Abstract
Bacterial infection with group B Streptococcus (GBS) represents a prominent threat to neonates and fetuses in the Western world, causing severe organ damage and even death. To improve current therapeutic strategies and to investigate new approaches, an appropriate in vivo model to study the immune response of a human immune system is needed. Therefore, we introduced humanized mice as a new model for GBS-induced sepsis. Humanized mice feature deficiencies similar to those found in neonates, such as lower immunoglobulin levels and myeloid cell dysfunction. Due to the husbandry in specific-pathogen-free (SPF) facilities, the human immune cells in these mice also exhibit a naive phenotype which mimics the conditions in fetuses/neonates. Following infection, cytokine release and leukocyte trafficking from the bone marrow to the lymphoid organ (spleen) and into the peritoneum (site of infection) as well as bacterial spreading and clearance were traceable in the humanized mice. Furthermore, we investigated the effects of betamethasone and indomethacin treatment using this novel sepsis model. Although both drugs are commonly used in perinatal care, little is known about their effects on the neonatal immune system. Treatment of infected humanized mice not only induced the reduction of human leukocytes in the spleen but also increased the bacterial load in all analyzed organs, including the brain, which did not show infiltration of live GBS in untreated controls. These studies demonstrate the utility of the humanized mice as a new model to study an immature human immune response during bacterial infection and allow the investigation of side effects induced by various treatments.
INTRODUCTION
Streptococcus agalactiae (group B streptococcus [GBS]) is a leading cause of invasive neonatal infection, inducing sepsis, pneumonia, and meningitis (1–3). This problem is aggravated by the emergence of strains with antibiotic resistance against clindamycin, erythromycin, and penicillin which are routinely used in the clinic for intrapartum antibiotic prophylaxis (4, 5).
While GBS-colonized healthy adults do not show any signs of infection, this pathogen can cause severe invasive bacterial infections in immunocompromised hosts, in elderly patients, and especially in neonates. Primarily responsible for the rapid disease progression in neonates is the inexperienced and immature immune system, which features various deficiencies. To mimic the naive and immature human immune system in vivo, we introduced the humanized mouse model in our studies: transplantation of CD34+ hematopoietic stem cells (e.g., isolated from human cord blood) into immunodeficient mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ [NSG]) induces the development of a human immune system (6, 7) and allows the investigation of human diseases in a small animal model (8–11). However, some human immune cell populations are weakly present or exhibit functional deficiencies in these mice which mimic the impaired neonatal immune system in many cases. For example, the low number of human granulocytes in humanized mice is comparable to the situation in neonates with a limited neutrophil storage pool in the bone marrow (12) and multiple dysfunctions of neutrophils such as reduced chemotaxis, phagocytosis, and bactericidal activity (13).
Likewise, monocytes from humanized NSG mice and human cord blood-derived neonatal monocytes displayed a similar phenotype concerning their phagocytic activity and expression of costimulatory molecules (14).
Furthermore, the protective environment of the amniotic cavity of neonates and humanized mouse husbandry in a specific-pathogen-free (SPF) facility induces a similar naive phenotype of the adaptive immune cells in neonates (15–18) and humanized mice (11). The maturation of human T cells in the mouse thymus is dependent on the murine major histocompatibility complex (MHC) (lack of positive selection on human MHC). As a result, the induction of T cell-dependent B cell activation, the production of human immunoglobulins (Ig), and the class switch toward antigen-specific IgG are generally poor in humanized mice (19). Similar findings are observed in neonates, including reduced MHC II and CD86 expression (20, 21) and low Ig levels (16, 18, 22). Neonatal B cells also exhibit a reduced class switch capacity toward antigen-specific IgG because of lower interleukin-4 (IL-4), IL-5 receptor, and IL-2 receptor γ chain (IL-2RG) expression (23–25).
Another example of immunological similarities found in both neonates and humanized mice is complement deficiency. Several studies described reduced amounts of complement factors with weak activity in newborns (26–29), impairing the clearance of bacterial infections. Similarly, there is a lack of human complement as well as dysfunctional mouse complement in humanized mice (30–32).
On the basis of these criteria, we selected the humanized mouse model for our studies using NSG newborns transplanted with cord blood-derived hematopoietic stem cells. These mice developed a naive human immune system due to the SPF husbandry. Subsequently, mice were inoculated with GBS and the effects of two different agents which are already applied in the clinic in situations of threatened preterm delivery were investigated: betamethasone, a glucocorticoid which is used to prevent neonatal respiratory distress syndrome and decreases neonatal mortality in infants born at 24 to 34 weeks of gestation (33), and indomethacin, a nonsteroidal anti-inflammatory drug which is applied in the clinic as a second-line tocolytic agent (33, 34). Since more than 40% of preterm births are associated with infection (35, 36), both drugs sometimes counteract an ongoing bacterial infection in the fetus. Studies using neonatal rats and piglets investigated the effect of indomethacin and glucocorticoids during GBS infection, giving valuable insights into inflammation alteration in a neonatal immune system (37–40). However, the immune responses in infection and after treatment sometimes exhibit severe differences between species (41, 42). Hence, the objective of this study was to establish a new neonatal humanized sepsis model to investigate the effects of treatment on a human immune system in vivo in detail.
MATERIALS AND METHODS
Animals.
NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice were obtained from Jackson Laboratories (Bar Harbor, ME) and kept and bred in a specific-pathogen-free animal facility at the University of Regensburg. In order to generate humanized mice, newborn NSG mice received sublethal irradiation (1 Gy) and were subsequently injected intrahepatically with 2 × 105 CD34+ stem cells, isolated from human cord blood using the MACS cell separation system according to the manufacturer's protocol (Miltenyi Biotech, Bergisch Gladbach, Germany).
Ethics statements.
All animal work was approved by the local veterinary authorities from the district government based on the international European guidelines and national regulations of the German animal protection act (permission no. 54-2532.1-18/10).
Cord blood samples were taken with approval from the Ethics Committee of the University of Regensburg (permission no. 11-101-0231). All patients included in the experiments provided written informed consent. All experiments were performed in accordance with relevant institutional and national guidelines, regulations, and approvals.
Bacterial strain.
The group B streptococcus (GBS) reference strain ATCC 13813 (generously provided by the Department of Medical Microbiology and Hygiene, University Hospital of Regensburg) was grown from frozen stocks on 8% sheep blood agar plates. Subsequently, bacteria were adjusted to an optical density of 0.4 corresponding to 2 × 108 CFU/ml. For injection, appropriate numbers of CFU were adjusted to 1 ml in phosphate-buffered saline (PBS) (Pan Biotech, Aidenbach, Germany).
Infection model.
Mice were infected with GBS at the age of 3 to 5 months. In order to determine appropriate infectious doses for humanized mice, 105 to 108 CFU was injected intraperitoneally (i.p.) (n = 56 humanized mice, n = 11 irradiated control mice).
For the majority of experiments, two doses were defined. A moderate dose of infection with GBS (106 CFU; animals sacrificed after 3 [n = 10] and 7 [n = 7] days, respectively) was chosen to study the human immune response with high rates of survival. In order to analyze more pronounced short-time effects of GBS infection, a high dose of GBS (107 CFU) was inoculated i.p. and animals were sacrificed and analyzed after 24 h postinfection (n = 10).
