eid J, Rank D, Redman JC, Steyert SR, Frimodt-Moller J, Struve C, Petersen AM, Krogfelt KA, Nataro JP, Schadt EE, Waldor MK. 2011. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N. Engl. J. Med. 365:709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bielaszewska M, Mellmann A, Zhang W, Kock R, Fruth A, Bauwens A, Peters G, Karch H. 2011. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect. Dis. 11:671–676 [DOI] [PubMed] [Google Scholar]
- 13. Cheung MK, Li L, Nong W, Kwan HS. 2011. 2011 German Escherichia coli O104:H4 outbreak: whole-genome phylogeny without alignment. BMC Res. Notes 4:533 doi:10.1186/1756-0500-4-533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rohde H, Qin J, Cui Y, Li D, Loman NJ, Hentschke M, Chen W, Pu F, Peng Y, Li J, Xi F, Li S, Li Y, Zhang Z, Yang X, Zhao M, Wang P, Guan Y, Cen Z, Zhao X, Christner M, Kobbe R, Loos S, Oh J, Yang L, Danchin A, Gao GF, Song Y, Yang H, Wang J, Xu J, Pallen MJ, Aepfelbacher M, Yang R. 2011. Open-source genomic analysis of Shiga-toxin-producing E. coli O104:H4. N. Engl. J. Med. 365:718–724 [DOI] [PubMed] [Google Scholar]
- 15. Bockemuhl J, Aleksic S, Karch H. 1992. Serological and biochemical properties of Shiga-like toxin (verocytotoxin)-producing strains of Escherichia coli, other than O-group 157, from patients in Germany. Zentralbl. Bakteriol. 276:189–195 [DOI] [PubMed] [Google Scholar]
- 16. Mellmann A, Bielaszewska M, Kock R, Friedrich AW, Fruth A, Middendorf B, Harmsen D, Schmidt MA, Karch H. 2008. Analysis of collection of hemolytic uremic syndrome-associated enterohemorrhagic Escherichia coli. Emerg. Infect. Dis. 14:1287–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boudailliez B, Berquin P, Mariani-Kurkdjian P, Ilef D, Cuvelier B, Capek I, Tribout B, Bingen E, Piussan C. 1997. Possible person-to-person transmission of Escherichia coli O111-associated hemolytic uremic syndrome. Pediatr. Nephrol. 11:36–39 [DOI] [PubMed] [Google Scholar]
- 18. Morabito S, Karch H, Mariani-Kurkdjian P, Schmidt H, Minelli F, Bingen E, Caprioli A. 1998. Enteroaggregative, Shiga toxin-producing Escherichia coli O111:H2 associated with an outbreak of hemolytic-uremic syndrome. J. Clin. Microbiol. 36:840–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Willshaw GA, Scotland SM, Smith HR, Rowe B. 1992. Properties of Vero cytotoxin-producing Escherichia coli of human origin of O serogroups other than O157. J. Infect. Dis. 166:797–802 [DOI] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention 2011. Investigation update: outbreak of Shiga toxin-producing E. coli O104 (STEC O104:H4) infections associated with travel to Germany. Centers for Disease Control and Prevention, Atlanta, GA: [DOI] [PubMed] [Google Scholar]
- 21. Iyoda S, Tamura K, Itoh K, Izumiya H, Ueno N, Nagata K, Togo M, Terajima J, Watanabe H. 2000. Inducible stx2 phages are lysogenized in the enteroaggregative and other phenotypic Escherichia coli O86:HNM isolated from patients. FEMS Microbiol. Lett. 191:7–10 [DOI] [PubMed] [Google Scholar]
- 22. Smith HR, Cheasty T, Rowe B. 1997. Enteroaggregative Escherichia coli and outbreaks of gastroenteritis in UK. Lancet 350:814–815 [DOI] [PubMed] [Google Scholar]
- 23. Tompkins DS, Hudson MJ, Smith HR, Eglin RP, Wheeler JG, Brett MM, Owen RJ, Brazier JS, Cumberland P, King V, Cook PE. 1999. A study of infectious intestinal disease in England: microbiological findings in cases and controls. Commun. Dis. Public Health 2:108–113 [PubMed] [Google Scholar]
