Abstract
Francisella tularensis, the causative agent of tularemia, is a category A bioterrorism agent. A vaccine that is safer and more effective than the currently available unlicensed F. tularensis live vaccine strain (LVS) is needed to protect against intentional release of aerosolized F. tularensis, the most dangerous type of exposure. In this study, we employed a heterologous prime-boost vaccination strategy comprising intradermally administered LVS ΔcapB (highly attenuated capB-deficient LVS mutant) as the primer vaccine and rLm/iglC (recombinant attenuated Listeria monocytogenes expressing the F. tularensis immunoprotective antigen IglC) as the booster vaccine. Boosting LVS ΔcapB-primed mice with rLm/iglC significantly enhanced T cell immunity; their splenic T cells secreted significantly more gamma interferon (IFN-γ) and had significantly more cytokine (IFN-γ and/or tumor necrosis factor [TNF] and/or interleukin-2 [IL-2])-producing CD4+ and CD8+ T cells upon in vitro IglC stimulation. Importantly, mice primed with LVS ΔcapB or rLVS ΔcapB/IglC, boosted with rLm/iglC, and subsequently challenged with 10 50% lethal doses (LD50) of aerosolized highly virulent F. tularensis Schu S4 had a significantly higher survival rate and mean survival time than mice immunized with only LVS ΔcapB (P < 0.0001); moreover, compared with mice immunized once with LVS, primed-boosted mice had a higher survival rate (75% versus 62.5%) and mean survival time during the first 21 days postchallenge (19 and 20 days for mice boosted after being primed with LVS ΔcapB and rLVS ΔcapB/IglC, respectively, versus 17 days for mice immunized with LVS) and maintained their weight significantly better (P < 0.01). Thus, the LVS ΔcapB-rLm/iglC prime-boost vaccination strategy holds substantial promise for a vaccine that is safer and at least as potent as LVS.
INTRODUCTION
Francisella tularensis is the causative agent of tularemia. There are four subspecies of F. tularensis: F. tularensis subsp. tularensis (type A), F. tularensis subsp. holarctica (type B), F. tularensis subsp. mediasiatica, and F. tularensis subsp. novicida (1), although F. tularensis subsp. novicida is considered by some to be a separate species (2). F. tularensis subsp. tularensis and F. tularensis subsp. holarctica are two clinically important entities, both of which can be transmitted among animals and from animals to humans by arthropod bites or by aerosol. F. tularensis subsp. tularensis, found in the United States, is highly virulent in rodents (3), nonhuman primates (4), and humans (5, 6), causes the most severe disease, and is the subspecies of greatest concern as a weapon of bioterrorism. F. tularensis subsp. holarctica, found in Eurasia and in North America, causes less severe disease than F. tularensis subsp. tularensis in humans. Following cutaneous exposure, tularemia typically presents as an ulceronodular disease with painful, ulcerated skin lesions and swollen lymph nodes. In 15% of untreated cases, the ulceronodular disease spreads hematogenously to involve lungs and pleura. Following inhalation exposure, tularemia presents with acute flu-like symptoms followed by pleuro-pneumonic and typhoidal illness. Pneumonic tularemia is difficult to diagnose (7), and delay in treatment can be fatal (8). Even when successfully treated with appropriate antibiotics, pneumonic tularemia causes substantial morbidity; most patients require hospitalization and administration of parenteral antibiotics (7), typically in intensive care units. Because of its high degree of pathogenicity in humans, its low infectious dose, and the relative ease with which it can be cultured and aerosolized, F. tularensis is classified as a category A agent of bioterrorism and is considered one of the most likely pathogens to be employed in a bioterrorist attack.
Currently, there is no licensed vaccine available against F. tularensis. The F. tularensis live vaccine strain (LVS), a multideletional mutant of virulent F. tularensis subsp. holarctica, is the only vaccine against tularemia used in the United States, and then only under special circumstances. Among 17 unique genetic regions found in the highly virulent F. tularensis subsp. tularensis Schu S4 strain, 12 are absent from the F. tularensis subsp. holarctica LVS genome (9), 2 of which encode the major virulence factors FTT0918 and PilA (10, 11); complementation of LVS with these two genes fully restores virulence of LVS to that of the virulent parental F. tularensis subsp. holarctica (12). LVS has major limitations, including the following: (i) it retains significant toxicity (5), (ii) it provides poor protection against high-dose aerosol challenge (13), and (iii) it displays mixed (blue-gray) colony morphology, with only one colony type inducing protective immunity (14, 15). Nevertheless, from the standpoint of efficacy, LVS, administered by scarification as a single dose, is the current standard for protection against challenge with F. tularensis.
In searching for a vaccine that is safer than LVS, we previously developed LVS ΔcapB, an LVS mutant with a targeted deletion in a putative capsular gene, capB. Thus, LVS ΔcapB has three major attenuating deletions, in capB, FTT0918, and pilA (12). LVS ΔcapB is significantly attenuated in mice. Whereas the 50% lethal dose (LD50) for LVS by the intranasal (i.n.) route is 700 CFU, the LD50 for LVS ΔcapB is >107 CFU (16). Mice immunized with LVS ΔcapB i.n. or by the intradermal (i.d.) route develop humoral and cellular immune responses comparable to those of mice immunized with LVS, and when these mice are challenged with a lethal dose of LVS i.n. 4 or 8 weeks later, they are 100% protected from illness and death and have significantly lower levels (3 to 5 log) of LVS in the lung, liver, and spleen than unimmunized mice. Most importantly, mice immunized i.n. or i.d. with LVS ΔcapB and then challenged 6 weeks later by aerosol with 10 LD50 of the highly virulent F. tularensis subsp. tularensis Schu S4 strain are significantly protected. By the i.n. route, mice immunized with LVS ΔcapB are 100% protected, as are mice immunized with LVS; however, whereas LVS ΔcapB is nontoxic, LVS immunization kills ∼25% of i.n. vaccinated mice. By the i.d. route, LVS ΔcapB is less potent than LVS (16).
In further search for a better vaccine alternative to LVS, we previously developed a recombinant Listeria monocytogenes vaccine expressing F. tularensis immunoprotective proteins as a vaccine against F. tularensis. L. monocytogenes, like F. tularensis, is a facultative intracellular bacterium; moreover, it shares a similar intracellular lifestyle with F. tularensis, escaping the phagosome and multiplying free in the cytoplasm of the host cell (17, 18). L. monocytogenes is known to induce innate and adaptive immune responses (19) and has been developed as a cancer vaccine vector (20). In a previous study, we chose a highly attenuated L. monocytogenes mutant with a deletion in actA, L. monocytogenes ΔactA, as a vector, and constructed a recombinant L. monocytogenes vaccine expressing F. tularensis IglC (rLm/iglC) (21). IglC (intracellular growth locus subunit C) is encoded by genes (FTT1712 and FTT1357) on the Francisella pathogenicity island (FPI), which is highly upregulated during macrophage intracellular infection, required for intracellular survival, growth, and phagosome escape (22–24), and essential for virulence (25). It is highly immunogenic (21, 26–28) and was previously identified by our group as an immunoprotective antigen (21). Our previous study showed that mice immunized i.d. with rLm/iglC developed significantly higher IglC-specific T cell immune responses than sham-immunized mice, and when these mice were challenged i.n. with F. tularensis LVS (6 LD50), they had a significantly lower mean tissue bacterial burden and a higher survival rate than those of animals immunized with saline or the vector control. Most importantly, mice immunized with rLm/iglC i.d. were protected against aerosol challenge with F. tularensis Schu S4. However, in subsequent studies using higher aerosol challenge doses of F. tularensis Schu S4, rLm/iglC was not as potent as LVS. This prompted us to develop a more consistently potent vaccine—one that was not only safer than LVS but at least as potent.
In this study, to develop a more potent vaccine than LVS without sacrificing safety, we utilized a heterologous prime-boost vaccination strategy with LVS ΔcapB or LVS ΔcapB overexpressing an F. tularensis antigen as the prime vaccine and rLm/iglC as the booster vaccine. We show that the LVS ΔcapB-rLm/iglC prime-boost vaccine induces strong cellular immune responses and confers protective immunity against F. tularensis Schu S4 aerosol challenge that is comparable to or greater than that conferred by LVS.
MATERIALS AND METHODS
Bacteria and vaccines.
F. tularensis LVS and Schu S4 strains were obtained from the Centers for Disease Control and Prevention (Atlanta, GA). LVS ΔcapB, LVS ΔwbtDEF (LVS ΔLPS), and rLm/iglC were constructed in our laboratory as described previously (16, 21, 29). To prepare the LVS stocks, we passaged the bacteria once on phorbol-12-myristate-13-acetate (PMA)-differentiated monolayers of THP-1 cells, amplified them on chocolate II agar (BD BBL, Sparks, MD) for 2 to 3 days, scraped the colonies into sterile phosphate-buffered saline (PBS), resuspended the bacteria in the presence of 20% glycerol, and stored them frozen at −80°C. In some experiments, we used chocolate agar made with GC medium base (BD Difco) supplemented with 1% hemoglobin and 1% IsoVitalex enrichment (BD BBL). Before use in animals, one vial of the LVS or Schu S4 strain was removed from the freezer, immediately thawed in a 37°C water bath, diluted in sterile saline, and kept on ice until use. To prepare heat-inactivated (HI) bacteria, we propagated the LVS on chocolate agar for 2 to 3 days, resuspended the bacteria in PBS to a final optical density at 540 nm (OD540) of 1 (equivalent to 2 × 109 CFU/ml), killed the bacteria by incubation at 80°C for 1 h, and stored the bacteria frozen at −80°C until use. Immediately after it was heat inactivated, LVS was plated on chocolate agar and incubated for 3 days at 37°C to verify that there were no live bacteria remaining.
The LVS ΔcapB and LVS ΔLPS stocks were prepared by culturing on chocolate agar as described for LVS and were stored frozen at −80°C. The L. monocytogenes ΔactA and rLm/iglC stocks were prepared by culturing the bacteria in brain heart infusion (BHI) broth (BD BBL) overnight at 37°C with agitation, subculturing them in BHI medium until late log phase, harvesting them by centrifugation, washing the bacterial pellet once with PBS, resuspending the bacteria in PBS in the presence of 20% glycerol, and storing them frozen at −80°C. The stocks were thawed periodically, cultured on agar to verify the titer of viable bacteria, and prepared for animal use as described above for the LVS and Schu S4 strains.
Mice.
Six- to 8-week-old specific-pathogen-free female BALB/c mice were purchased from Charles River Laboratory (Wilmington, MA) and used according to protocols approved by the animal research committees of UCLA and Colorado State University.
Construction of recombinant attenuated LVS strains expressing F. tularensis proteins.