For the treatment studies, infected animals received either betamethasone (5 mg/kg; n = 28), indomethacin (3 mg/kg; n = 18), or vehicle (PBS; n = 42) alone. High-dose-infected animals were treated 3 h postinfection. In the moderate-dose model, animals received two treatments i.p.: the first one after 24 h, the second one 48 h postinfection.
Preparation of mononuclear cells from various tissues and peripheral blood.
Mononuclear cells (MNC) were isolated from peripheral blood (pb), spleen, mesenteric lymph node (mLN), liver, lung, kidney, brain, bone marrow (bm), and peritoneum. For preparation of MNC from pb, mice were anesthetized and bled retrobulbarly. Blood (100 μl) was mixed with 20 μl 0.5 M EDTA to prevent clotting and stained for flow cytometric analysis. Subsequently, animals were killed by cervical dislocation and cells from various tissues were extracted. The peritoneal cavity of each mouse was rinsed with 10 ml PBS plus 2 mM EDTA plus 2% fetal calf serum to isolate peritoneal exudate cells (PEC). To obtain MNC of bm, femurs were removed and the ends were clipped off and rinsed with 20 ml PBS plus 2 mM EDTA using a syringe with a 27-gauge needle (BD Bioscience, Franklin Lakes, NJ). The spleen, mLN, liver, and lung were weighted, passed through a 40-μm-pore-size cell strainer (BD), and rinsed with 20 ml PBS plus 2 mM EDTA. The resulting MNC suspensions were either used directly to determine the number of CFU in the corresponding organ or analyzed by flow cytometry.
Cells from lung and liver were resuspended using 5 ml 40% Percoll (GE Healthcare, Uppsala, Sweden) diluted with RPMI medium (Pan Biotech, Aidenbach, Germany) and underlayered using 5 ml 70% Percoll/RPMI. Gradients were centrifuged for 20 min at 800 relative centrifugal force (RCF) at room temperature, and the interphase was collected and washed with PBS. Liver and lung stromal cells from the upper layer and erythrocytes from the bottom layer were discarded. Subsequently, MNC from the interphase were counted and 106 cells were stained for flow cytometric analysis.
CFU in various organs.
Organs (spleen, liver, lung, kidneys, brain, and bm) were homogenized and serially diluted 1:10 down to 10−8 using PBS, plated on 8% sheep blood agar plates, and grown overnight at 37°C. Colonies were counted, and the number of CFU per g of organ was calculated (for bm and peritoneal lavage, the number of CFU per ml was determined).
Flow cytometric analysis of MNC.
Reconstitution of humanized mice with human leukocytes and leukocyte subpopulations were analyzed with an LSR-II flow cytometer (BD Bioscience, San Jose, CA). Cells from pb, spleen, liver, lung, peritoneum, mLN, and bm were stained using the following human-specific monoclonal antibodies: anti-CD45-APC (clone HI30), anti-CD19-Pe (clone HIB19), anti-CD4-FITC (clone SK3), anti-CD66-Pe (clone B1.1/CD66), and anti-CD33-PerCP-Cy5.5 (clone P67.6) from BD Bioscience, anti-HLA-DR-Pe-Cy7 (clone LN3), anti-CD80-Biotin (clone 2D10.4), anti-CD3-PerCP-Cy5.5 (clone OKT3), anti-CD45RA-Pe-Cy7 (clone HI100), and anti-CD27-Biotin (clone 0323) from eBioscience (San Diego, CA), and anti-CD8a-APC (clone HIT8a) from Biolegend (San Diego, CA). For isotype control stainings, the appropriate monoclonal mouse antibodies were used.
Cytokine detection.
To determine cytokine production of human leukocytes, 100 μl blood was drawn retrobulbarly from humanized mice. Blood was allowed to clot at 4°C for 20 min, and serum was collected after two succeeding centrifugations (3,000 RCF and 10,000 RCF, 10 min at 4°C each). The resulting serum was stored at −80°C. Human cytokine concentrations (pg/ml) of serum samples were analyzed by Multimetrix GmbH (Regensburg, Germany) using the cytokine human 6 Plex Panel (interleukin [IL]-1β, IL-6, IL-8, IL-10, gamma interferon [IFN-γ], and tumor necrosis factor [TNF]) (Life Technologies GmbH, Darmstadt, Germany). For acquisition of the mean fluorescence intensity, the Luminex xMAP 100 system (Luminex Corp., Austin, TX) was used and the data were analyzed using the Liquichip Analyzer software (Qiagen, Hilden, Germany).
Histology.
Tissue specimens (spleen, liver, brain, thymus, lymph nodes, and lung) were fixed with 4% formalin, embedded in paraffin, and stained as previously described (11). Briefly, specimens were deparaffinized and pretreated by microwave heating for 30 min at 320 W in 0.1 M citrate buffer adjusted to pH 7.3. The immunostaining was automatically performed on a Ventana Nexes autostainer (Ventana, Tucson, AZ) by using the streptavidin-biotin-peroxidase complex method with 3,3′-diaminobenzidine as a chromogen. The following antibodies were used: anti-CD3 clone SP (Thermo Fisher Scientific Inc., Fremont, CA) and anti-CD79a clone JCB117 and anti-CD45/LCA clone 2B11-PD7/26 (Dako, Glostrup, Denmark).
Statistics.
Statistical analysis was performed using GraphPadPrism (GraphPad Software, La Jolla, CA). All data are represented as mean ± standard error of the mean (SEM) and were tested for statistical significance using Student's t test, analysis of variance (ANOVA), Bonferroni posttest, log rank, or Tukey's multiple comparison test, as indicated in the figure legends.
RESULTS
Dose-dependent disease progression in GBS-infected humanized mice.
The transplantation of CD34+ stem cells resulted in high levels of human immune cell reconstitution (percentage of human CD45/isolated cells) in various organs, including the spleen, liver, bone marrow, and lymph nodes of humanized mice 3 to 5 months posttransplantation (see Fig. S1A in the supplemental material). Lower levels of reconstitution (percentage of human CD45/isolated cells) were measured in the lung and peritoneal exudate cells (PEC). Of note, lung and liver stromal cells and erythrocytes were removed before the reconstitution levels were calculated. To monitor the development of human lymphocytes, the mice were bled and analyzed by flow cytometry at different time points (see Fig. S1B). Human T cell differentiation in the thymus of these mice requires a longer period of time than human B cell maturation in the bone marrow, and therefore T cell numbers increase steadily in the peripheral blood (see Fig. S1B). To ensure a reliable T cell population during infection, we performed all experiments in humanized mice older than 15 weeks. The presence of human immune cells in the different organs obtained from flow cytometric analysis (see Fig. S1) was confirmed using immunohistochemistry (see Fig. S2 in the supplemental material).
