Abstract
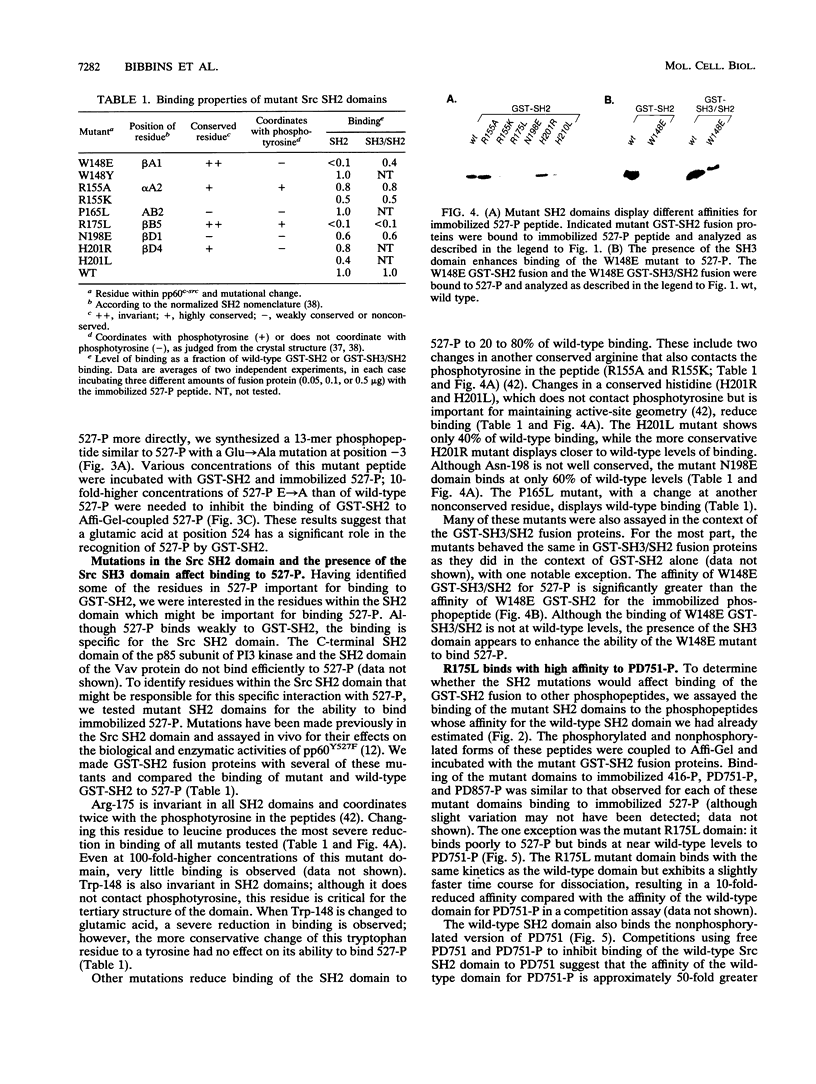
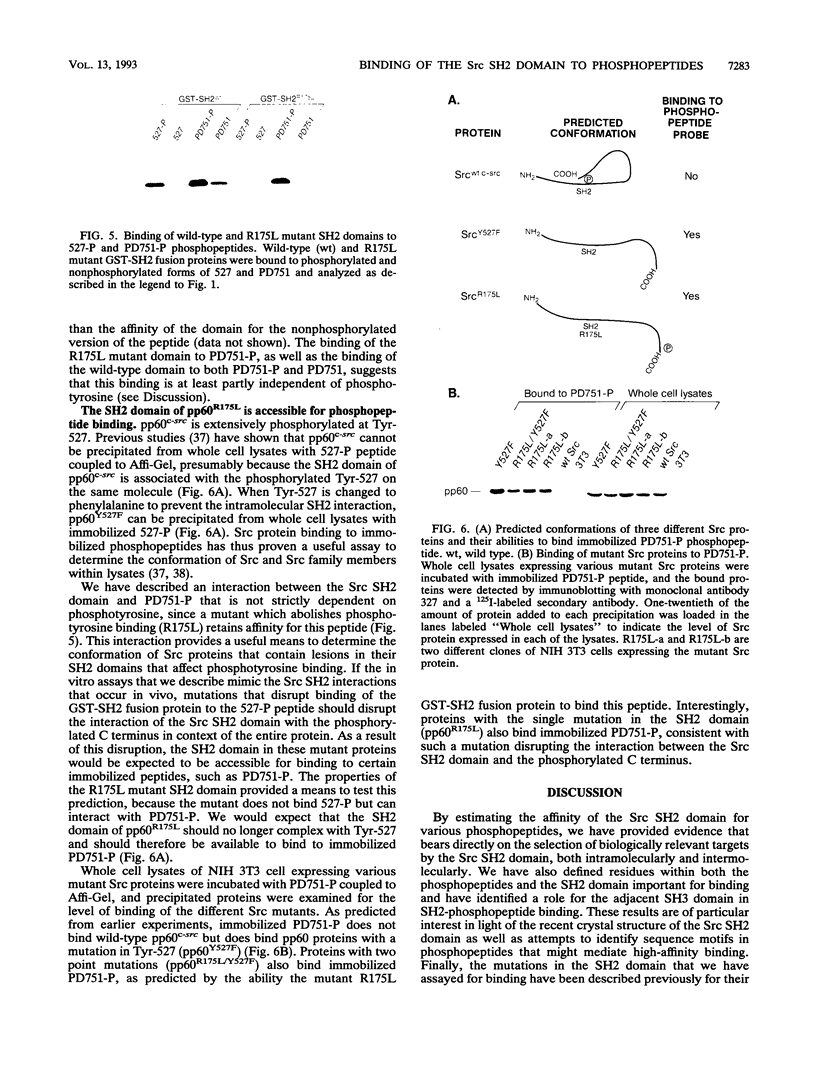
Src homology 2 (SH2) domains are found in a variety of signaling proteins and bind phosphotyrosine-containing peptide sequences. To explore the binding properties of the SH2 domain of the Src protein kinase, we used immobilized phosphopeptides to bind purified glutathione S-transferase-Src SH2 fusion proteins. With this assay, as well as a free-peptide competition assay, we have estimated the affinities of the Src SH2 domain for various phosphopeptides relative to a Src SH2-phosphopeptide interaction whose Kd has been determined previously (YEEI-P; Kd = 4 nM). Two Src-derived phosphopeptides, one containing the regulatory C-terminal Tyr-527 and another containing the autophosphorylation site Tyr-416, bind the Src SH2 domain in a specific though low-affinity manner (with about 10(4)-lower affinity than the YEEI-P peptide). A platelet-derived growth factor receptor (PDGF-R) phosphopeptide containing Tyr-857 does not bind appreciably to the Src SH2 domain, suggesting it is not the PDGF-R binding site for Src as previously reported. However, another PDGF-R-derived phosphopeptide containing Tyr-751 does bind the Src SH2 domain (with an affinity approximately 2 orders of magnitude lower than that of YEEI-P). All of the phosphopeptides which bind to the Src SH2 domain contain a glutamic acid at position -3 or -4 with respect to phosphotyrosine; changing this residue to alanine greatly diminishes binding. We have also tested Src SH2 mutants for their binding properties and have interpreted our results in light of the recent crystal structure solution for the Src SH2 domain. Mutations in various conserved and nonconserved residues (R155A, R155K, N198E, H201R, and H201L) cause slight reductions in binding, while two mutations cause severe reductions. The W148E mutant domain, which alters the invariant tryptophan that marks the N-terminal border of the SH2 domain, binds poorly to phosphopeptides. Inclusion of the SH3 domain in the fusion protein partially restores the binding by the W148E mutant. A change in the invariant arginine that coordinates twice with phosphotyrosine in the peptide (R175L) results in a nearly complete loss of binding. The R175L mutant does display high affinity for the PDGF-R peptide containing Tyr-751, via an interaction that is at least partly phosphotyrosine independent. We have used this interaction to show that the R175L mutation also disrupts the intramolecular interaction between the Src SH2 domain and the phosphorylated C terminus within the context of the entire Src protein; thus, the binding properties observed for mutant domains in an in vitro assay appear to mimic those that occur in vivo.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D., Koch C. A., Grey L., Ellis C., Moran M. F., Pawson T. Binding of SH2 domains of phospholipase C gamma 1, GAP, and Src to activated growth factor receptors. Science. 1990 Nov 16;250(4983):979–982. doi: 10.1126/science.2173144. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Cheng S. H., Piwnica-Worms H., Harvey R. W., Roberts T. M., Smith A. E. The carboxy terminus of pp60c-src is a regulatory domain and is involved in complex formation with the middle-T antigen of polyomavirus. Mol Cell Biol. 1988 Apr;8(4):1736–1747. doi: 10.1128/mcb.8.