LVS ΔcapB strains overexpressing F. tularensis IglC or IglA were constructed by electroporating a shuttle plasmid carrying an IglA or IglC expression cassette into LVS ΔcapB. To construct the shuttle plasmid, the coding sequence for F. tularensis IglC or IglA was amplified by PCR, using the genomic DNA of a virulent F. tularensis subsp. tularensis recent clinical isolate (RCI) as a template, with the primer pair 5′-GGATCATATGATGATTATGAGTGAGATGAT-3′ and 5′-GAACGGATCCCTATGCAGCTGCAATATATC-3′ for IglC and 5′-GGATCATATGCTTATAAGGTGTTGTGAAAAAAAGG-3′ and 5′-GAACGGATCCCTACTTACCATCTACTTGTTGATTA-3′ for IglA, incorporating NdeI and BamHI sites at the 5′ and 3′ ends, respectively. The PCR product, NdeI-IglC-BamHI or NdeI-IglA-BamHI, was cloned into the pFNLTP6 gro-gfp shuttle plasmid, replacing gfp downstream of the groE promoter (30). The inserted DNA sequences were verified by restriction enzyme digestion and nucleotide sequencing. The resultant plasmid carrying the F. tularensis IglC or IglA expression cassette, pFNL/gro-IglC or pFNL/gro-IglA, was electroporated into LVS ΔcapB, and transformants were selected as kanamycin-resistant clones. The selected clones were verified by colony PCR, amplifying the sequence encoding F. tularensis protein by use of primers specific to the pFNLTP6 vector. The resultant strains, rLVS ΔcapB/IglC and rLVS ΔcapB/IglA, were evaluated for levels of F. tularensis protein expression. The stocks used for in vitro and in vivo experiments were prepared similarly to LVS stocks.
Vaccination and challenge of mice.
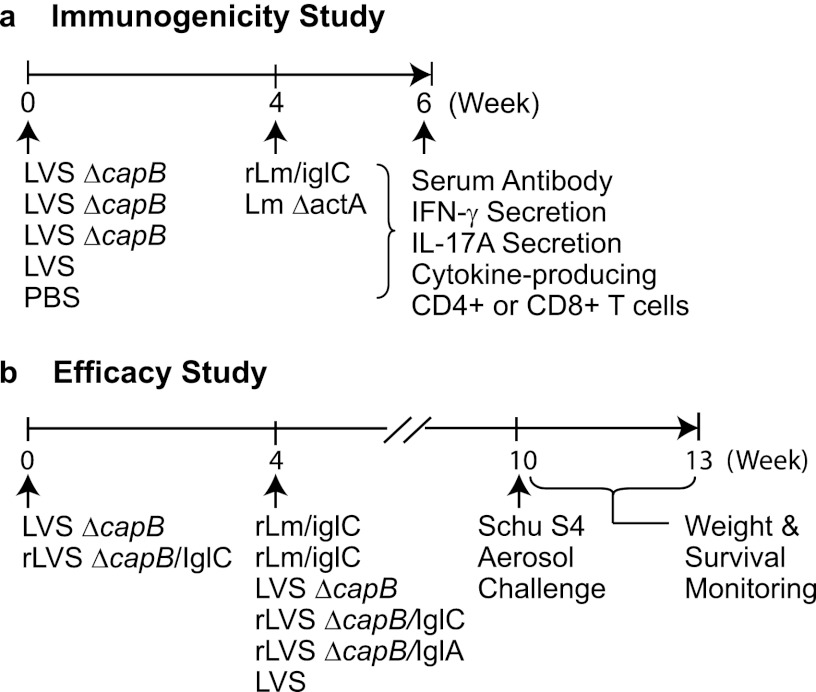
Vaccination and challenge of mice were conducted as described previously (21). For immunology studies (Fig. 1a), mice were primed i.d. at week 0 with 104 CFU LVS, 106 CFU LVS ΔcapB, or PBS (sham control), either not boosted or boosted at week 4 with 106 rLm/iglC or 106 L. monocytogenes ΔactA (vector control), bled, and then euthanized at week 6. Sera were prepared and assayed for antibody endpoint titer. Spleens were removed, and a single-cell suspension of splenocytes was prepared for assaying T cell immune responses. For study of the efficacy of prime-boost vaccination utilizing LVS ΔcapB (Fig. 1b and Fig. 6), mice were primed i.d. with 106 CFU LVS ΔcapB or rLVS ΔcapB/IglC at week 0, boosted i.d. with 106 CFU rLm/iglC at week 4, and challenged by aerosol with 10 LD50 of F. tularensis subsp. tularensis Schu S4 at week 10, at Colorado State University. Mice not immunized or immunized once at week 4 with LVS, LVS ΔcapB, rLVS ΔcapB/IglC, or rLVS ΔcapB/IglA and challenged at week 10 (so as to keep the immunization-challenge interval constant) served as controls. For study of the efficacy of prime-boost vaccination utilizing rLVS ΔLPS/IglC (see Fig. S4 in the supplemental material), mice were primed i.d. with 106 CFU rLVS ΔLPS/IglC at week 0 and boosted with rLm/iglC twice, at weeks 3 and 6, or primed once at week 3 and boosted once at week 6, and then challenged by aerosol with 3 or 10 LD50 of the F. tularensis subsp. tularensis Schu S4 strain at week 12 at Colorado State University. Mice immunized twice, at weeks 3 and 6, with normal saline (sham), LVS, LVS ΔLPS, or rLVS ΔLPS/IglC and challenged at week 12 served as homologous prime-boost controls. The aerosol challenge was conducted in a chamber of 5 cubic feet, with conscious and active mice, using a Glas-Col inhalation exposure system (Glas-Col, LLC, Terre Haute, IN). The doses of 3 and 10 LD50 for the Schu S4 strain were obtained by aerosolizing 5 ml of a suspension containing 3.2 × 106 to 3.4 × 106 and 1.1 × 107 to 1.2 × 107 CFU/ml, respectively, over a period of 15 min. The actual number of bacteria in the nebulizer was confirmed by culturing the bacterial suspension in duplicate on Mueller-Hinton agar. Challenged mice were weighed and monitored for illness and death for 3 weeks. Mice that met predetermined humane endpoints for euthanasia were euthanized and counted as a death. Mean survival time (MST) was calculated by dividing the sum of the surviving days of all mice by the total number of mice examined, with animals surviving until the end of the experiment given a survival time of 21 days, when the experiment was terminated.
Fig 1.
Immunization and challenge protocols. (a) Immunogenicity study. BALB/c mice were primed i.d. with LVS ΔcapB at week 0 and boosted i.d. with rLm/iglC or with L. monocytogenes ΔactA (vector control) at week 4. Mice sham immunized with PBS or immunized once with LVS or LVS ΔcapB at week 0 and not subsequently boosted served as controls. At week 6, all mice were euthanized, their sera were assayed for antibody titer, and their splenic lymphocytes were assayed for antigen-specific production of IFN-γ and IL-17A or for cytokine-producing CD4+ or CD8+ T cells by multiparameter intracellular cytokine staining and flow cytometry. (b) Efficacy study. Mice were primed with LVS ΔcapB or rLVS ΔcapB/IglC at week 0 and boosted with rLm/iglC at week 4. Mice not immunized (not shown) or immunized once with LVS ΔcapB, rLVS ΔcapB/IglC, rLVS ΔcapB/IglA, or LVS at week 4 served as controls. At week 10, 6 weeks after the last or only immunization, such that the immunization-challenge interval was held constant, all mice were challenged with the F. tularensis subsp. tularensis Schu S4 strain by aerosol, after which the mice were monitored for weight loss, signs of illness, and survival for 3 weeks.
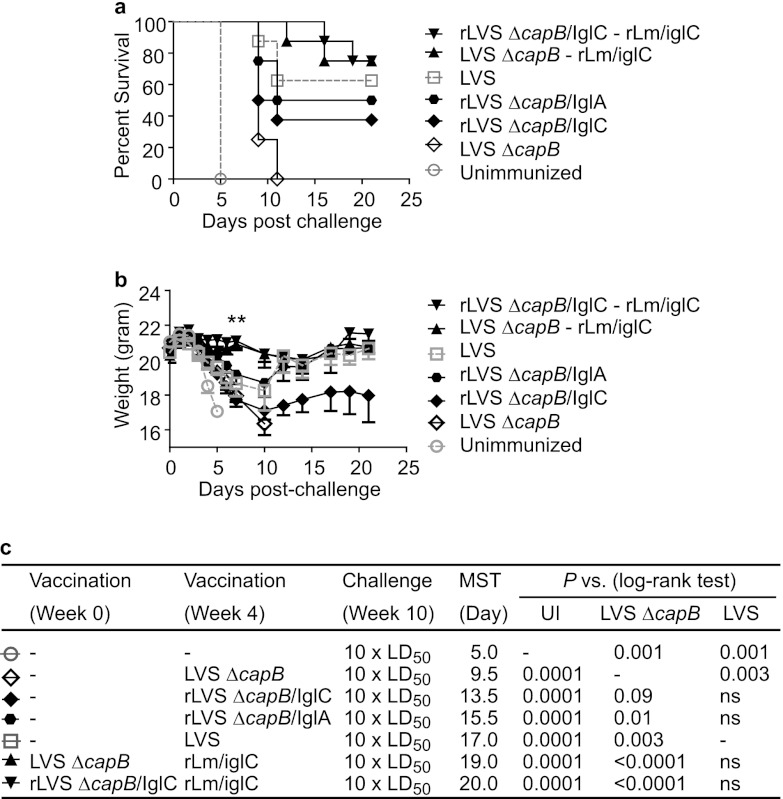
Fig 6.
Boosting of LVS ΔcapB-primed mice with rLm/IglC enhances protective immunity against aerosol challenge with F. tularensis Schu S4, and the overall efficacy of prime-boost vaccination is superior to that of immunization with one dose of LVS. Mice (n = 8 mice/group) were primed with LVS ΔcapB or rLVS ΔcapB/IglC and boosted with rLm/iglC. Unimmunized mice or mice vaccinated with LVS ΔcapB, rLVS ΔcapB/IglC, rLVS ΔcapB/IglA, or LVS and not boosted served as controls. All mice were challenged by aerosol with 10 LD50 of the F. tularensis Schu S4 strain. The survival (a) and mean weight (b) of mice in each group were monitored for 21 days postchallenge. The MST of mice in each group (column 5) and statistical analyses of differences in survival between each group of mice and unimmunized mice (UI; column 6), LVS ΔcapB-immunized mice (column 7), or LVS-immunized mice (column 8) are shown in panel c. **, P < 0.01 by Student t test analysis of the difference between the weights of either LVS ΔcapB-rLm/iglC- or rLVS ΔcapB/IglC-rLm/iglC-primed and -boosted mice and LVS-immunized mice at 7 days postchallenge. The data for unimmunized mice and mice immunized with only LVS ΔcapB or LVS were published previously (16). ns, not significant.