To identify the optimal dose of infection, different doses of bacteria were injected intraperitoneally. Lower doses of GBS (1 × 105 to 5 × 105) led to the survival of 90 to 100% of infected animals (Fig. 1A), whereas injection of 107 GBS resulted in the death of 80% of the animals 3 days postinfection. Higher doses led to a more rapid and pronounced disease progression with ∼75% (5 × 107) to 100% (5 × 108) lethality. In order to ensure that the human and not the residual mouse leukocytes play a prominent role in the immune response to the GBS infection, conditioned NSG mice (without human immune cells) as well as humanized littermates were infected with 106 CFU. After 2 days, 10 of 11 infected radiated NSG mice died, whereas all (11/11) humanized littermates survived (Fig. 1B). CFU concentration for each organ was determined for humanized mice infected with high and moderate doses of GBS (Fig. 1C).
Fig 1.
Dose-dependent survival of infected humanized mice and controls. (A) Survival curves of Streptococcus agalactiae (GBS)-infected humanized mice. (B) Survival curves of 1 × 106 GBS-infected humanized mice and conditioned control mice (irradiated but not reconstituted with human CD34+ cells). Numbers of animals are indicated in parentheses. Statistical differences were calculated using Mantel-Cox (log rank) test (***, P < 0.001). (C) CFU count for each organ was determined after 1 day of high-dose infection (left graph, 107 CFU GBS; n = 10) and 3 days (middle graph, n = 10) and 7 days (right graph, n = 6) of moderate-dose (1 × 106) infection.
For the following experiments, two doses were defined. A moderate dose of infection (106 CFU; animals sacrificed after 3 and 7 days) was chosen to study the human immune response with high rates of survival. In order to analyze more pronounced short-term effects of GBS infection, a high-dose infection (107 CFU) was inoculated and animals were sacrificed after 24 h.
Although intraperitoneal (i.p.) injection of 106 CFU led to a rapid systemic spread of GBS (data not shown), some humanized mice were able to clear the infection completely after 3 (4/10) and 7 (4/6) days. These animals were free of live bacteria in all tested organs (lung, liver, peritoneum, bm, kidney, and spleen), were in generally good health, and gained weight (data not shown). Infection with 107 CFU led to rapid systemic dissemination of live bacteria. While the highest concentration of CFU was at the site of injection (peritoneum), high numbers of bacteria were found in all organs investigated, even in the brain of 5/10 animals (Fig. 1C).
Migration of immune cells after Streptococcus agalactiae infection in humanized mice.
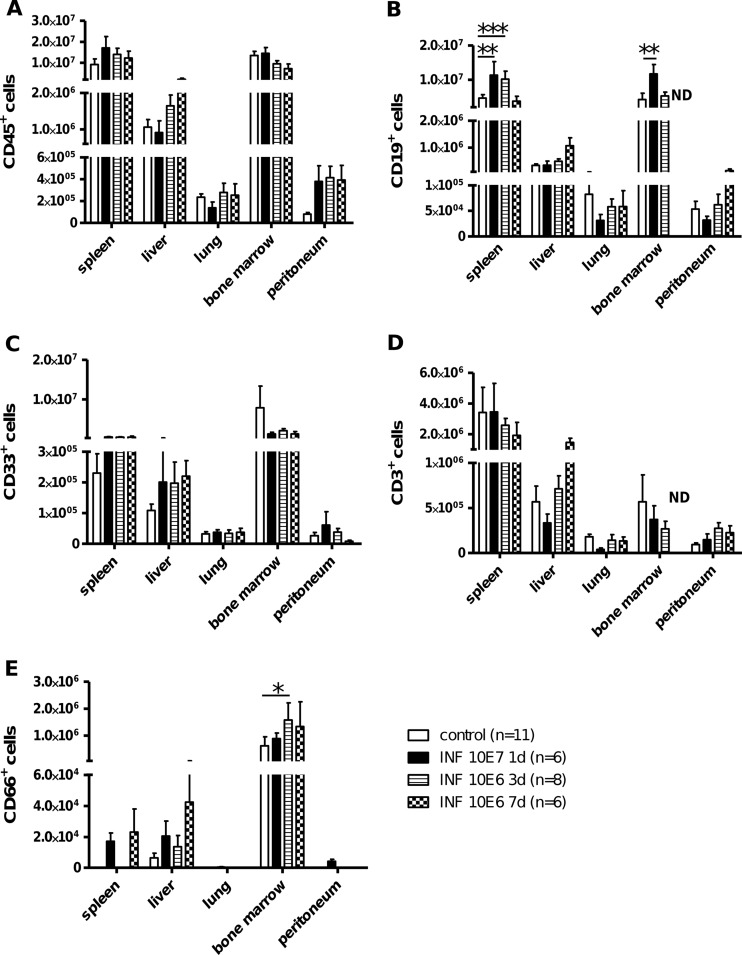
To analyze the effect of GBS infection on migration of cells in humanized mice after bacterial infection, the total numbers of cells from different organs (lung, liver, spleen, peritoneum, and bm) were determined. Most organs did not show significant changes in the total leukocyte count (without differentiation of human or mouse origin) after GBS infection (data not shown). However, there was a marked decrease in the total number of cells in the bm 3 days postinfection, which vanished 4 days later. A trend toward higher numbers of splenocytes could be detected 1 and 3 days postinfection, which became significant on day 7. Further investigation of the total numbers of different human immune cell subsets in various organs after infection revealed no significant changes in the total amount of human leukocytes (CD45), myeloid cells (CD33), or T cells (CD3) (Fig. 2A, C, and D). No significant changes were detectable in the CD4-CD8 distribution or their phenotype (naive versus memory) (data not shown). Only human B cells (CD19) significantly increased in the bm (3 days postinfection) and in the spleen (3 and 7 days postinfection) after a moderate dose of GBS inoculation (Fig. 2B). CD66+ granulocyte levels were low in all organs but were significantly increased after 3 days of a moderate dose of infection in the bm (Fig. 2E).
Fig 2.
Human immune cell trafficking in GBS-infected humanized mice. Humanized mice were infected with a moderate dose of GBS (1 × 106 CFU) for 3 days or 7 days or a high dose (1 × 107 CFU) for 1 day. The total numbers of human immune cells (CD45+) (A), human B cells (CD19+) (B), human myeloid cells (CD33+) (C), human T cells (CD3+) (D), and human granulocytes (CD66+) (E) were calculated from different organs, including peritoneal exudate cells (PEC). Significances between groups were analyzed using the Bonferroni posttest: *, P < 0.05; **, P < 0.01; ***, P < 0.001. ND, not done.
Further, we investigated whether leukocyte trafficking in peripheral blood occurred. On day 3, the percentages of human CD45+ cells (Fig. 3) and human B cells (data not shown) were significantly reduced whereas the percentage of myeloid cells (CD33) was significantly elevated on day 3 and on day 7 (106 CFU) and also after 24 h of high-dose infection (107 CFU) (Fig. 3). The percentage of human T cells (CD3) was significantly increased after high-dose infection but not after moderate infection (data not shown).
Fig 3.
Influence of GBS infection on peripheral blood leukocyte populations in humanized mice. Humanized mice were infected with a moderate dose of GBS (1 × 106 CFU) for 3 days and 7 days as well as 1 day with a high dose of GBS (1 × 107 CFU). The percentage of human leukocytes (CD45+), human myeloid cells (CD33+), and human granulocytes (CD66+) in pb before (pre INF) and after (post INF) the infection was determined using flow cytometry. Values were analyzed using Student's t test: *, P < 0.05.