- 24. Pabst WL, Altwegg M, Kind C, Mirjanic S, Hardegger D, Nadal D. 2003. Prevalence of enteroaggregative Escherichia coli among children with and without diarrhea in Switzerland. J. Clin. Microbiol. 41:2289–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Itoh Y, Nagano I, Kunishima M, Ezaki T. 1997. Laboratory investigation of enteroaggregative Escherichia coli O untypeable:H10 associated with a massive outbreak of gastrointestinal illness. J. Clin. Microbiol. 35:2546–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang DB, Dupont HL. 2004. Enteroaggregative Escherichia coli: an emerging pathogen in children. Semin. Pediatr. Infect. Dis. 15:266–271 [DOI] [PubMed] [Google Scholar]
- 27. Bhan MK, Bhandari N, Sazawal S, Clemens J, Raj P, Levine MM, Kaper JB. 1989. Descriptive epidemiology of persistent diarrhoea among young children in rural northern India. Bull. World Health Organ. 67:281–288 [PMC free article] [PubMed] [Google Scholar]
- 28. Okeke IN, Lamikanra A, Steinruck H, Kaper JB. 2000. Characterization of Escherichia coli strains from cases of childhood diarrhea in provincial southwestern Nigeria. J. Clin. Microbiol. 38:7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ruttler ME, Renna NF, Balbi L, Garcia B, Guidone L, Fernandez R, Puig O, Ortiz A. 2002. Characterization of enteroaggregative Escherichia coli strains isolated from children with acute diarrhea, in Mendoza, Argentina. Rev. Argent. Microbiol. 34:167–170 [PubMed] [Google Scholar]
- 30. Cobeljic M, Miljkovic-Selimovic B, Paunovic-Todosijevic D, Velickovic Z, Lepsanovic Z, Zec N, Savic D, Ilic R, Konstantinovic S, Jovanovic B, Kostic V. 1996. Enteroaggregative Escherichia coli associated with an outbreak of diarrhoea in a neonatal nursery ward. Epidemiol. Infect. 117:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huppertz HI, Rutkowski S, Aleksic S, Karch H. 1997. Acute and chronic diarrhoea and abdominal colic associated with enteroaggregative Escherichia coli in young children living in western Europe. Lancet 349:1660–1662 [DOI] [PubMed] [Google Scholar]
- 32. Mossoro C, Glaziou P, Yassibanda S, Lan NT, Bekondi C, Minssart P, Bernier C, Le Bouguenec C, Germani Y. 2002. Chronic diarrhea, hemorrhagic colitis, and hemolytic-uremic syndrome associated with HEp-2 adherent Escherichia coli in adults infected with human immunodeficiency virus in Bangui, Central African Republic. J. Clin. Microbiol. 40:3086–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mayer HB, Wanke CA. 1995. Enteroaggregative Escherichia coli as a possible cause of diarrhea in an HIV-infected patient. N. Engl. J. Med. 332:273–274 [DOI] [PubMed] [Google Scholar]
- 34. Mathewson JJ, Jiang ZD, Zumla A, Chintu C, Luo N, Calamari SR, Genta RM, Steephen A, Schwartz P, DuPont HL. 1995. HEp-2 cell-adherent Escherichia coli in patients with human immunodeficiency virus-associated diarrhea. J. Infect. Dis. 171:1636–1639 [DOI] [PubMed] [Google Scholar]
- 35. Jiang ZD, Greenberg D, Nataro JP, Steffen R, DuPont HL. 2002. Rate of occurrence and pathogenic effect of enteroaggregative Escherichia coli virulence factors in international travelers. J. Clin. Microbiol. 40:4185–4190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mathewson JJ, Johnson PC, DuPont HL, Morgan DR, Thornton SA, Wood LV, Ericsson CD. 1985. A newly recognized cause of travelers' diarrhea: enteroadherent Escherichia coli. J. Infect. Dis. 151:471–475 [DOI] [PubMed] [Google Scholar]