Isolation of lymphocytes from mouse spleen.
Groups of four BALB/c mice were sham immunized or immunized i.d. as described previously (16). At various times postvaccination, mice were anesthetized by intraperitoneal (i.p.) injection of ketamine (80 mg/kg of body weight) and xylazine (10 mg/kg), bled, and then euthanized. Spleens were removed, placed in a petri dish containing 5 ml T cell medium (Dulbecco's modified Eagle's medium [DMEM] supplemented with 10% heat-inactivated fetal bovine serum [HI-FBS], penicillin [100 IU/ml]-streptomycin [100 μg/ml], 0.1 mM nonessential amino acids, 4 mM l-glutamine, 1 mM sodium pyruvate, and 0.05 mM β-mercaptoethanol), and gently pressed with the flat end of a 5-ml syringe to release the splenocytes into the medium. Erythrocytes were subsequently lysed using PharmLyse (BD Pharmingen), and tissue debris and cell aggregates were cleared by filtration through cell strainers with 70-μm-pore-size nylon membranes (Falcon). Single-cell suspensions of splenocytes in T cell medium were used for assaying T cell immune responses as described below.
Production of IFN-γ, IL-17A, and TNF by immune splenocytes.
Single-cell suspensions of splenocytes from mice that were sham immunized or immunized with various vaccines were stimulated with IglC protein (10 μg/ml) (21), HI-LVS (5 × 106/ml), or concanavalin A (ConA; 5 μg/ml) for 3 days. After 3 days, the culture supernatant fluid was collected, cell debris was removed by centrifugation, and the supernatant fluid was either assayed immediately or stored in assay diluent (BD Biosciences) at −80°C until use. The production of mouse gamma interferon (IFN-γ), interleukin-17A (IL-17A), and tumor necrosis factor (TNF) in the culture supernatant fluid was assayed by using a mouse cytokine enzyme immunoassay (EIA) kit (BD Biosciences) following the manufacturer's instructions.
Intracellular cytokine staining and flow cytometry analysis.
Splenocytes (106 cells per well) were seeded in U-bottom 96-well plates and incubated with medium alone or with medium containing IglC protein, an IglC peptide (TDEAWGIMIDLSNLE; kindly provided by Justin Skoble of Aduro Biotech) (2 μg/ml), or HI-LVS overnight. GolgiPlug (protein transport inhibitor containing brefeldin A) diluted in T cell medium was then added to all wells; PMA was additionally added to positive-control wells. Five hours after addition of GolgiPlug, cells were harvested, washed with PBS, and stained with Live/Dead fixable violet dead cell stain (Invitrogen) for 10 min at room temperature. Subsequently, cells were incubated with Fc block for 15 min, followed by incubation with allophycocyanin (APC)-Cy7-conjugated anti-CD4 (clone RM-4-5) and peridinin chlorophyll protein (PerCp)-Cy5.5-conjugated anti-CD8 (clone 53-6.7) antibodies in Fc block for an additional 15 min. The cells were then washed, fixed, permeabilized with Cytofix/Cytoperm, and stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (clone 17-A2) and panels of antibodies against the intracellular markers IFN-γ (phycoerythrin [PE] conjugated; clone XMG 17A2), TNF (PE-Cy7 conjugated; clone MP6-XT22), and IL-2 (APC conjugated; clone JES6-5H4). All intracellular cytokine staining reagents were purchased from BD Biosciences, except where noted. A total of 100,000 lymphocytes per sample were acquired with an LSRII-HT (BD) flow cytometer. The frequencies of live CD4+ and CD8+ T cells expressing each of the seven different possible combinations of IFN-γ, TNF, and IL-2 were determined using FACSDiva (BD) software. Background numbers of cells producing cytokines without antigen stimulation were subtracted.
Serum antibody detection by enzyme-linked immunosorbent assay (ELISA).
Sera collected from sham-immunized mice or mice immunized with various vaccines were analyzed for levels of IgG and subtype IgG1 and IgG2a antibodies specific for HI-LVS as described previously (16). The endpoint antibody titer was calculated as the reciprocal of the highest serum dilution that was a minimum of 0.05 optical density units above the mean plus 3 standard errors of the sham-immunized animals.
Statistics.
One-way analysis of variance (ANOVA) with Tukey's multiple-comparison test or two-way ANOVA with Bonferroni's posttest was performed using GraphPad Prism 5 (San Diego, CA) to determine the significance of differences in comparisons of mean mouse cytokine production, mean serum antibody endpoint titers, and mean frequencies of cytokine-producing CD4+ and CD8+ T cells among mice in vaccinated and control groups. A log-rank analysis (Mantel-Cox test) in GraphPad Prism 5 was used to determine the significance of differences in survival curves among mice in immunized and control groups.
RESULTS
Boosting of LVS ΔcapB-immunized mice with rLm/iglC induces antigen-specific cytokine production and a Th1-type antibody response.
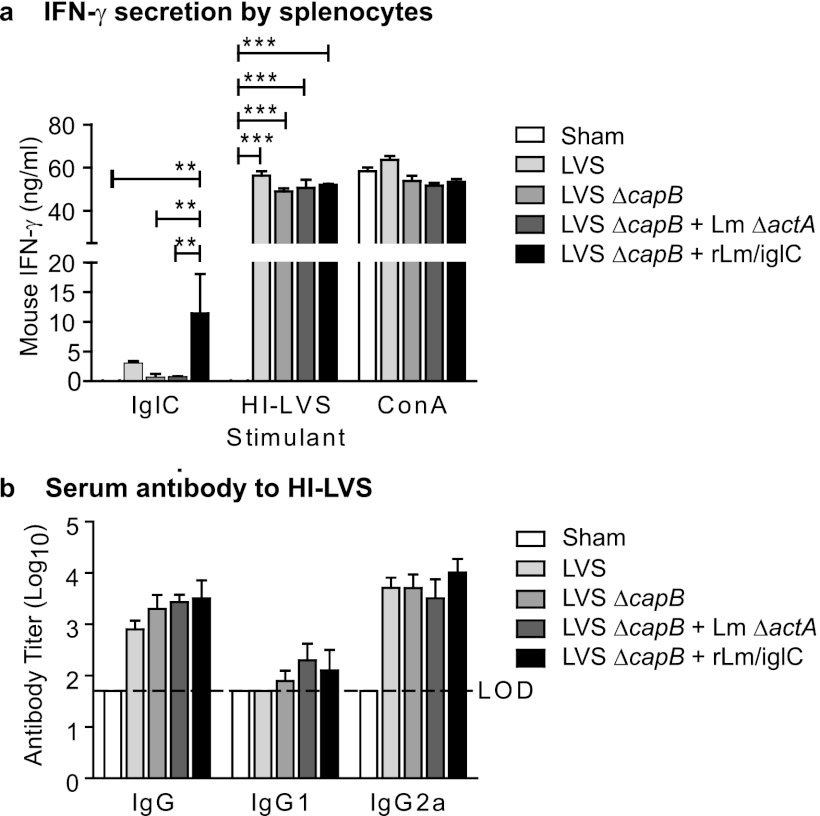
Previously, we showed that immunization with LVS ΔcapB induces protective immunity against F. tularensis challenge in mice (16). We also showed that immunization with rLm/iglC elicits IglC-specific T cell-mediated protective immunity against F. tularensis challenge in mice (21). To examine whether priming with LVS ΔcapB and boosting with rLm/iglC enhances T cell-mediated immunogenicity, we primed mice with LVS ΔcapB and boosted them 4 weeks later with rLm/iglC or its vector control, L. monocytogenes ΔactA. Mice immunized with PBS (sham), LVS, or LVS ΔcapB once, at week 0, served as controls (Fig. 1a). At week 6, mice were euthanized, their spleens were removed, and single-cell suspensions of splenocytes were prepared. The splenocytes were stimulated with IglC protein, HI-LVS, or ConA for 3 days, and the culture supernatants were assayed for IFN-γ. As shown in Fig. 2a, when stimulated with IglC protein, the immune splenocytes from mice primed with LVS ΔcapB and boosted with rLm/iglC secreted IFN-γ at a significantly higher level than that of sham-immunized mice, mice immunized with LVS ΔcapB alone, or mice primed with LVS ΔcapB and boosted with the L. monocytogenes ΔactA vector (P < 0.01). Similar results were obtained in a separate experiment, except that the difference in IFN-γ production between mice primed-boosted with LVS ΔcapB-rLm/iglC and mice immunized with LVS ΔcapB did not reach statistical significance (see Fig. S1a in the supplemental material). Mice primed with LVS ΔcapB and boosted with rLm/iglC also secreted IFN-γ at a higher level than mice immunized once with LVS in the experiment shown in Fig. 2a, although the difference did not reach statistical significance; in a separate experiment, there was no difference between these groups (see Fig. S1a). When stimulated with HI-LVS, the immune splenocytes from all immunized mice secreted IFN-γ at significantly higher levels than those in sham-immunized mice (P < 0.001) (Fig. 2a); this was also seen in a separate experiment (see Fig. S1a). When cells were stimulated with ConA, there were no significant differences in the levels of IFN-γ secretion among sham-immunized mice and mice immunized with various vaccines (Fig. 2a). These results show that boosting of LVS ΔcapB-primed mice with rLm/iglC enhances IglC-specific IFN-γ secretion by immune lymphocytes.
Fig 2.
Priming with LVS ΔcapB and boosting with rLm/iglC induce antigen-specific Th1-type antibody and cytokine production. Mice (n = 4 mice/group) were vaccinated, bled, and euthanized as described in the legend to Fig. 1a and then tested for IFN-γ secretion and serum antibody production. (a) IFN-γ secretion by splenic lymphocytes. Single-cell suspensions of mouse splenic lymphocytes were prepared and stimulated with the IglC protein, heat-inactivated LVS (HI-LVS), or ConA for 3 days. Production of IFN-γ in the culture supernatant fluid was determined by ELISA. Data shown are mean values and standard errors for 4 mice. A line above the bars indicates a statistical comparison between the bar beneath the left end of the line and the bar beneath the right end of the line. Only comparisons where differences are statistically significant are shown. **, P < 0.01; ***, P < 0.001 (by one-way ANOVA with Tukey's multiple-comparison test). (b) Serum antibody titers. Sera were analyzed for IgG and subtype IgG1 and IgG2a antibodies specific to HI-LVS. The antibody level was calculated as the log10 of the reciprocal of the endpoint dilution of the test serum. LOD, limit of detection. The experiment was repeated twice, and similar results were obtained (data for one repeat experiment are shown in Fig. S1 in the supplemental material).