To investigate the migration capacity of human immune cells to the site of infection, PEC were further analyzed and revealed a significant increase in the percentage of human leukocytes at each time point and dose of infection compared to those of uninfected controls (Fig. 4A). On day 3, a marked increase of T cells was observed (Fig. 4B). An increase of the myeloid cell population was obvious after high dose and after 3 days of a moderate dose of infection but was reduced again on day 7. Granulocytes were only present 24 h after high-dose inoculation with GBS.
Fig 4.
Influx of human immune cells into the peritoneal cavity of GBS-infected humanized mice increased over time. Humanized mice were infected (INF) for 1 day with a high dose (1 × 107 CFU) as well as 3 or 7 days with a moderate dose (1 × 106 CFU) of GBS. The percentage of human leukocytes (CD45+) (A) as well as that of human B cells (CD19+), T cells (CD3+), myeloid cells (CD33+), and granulocytes (CD66+) (B) was analyzed and compared to levels in noninfected humanized mice. Numbers of animals are indicated in parentheses. Significances between groups were analyzed with 1-way (A) or 2-way (B) ANOVA: A***, P < 0.001; A**, P < 0.01. Additionally, significances between groups were analyzed with Tukey's multiple comparison test (1-way ANOVA) (*, P < 0.05; **, P < 0.01) and the Bonferroni posttest (2-way ANOVA; significances are marked within the columns in the same shading style for corresponding comparisons) (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Increased serum cytokine levels in GBS-infected humanized mice.
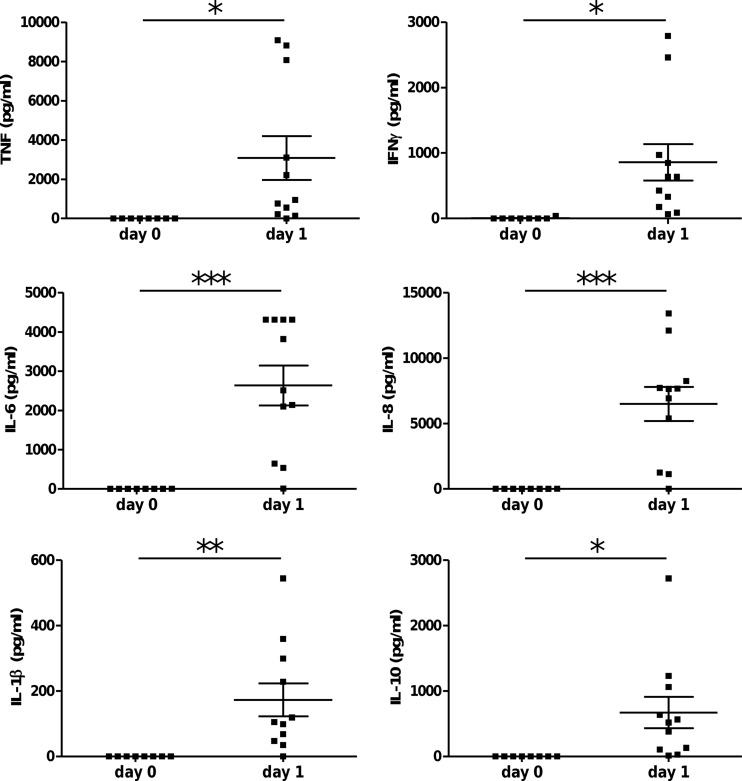
Serum was analyzed during the acute inflammation after high-dose infection (107 CFU; 1 day) and revealed the induction of human proinflammatory cytokines (IFN-γ, TNF, IL-1β, and IL-6), chemokines (IL-8), and counterregulatory (anti-inflammatory) cytokines (IL-10) (Fig. 5). A trend of increased cytokine levels (except IL-1β) was also found in humanized mice 2 and 3 days postinfection with moderate doses of GBS (106) (data not shown).
Fig 5.
Human cytokine response in GBS-infected humanized mice. Humanized mice were infected with a high dose (1 × 107 CFU; n = 11) of GBS, and 24 h later the serum levels of human TNF, IFN-γ, IL-1β, IL-6, IL-8, and IL-10 were determined. Humanized mice without infection served as controls (n = 8). Significant differences were calculated using the Student t test: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Indomethacin and betamethasone influence bacterial load and immune cell distribution.
To study the influence of both treatments on the infection in humanized mice, animals were injected with GBS (107 or 106 CFU) and treated with either betamethasone or indomethacin in the high-dose (1 day) or moderate-dose (3 or 7 days) infection model.
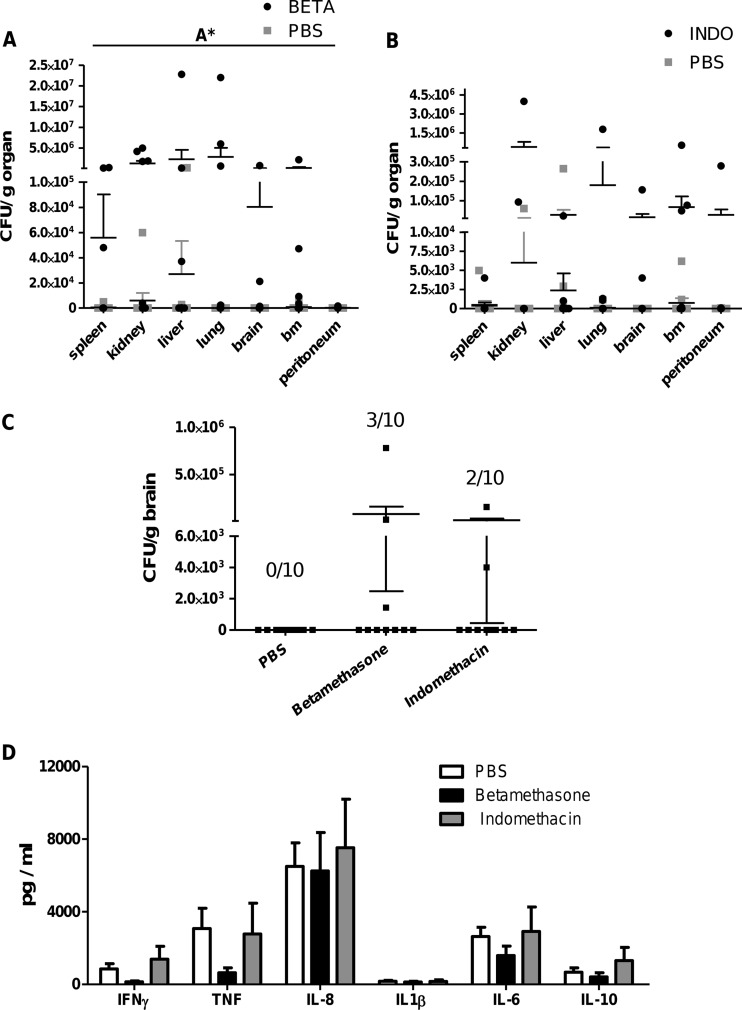
In order to successfully fight off bacterial infections, the ability to inhibit the systemic spreading of bacteria is crucial. Therefore, the effect of treatment on systemic bacterial clearance (spleen, kidney, liver, lung, brain, bm, and peritoneum) was analyzed in all experiments. In the moderate-dose model (3 days postinfection), betamethasone significantly reduced systemic clearance compared to vehicle (PBS)-treated animals (Fig. 6A). Although indomethacin-treated animals also displayed a trend toward reduced clearance in nearly all organs, the differences were not significant (Fig. 6B). Importantly, the presence of live bacteria in the brain after infection with 106 CFU was detected only in animals receiving treatment and never observed in untreated controls (Fig. 6C).