- 37. Adachi JA, Ericsson CD, Jiang ZD, DuPont MW, Pallegar SR, DuPont HL. 2002. Natural history of enteroaggregative and enterotoxigenic Escherichia coli infection among US travelers to Guadalajara, Mexico. J. Infect. Dis. 185:1681–1683 [DOI] [PubMed] [Google Scholar]
- 38. Adachi JA, Jiang ZD, Mathewson JJ, Verenkar MP, Thompson S, Martinez-Sandoval F, Steffen R, Ericsson CD, DuPont HL. 2001. Enteroaggregative Escherichia coli as a major etiologic agent in traveler's diarrhea in 3 regions of the world. Clin. Infect. Dis. 32:1706–1709 [DOI] [PubMed] [Google Scholar]
- 39. Gascon J, Vargas M, Quinto L, Corachan M, Jimenez de Anta MT, Vila J. 1998. Enteroaggregative Escherichia coli strains as a cause of traveler's diarrhea: a case-control study. J. Infect. Dis. 177:1409–1412 [DOI] [PubMed] [Google Scholar]
- 40. Boisen N, Scheutz F, Rasko DA, Redman JC, Persson S, Simon J, Kotloff KL, Levine MM, Sow S, Tamboura B, Toure A, Malle D, Panchalingam S, Krogfelt KA, Nataro JP. 2012. Genomic characterization of enteroaggregative Escherichia coli from children in Mali. J. Infect. Dis. 205:431–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Czeczulin JR, Whittam TS, Henderson IR, Navarro-Garcia F, Nataro JP. 1999. Phylogenetic analysis of enteroaggregative and diffusely adherent Escherichia coli. Infect. Immun. 67:2692–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kang G, Pulimood AB, Mathan MM, Mathan VI. 2001. Enteroaggregative Escherichia coli infection in a rabbit model. Pathology 33:341–346 [PubMed] [Google Scholar]
- 43. Sainz T, Perez J, Fresan MC, Flores V, Jimenez L, Hernandez IHU, Eslava C. 2002. Histological alterations and immune response induced by Pet toxin during colonization with enteroaggregative Escherichia coli (EAEC) in a mouse model infection. J. Microbiol. 40:91–97 [Google Scholar]
- 44. Harrington SM, Sheikh J, Henderson IR, Ruiz-Perez F, Cohen PS, Nataro JP. 2009. The Pic protease of enteroaggregative Escherichia coli promotes intestinal colonization and growth in the presence of mucin. Infect. Immun. 77:2465–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morin N, Tirling C, Ivison SM, Kaur AP, Nataro JP, Steiner TS. 2010. Autoactivation of the AggR regulator of enteroaggregative Escherichia coli in vitro and in vivo. FEMS Immunol. Med. Microbiol. 58:344–355 [DOI] [PubMed] [Google Scholar]
- 46. Martinez-Jehanne V, du Merle L, Bernier-Febreau C, Usein C, Gassama-Sow A, Wane AA, Gouali M, Damian M, Aidara-Kane A, Germani Y, Fontanet A, Coddeville B, Guerardel Y, Le Bouguenec C. 2009. Role of deoxyribose catabolism in colonization of the murine intestine by pathogenic Escherichia coli strains. Infect. Immun. 77:1442–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Torres AG, Cieza RJ, Rojas-Lopez M, Blumentritt CA, Souza CS, Johnston RK, Strockbine N, Kaper JB, Sbrana E, Popov VL. 2012. In vivo bioluminescence imaging of Escherichia coli O104:H4 and role of aerobactin during colonization of a mouse model of infection. BMC Microbiol. 