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Kashishian A. In vivo binding properties of SH2 domains from GTPase-activating protein and phosphatidylinositol 3-kinase. Mol Cell Biol. 1993 Mar;13(3):1737–1745. doi: 10.1128/mcb.13.3.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Dhand R., Pilat D., Twamley G. M., Waterfield M. D., Roussel M. F. Activation of Src family kinases by colony stimulating factor-1, and their association with its receptor. EMBO J. 1993 Mar;12(3):943–950. doi: 10.1002/j.1460-2075.1993.tb05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Kypta R. M., Cooper J. A., Kazlauskas A. Platelet-derived growth factor receptor sequences important for binding of src family tyrosine kinases. Cell Growth Differ. 1991 Oct;2(10):483–486. [PubMed] [Google Scholar]
- DeClue J. E., Martin G. S. Linker insertion-deletion mutagenesis of the v-src gene: isolation of host- and temperature-dependent mutants. J Virol. 1989 Feb;63(2):542–554. doi: 10.1128/jvi.63.2.542-554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo J. A., Kaplan D. R., Kavanaugh W. M., Turck C. W., Williams L. T. A phosphatidylinositol-3 kinase binds to platelet-derived growth factor receptors through a specific receptor sequence containing phosphotyrosine. Mol Cell Biol. 1991 Feb;11(2):1125–1132. doi: 10.1128/mcb.11.2.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl W. J., Escobedo J. A., Martin G. A., Turck C. W., del Rosario M., McCormick F., Williams L. T. Distinct phosphotyrosines on a growth factor receptor bind to specific molecules that mediate different signaling pathways. Cell. 1992 May 1;69(3):413–423. doi: 10.1016/0092-8674(92)90444-h. [DOI] [PubMed] [Google Scholar]
- Hirai H., Varmus H. E. Mutations in src homology regions 2 and 3 of activated chicken c-src that result in preferential transformation of mouse or chicken cells. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8592–8596. doi: 10.1073/pnas.87.21.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H., Varmus H. E. Site-directed mutagenesis of the SH2- and SH3-coding domains of c-src produces varied phenotypes, including oncogenic activation of p60c-src. Mol Cell Biol. 1990 Apr;10(4):1307–1318. doi: 10.1128/mcb.10.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Morrison D. K., Wong G., McCormick F., Williams L. T. PDGF beta-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell. 1990 Apr 6;61(1):125–133. doi: 10.1016/0092-8674(90)90220-9. [DOI] [PubMed] [Google Scholar]
- Kashishian A., Kazlauskas A., Cooper J. A. Phosphorylation sites in the PDGF receptor with different specificities for binding GAP and PI3 kinase in vivo. EMBO J. 1992 Apr;11(4):1373–1382. doi: 10.1002/j.1460-2075.1992.tb05182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Autophosphorylation of the PDGF receptor in the kinase insert region regulates interactions with cell proteins. Cell. 1989 Sep 22;58(6):1121–1133. doi: 10.1016/0092-8674(89)90510-2. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Ellis C., Pawson T., Cooper J. A. Binding of GAP to activated PDGF receptors. Science. 1990 Mar 30;247(4950):1578–1581. doi: 10.1126/science.2157284. [DOI] [PubMed] [Google Scholar]