Fig 6.
Influence of betamethasone (BETA) and indomethacin (INDO) treatment on bacterial load and cytokine release in GBS-infected humanized mice. Humanized mice were infected with a moderate dose (1 × 106 CFU) of GBS and treated twice (24 and 48 h p.i.) with betamethasone (A) (n = 10), indomethacin (B) (n = 10), or vehicle alone (PBS) (n = 10). On day 3, mice were sacrificed and CFU per organ were analyzed for each animal. (C) CFU/g brain after 3 days of infection with or without indomethacin or betamethasone treatment. Numbers indicate CFU+ mice/total number of analyzed animals. (D) Humanized mice were infected with a high dose of GBS (1 × 107 CFU). Three hours postinfection, animals received betamethasone (n = 8), indomethacin (n = 4), or vehicle (PBS) alone (n = 11). After 24 h of infection, the serum cytokine levels were analyzed. Significant differences between groups were calculated using 2-way ANOVA (A*, P < 0.05).
After 7 days of moderate-dose infection, a trend toward larger amounts of CFU in betamethasone- as well as indomethacin-injected mice was detectable, indicating a lower rate of clearance in treated animals at this time point as well (data not shown).
Characterizing the influence of treatment on cytokine production during acute infection (high dose) revealed a reduction in levels of the proinflammatory cytokines TNF, IFN-γ, and IL-6 after betamethasone treatment (Fig. 6D). Indomethacin treatment of a moderate 3-day infection increased IL-8 significantly but did not alter other cytokine levels (data not shown).
To determine the effect of betamethasone and indomethacin on the migration of leukocytes during GBS infection, the percentage of the cell populations in the pb (Fig. 7A) and the total number of immune cell populations in the organs (Fig. 7B) were analyzed in moderate-dose-infected humanized mice 3 days postinfection. In pb, a reduction of the percentage of CD45+ cells occurred after infection but was not changed by either treatment whereas both treatments significantly decreased the percentage of B cells (CD19) (Fig. 7A). Betamethasone induced an increase in the CD33+ myeloid cell population after infection. This effect was even more pronounced when animals were treated with indomethacin (Fig. 7A).
Fig 7.
Influence of indomethacin (INDO) and betamethasone (BETA) treatment on human leukocyte populations in GBS-infected humanized mice. Humanized mice were infected with a moderate dose (1 × 106 CFU) of GBS and treated twice (24 and 48 h) with betamethasone (n = 9), indomethacin (n = 6), or vehicle alone (PBS: n = 8). On day 3, mice were sacrificed and leukocyte populations in pb (A) and organs (spleen, liver, lung, bm, and peritoneal cavity) (B) were analyzed. Significant differences were calculated using 1-way (A) or 2-way (B) ANOVA: A*, P < 0.05; A**, P < 0.01; A***, P < 0.001. In addition, significant differences between different treatment groups were calculated using Tukey's multiple comparison test (1-way ANOVA) and the Bonferroni posttest (2-way ANOVA): *, P < 0.05; **, P < 0.01.
In the spleen, betamethasone treatment reduced the total number of human CD45+ cells, human B cells (CD19), and human T cells (CD3) (Fig. 7B, panels I, II, and III). In the bm, glucocorticoid treatment significantly increased the number of human B cells (Fig. 7B, panel II) and decreased the number of human myeloid cells (Fig. 7B, panel IV) compared to PBS-treated mice.
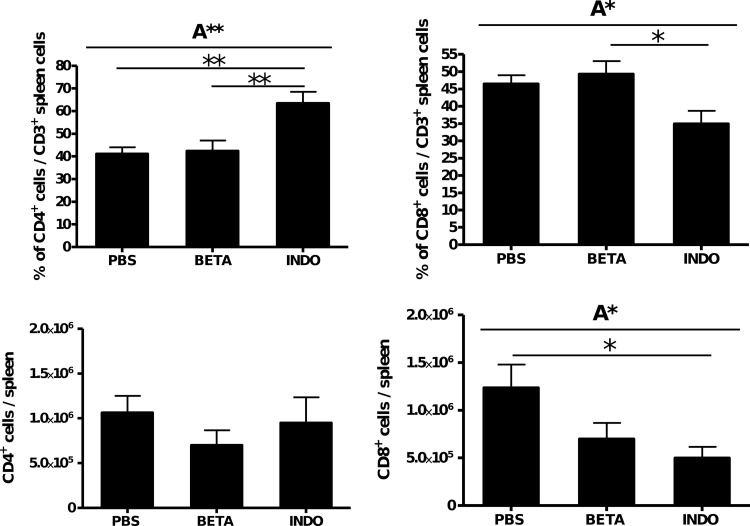
Indomethacin significantly reduced the levels of human leukocytes (Fig. 7B, panel I), human B cells (Fig. 7B, panel II), and human T cells in the spleen (Fig. 7B, panel III) and significantly decreased the amount of human CD8+ T cells (Fig. 8) compared to PBS-treated mice. No differences in immune cell distribution were found after 7 days of infection (data not shown).
Fig 8.
Influence of indomethacin and betamethasone treatment on human T cell population in GBS-infected humanized mice. Humanized mice were infected with a moderate dose (1 × 106 CFU) of GBS and treated twice (24 and 48 h) with betamethasone (n = 9), indomethacin (n = 6), or vehicle alone (PBS: n = 8). On day 3, mice were sacrificed and CD4 and CD8 T cell populations in the spleen were analyzed. Significant differences were calculated using 1-way ANOVA: A*, P < 0.05; A**, P < 0.01. In addition, significant differences between different treatment groups were calculated using Tukey's multiple comparison test: *, P < 0.05; **, P < 0.01.
DISCUSSION
Bacterial infections, especially GBS infections, continue to present a major threat to neonates and fetuses, and an appropriate model to study the effect of new or already existing treatments in the context of an immature human immune system is still missing.
In this study, we introduced humanized mice as a new animal model for neonatal GBS infection. The human immune system in the animals was generated by transplanting CD34+ hematopoietic stem cells from human cord blood into newborn NSG mice which developed a naive human immune system due to SPF husbandry. The ongoing human immune response against GBS infection was characterized, and the feasibility of testing side effects of treatments in this humanized sepsis model was investigated exemplarily using betamethasone and indomethacin.