Recent studies showed that IL-17A is also important in control of primary infection and vaccine-induced protection against F. tularensis (31, 32). To examine IL-17A production after prime-boost immunization with LVS ΔcapB-rLm/iglC, we assayed IL-17A in the culture supernatant fluid of immune splenocytes upon in vitro stimulation with IglC protein or HI-LVS, as shown in Fig. S1b in the supplemental material. We found that in response to the IglC protein, splenocytes from mice primed with LVS ΔcapB and boosted with rLm/iglC produced a larger amount of IL-17A than splenocytes from sham-immunized mice and mice primed with LVS ΔcapB only, although the differences did not reach statistical significance; the amount of IL-17A produced by the splenocytes of primed-boosted mice was comparable to that produced by the splenocytes of LVS-immunized mice. In response to HI-LVS, splenocytes from mice immunized with LVS ΔcapB or LVS ΔcapB-rLm/iglC produced significantly more IL-17A than that in sham-immunized mice (P < 0.001), albeit less than that in mice immunized with LVS.
It has been demonstrated that the humoral immune response plays a role in protection against pulmonary F. tularensis infection (33, 34). To examine the humoral immune response induced by prime-boost immunization, we immunized mice with the various vaccines as described above for cytokine production. At week 6, sera were assayed for antibodies specific to HI-LVS and IglC. Mice immunized with LVS or LVS ΔcapB or primed with LVS ΔcapB and boosted with L. monocytogenes ΔactA or rLm/iglC produced significantly more HI-LVS-specific IgG antibody than did sham-immunized mice (Fig. 2b; see Fig. S1c in the supplemental material). Isotype analysis revealed that Th1-type IgG2a, not Th2-type IgG1, was the dominant subtype. Mice primed with LVS ΔcapB and boosted with rLm/iglC produced a larger amount of IgG2a than mice primed only with LVS ΔcapB, although the difference did not reach statistical significance (Fig. 2b; see Fig. S1c). IglC-specific antibody was not detected in any group of mice (data not shown). These results show that LVS ΔcapB-rLm/iglC prime-boost immunization induces an HI-LVS-specific Th1-type antibody response.
Boosting of LVS ΔcapB-immunized mice with rLm/iglC induces elevated cytokine expression in both CD4+ and CD8+ T cells upon in vitro antigen stimulation.
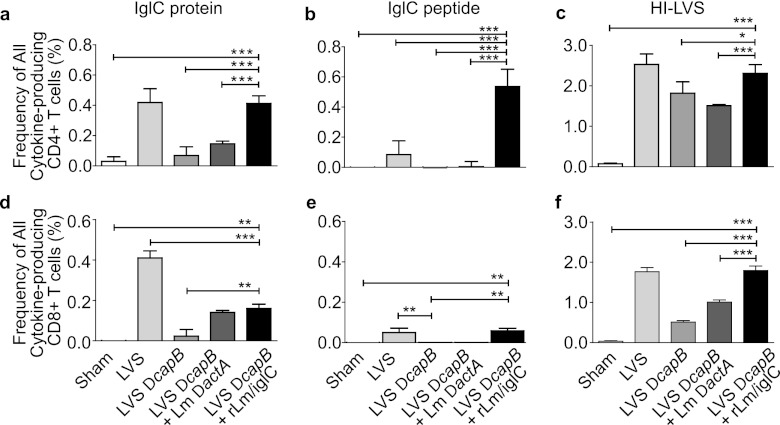
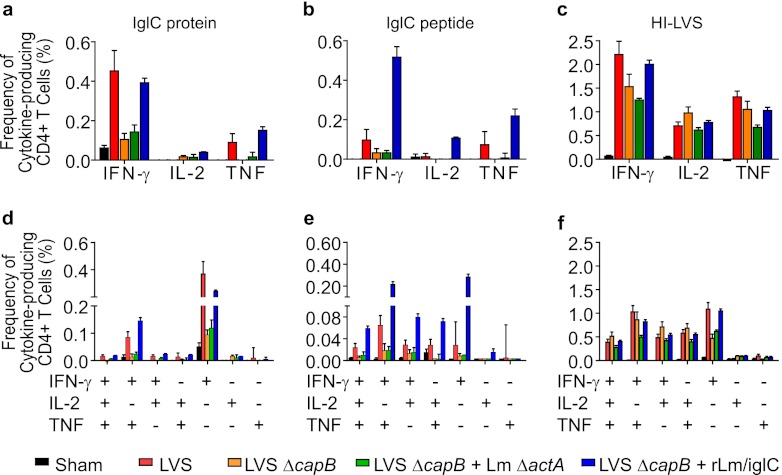
Immunization with LVS ΔcapB or rLm/iglC induces F. tularensis antigen-specific lymphocyte proliferation, and immunization with rLm/iglC induces IFN-γ expression by both CD4+ and CD8+ T cells (16, 21). To examine whether boosting with rLm/iglC enhances cytokine expression by CD4+ and CD8+ T cells, we performed multiparameter intracellular cytokine staining and flow cytometry analysis of the immune splenic lymphocytes, using the gating strategy described in Fig. S2 in the supplemental material. As shown in Fig. 3, mice primed with LVS ΔcapB and boosted with rLm/iglC had a significantly higher frequency of cytokine (IFN-γ and/or IL-2 and/or TNF)-producing CD4+ T cells in their spleens after in vitro stimulation with IglC protein (Fig. 3a), IglC peptide (Fig. 3b), or HI-LVS (Fig. 3c) than did sham-immunized mice, mice immunized with only LVS ΔcapB, or mice primed with LVS ΔcapB and boosted with the L. monocytogenes ΔactA vector. The frequency of cytokine-producing CD4+ T cells for mice primed with LVS ΔcapB and boosted with rLm/iglC was comparable to that of LVS-immunized mice in response to IglC and HI-LVS but significantly greater than that of LVS-immunized mice in response to IglC peptide. Similarly, in comparison with sham-immunized mice or mice immunized with LVS ΔcapB only, mice primed with LVS ΔcapB and boosted with rLm/iglC had a significantly higher frequency of cytokine-producing CD8+ T cells in the spleen after in vitro stimulation with IglC protein (Fig. 3d), IglC peptide (Fig. 3e), or HI-LVS (Fig. 3f). Interestingly, the IglC peptide used in this study was identified mainly as a CD4+ T cell epitope in BALB/c mice, the strain used in this study, and as a CD8+ T cell epitope in C57BL/6 mice, after single immunization with an attenuated L. monocytogenes strain expressing IglC (Justin Skoble, personal communication). For mice primed with LVS ΔcapB and boosted with rLm/iglC, the frequency of cytokine-producing CD4+ T cells specific for the IglC peptide was ∼5-fold greater than that for LVS-immunized mice, the only other group to respond significantly to the peptide (Fig. 3b). Although the IglC peptide used in this study is not known to be a CD8+ T cell epitope in BALB/c mice, the frequency of CD8+ T cells responding to the peptide was significantly elevated for both LVS-immunized and LVS ΔcapB-rLm/iglC-primed and -boosted mice (Fig. 3e); however, in contrast to the case with CD4+ T cells (Fig. 3b), the frequencies of CD8+ T cells responding to this peptide (Fig. 3e) were relatively low and comparable for the LVS-immunized and LVS ΔcapB-rLm/iglC-primed and -boosted mice. These results showed that the heterologous prime-boost vaccination protocol induces antigen-specific functional CD4+ and CD8+ T cell immune responses.
Fig 3.
Priming with LVS ΔcapB and boosting with rLm/iglC enhance IglC-specific cytokine-producing CD4+ and CD8+ T cells. Splenic lymphocytes were prepared from sham-immunized mice or mice immunized with various vaccines as shown in Fig. 1a, stained for cell surface and intracellular markers, and analyzed for the frequency of CD4+ or CD8+ T cells producing any of the cytokines among IFN-γ, IL-2, and TNF, using multiparameter flow cytometry by the strategy depicted in Fig. S2 in the supplemental material. Data shown are mean values and standard errors for the total frequencies of CD4+ (a to c) and CD8+ (d to f) T cells producing one, two, or three of the cytokines among IFN-γ, IL-2, and TNF. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (by two-way ANOVA). The experiment was repeated once, with similar results.
Priming with LVS ΔcapB and boosting with rLm/iglC induces multifunctional Th1-type CD4+ T cells upon in vitro antigen stimulation.
Multifunctional Th-1 type CD4+ T cells have been demonstrated to play a role in protective immunity against intracellular pathogens such as Leishmania (35) and Mycobacterium tuberculosis (in some studies [36–39]). Therefore, we analyzed the frequency of splenic CD4+ T cells expressing the intracellular cytokines IFN-γ, TNF, and IL-2 as described and illustrated in Fig. S2 in the supplemental material. After in vitro stimulation with the IglC protein (Fig. 4a), mice primed with LVS ΔcapB and boosted with rLm/iglC had a significantly higher frequency of IFN-γ- or TNF-expressing CD4+ T cells in their spleens than sham-immunized mice (P < 0.001), mice immunized with LVS ΔcapB only (P < 0.001), and mice primed with LVS ΔcapB and boosted with the L. monocytogenes ΔactA vector (P < 0.001); the frequency of CD4+ T cells expressing IFN-γ or TNF for the LVS ΔcapB-rLm/iglC-primed and -boosted mice was comparable to that of LVS-immunized mice. The frequency of CD4+ T cells expressing IFN-γ was examined in a separate study, and similar results were obtained (see Fig. S3a). The frequency of IL-2-expressing CD4+ T cells was low in all tested groups. After in vitro stimulation with the IglC peptide (Fig. 4b), mice primed with LVS ΔcapB and boosted with rLm/iglC had significantly higher frequencies of IFN-γ (P < 0.001)-, IL-2 (P < 0.05)-, and TNF (P < 0.001)-expressing CD4+ T cells in their spleens than all other groups, including mice immunized with LVS. After in vitro stimulation with HI-LVS (Fig. 4c), all immunized mice had significantly higher frequencies of IFN-γ-, IL-2-, and TNF-expressing CD4+ T cells than sham-immunized mice (P < 0.001). Mice primed with LVS ΔcapB and boosted with rLm/iglC had a higher frequency of IFN-γ-expressing CD4+ T cells after HI-LVS stimulation than mice immunized with LVS ΔcapB only (P < 0.001) or with LVS ΔcapB followed by boosting with the L. monocytogenes ΔactA vector (P < 0.001) (Fig. 4c), although the primed-boosted mice did not show a higher frequency of IFN-γ-expressing CD4+ T cells after HI-LVS stimulation than LVS ΔcapB-immunized mice in a separate experiment (see Fig. S3a).