12:112 doi:10.1186/1471-2180-12-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roche JK, Cabel A, Sevilleja J, Nataro J, Guerrant RL. 2010. Enteroaggregative Escherichia coli (EAEC) impairs growth while malnutrition worsens EAEC infection: a novel murine model of the infection malnutrition cycle. J. Infect. Dis. 202:506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Al Safadi R, Abu-Ali GS, Sloup RE, Rudrik JT, Waters CM, Eaton KA, Manning SD. 2012. Correlation between in vivo biofilm formation and virulence gene expression in Escherichia coli O104:H4. PLoS One 7:e41628 doi:10.1371/journal.pone.0041628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Garcia A, Fox JG. 2003. The rabbit as a new reservoir host of enterohemorrhagic Escherichia coli. Emerg. Infect. Dis. 9:1592–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Panda A, Tatarov I, Melton-Celsa AR, Kolappaswamy K, Kriel EH, Petkov D, Coksaygan T, Livio S, McLeod CG, Nataro JP, O'Brien AD, DeTolla LJ. 2010. Escherichia coli O157:H7 infection in Dutch belted and New Zealand white rabbits. Comp. Med. 60:31–37 [PMC free article] [PubMed] [Google Scholar]
- 52. Garcia A, Bosques CJ, Wishnok JS, Feng Y, Karalius BJ, Butterton JR, Schauer DB, Rogers AB, Fox JG. 2006. Renal injury is a consistent finding in Dutch belted rabbits experimentally infected with enterohemorrhagic Escherichia coli. J. Infect. Dis. 193:1125–1134 [DOI] [PubMed] [Google Scholar]
- 53. Melton-Celsa AR, Zangari T, Panda A, Smith MA, Boisen N, Taratov I, Lipsky MM, DeTolla LJ, Kaper JP, O'Brien AD. 2012. Abstr. 8th Int. Symp. Shiga Toxin (Verocytotoxin) Producing Escherichia coli Infect., Amsterdam, The Netherlands, abstr. D2-7 [Google Scholar]
- 54. National Research Council, Committee for the Update of the Guide for the Care and Use of Laboratory Animals 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC [Google Scholar]
- 55. Centers for Disease Control and Prevention and National Institutes of Health 2007. Biosafety in microbiological and biomedical laboratories (BMBL), 5th ed U.S. Government Printing Office, Washington, DC [Google Scholar]
- 56. Shaikh N, Tarr PI. 2003. Escherichia coli O157:H7 Shiga toxin-encoding bacteriophages: integrations, excisions, truncations, and evolutionary implications. J. Bacteriol. 185:3596–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scheutz F, Teel LD, Beutin L, Pierard D, Buvens G, Karch H, Mellmann A, Caprioli A, Tozzoli R, Morabito S, Strockbine NA, Melton-Celsa AR, Sanchez M, Persson S, O'Brien AD. 2012. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing stx nomenclature. J. Clin. Microbiol. 50:2951–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roche Applied Sciences 2009. DIG application manual for filter hybridization. Roche Applied Sciences, Mannheim, Germany [Google Scholar]
- 59. Lindgren SW, Melton AR, O'Brien AD. 1993. Virulence of enterohemorrhagic Escherichia coli O91:H21 clinical isolates in an orally infected mouse model. Infect. Immun. 61:3832–3842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gentry MK, Dalrymple JM. 1980. Quantitative microtiter cytotoxicity assay for Shigella toxin. J. Clin. Microbiol. 12:361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schmitt CK, McKee ML, O'Brien AD. 1991. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H− strain E32511. Infect. Immun. 59:1065–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wadolkowski EA, Burris JA, O'Brien AD. 1990. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 58:2438–2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mohawk KL, Melton-Celsa AR, Zangari T, Carroll EE, O'Brien AD. 2010. Pathogenesis of Escherichia coli O157:H7 strain 86-24 following oral infection of BALB/c mice with an intact commensal flora. Microb. Pathog. 