During the last decades, studies in mice have helped to elucidate the role of the immature neonatal immune system during bacterial and parasitic infection (43–48). Nevertheless, many significant differences between the mouse and human immune systems have been described (41), including differential expression of TLR, different immunoglobulin isotypes and receptors, and various proportions of leukocyte populations, as well as effects of certain proteins and factors (e.g., nitric oxide production). These differences between mice and humans might explain why virtually none of the treatments that had a significant impact in murine models of sepsis worked in the following 25 clinical trials in patients (49, 50). In comparison with humans, rodents are generally highly resistant to most types of induced inflammation. For example, the sensitivity to lipopolysaccharide (LPS) is 1,000 to 10,000 times higher in humans (51, 52) than in mice (53). These distinctly different immune responses are most likely due to diverse reactions in humans and mice, such as, e.g., cytokine release. This aspect became clearly recognizable during the first clinical trials in which TNF was injected for cancer treatment and did not induce symptoms in mice (54) but triggered severe side effects in humans (55). Similarly, the manipulation of immune responses using monoclonal antibodies can result in a completely different outcome in both species. The latest example is the agonistic monoclonal antibody TGN1412, which did not show critical side effects in mice (56, 57), whereas human volunteers receiving this antibody displayed respiratory distress, pulmonary infiltrates, cytokine storm, and lymphopenia (58). In addition, mice often clear infections more rapidly without systemic spreading, inflammation, or kidney, lung, and liver damage, which are an important part of the sepsis syndrome in humans leading to the death of the patient.
Unsinger and colleagues used humanized mice to study apoptosis induction in human immune cells following cecal ligation and puncture (CLP). The study revealed that the immune response (e.g., cytokine release and lymphocyte apoptosis induction) after bacterial challenge in these animals is comparable to that in humans (59).
We extended this study and investigated human immune cell trafficking in GBS-infected humanized mice. Despite certain deficiencies, the human immune cells displayed migration capacity, as shown by the emigration out of the bm (reduced cell count after infection) into the peritoneal cavity (site of infection) and the lymphoid organs (spleen) passing the transport medium (pb). In addition, the induction of proliferation and maturation was reflected by a significantly increased population of CD66+ granulocytes in the bm at 3 and 7 days postinfection. One day after high-dose infection, human CD66+ granulocytes were able to migrate into the peritoneum but were unable to control the infection or prevent systemic spreading.
The influence of treatment with betamethasone or indomethacin on migration and/or proliferation in various organs was visible in infected humanized mice as observed in the overall reduction of CD45+ leukocytes in the spleen (caused mainly by a reduction of human T cells). In the bone marrow, the number of myeloid cells decreased but the number of human B cells increased after both treatments.
Induction of human proinflammatory as well as counterregulating anti-inflammatory cytokines and chemokines in the serum proved the functional activation of human immune cells in these mice. The plasma cytokine levels in infected humanized mice are comparable with those of septic neonatal mice (60–63). Septic human adults and neonates also display increased plasma levels of the cytokines measured in this study; however, they do not reach the plasma concentrations of existing animal models for sepsis or infected humanized mice (64, 65). This might be due to a lower bacterial burden in human patients. Glucocorticoid treatment had no prominent effect on the cytokine response but resulted in systemic elevation of CFU counts after 3 days of infection. An increased bacterial load following glucocorticoid treatment was also described by Tran and Weisman after infecting newborn rats with GBS (39).
Animals receiving indomethacin only displayed a tendency toward reduced bacterial clearance but showed treatment-induced changes in leukocyte populations similar to those in glucocorticoid-treated humanized mice. Importantly, only in indomethacin (2/10) and betamethasone (3/10)-treated animals infected with a moderate dose was GBS able to translocate into the brain tissue. Our data suggest that indomethacin treatment might have detrimental effects on human neonates suffering from GBS infection. However, studies using neonatal animal models revealed that treatment with this anti-inflammatory drug also has beneficial effects during GBS infection. Indomethacin reduces sepsis-related pulmonary hypertension, increases arterial partial pressure of oxygen as well as cardiac output, prevents metabolic acidosis, and augments hemodynamics, improving the outcome in GBS-induced sepsis (37, 38, 40, 66).
Our results indicate that in the situation of imminent preterm delivery induced by GBS infection, treatment with betamethasone or indomethacin may have the potential to trigger the course of disease. However, since both therapeutic options (prevention of preterm birth by indomethacin and induction of lung maturation by betamethasone) are necessary for neonatal survival, antibiotic treatment should always be combined with both treatments in the case of suspected bacterial infection to prevent the increase of bacterial load and therefore avoid the possible drug-induced side effects described above.
As seen in the individual differences in bacterial load and immune cell distribution, heterogeneity (induced by different stem cell donors) sometimes interfered with the pooling of data and impeded significant differences between groups. Nevertheless, this model corresponds to the situation in humans more accurately than that of inbred mice with their identical genetic background. To assess the effects of new treatment strategies, a higher number of humanized mice (compared to inbred mice) might be necessary.
Of note, humanized NOD/scid and NSG mice still possess mouse macrophages and granulocytes, but these cells exhibit dysfunctions in maturation and activation (7, 32). These deficient myeloid cells do not seem to play a prominent role in fighting off bacterial infections, since we found that only 1/11 conditioned NSG mice survive GBS infection compared to 11/11 humanized littermates. Nevertheless, mouse cell activity most likely contributes to the immunological processes to a certain degree.
In conclusion, humanized mice feature deficiencies in their immune system similar to those in human neonates. Therefore, they offer a new opportunity to investigate the effects of GBS infection in combination with treatment on a human immune system in a small animal model. Although an animal model will never be able to mimic the situation in human neonates entirely, humanized mice offer a new—and in certain aspects improved—approach to investigate sepsis in the context of human immune responses.
Mouse models remain to be the first choice to test the efficiency of new therapies in high-throughput studies under standardized conditions. However, humanized mice might help to bridge the gap between in vitro as well as in vivo mouse models and clinical trials to elucidate possible side effects before they enter the stage of clinical testing.
Supplementary Material
ACKNOWLEDGMENTS
We especially thank Nancie Archin (University of North Carolina at Chapel Hill School of Medicine) for proofreading the manuscript. We also thank O. Kölbl (Department of Radiotherapy, Regensburg) for giving us access to the linear accelerators and L. Shultz for providing the NSG mice. We are grateful to M. Siebörger, E. Wiesler, M. Bock, R. Kromas, and I. Fink for excellent assistance.
Footnotes
Published ahead of print 25 February 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01235-12.
