Abstract
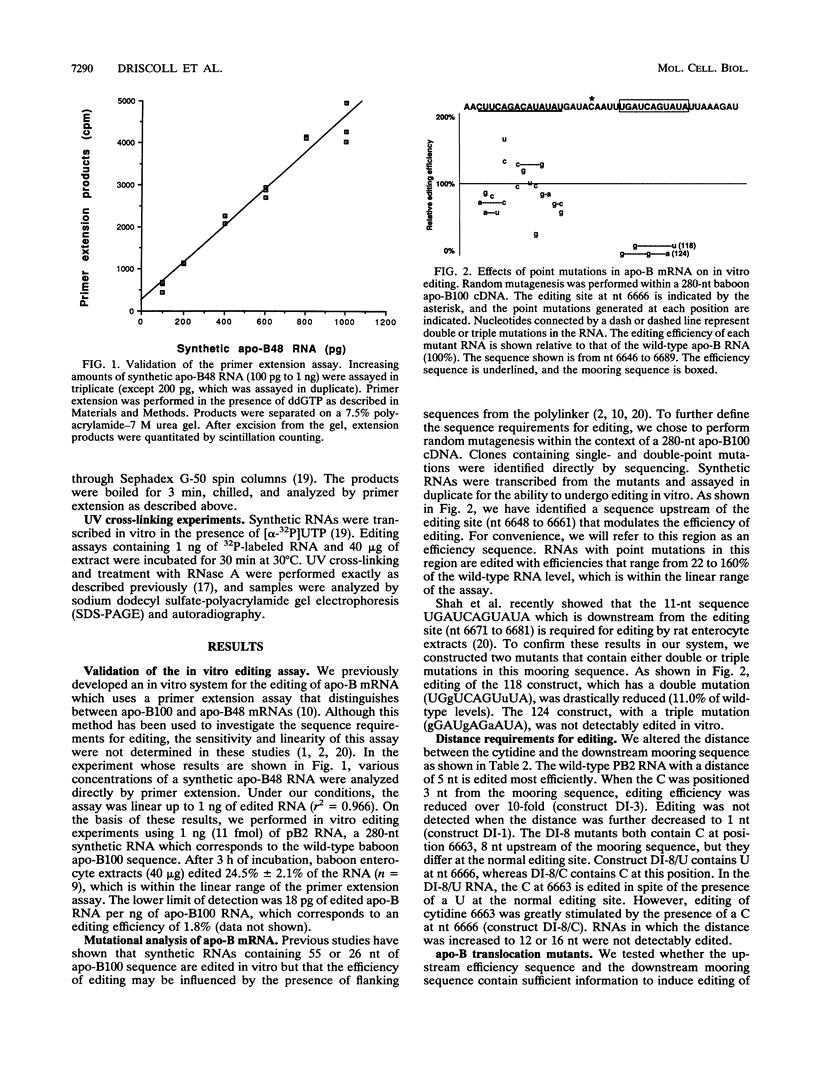
An RNA editing mechanism modifies apolipoprotein B (apo-B) mRNA in the intestine by converting cytosine at nucleotide (nt) 6666 to uracil. To define the sequence requirements for editing, mutant apo-B RNAs were analyzed for the ability to be edited in vitro by enterocyte extracts. Editing was detected by a sensitive and linear primer extension assay. An upstream region (nt 6648 to 6661) which affected the efficiency of editing was identified. RNAs with mutations in this efficiency sequence were edited at 22 to 160% of wild-type levels. Point mutations in a downstream 11-nt mooring sequence (nt 6671 to 6681) abolished editing, confirming previous studies (R. R. Shah, T. J. Knott, J. E. Legros, N. Navaratnam, J. C. Greeve, and J. Scott, J. Biol. Chem. 266:16301-16304, 1991). The optimal distance between the editing site and the mooring sequence is 5 nt, but a C positioned 8 nt upstream is edited even when nt 6666 contains U. The efficiency and mooring sequences were inserted individually and together adjacent to a heterologous C in apo-B mRNA. The mooring sequence alone induced editing of the C at nt 6597 both in vitro and in transfected rat hepatoma cells. Editing at nt 6597 was specific, was independent of editing at nt 6666, and was stimulated to wild-type levels when the efficiency sequence was also inserted. Introduction of the mooring sequence into a heterologous mRNA, luciferase mRNA, induced editing of an upstream cytidine. Although UV cross-linking studies have previously shown that proteins of 60 to 66 kDa cross-link to apo-B mRNA, these proteins did not cross-link to the luciferase translocation mutants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backus J. W., Smith H. C. Apolipoprotein B mRNA sequences 3' of the editing site are necessary and sufficient for editing and editosome assembly. Nucleic Acids Res. 1991 Dec 25;19(24):6781–6786. doi: 10.1093/nar/19.24.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus J. W., Smith H. C. Three distinct RNA sequence elements are required for efficient apolipoprotein B (apoB) RNA editing in vitro. Nucleic Acids Res. 1992 Nov 25;20(22):6007–6014. doi: 10.1093/nar/20.22.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström K., Garcia Z., Poksay K. S., Johnson D. F., Lusis A. J., Innerarity T. L. Apolipoprotein B mRNA editing. Direct determination of the edited base and occurrence in non-apolipoprotein B-producing cell lines. J Biol Chem. 1990 Dec 25;265(36):22446–22452. [PubMed] [Google Scholar]
- Chen S. H., Li X. X., Liao W. S., Wu J. H., Chan L. RNA editing of apolipoprotein B mRNA. Sequence specificity determined by in vitro coupled transcription editing. J Biol Chem. 1990 Apr 25;265(12):6811–6816. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Davidson N. O., Powell L. M., Wallis S. C., Scott J. Thyroid hormone modulates the introduction of a stop codon in rat liver apolipoprotein B messenger RNA. J Biol Chem. 1988 Sep 25;263(27):13482–13485. [PubMed] [Google Scholar]
- Davies M. S., Wallis S. C., Driscoll D. M., Wynne J. K., Williams G. W., Powell L. M., Scott J. Sequence requirements for apolipoprotein B RNA editing in transfected rat hepatoma cells. J Biol Chem. 1989 Aug 15;264(23):13395–13398. [PubMed] [Google Scholar]