Fig 4.
Priming with LVS ΔcapB and boosting with rLm/iglC enhance IglC-specific multifunctional CD4+ T cells. Splenic lymphocytes from sham-immunized mice and mice immunized with various vaccines (n = 4 mice/group) were stimulated with F. tularensis IglC protein (a and d), IglC peptide (b and e), or HI-LVS (c and f) and analyzed for cytokine-producing CD4+ T cells by multiparameter flow cytometry. (a to c) Total frequencies of CD4+ T cells expressing IFN-γ, IL-2, or TNF (i.e., all T cell subsets producing a given cytokine either alone or with other cytokines). (d to f) Multiparameter analysis of CD4+ T cells. Data shown are the frequencies of the 7 subpopulations of CD4+ T cells expressing one, two, or three cytokines among IFN-γ, IL-2, and TNF. Values are means and standard errors for 4 mice. The experiment was repeated once, with similar results.
After analysis of the 7 possible combinations of CD4+ T cells producing one or more cytokines among IFN-γ, IL-2, and TNF, we found that in response to stimulation with the IglC protein (Fig. 4d), the largest subsets of T cells were those producing only IFN-γ or producing both IFN-γ and TNF. Mice primed with LVS ΔcapB and boosted with rLm/iglC had a significantly higher frequency of CD4+ T cells producing only IFN-γ or producing both IFN-γ and TNF than sham-immunized mice (P < 0.001), mice immunized with LVS ΔcapB only (P < 0.001), or mice immunized with LVS ΔcapB and boosted with the L. monocytogenes ΔactA vector (P < 0.001). Note that the frequency of CD4+ T cells expressing only IFN-γ was significantly lower in mice primed with LVS ΔcapB and boosted with rLm/iglC than in mice immunized with LVS (P < 0.001); however, the frequency of CD4+ T cells expressing both IFN-γ and TNF was significantly higher in mice primed with LVS ΔcapB and boosted with rLm/iglC than in mice immunized with LVS (P < 0.001). In response to IglC peptide stimulation (Fig. 4e), mice primed with LVS ΔcapB and boosted with rLm/iglC had a significantly higher frequency of multifunctional CD4+ T cells producing all 3 cytokines (IFN-γ, IL-2, and TNF) than sham-immunized mice (P < 0.05) and mice immunized with LVS ΔcapB only (P < 0.05); the frequencies of CD4+ T cells expressing all the combinations of any two cytokines or IFN-γ only were significantly higher in LVS ΔcapB-rLm/iglC-primed and -boosted mice than in sham-immunized mice, mice immunized with LVS ΔcapB only, and mice primed with LVS ΔcapB and boosted with L. monocytogenes ΔactA (P < 0.001 to 0.05). Note that the frequencies of CD4+ T cells producing IFN-γ and TNF, IFN-γ and IL-2, or IFN-γ only were significantly higher in LVS ΔcapB-rLm/iglC-primed and -boosted mice than in LVS-immunized mice (P < 0.05 to P < 0.001); the frequency of CD4+ T cells producing all three cytokines or the combination of IL-2 and TNF was also greater for LVS ΔcapB-rLm/iglC-primed and -boosted mice than for LVS-immunized mice, but the difference did not reach statistical significance. In response to HI-LVS (Fig. 4f), multicytokine-producing CD4+ T cells were detected in all groups of mice except sham-immunized mice. These results demonstrate that LVS ΔcapB-rLm/iglC prime-boost vaccination induces IglC-specific multifunctional CD4+ T cells.
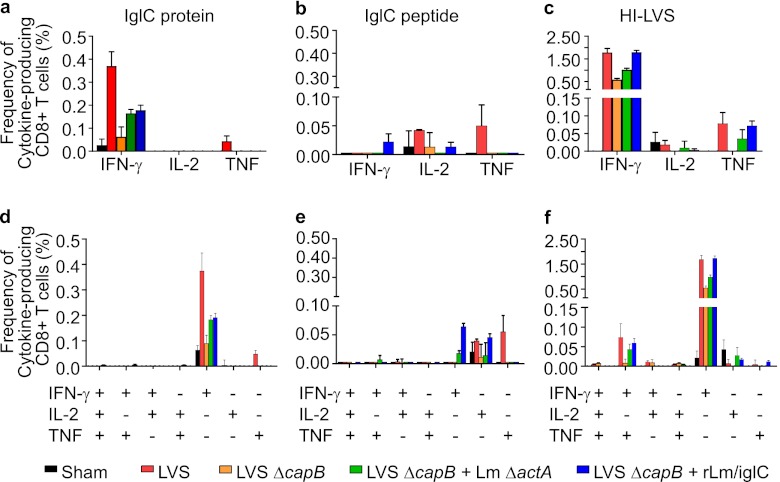
Priming with LVS ΔcapB and boosting with rLm/iglC induces CD8+ cells producing primarily IFN-γ upon in vitro antigen stimulation.
Th1-type cytokine-producing CD8+ T cells have been shown to play an important role in vaccine-induced protection against F. tularensis (40, 41). Therefore, we analyzed the frequency of CD8+ T cells expressing Th1-type cytokines, including IFN-γ, TNF, and IL-2. In contrast to CD4+ T cells, immune CD8+ T cells expressed primarily IFN-γ in response to the IglC protein (Fig. 5a and d). Mice primed with LVS ΔcapB and boosted with rLm/iglC had a significantly higher frequency of IFN-γ-expressing CD8+ T cells in their spleens than sham-immunized mice (P < 0.001) and mice immunized with LVS ΔcapB only (P < 0.001). Similar results were obtained in a separate experiment (see Fig. S3b in the supplemental material). The frequency of IglC-specific CD8+ T cells expressing IFN-γ for the LVS ΔcapB-rLm/iglC-primed and -boosted mice was comparable to that for mice primed-boosted with LVS ΔcapB-L. monocytogenes ΔactA vector (Fig. 5a). The response of CD8+ T cells to the IglC peptide was paltry (Fig. 5b and e), which was not surprising, because the IglC peptide was identified as a CD4+, not CD8+, T cell epitope. In response to HI-LVS stimulation, immunized mice produced CD8+ T cells expressing primarily IFN-γ or TNF (Fig. 5c and f). Mice primed with LVS ΔcapB and boosted with rLm/iglC had a significantly larger number of IFN-γ expressing CD8+ T cells in response to HI-LVS than sham-immunized mice (P < 0.001), mice immunized with LVS ΔcapB (P < 0.001), or mice primed with LVS ΔcapB and boosted with the L. monocytogenes ΔactA vector (P < 0.01). Similar results were obtained in a separate experiment (see Fig. S3b). The frequency of IFN-γ-expressing CD8+ T cells for the LVS ΔcapB-rLm/iglC-primed and -boosted mice was comparable to that of mice immunized with LVS (Fig. 5c), although it was lower in a separate experiment (see Fig. S3b). The IFN-γ-expressing CD8+ T cells were comprised primarily of CD8+ T cells expressing IFN-γ only and, to a lesser extent, CD8+ T cells expressing both IFN-γ and TNF (Fig. 5f).
Fig 5.
Priming with LVS ΔcapB and boosting with rLm/iglC enhance antigen-specific cytokine-producing CD8+ T cells. Splenic lymphocytes from sham-immunized mice and mice immunized with various vaccines (n = 4 mice/group) were stimulated with F. tularensis IglC protein (a and d), IglC peptide (b and e), or HI-LVS (c and f) and analyzed for cytokine-producing CD8+ T cells by multiparameter flow cytometry. (a to c) Total frequencies of CD8+ T cells expressing IFN-γ, IL-2, or TNF. (d to f) Multiparameter analysis of CD8+ T cells. Data shown are the frequencies of the 7 subpopulations of CD8+ T cells expressing one, two, or three cytokines among IFN-γ, IL-2, and TNF. Values are means and standard errors for 4 mice. The experiment was repeated once, with similar results.
Priming with LVS ΔcapB and boosting with rLm/iglC induces protective immunity against aerosol challenge with an F. tularensis Schu S4 strain that is superior to that induced by single LVS immunization.
To examine the protective efficacy of the prime-boost vaccination strategy, we utilized LVS ΔcapB or rLVS ΔcapB/IglC as a primer vaccine and rLm/iglC as a heterologous booster vaccine. rLVS ΔcapB/IglC was constructed to overexpress the immunoprotective antigen IglC. By Western blotting of broth-grown bacteria, using a polyclonal antibody to IglC, rLVS ΔcapB/IglC expressed approximately 2-fold more IglC than the parental LVS ΔcapB strain (data not shown). Similarly, rLVS ΔcapB/IglA was constructed to overexpress IglA, and by Western blotting using a polyclonal antibody to IglA, rLVS ΔcapB/IglA expressed approximately 2-fold more IglA than the parental LVS ΔcapB strain in broth culture (data not shown). IglA, encoded by FTT1714 and FTT1359 in the FPI, like IglC, is also required for intramacrophage growth and virulence in mice (25, 42) and is immunogenic in murine models and human tularemia (28, 43, 44). Mice (8 per group) were immunized i.d. with LVS ΔcapB or rLVS ΔcapB/IglC at week 0 and boosted with rLm/iglC at week 4. Mice not vaccinated or mice primed i.d. with LVS, LVS ΔcapB, rLVS ΔcapB/IglC, or rLVS ΔcapB/IglA at week 4 and not boosted served as controls. All mice were challenged at week 10 (6 weeks after the only or last immunization) with aerosolized F. tularensis Schu S4 (10 LD50) as shown in Fig. 1b and 6c; the immunization-challenge interval was kept constant for all immunized groups so as not to bias the experiment in favor of primed-boosted animals. After challenge, mice were monitored for weight change, signs of illness, and death for 3 weeks. As previously reported, mice immunized with the parental LVS ΔcapB vaccine had a higher survival rate and survived significantly longer than the unimmunized mice (16). Mice immunized once with rLVS ΔcapB/IglC or rLVS ΔcapB/IglA had a higher survival rate and MST than mice immunized with the parental LVS ΔcapB vaccine (Fig. 6a and c) (P = 0.09 and 0.01, respectively); the survival of mice in these groups was not significantly different from that of mice immunized with LVS. Most importantly, mice primed i.d. with LVS ΔcapB or rLVS ΔcapB/IglC and boosted with rLm/iglC had a significantly higher survival rate and MST than mice immunized with parental LVS ΔcapB only (Fig. 6a and c) (P < 0.0001). None of the surviving mice primed with LVS ΔcapB or rLVS ΔcapB/IglC and boosted with rLm/iglC showed weight loss (Fig. 6b), indicating high-level protection. Importantly, the survival rate and MST for these primed-boosted mice were higher than those for mice immunized with LVS, although the difference did not reach statistical significance (Fig. 6c). However, in contrast to mice immunized with the prime-boost vaccines (LVS ΔcapB-rLm/iglC or rLVS ΔcapB/IglC-rLm/iglC), mice immunized with LVS suffered significant weight loss at day 7 postchallenge (P < 0.01 versus each of these prime-boost vaccines) (Fig. 6b). Among the primed-boosted animals, there was no significant difference between mice primed with LVS ΔcapB and mice primed with rLVS ΔcapB/IglC.