REFERENCES
- 1. Maisey HC, Doran KS, Nizet V. 2009. Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert Rev. Mol. Med. 10:e27 doi:10.1017/S1462399408000811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koenig JM, Keenan WJ. 2009. Group B streptococcus and early-onset sepsis in the era of maternal prophylaxis. Pediatr. Clin. North Am. 56:689–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Henneke P, Berner R. 2006. Interaction of neonatal phagocytes with group B streptococcus: recognition and response. Infect. Immun. 74:3085–3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shet A, Ferrieri P. 2004. Neonatal & maternal group B streptococcal infections: a comprehensive review. Indian J. Med. Res. 120:141–150 [PubMed] [Google Scholar]
- 5. Rajagopal L. 2009. Understanding the regulation of group B streptococcal virulence factors. Future Microbiol. 4:201–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. 2005. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 106:1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. 2005. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174:6477–6489 [DOI] [PubMed] [Google Scholar]
- 8. Mota J, Rico-Hesse R. 2009. Humanized mice show clinical signs of dengue fever according to infecting virus genotype. J. Virol. 83:8638–8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joseph A, Zheng JH, Chen K, Dutta M, Chen C, Stiegler G, Kunert R, Follenzi A, Goldstein H. 2010. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. J. Virol. 84:6645–6653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wege AK, Florian C, Ernst W, Zimara N, Schleicher U, Hanses F, Schmid M, Ritter U. 2012. Leishmania major infection in humanized mice induces systemic infection and provokes a nonprotective human immune response. PLoS Negl. Trop. Dis. 6:e1741 doi:10.1371/journal.pntd.0001741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wege AK, Ernst W, Eckl J, Frankenberger B, Vollmann-Zwerenz A, Männel DN, Ortmann O, Kroemer A, Brockhoff G. 2011. Humanized tumor mice—a new model to study and manipulate the immune response in advanced cancer therapy. Int. J. Cancer 129:2194–2206 [DOI] [PubMed] [Google Scholar]
- 12. Chirico G. 2005. Development of the immune system in neonates. J. Arab Neonatol. Forum 2(2):5–11 [Google Scholar]
- 13. Wolach B, Sonnenschein D, Gavrieli R, Chomsky O, Pomeranz A, Yuli I. 1998. Neonatal neutrophil inflammatory responses: parallel studies of light scattering, cell polarization, chemotaxis, superoxide release, and bactericidal activity. Am. J. Hematol. 58:8–15 [DOI] [PubMed] [Google Scholar]
- 14. Gille C, Orlikowsky TW, Spring B, Hartwig UF, Wilhelm A, Wirth A, Goecke B, Handgretinger R, Poets CF, André MC. 2012. Monocytes derived from humanized neonatal NOD/SCID/IL2Rγnull mice are phenotypically immature and exhibit functional impairments. Hum. Immunol. 73:346–354 [DOI] [PubMed] [Google Scholar]
- 15. Yamada A, Kaneyuki T, Hara A, Rothstein DM, Yokoyama MM. 1992. CD45 isoform expression on human neonatal T cells: expression and turnover of CD45 isoforms on neonatal versus adult T cells after activation. Cell. Immunol. 142:114–124 [DOI] [PubMed] [Google Scholar]
- 16. Schelonka RL, Infante AJ. 1998. Neonatal immunology. Semin. Perinatol. 22:2–14 [DOI] [PubMed] [Google Scholar]
- 17. Marodi L. 2002. Down-regulation of Th1 responses in human neonates. Clin. Exp. Immunol. 128:1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adkins B, Leclerc C, Marshall-Clarke S. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553–564 [DOI] [PubMed] [Google Scholar]
- 19. McDermott SP, Eppert K, Lechman ER, Doedens M, Dick JE. 2010. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood 116:193–200 [DOI] [PubMed] [Google Scholar]
- 20. Marshall-Clarke S, Reen D, Tasker L, Hassan J. 2000. Neonatal immunity: how well has it grown up. Immunol. Today 21:35–41 [DOI] [PubMed] [Google Scholar]
- 21. Jones CA, Holloway JA, Warner JO. 2002. Phenotype of fetal monocytes and B lymphocytes during the third trimester of pregnancy. J. Reprod. Immunol. 56:45–60 [DOI] [PubMed] [Google Scholar]
- 22. Splawski JB, Jelinek DF, Lipsky PE. 1991. Delineation of the functional capacity of human neonatal lymphocytes. J. Clin. Invest. 87:545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Recher M, Berglund LJ, Avery DT, Cowan MJ, Gennery AR, Smart J, Peake J, Wong M, Pai SY, Baxi S, Walter JE, Palendira U, Tangye GA, Rice M, Brothers S, Al-Herz W, Oettgen H, Eibel H, Puck JM, Cattaneo F, Ziegler JB, Giliani S, Tangye SG, Notarangelo LD. 2011. IL-21 is the primary common γ chain-binding cytokine required for human B-cell differentiation in vivo. Blood 118:6824–6835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zola H, Fusco M, Macardle PJ, Flego L, Roberton D. 1995. Expression of cytokine receptors by human cord blood lymphocytes: comparison with adult blood lymphocytes. Pediatr. Res. 38:397–403 [DOI] [PubMed] [Google Scholar]
- 25. Saito S, Morii T, Umekage H, Makita K, Nishikawa K, Narita N, Ichijo M, Morikawa H, Ishii N, Nakamura M, Sugamura K. 1996. Expression of the interleukin-2 receptor gamma chain on cord blood mononuclear cells. Blood 87:3344–3350 [PubMed] [Google Scholar]
- 26. Zilow G, Zilow EP, Burger R, Linderkamp O. 1993. Complement activation in newborn infants with early onset infection. Pediatr. Res. 34:199–203 [DOI] [PubMed] [Google Scholar]
- 27. Sonntag J, Brandenburg U, Polzehl D, Strauss E, Vogel M, Dudenhausen JW, Obladen M. 1998. Complement system in healthy term newborns: reference values in umbilical cord blood. Pediatr. Dev. Pathol. 1:131–135 [DOI] [PubMed] [Google Scholar]
- 28. Drew JH, Arroyave CM. 1980. The complement system of the newborn infant. Biol. Neonate 37:209–217 [DOI] [PubMed] [Google Scholar]
- 29. Lassiter HA, Watson SW, Seifring ML, Tanner JE. 1992. Complement factor 9 deficiency in serum of human neonates. J. Infect. Dis. 166:53–57 [DOI] [PubMed] [Google Scholar]
- 30. Harvey BS, Baker CJ, Edwards MS. 1992. Contributions of complement and immunoglobulin to neutrophil-mediated killing of enterococci. Infect. Immun. 60:3635–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Foreman O, Kavirayani AM, Griffey SM, Reader R, Shultz LD. 2011. Opportunistic bacterial infections in breeding colonies of the NSG mouse strain. Vet. Pathol. 48:495–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV, Greiner DL. 1995. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 154:180–191 [PubMed] [Google Scholar]
- 33. Goldenberg RL. 2002. The management of preterm labor. Obstet. Gynecol. 100:1020–1037 [DOI] [PubMed] [Google Scholar]
- 34. Suarez RD, Grobman WA, Parilla D. 2001. Indomethacin tocolysis and intraventricular hemorrhage. Obstet. Gynecol. 97:921–925 [DOI] [PubMed] [Google Scholar]
- 35. Locksmith G, Duff P. 2001. Infection, antibiotics, and preterm delivery. Semin. Perinatol. 25:295–309 [DOI] [PubMed] [Google Scholar]