- Diamond A. M., Montero-Puerner Y., Lee B. J., Hatfield D. Selenocysteine inserting tRNAs are likely generated by tRNA editing. Nucleic Acids Res. 1990 Nov 25;18(22):6727–6727. doi: 10.1093/nar/18.22.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll D. M., Casanova E. Characterization of the apolipoprotein B mRNA editing activity in enterocyte extracts. J Biol Chem. 1990 Dec 15;265(35):21401–21403. [PubMed] [Google Scholar]
- Driscoll D. M., Wynne J. K., Wallis S. C., Scott J. An in vitro system for the editing of apolipoprotein B mRNA. Cell. 1989 Aug 11;58(3):519–525. doi: 10.1016/0092-8674(89)90432-7. [DOI] [PubMed] [Google Scholar]
- Garcia Z. C., Poksay K. S., Boström K., Johnson D. F., Balestra M. E., Shechter I., Innerarity T. L. Characterization of apolipoprotein B mRNA editing from rabbit intestine. Arterioscler Thromb. 1992 Feb;12(2):172–179. doi: 10.1161/01.atv.12.2.172. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Harris S. G., Sabio I., Mayer E., Steinberg M. F., Backus J. W., Sparks J. D., Sparks C. E., Smith H. C. Extract-specific heterogeneity in high-order complexes containing apolipoprotein B mRNA editing activity and RNA-binding proteins. J Biol Chem. 1993 Apr 5;268(10):7382–7392. [PubMed] [Google Scholar]
- Hodges P. E., Navaratnam N., Greeve J. C., Scott J. Site-specific creation of uridine from cytidine in apolipoprotein B mRNA editing. Nucleic Acids Res. 1991 Mar 25;19(6):1197–1201. doi: 10.1093/nar/19.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges P., Scott J. Apolipoprotein B mRNA editing: a new tier for the control of gene expression. Trends Biochem Sci. 1992 Feb;17(2):77–81. doi: 10.1016/0968-0004(92)90506-5. [DOI] [PubMed] [Google Scholar]
- Knott T. J., Pease R. J., Powell L. M., Wallis S. C., Rall S. C., Jr, Innerarity T. L., Blackhart B., Taylor W. H., Marcel Y., Milne R. Complete protein sequence and identification of structural domains of human apolipoprotein B. Nature. 1986 Oct 23;323(6090):734–738. doi: 10.1038/323734a0. [DOI] [PubMed] [Google Scholar]
- Navaratnam N., Shah R., Patel D., Fay V., Scott J. Apolipoprotein B mRNA editing is associated with UV crosslinking of proteins to the editing site. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):222–226. doi: 10.1073/pnas.90.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell L. M., Wallis S. C., Pease R. J., Edwards Y. H., Knott T. J., Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987 Sep 11;50(6):831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- Shah R. R., Knott T. J., Legros J. E., Navaratnam N., Greeve J. C., Scott J. Sequence requirements for the editing of apolipoprotein B mRNA. J Biol Chem. 1991 Sep 5;266(25):16301–16304. [PubMed] [Google Scholar]
- Smith H. C., Kuo S. R., Backus J. W., Harris S. G., Sparks C. E., Sparks J. D. In vitro apolipoprotein B mRNA editing: identification of a 27S editing complex. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1489–1493. doi: 10.1073/pnas.88.4.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B., Köhler M., Sprengel R., Seeburg P. H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991 Oct 4;67(1):11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Stuart K., Feagin J. E., Abraham J. M. RNA editing: the creation of nucleotide sequences in mRNA--a minireview. Gene. 1989 Oct 15;82(1):155–160. doi: 10.1016/0378-1119(89)90040-1. [DOI] [PubMed] [Google Scholar]
- Teng B., Burant C. F., Davidson N. O. Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science. 1993 Jun 18;260(5115):1816–1819. doi: 10.1126/science.8511591. [DOI] [PubMed] [Google Scholar]