In another efficacy study, we compared the efficacy of heterologous prime-boost vaccination with that of homologous prime-boost vaccination, using a different and by itself less potent version of an attenuated LVS strain—LVS ΔLPS (16), overexpressing IglC (LVS ΔLPS/IglC)—as the primer vaccine (see Fig. S4 in the supplemental material); LVS ΔLPS/IglC expressed IglC at a level ∼2-fold higher than that of the parental LVS ΔLPS strain (data not shown). As illustrated in Fig. S4e, mice were primed with LVS ΔLPS overexpressing IglC (rLVS ΔLPS/IglC) at week 0 and boosted twice, at weeks 3 and 6, with rLm/iglC, or they were primed with rLVS ΔLPS/IglC at week 3 and boosted once at week 6. Mice immunized with normal saline (sham), LVS ΔLPS, rLVS ΔLPS/IglC, or LVS twice, at weeks 3 and 6, served as controls. At week 12, 6 weeks after the last immunization for all vaccine regimens, we challenged mice with either 3 LD50 (4 mice per group) or 10 LD50 (8 mice per group) of F. tularensis Schu S4 by aerosol, as in the experiment shown in Fig. 6, and monitored the animals for weight change, signs of illness, and death for 3 weeks (the immunization-challenge interval was also kept constant for all immunized groups so as not to bias the experiment in favor of primed-boosted animals). After a 3-LD50 (see Fig. S4a and e, upper section) or 10-LD50 (see Fig. S4b and e, lower section) F. tularensis Schu S4 aerosol challenge, mice primed with rLVS ΔLPS/IglC and boosted once or twice with rLm/iglC had significantly higher survival rates and MST than those of sham-immunized mice and mice immunized twice (homologously primed-boosted) with the primer vaccine (rLVS ΔLPS/IglC) only (see Fig. S4e, columns 7 and 8). Mice primed with rLVS ΔLPS/IglC and boosted twice with rLm/iglC had a higher survival rate and MST than mice primed with rLVS ΔLPS/IglC and boosted just once against both a 3-LD50 and 10-LD50 challenge with F. tularensis Schu S4, although the differences were not statistically significant. Mice primed with rLVS ΔLPS/IglC and boosted twice with rLm/iglC had a 100% survival rate in response to a 3-LD50 F. tularensis Schu S4 challenge, the same as for LVS-immunized mice; however, against a 10-LD50 F. tularensis Schu S4 challenge, their survival rate and MST were inferior to those immunized with LVS (62.5% survival rate versus 100% for LVS; MST of 15.3 days versus 21.0 days for LVS). Consistently, the mice that received a prime-boost vaccination and survived the F. tularensis Schu S4 challenge had no significant weight loss after day 6 postchallenge (see Fig. S4c and d).
These results indicate that boosting with rLm/iglC significantly enhances protective immunity induced by i.d. immunization with the parental LVS ΔcapB or LVS ΔLPS strain and that the heterologous prime-boost vaccination strategy holds substantial promise for producing a vaccine that is both safer and more potent than LVS.
DISCUSSION
Our studies show that a heterologous prime-boost vaccination strategy comprising an attenuated LVS mutant as the primer vaccine and an L. monocytogenes strain expressing an F. tularensis protein as the booster vaccine induces elevated antigen-specific T cell-mediated immune responses and potent protective immunity against virulent F. tularensis subsp. tularensis Schu S4 aerosol challenge. The best heterologous prime-boost vaccines tested, comprising either LVS ΔcapB or rLVS ΔcapB/IglC as the primer vaccine and rLm/iglC as the booster vaccine, were nontoxic and induced protective immunity to F. tularensis Schu S4 aerosol challenge that was superior to that obtained with LVS.
LVS, the only currently available yet unlicensed vaccine against tularemia, has several shortfalls. While highly efficacious by the aerosol route in humans (45) and by the i.n. route in animal models (46), it is much more virulent via these routes, with most human volunteers developing typhoidal tularemia after inhaling 106 CFU LVS (45) and with mice dying after i.n. immunization with only a few hundred organisms (47). LVS is safer, but also less efficacious, when administered by the i.d. route (45, 46). In humans, it currently must be administered by scarification (48). Given the drawbacks of LVS, a safer and more potent alternative vaccine is needed. The heterologous prime-boost vaccination strategy described here can fulfill this need.
With respect to safety, the two vaccines comprising our heterologous prime-boost vaccination strategy are much less toxic than LVS. The LVS ΔcapB vaccine, while untested in humans, is >10,000-fold less virulent than LVS in the mouse model (16). Even when the dose of LVS is reduced to 200 CFU i.n., where it retains its immunogenicity and protective efficacy, ∼25% of mice die from the immunization itself, versus 0% for the LVS ΔcapB vaccine, even at very high doses (16). Similarly, the rLm/iglC vaccine is nontoxic in mice at the doses used. Moreover, the L. monocytogenes vector has demonstrated safety in human clinical trials (20, 49–51).
With respect to efficacy, the heterologous prime-boost vaccine was found to be at least as potent as LVS in our mouse challenge study. Whereas the mean survival time for one-dose LVS-immunized mice during the 21-day period immediately after challenge with a 10-LD50 aerosol dose of F. tularensis Schu S4 was 17 days, the mean survival time for the mice immunized with the prime-boost vaccine was 19 days when LVS ΔcapB served as the primer vaccine and 20 days when rLVS ΔcapB/IglC served as the primer vaccine—the differences, however, did not reach statistical significance. Equally important, whereas LVS-immunized mice became ill and suffered substantial weight loss after challenge, losing over 10% of their body weight by day 10 postchallenge, the primed-boosted animals did not become ill and maintained their weight after challenge; differences in weight between the LVS-immunized and primed-boosted animals were statistically significant at day 7 after challenge. Thus, considering the overall protective capacity of the vaccines against both illness and death, the prime-boost vaccination strategy offered protection superior to that of LVS.
Note that the enhanced potency of the prime-boost vaccine versus LVS was demonstrated under conditions in which the immunization-challenge interval was held constant so as not to bias the study in favor of the prime-boost strategy. As vaccine efficacy against F. tularensis aerosol challenge wanes with time (46), not maintaining a constant immunization-challenge interval, defined as the time between the last (or only) immunization and the challenge, can bias the results in favor of the most recently administered vaccine, typically the booster vaccine in prime-boost studies; if anything, considering that the primer vaccines in our studies are multigenic for F. tularensis proteins, maintaining a constant immunization-challenge interval biases the results in favor of the prime-only vaccine (e.g., LVS).
Aside from the prime-boost vaccine described here, only single deletion mutants of wild-type type A F. tularensis have demonstrated efficacy comparable to or greater than that of LVS (52). However, as these single deletion mutants are one mutation away from reversion to full virulence, a second major attenuating deletion mutation is needed, at minimum, to ensure the safety of these vaccines. A second major attenuating deletion often renders these vaccines overattenuated and, consequently, ineffective. An instructive exception is the double deletion mutant Schu S4 ΔFTT0918 ΔcapB; this vaccine, while still potent, retained high virulence (i.e., was underattenuated), killing 1 of 5 i.d.-immunized BALB/c mice, the only species protected by the vaccine, and 93% and 47% of C3H/HeN mice immunized i.d. with 105 and 103 CFU, respectively (52). The experience to date suggests that by starting with a wild-type type A strain, it is difficult to engineer a deletion mutant that is neither overattenuated nor underattenuated. In contrast to the deletion mutants of highly virulent type A strains, our LVS ΔcapB mutant is neither underattenuated nor overattenuated. LVS ΔcapB is a single deletion mutant of the LVS strain, which was gradually attenuated via passages on artificial medium. Consequently, LVS ΔcapB has acquired three major attenuating deletions and several additional minor deletions versus its wild-type type B parent, making reversion to full type B virulence, which is already markedly less than the virulence of type A strains, virtually impossible. Thus, the prime-boost vaccine described here is the first vaccine demonstrated to be more potent than LVS that is suitable for human testing.
The safety and potency of the heterologous prime-boost vaccination strategy come at the price of requiring commercial development and regulatory approval of two distinct vaccines—the primer and booster vaccines. However, given the remarkable potency of the heterologous prime-boost vaccination strategy, this disadvantage might be offset by employing these primer and booster vaccine vectors as a platform technology for use against multiple target pathogens. For example, these vectors potentially could be used to express antigens of the agents of anthrax, plague, and other bioterrorist threats and so constitute a broad-spectrum vaccine against bioterrorism agents. Thus, under this scenario, while two vaccines would still need to be developed, the two vaccines could potentially replace multiple individual vaccines.
The heterologous prime-boost vaccination strategy has been employed against other especially challenging diseases, including HIV, malaria, and tuberculosis. Primer vaccines have frequently comprised DNA, a viral vector, or Mycobacterium bovis bacillus Calmette-Guérin (BCG), and booster vaccines have frequently comprised recombinant proteins in adjuvant, inactivated vaccines, or viral vectors (53). Our choice of an attenuated LVS ΔcapB vaccine as the primer vaccine stemmed from observations in our laboratory and others that subunit vaccines are relatively ineffective against highly virulent F. tularensis (21, 54, 55). Our choice of L. monocytogenes as the vector for the heterologous booster vaccine stemmed from our appreciation of this vector as sharing an intracytoplasmic lifestyle with F. tularensis (17); consequently, protein antigens expressed by L. monocytogenes should be processed and presented to the immune system similarly to those expressed by F. tularensis, and T cells so induced by recombinant L. monocytogenes should therefore have the capability of recognizing F. tularensis-infected host cells via the surface expression of similar major histocompatibility complex (MHC)-peptide complexes in the event of a subsequent infection of the vaccinated host with F. tularensis.
As an alternative to the prime-boost vaccination strategy, a single vaccine that is safer and more potent than LVS could potentially be developed that could be administered once or, more likely, multiple times via homologous boosting. Such a vaccine will almost certainly need to be comprised of a vaccine homologous with F. tularensis, such as an LVS ΔcapB vectored vaccine, as other types of vaccines have not shown sufficient potency to date. The LVS ΔcapB vaccine used in this study was rendered more potent by using it as a vector to express F. tularensis immunoprotective antigens, in this case IglA and IglC. The mean survival time of mice immunized with LVS ΔcapB—9.5 days—was increased to 13.5 days for mice immunized with rLVS ΔcapB/IglC and 15.5 days for mice immunized with rLVS ΔcapB/IglA. Indeed, these recombinant vaccines did not differ significantly in potency from LVS (mean survival time, 17 days) (Fig. 6). With further improvement, e.g., enhanced overexpression of recombinant proteins or overexpression of multiple proteins, such a single vaccine type may match the safety and potency of the heterologous prime-boost vaccine.