- Klippel A., Escobedo J. A., Fantl W. J., Williams L. T. The C-terminal SH2 domain of p85 accounts for the high affinity and specificity of the binding of phosphatidylinositol 3-kinase to phosphorylated platelet-derived growth factor beta receptor. Mol Cell Biol. 1992 Apr;12(4):1451–1459. doi: 10.1128/mcb.12.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiecik T. E., Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987 Apr 10;49(1):65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Kypta R. M., Goldberg Y., Ulug E. T., Courtneidge S. A. Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell. 1990 Aug 10;62(3):481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- Liebl E. C., England L. J., DeClue J. E., Martin G. S. Host range mutants of v-src: alterations in kinase activity and substrate interactions. J Virol. 1992 Jul;66(7):4315–4324. doi: 10.1128/jvi.66.7.4315-4324.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Brodeur S. R., Gish G., Songyang Z., Cantley L. C., Laudano A. P., Pawson T. Regulation of c-Src tyrosine kinase activity by the Src SH2 domain. Oncogene. 1993 May;8(5):1119–1126. [PubMed] [Google Scholar]
- Lowenstein E. J., Daly R. J., Batzer A. G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E. Y., Bar-Sagi D., Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992 Aug 7;70(3):431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- MacAuley A., Cooper J. A. Structural differences between repressed and derepressed forms of p60c-src. Mol Cell Biol. 1989 Jun;9(6):2648–2656. doi: 10.1128/mcb.9.6.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengere L. E., Pawson T. Identification of residues in GTPase-activating protein Src homology 2 domains that control binding to tyrosine phosphorylated growth factor receptors and p62. J Biol Chem. 1992 Nov 15;267(32):22779–22786. [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Mayer B. J., Fukui Y., Hanafusa H. Binding of transforming protein, P47gag-crk, to a broad range of phosphotyrosine-containing proteins. Science. 1990 Jun 22;248(4962):1537–1539. doi: 10.1126/science.1694307. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Baltimore D. Signalling through SH2 and SH3 domains. Trends Cell Biol. 1993 Jan;3(1):8–13. doi: 10.1016/0962-8924(93)90194-6. [DOI] [PubMed] [Google Scholar]
- Mori S., Rönnstrand L., Yokote K., Engström A., Courtneidge S. A., Claesson-Welsh L., Heldin C. H. Identification of two juxtamembrane autophosphorylation sites in the PDGF beta-receptor; involvement in the interaction with Src family tyrosine kinases. EMBO J. 1993 Jun;12(6):2257–2264. doi: 10.1002/j.1460-2075.1993.tb05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rhee S. G., Williams L. T. Platelet-derived growth factor (PDGF)-dependent association of phospholipase C-gamma with the PDGF receptor signaling complex. Mol Cell Biol. 1990 May;10(5):2359–2366. doi: 10.1128/mcb.10.5.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien M. C., Fukui Y., Hanafusa H. Activation of the proto-oncogene p60c-src by point mutations in the SH2 domain. Mol Cell Biol. 1990 Jun;10(6):2855–2862. doi: 10.1128/mcb.10.6.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Gish G. D. SH2 and SH3 domains: from structure to function. Cell. 1992 Oct 30;71(3):359–362. doi: 10.1016/0092-8674(92)90504-6. [DOI] [PubMed] [Google Scholar]