48:131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tzipori S, Montanaro J, Robins-Browne RM, Vial P, Gibson R, Levine MM. 1992. Studies with enteroaggregative Escherichia coli in the gnotobiotic piglet gastroenteritis model. Infect. Immun. 60:5302–5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hicks S, Candy DC, Phillips AD. 1996. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect. Immun. 64:4751–4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wadolkowski EA, Sung LM, Burris JA, Samuel JE, O'Brien AD. 1990. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect. Immun. 58:3959–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Maura D, Morello E, du Merle L, Bomme P, Le Bouguenec C, Debarbieux L. 2012. Intestinal colonization by enteroaggregative Escherichia coli supports long-term bacteriophage replication in mice. Environ. Microbiol. 14:1844–1854 [DOI] [PubMed] [Google Scholar]
- 68. Maura D, Galtier M, Le Bouguenec C, Debarbieux L. 2012. Virulent bacteriophages can target O104:H4 enteroaggregative Escherichia coli in the mouse intestine. Antimicrob. Agents Chemother. 56:6235–6242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wells CL, Maddaus MA, Jechorek RP, Simmons RL. 1988. Role of intestinal anaerobic bacteria in colonization resistance. Eur. J. Clin. Microbiol. Infect. Dis. 7:107–113 [DOI] [PubMed] [Google Scholar]
- 70. Schlessinger D. 1988. Failure of aminoglycoside antibiotics to kill anaerobic, low-pH, and resistant cultures. Clin. Microbiol. Rev. 1:54–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. van der Waaij D, Berghuis-de Vries JM, Lekkerkerk-van der Wees JEC. 1971. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J. Hyg. (Lond.) 69:405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van der Waaij D, Vossen JM, Altes CK, Hartgrink C. 1977. Reconventionalization following antibiotic decontamination in man and animals. Am. J. Clin. Nutr. 30:1887–1895 [DOI] [PubMed] [Google Scholar]
- 73. Ake JA, Jelacic S, Ciol MA, Watkins SL, Murray KF, Christie DL, Klein EJ, Tarr PI. 2005. Relative nephroprotection during Escherichia coli O157:H7 infections: association with intravenous volume expansion. Pediatrics 115:e673–e680 doi:10.1542/peds.2004-2236 [DOI] [PubMed] [Google Scholar]
- 74. Karpman D, Hakansson A, Perez MT, Isaksson C, Carlemalm E, Caprioli A, Svanborg C. 1998. Apoptosis of renal cortical cells in the hemolytic-uremic syndrome: in vivo and in vitro studies. Infect. Immun. 66:636–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kaneko K, Kiyokawa N, Ohtomo Y, Nagaoka R, Yamashiro Y, Taguchi T, Mori T, Fujimoto J, Takeda T. 2001. Apoptosis of renal tubular cells in Shiga-toxin-mediated hemolytic uremic syndrome. Nephron 87:182–185 [DOI] [PubMed] [Google Scholar]
- 76. Meyers KE, Kaplan BS. 2000. Many cell types are Shiga toxin targets. Kidney Int. 57:2650–2651 [DOI] [PubMed] [Google Scholar]
- 77. Robinson CM, Sinclair JF, Smith MJ, O'Brien AD. 2006. Shiga toxin of enterohemorrhagic Escherichia coli type O157:H7 promotes intestinal colonization. Proc. Natl. Acad. Sci. U. S. A. 103:9667–9672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sinclair JF, O'Brien AD. 2002. Cell surface-localized nucleolin is a eukaryotic receptor for the adhesin intimin-gamma of enterohemorrhagic Escherichia coli O157:H7. J. Biol. Chem. 277:2876–2885 [DOI] [PubMed] [Google Scholar]