Various immune mechanisms against F. tularensis have been elucidated in humans and in animal models. In humans, both humoral and T cell-mediated immune responses are induced within 2 weeks following vaccination with LVS or natural infection with tularemia (56). T cell-mediated immune responses persist for at least 25 years, and antibody responses last for at least 11 years (57, 58). Upon F. tularensis antigen stimulation, CD4+ and CD8+ T cells from F. tularensis-immune individuals produce Th1-type cytokines, including IFN-γ, TNF, and IL-2 (57–59). In murine models, CD4+ T cells, CD8+ T cells, and Th1-type cytokines such as IFN-γ and TNF are important for LVS-induced protection against F. tularensis (40, 41). Antibodies induced by LVS can provide prophylactic and therapeutic protection against pulmonary F. tularensis infection, but only in the presence of active cell-mediated immunity (33, 34). Recent studies show that the Th17-type cytokine IL-17A also plays an important role in protective immunity against F. tularensis infection (31, 32).
Although a great deal has been learned about the immune responses induced in natural infection and after vaccination, correlates of protective immunity against F. tularensis are still not well understood. One study in mice showed that the strength of vaccine-induced protection against LVS intraperitoneal challenge correlates with the capacity of lymphocytes to control intramacrophage LVS growth in vitro and with the degree of upregulation of a panel of cytokine genes (15). A human study showed that secretion of cytokines, including IFN-γ and macrophage inflammatory protein 1β (MIP-1β), by T lymphocytes and expression of IFN-γ, MIP-1β, and CD107 by CD4+ and CD8+ memory T cells correlate with LVS immunization and naturally acquired tularemia (60). In studies of other intracellular pathogens, such as Leishmania major (35) and M. tuberculosis (36–39), IFN-γ-, TNF-, and IL-2-expressing multifunctional T cells have been found to correlate with vaccine potency, although this has not been observed uniformly (61, 62).
To explore potential immune correlates of protective immunity for the heterologous prime-boost vaccine, we measured T cell-mediated immune responses—IFN-γ, TNF, and IL-17A production by splenic lymphocytes and the intracellular expression of IFN-γ, TNF, and IL-2 by CD4+ and CD8+ T cells—and humoral immune responses, i.e., serum antibody levels. T cells from LVS- and LVS ΔcapB-rLm/iglC-immunized mice recognized the IglC protein; T cells from mice primed with LVS ΔcapB and boosted with rLm/iglC secreted a significantly larger amount of IFN-γ in response to the IglC protein than T cells from sham-immunized mice and mice primed with LVS ΔcapB and boosted with the L. monocytogenes ΔactA vector (Fig. 2a; see Fig. S1a in the supplemental material). In contrast, none of the T cells from vaccinated mice stimulated with IglC or HI-LVS secreted appreciable amounts of TNF (data not shown). In a separate experiment, we found that the T cells from the primed-boosted mice also secreted more IL-17A than T cells from sham-immunized mice and mice immunized with LVS ΔcapB, although the difference was not statistically significant (see Fig. S1b). Our multiparameter flow cytometry analysis showed that mice primed with LVS ΔcapB and boosted with rLm/iglC induced a significantly higher frequency of splenic CD4+ T cells producing both IFN-γ and TNF upon stimulation with the IglC protein or IglC peptide than sham-immunized mice, mice primed with LVS ΔcapB only, and mice primed with LVS ΔcapB and boosted with the L. monocytogenes ΔactA vector (Fig. 4). The LVS ΔcapB-rLm/iglC-primed and -boosted mice also induced a significantly higher frequency of CD8+ T cells expressing only IFN-γ upon stimulation with HI-LVS than mice primed with LVS ΔcapB only or mice primed with LVS ΔcapB and boosted with the L. monocytogenes ΔactA vector (Fig. 5; see Fig. S3). With respect to humoral immune responses, we found that all immunized mice produced significantly elevated IgG antibody, predominantly IgG2a, compared with sham-immunized mice. The elevated intracellular expression of IFN-γ by T cells from LVS ΔcapB-rLm/iglC-primed and -boosted mice (Fig. 3 to 5) was consistent with the high levels of secreted IFN-γ measured in the supernatants of these cells and with elevated Th1-type (IgG2a) serum antibody titers (Fig. 2; see Fig. S1). The fact that the frequencies of antigen-specific multifunctional CD4+ T cells and CD8+ T cells producing IFN-γ only or both IFN-γ and TNF in LVS ΔcapB-rLm/iglC-primed and -boosted mice and in LVS-immunized mice were higher than those in other groups and correlated with vaccine potency suggests that these T cells may play important roles in protective immunity to F. tularensis. We are further exploring multifunctional CD4+ and IFN-γ- and/or TNF-producing CD8+ T cells as potential correlates of protection against F. tularensis in studies of immune responses generated by vaccines of escalating potency against aerosol challenge with virulent F. tularensis.
In summary, we have shown that a heterologous prime-boost vaccination strategy comprising LVS ΔcapB or LVS ΔcapB secreting a key F. tularensis protein as the primer vaccine and rLm/iglC as the booster vaccine has substantial promise as a safer and more potent replacement vaccine for the LVS vaccine. The LVS ΔcapB and L. monocytogenes vectors may also serve as vectors for a broad-spectrum vaccine targeting especially challenging intracellular pathogens, including multiple potential agents of bioterrorism.
Supplementary Material
ACKNOWLEDGMENTS
The UCLA Flow Cytometry Core Facility is supported by NIH grants AI028697 and CA016042. This study was supported by grants AI084908 and AI101189 (M.A.H.) from the National Institutes of Health.
We thank Saša Masleša-Galić for technical assistance and Nicole Marlenee for assistance with the animal studies.
Footnotes
Published ahead of print 25 February 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01013-12.
REFERENCES
- 1. Sjostedt A. 2007. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann. N. Y. Acad. Sci. 1105:1–29 [DOI] [PubMed] [Google Scholar]
- 2. Johansson A, Celli J, Conlan W, Elkins KL, Forsman M, Keim PS, Larsson P, Manoil C, Nano FE, Petersen JM, Sjostedt A. 2010. Objections to the transfer of Francisella novicida to the subspecies rank of Francisella tularensis. Int. J. Syst. Evol. Microbiol. 60:1717–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bell JF, Owen CR, Larson CL. 1955. Virulence of bacterium tularense. I. A study of the virulence of bacterium tularense in mice, guinea pigs, and rabbits. J. Infect. Dis. 97:162–166 [DOI] [PubMed] [Google Scholar]
- 4. Day WC, Berendt RF. 1972. Experimental tularemia in Macaca mulatta: relationship of aerosol particle size to the infectivity of airborne Pasteurella tularensis. Infect. Immun. 5:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saslaw S, Eigelsbach HT, Wilson HE, Prior JA, Carhart S. 1961. Tularemia vaccine study. I. Intracutaneous challenge. Arch. Intern. Med. 107:689–701 [DOI] [PubMed] [Google Scholar]
- 6. Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. 1961. Tularemia vaccine study. II. Respiratory challenge. Arch. Intern. Med. 107:702–714 [DOI] [PubMed] [Google Scholar]
- 7. Matyas BT, Nieder HS, Telford SR., 3rd 2007. Pneumonic tularemia on Martha's Vineyard: clinical, epidemiologic, and ecological characteristics. Ann. N. Y. Acad. Sci. 1105:351–377 [DOI] [PubMed] [Google Scholar]
- 8. Feldman KA, Enscore RE, Lathrop SL, Matyas BT, McGuill M, Schriefer ME, Stiles-Enos D, Dennis DT, Petersen LR, Hayes EB. 2001. An outbreak of primary pneumonic tularemia on Martha's Vineyard. N. Engl. J. Med. 345:1601–1606 [DOI] [PubMed] [Google Scholar]
- 9. Svensson K, Larsson P, Johansson D, Bystrom M, Forsman M, Johansson A. 2005. Evolution of subspecies of Francisella tularensis. J. Bacteriol. 187:3903–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forslund AL, Kuoppa K, Svensson K, Salomonsson E, Johansson A, Bystrom M, Oyston PC, Michell SL, Titball RW, Noppa L, Frithz-Lindsten E, Forsman M, Forsberg A. 2006. Direct repeat-mediated deletion of a type IV pilin gene results in major virulence attenuation of Francisella tularensis. Mol. Microbiol. 59:1818–1830 [DOI] [PubMed] [Google Scholar]
- 11. Twine S, Bystrom M, Chen W, Forsman M, Golovliov I, Johansson A, Kelly J, Lindgren H, Svensson K, Zingmark C, Conlan W, Sjostedt A. 2005. A mutant of Francisella tularensis strain SCHU S4 lacking the ability to express a 58-kilodalton protein is attenuated for virulence and is an effective live vaccine. Infect. Immun. 73:8345–8352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salomonsson E, Kuoppa K, Forslund AL, Zingmark C, Golovliov I, Sjostedt A, Noppa L, Forsberg A. 2009. Reintroduction of two deleted virulence loci restores full virulence to the live vaccine strain of Francisella tularensis. Infect. Immun. 77:3424–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCrumb FR. 1961. Aerosol infection of man with Pasteurella tularensis. Bacteriol. Rev. 25:262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eigelsbach HT, Downs CM. 1961. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J. Immunol. 87:415–425 [PubMed] [Google Scholar]
- 15. De Pascalis R, Chou AY, Bosio CM, Huang CY, Follmann DA, Elkins KL. 2012. Development of functional and molecular correlates of vaccine-induced protection for a model intracellular pathogen, F. tularensis LVS. PLoS Pathog. 8:e1002494 doi:10.1371/journal.ppat.1002494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jia Q, Lee BY, Bowen R, Dillon BJ, Som SM, Horwitz MA. 2010. A Francisella tularensis live vaccine strain (LVS) mutant with a deletion in capB, encoding a putative capsular biosynthesis protein, is significantly more attenuated than LVS yet induces potent protective immunity in mice against F. tularensis challenge. Infect. Immun. 78:4341–4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clemens DL, Lee BY, Horwitz MA. 2005. Francisella tularensis enters macrophages via a novel process involving pseudopod loops. Infect. Immun. 73:5892–5902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Golovliov I, Baranov V, Krocova Z, Kovarova H, Sjostedt A. 2003. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect. Immun. 71:5940–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pamer EG. 2004. Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 4:812–823 [DOI] [PubMed] [Google Scholar]