- Pendergast A. M., Muller A. J., Havlik M. H., Maru Y., Witte O. N. BCR sequences essential for transformation by the BCR-ABL oncogene bind to the ABL SH2 regulatory domain in a non-phosphotyrosine-dependent manner. Cell. 1991 Jul 12;66(1):161–171. doi: 10.1016/0092-8674(91)90148-r. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Reynolds A. B., Vila J., Lansing T. J., Potts W. M., Weber M. J., Parsons J. T. Activation of the oncogenic potential of the avian cellular src protein by specific structural alteration of the carboxy terminus. EMBO J. 1987 Aug;6(8):2359–2364. doi: 10.1002/j.1460-2075.1987.tb02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D., Margolis B., Mohammadi M., Daly R. J., Daum G., Li N., Fischer E. H., Burgess W. H., Ullrich A., Schlessinger J. SH2 domains prevent tyrosine dephosphorylation of the EGF receptor: identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase C gamma. EMBO J. 1992 Feb;11(2):559–567. doi: 10.1002/j.1460-2075.1992.tb05087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel R. R., Brodeur S. R., Shalloway D., Laudano A. P. Selective binding of activated pp60c-src by an immobilized synthetic phosphopeptide modeled on the carboxyl terminus of pp60c-src. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10696–10700. doi: 10.1073/pnas.88.23.10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieh M., Bolen J. B., Weiss A. CD45 specifically modulates binding of Lck to a phosphopeptide encompassing the negative regulatory tyrosine of Lck. EMBO J. 1993 Jan;12(1):315–321. doi: 10.1002/j.1460-2075.1993.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Valius M., Bazenet C., Kazlauskas A. Tyrosines 1021 and 1009 are phosphorylation sites in the carboxy terminus of the platelet-derived growth factor receptor beta subunit and are required for binding of phospholipase C gamma and a 64-kilodalton protein, respectively. Mol Cell Biol. 1993 Jan;13(1):133–143. doi: 10.1128/mcb.13.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman G., Kominos D., Robertson S. C., Pant N., Baltimore D., Birge R. B., Cowburn D., Hanafusa H., Mayer B. J., Overduin M. Crystal structure of the phosphotyrosine recognition domain SH2 of v-src complexed with tyrosine-phosphorylated peptides. Nature. 1992 Aug 20;358(6388):646–653. doi: 10.1038/358646a0. [DOI] [PubMed] [Google Scholar]
- Waksman G., Shoelson S. E., Pant N., Cowburn D., Kuriyan J. Binding of a high affinity phosphotyrosyl peptide to the Src SH2 domain: crystal structures of the complexed and peptide-free forms. Cell. 1993 Mar 12;72(5):779–790. doi: 10.1016/0092-8674(93)90405-f. [DOI] [PubMed] [Google Scholar]
- Wang H. C., Parsons J. T. Deletions and insertions within an amino-terminal domain of pp60v-src inactivate transformation and modulate membrane stability. J Virol. 1989 Jan;63(1):291–302. doi: 10.1128/jvi.63.1.291-302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E. R., McDonald O. B., Sahyoun N. Quantitative analysis of SH2 domain binding. Evidence for specificity and competition. J Biol Chem. 1992 Jul 15;267(20):14138–14144. [PubMed] [Google Scholar]