- 36. Agrawal V, Hirsch E. 2012. Intrauterine infection and preterm labor. Semin. Fetal Neonatal Med. 17:12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gibson RL, Truog WE, Henderson WR, Jr, Redding GJ. 1992. Group B streptococcal sepsis in piglets: effect of combined pentoxifylline and indomethacin pretreatment. Pediatr. Res. 31:222–227 [DOI] [PubMed] [Google Scholar]
- 38. Pauly TH, Aziz SM, Horstman SJ, Gillespie MN. 1992. Impact of prostaglandin and thromboxane synthesis blockade on disposition of group B streptococcus in lung and liver of intact piglet. Pediatr. Res. 31:14–17 [DOI] [PubMed] [Google Scholar]
- 39. Tran TV, Weisman LE. 2004. Dexamethasone effects on group B streptococcal infection in newborn rats. Pediatr. Infect. Dis. J. 23:47–52 [DOI] [PubMed] [Google Scholar]
- 40. Short BL, Miller MK, Pan J. 1988. Group B streptococcal (GBSS) newborn septic shock model: the role of prostaglandins. Prog. Clin. Biol. Res. 264:333–336 [PubMed] [Google Scholar]
- 41. Mestas J, Hughes CC. 2004. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172:2731–2738 [DOI] [PubMed] [Google Scholar]
- 42. Bryant CE, Monie TP. 2012. Mice, men and the relatives: cross-species studies underpin innate immunity. Open Biol. 2:120015 doi:10.1098/rsob.120015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kronforst KD, Mancuso CJ, Pettengill M, Ninkovic J, Power Coombs MR, Stevens C, Otto M, Mallard C, Wang X, Goldmann D, Levy O. 2012. A neonatal model of intravenous Staphylococcus epidermidis infection in mice <24 h old enables characterization of early innate immune responses. PLoS One 7:e43897 doi:10.1371/journal.pone.0043897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mittal R, Gonzalez-Gomez I, Panigrahy A, Goth K, Bonnet R, Prasadarao NV. 2010. IL-10 administration reduces PGE-2 levels and promotes CR3-mediated clearance of Escherichia coli K1 by phagocytes in meningitis. J. Exp. Med. 207:1307–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Placencia FX, Kong L, Weisman LE. 2009. Treatment of methicillin-resistant Staphylococcus aureus in neonatal mice: lysostaphin versus vancomycin. Pediatr. Res. 65:420–424 [DOI] [PubMed] [Google Scholar]
- 46. Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, Ungaro R, Levy O, Moldawer LL. 2008. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood 112:1750–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Henneke P, Dramsi S, Mancuso G, Chraibi K, Pellegrini E, Theilacker C, Hübner J, Santos-Sierra S, Teti G, Golenbock DT, Poyart C, Trieu-Cuot P. 2008. Lipoproteins are critical TLR2 activating toxins in group B streptococcal sepsis. J. Immunol. 180:6149–6158 [DOI] [PubMed] [Google Scholar]
- 48. Kenzel S, Mancuso G, Malley R, Teti G, Golenbock DT, Henneke P. 2006. c-Jun kinase is a critical signaling molecule in a neonatal model of group B streptococcal sepsis. J. Immunol. 176:3181–3188 [DOI] [PubMed] [Google Scholar]
- 49. Natanson C, Hoffman WD, Suffredini AF, Eichacker PQ, Danner RL. 1994. Selected treatment strategies for septic shock based on proposed mechanisms of pathogenesis. Ann. Intern. Med. 120:771–783 [DOI] [PubMed] [Google Scholar]
- 50. Zeni F, Freeman B, Natanson C. 1997. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit. Care Med. 25:1095–1100 [DOI] [PubMed] [Google Scholar]
- 51. Sauter C, Wolfensberger C. 1980. Interferon in human serum after injection of endotoxin. Lancet ii:852–853 [DOI] [PubMed] [Google Scholar]
- 52. Warren HS. 2009. Editorial: mouse models to study sepsis syndrome in humans. J. Leukoc. Biol. 86:199–201 [DOI] [PubMed] [Google Scholar]
- 53. Ren Y, Xie Y, Jiang G, Fan J, Yeung J, Li W, Tam PK, Savill J. 2008. Apoptotic cells protect mice against lipopolysaccharide-induced shock. J. Immunol. 180:4978–4985 [DOI] [PubMed] [Google Scholar]
- 54. Asher A, Mulé JJ, Reichert CM, Shiloni E, Rosenberg SA. 1987. Studies on the anti-tumor efficacy of systemically administered recombinant tumor necrosis factor against several murine tumors in vivo. J. Immunol. 138:963–974 [PubMed] [Google Scholar]
- 55. Chapman PB, Lester TJ, Casper ES, Gabrilove JL, Wong GY, Kempin SJ, Gold PJ, Welt S, Warren RS, Starnes HF, et al. 1987. Clinical pharmacology of recombinant human tumor necrosis factor in patients with advanced cancer. J. Clin. Oncol. 5:1942–1951 [DOI] [PubMed] [Google Scholar]
- 56. Beyersdorf N, Gaupp S, Balbach K, Schmidt J, Toyka KV, Lin CH, Hanke T, Hünig T, Kerkau T, Gold R. 2005. Selective targeting of regulatory T cells with CD28 superagonists allows effective therapy of experimental autoimmune encephalomyelitis. J. Exp. Med. 202:445–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hunig T, Dennehy K. 2005. CD28 superagonists: mode of action and therapeutic potential. Immunol. Lett. 100:21–28 [DOI] [PubMed] [Google Scholar]
- 58. Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. 2006. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355:1018–1028 [DOI] [PubMed] [Google Scholar]
- 59. Unsinger J, McDonough JS, Shultz LD, Ferguson TA, Hotchkiss RS. 2009. Sepsis-induced human lymphocyte apoptosis and cytokine production in “humanized” mice. J. Leukoc. Biol. 86:219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mancuso G, Tomasello F, Migliardo M, Delfino D, Cochran J, Cook JA, Teti G. 1994. Beneficial effects of interleukin-6 in neonatal mouse models of group B streptococcal disease. Infect. Immun. 62:4997–5002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cusumano V, Mancuso G, Genovese F, Delfino D, Beninati C, Losi E, Teti G. 1996. Role of gamma interferon in a neonatal mouse model of group B streptococcal disease. Infect. Immun. 64:2941–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mancuso G, Cusumano V, Genovese F, Gambuzza M, Beninati C, Teti G. 1997. Role of interleukin 12 in experimental neonatal sepsis caused by group B streptococci. Infect. Immun. 65:3731–3735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wynn JL, Scumpia PO, Delano MJ, O'Malley KA, Ungaro R, Abouhamze A, Moldawer KL. 2007. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock 28:675–683 [DOI] [PubMed] [Google Scholar]
- 64. Kurt AN, Aygun AD, Godekmerdan A, Kurt A, Dogan Y, Yilmaz E. 2007. Serum IL-1beta, IL-6, IL-8, and TNF-alpha levels in early diagnosis and management of neonatal sepsis. Mediators Inflamm. 2007:31397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lvovschi V, Arnaud L, Parizot C, Freund Y, Juillien G, Ghillani-Dalbin P, Bouberima M, Larsen M, Riou B, Gorochov G, Hausfater P. 2011. Cytokine profiles in sepsis have limited relevance for stratifying patients in the emergency department: a prospective observational study. PLoS One 6:e28870 doi:10.1371/journal.pone.0028870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Runkle B, Goldberg RN, Streitfeld MM, Clark MR, Buron E, Setzer ES, Bancalari E. 1984. Cardiovascular changes in group B streptococcal sepsis in the piglet: response to indomethacin and relationship to prostacyclin and thromboxane A2. Pediatr. Res. 18:874–878 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.