- 20. Bruhn KW, Craft N, Miller JF. 2007. Listeria as a vaccine vector. Microbes Infect. 9:1226–1235 [DOI] [PubMed] [Google Scholar]
- 21. Jia Q, Lee BY, Clemens DL, Bowen RA, Horwitz MA. 2009. Recombinant attenuated Listeria monocytogenes vaccine expressing Francisella tularensis IglC induces protection in mice against aerosolized type A F. tularensis. Vaccine 27:1216–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Golovliov I, Ericsson M, Sandstrom G, Tarnvik A, Sjostedt A. 1997. Identification of proteins of Francisella tularensis induced during growth in macrophages and cloning of the gene encoding a prominently induced 23-kilodalton protein. Infect. Immun. 65:2183–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lai XH, Golovliov I, Sjostedt A. 2004. Expression of IglC is necessary for intracellular growth and induction of apoptosis in murine macrophages by Francisella tularensis. Microb. Pathog. 37:225–230 [DOI] [PubMed] [Google Scholar]
- 24. Santic M, Molmeret M, Klose KE, Jones S, Kwaik YA. 2005. The Francisella tularensis pathogenicity island protein IglC and its regulator MglA are essential for modulating phagosome biogenesis and subsequent bacterial escape into the cytoplasm. Cell. Microbiol. 7:969–979 [DOI] [PubMed] [Google Scholar]
- 25. Barker JR, Klose KE. 2007. Molecular and genetic basis of pathogenesis in Francisella tularensis. Ann. N. Y. Acad. Sci. 1105:138–159 [DOI] [PubMed] [Google Scholar]
- 26. Havlasova J, Hernychova L, Halada P, Pellantova V, Krejsek J, Stulik J, Macela A, Jungblut PR, Larsson P, Forsman M. 2002. Mapping of immunoreactive antigens of Francisella tularensis live vaccine strain. Proteomics 2:857–867 [DOI] [PubMed] [Google Scholar]
- 27. Havlasova J, Hernychova L, Brychta M, Hubalek M, Lenco J, Larsson P, Lundqvist M, Forsman M, Krocova Z, Stulik J, Macela A. 2005. Proteomic analysis of anti-Francisella tularensis LVS antibody response in murine model of tularemia. Proteomics 5:2090–2103 [DOI] [PubMed] [Google Scholar]
- 28. Janovska S, Pavkova I, Hubalek M, Lenco J, Macela A, Stulik J. 2007. Identification of immunoreactive antigens in membrane proteins enriched fraction from Francisella tularensis LVS. Immunol. Lett. 108:151–159 [DOI] [PubMed] [Google Scholar]
- 29. Clemens DL, Lee BY, Horwitz MA. 2012. O-antigen-deficient Francisella tularensis live vaccine strain mutants are ingested via an aberrant form of looping phagocytosis and show altered kinetics of intracellular trafficking in human macrophages. Infect. Immun. 80:952–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maier TM, Havig A, Casey M, Nano FE, Frank DW, Zahrt TC. 2004. Construction and characterization of a highly efficient Francisella shuttle plasmid. Appl. Environ. Microbiol. 70:7511–7519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, Onishi R, Nyugen N, Walter MJ, Pociask D, Randall TD, Gaffen SL, Iwakura Y, Kolls JK, Khader SA. 2009. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31:799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shen H, Harris G, Chen W, Sjostedt A, Ryden P, Conlan W. 2010. Molecular immune responses to aerosol challenge with Francisella tularensis in mice inoculated with live vaccine candidates of varying efficacy. PLoS One 5:e13349 doi:10.1371/journal.pone.0013349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mara-Koosham G, Hutt JA, Lyons CR, Wu TH. 2011. Antibodies contribute to effective vaccination against respiratory infection by type A Francisella tularensis strains. Infect. Immun. 79:1770–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cowley SC, Elkins KL. 2011. Immunity to francisella. Front. Microbiol. 2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843–850 [DOI] [PubMed] [Google Scholar]
- 36. Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di Carlo P, Titone L, Galati D, Bocchino M, Matarese A, Salerno A, Sanduzzi A, Franken WP, Ottenhoff TH, Dieli F. 2010. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur. J. Immunol. 40:2211–2220 [DOI] [PubMed] [Google Scholar]
- 37. Derrick SC, Yabe IM, Yang A, Morris SL. 2011. Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4 T cells. Vaccine 29:2902–2909 [DOI] [PubMed] [Google Scholar]
- 38. Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, Tchilian EZ. 2008. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J. Immunol. 181:4955–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lindenstrom T, Agger EM, Korsholm KS, Darrah PA, Aagaard C, Seder RA, Rosenkrands I, Andersen P. 2009. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J. Immunol. 182:8047–8055 [DOI] [PubMed] [Google Scholar]
- 40. Conlan WJ, Shen H, Kuolee R, Zhao X, Chen W. 2005. Aerosol-, but not intradermal-immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an alphabeta T cell- and interferon gamma-dependent mechanism. Vaccine 23:2477–2485 [DOI] [PubMed] [Google Scholar]
- 41. Sjostedt A, North RJ, Conlan JW. 1996. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology 142:1369–1374 [DOI] [PubMed] [Google Scholar]
- 42. Nano FE, Schmerk C. 2007. The Francisella pathogenicity island. Ann. N. Y. Acad. Sci. 1105:122–137 [DOI] [PubMed] [Google Scholar]
- 43. Eyles JE, Unal B, Hartley MG, Newstead SL, Flick-Smith H, Prior JL, Oyston PC, Randall A, Mu Y, Hirst S, Molina DM, Davies DH, Milne T, Griffin KF, Baldi P, Titball RW, Felgner PL. 2007. Immunodominant Francisella tularensis antigens identified using proteome microarray. Proteomics 7:2172–2183 [DOI] [PubMed] [Google Scholar]
- 44. Valentino MD, Maben ZJ, Hensley LL, Woolard MD, Kawula TH, Frelinger JA, Frelinger JG. 2011. Identification of T-cell epitopes in Francisella tularensis using an ordered protein array of serological targets. Immunology 132:348–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hornick RB, Eigelsbach HT. 1966. Aerogenic immunization of man with live tularemia vaccine. Bacteriol. Rev. 30:532–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen W, Shen H, Webb A, KuoLee R, Conlan JW. 2003. Tularemia in BALB/c and C57BL/6 mice vaccinated with Francisella tularensis LVS and challenged intradermally, or by aerosol with virulent isolates of the pathogen: protection varies depending on pathogen virulence, route of exposure, and host genetic background. Vaccine 21:3690–3700 [DOI] [PubMed] [Google Scholar]
- 47. Fortier AH, Slayter MV, Ziemba R, Meltzer MS, Nacy CA. 1991. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect. Immun. 59:2922–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Conlan WJ, Oyston PC. 2007. Vaccines against Francisella tularensis. Ann. N. Y. Acad. Sci. 1105:325–350 [DOI] [PubMed] [Google Scholar]
- 49. Maciag PC, Radulovic S, Rothman J. 2009. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine 27:3975–3983 [DOI] [PubMed] [Google Scholar]
- 50. Johnson PV, Blair BM, Zeller S, Kotton CN, Hohmann EL. 2011. Attenuated Listeria monocytogenes vaccine vectors expressing influenza A nucleoprotein: preclinical evaluation and oral inoculation of volunteers. Microbiol. Immunol. 55:304–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, Sterman DH, Hassan R, Lutz E, Moyer B, Giedlin M, Louis JL, Sugar EA, Pons A, Cox AL, Levine J, Murphy AL, Illei P, Dubensky TW, Jr, Eiden JE, Jaffee EM, Laheru DA. 2012. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin. Cancer Res. 18:858–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Conlan JW, Shen H, Golovliov I, Zingmark C, Oyston PC, Chen W, House RV, Sjostedt A. 2010. Differential ability of novel attenuated targeted deletion mutants of Francisella tularensis subspecies tularensis strain SCHU S4 to protect mice against aerosol challenge with virulent bacteria: effects of host background and route of immunization. Vaccine 28:1824–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lu S. 2009. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 21:346–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Conlan JW, Shen H, Webb A, Perry MB. 2002. Mice vaccinated with the O-antigen of Francisella tularensis LVS lipopolysaccharide conjugated to bovine serum albumin develop varying degrees of protective immunity against systemic or aerosol challenge with virulent type A and type B strains of the pathogen. Vaccine 20:3465–3471 [DOI] [PubMed] [Google Scholar]
- 55. Huntley JF, Conley PG, Rasko DA, Hagman KE, Apicella MA, Norgard MV. 2008. Native outer membrane proteins protect mice against pulmonary challenge with virulent type A Francisella tularensis. Infect. Immun. 76:3664–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Elkins KL, Cowley SC, Bosio CM. 2007. Innate and adaptive immunity to Francisella. Ann. N. Y. Acad. Sci. 1105:284–324 [DOI] [PubMed] [Google Scholar]
- 57. Eneslatt K, Rietz C, Ryden P, Stoven S, House RV, Wolfraim LA, Tarnvik A, Sjostedt A. 2011. Persistence of cell-mediated immunity three decades after vaccination with the live vaccine strain of Francisella tularensis. Eur. J. Immunol. 41:974–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ericsson M, Sandstrom G, Sjostedt A, Tarnvik A. 1994. Persistence of cell-mediated immunity and decline of humoral immunity to the intracellular bacterium Francisella tularensis 25 years after natural infection. J. Infect. Dis. 170:110–114 [DOI] [PubMed] [Google Scholar]
- 59. Koskela P, Herva E. 1980. Cell-mediated immunity against Francisella tularensis after natural infection. Scand. J. Infect. Dis. 12:281–287 [DOI] [PubMed] [Google Scholar]
- 60. Eneslatt K, Normark M, Bjork R, Rietz C, Zingmark C, Wolfraim LA, Stoven S, Sjostedt A. 2012. Signatures of T cells as correlates of immunity to Francisella tularensis. PLoS One 7:e32367 doi:10.1371/journal.pone.0032367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Connor LM, Harvie MC, Rich FJ, Quinn KM, Brinkmann V, Le Gros G, Kirman JR. 2010. A key role for lung-resident memory lymphocytes in protective immune responses after BCG vaccination. Eur. J. Immunol. 40:2482–2492 [DOI] [PubMed] [Google Scholar]
- 62. Kagina BM, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, Gamieldien H, Sidibana M, Hatherill M, Gelderbloem S, Mahomed H, Hawkridge A, Hussey G, Kaplan G, Hanekom WA. 2010. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am. J. Respir. Crit. Care Med. 182:1073–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.