Abstract
The expression and function of psoriasin in the brain have been insufficiently characterized. Here, we show the induction of psoriasin expression in the central nervous system (CNS) after bacterial and viral stimulation. We used a pneumococcal meningitis in vivo model that revealed S100A15 expression in astrocytes and meningeal cells. These results were confirmed by a cell-based in vivo assay using primary rat glial and meningeal cell cultures. We investigated psoriasin expression in glial and meningeal cells using polyinosinic-polycytidylic acid, a synthetic analog of double-stranded RNA that mimics viral infection. Furthermore, previous results showed that antimicrobial peptides have not only bactericidal but also immunomodulatory functions. To test this statement, we used recombinant psoriasin as a stimulus. Glial and meningeal cells were treated with recombinant psoriasin at concentrations from 25 to 500 ng/ml. Treated microglia and meningeal cells showed phosphorylation of the extracellular signal-regulated kinase 1 (ERK1)/ERK2 (ERK1/2) signal transduction pathway. We demonstrated that this activation of ERK depends on RAGE, the receptor for advanced glycation end products. Furthermore, microglia cells treated with recombinant psoriasin change their phenotype to an enlarged shape. In conclusion, our results indicate an occurrence of psoriasin in the brain. An involvement of psoriasin as an antimicrobial protein that modulates the innate immune system after bacterial or viral stimulation is possible.
INTRODUCTION
Bacterial meningitis is a serious inflammatory disease of the central nervous system (CNS) that is considered to be one of the leading causes of infection-related death worldwide (1). Bacterial meningitis is characterized by an intense inflammatory response of the meningeal cells and the subarachnoid space, and it implies a breakdown of the blood-brain barrier (BBB) (2, 3). Glial cells, e.g., astrocytes and microglia, are key regulators of the innate immune response in the CNS. These cells are the primary responders to cellular stress and infection, which cause them to induce, produce, and release molecular signals that initiate glial responses, leading to inflammation, neurodegeneration, and apoptosis (4). Microglia cells are classified as specialized macrophages of the CNS because they release proinflammatory cytokines and chemokines. A transformation of resting microglia to reactive states in response to pathology has been previously reported (5, 6). Astrocytes play a major role in inflammatory response within the CNS, because they are also required for structural support and maintenance of the BBB (7). Meningeal cells as well as glial cells respond to bacterial pathogens by secreting inflammatory mediators (8). These mediators include antimicrobial peptides (AMP), which are a part of the innate immune system and are involved in the first line of defense (9, 10). Previous studies have suggested that AMPs play an important role in combating bacterial infection of the CNS (11, 12). Brandenburg et al. (13) have demonstrated that glial cells produce CRAMP, an AMP important in combating pathogens in the CNS. AMPs are also involved in inflammation, wound healing, and angiogenesis as well as in the regulation of the adaptive immune system and maintenance of homeostasis (14). A diverse set of mammalian AMPs have been described: defensins, cathelicidins, histatins, and neuroantimicrobial peptides such as proenkephalin and bombesin (15, 16). But in recent years the S100 antimicrobial protein family has received increasing scrutiny (17–19). Psoriasin (S100A7) is an important member of the S100 family, which are EF-hand calcium-binding proteins (20). Psoriasin is upregulated in primary keratinocytes (21) and is a major player in the local innate defense of healthy skin against bacteria (22). Furthermore, it plays a role in wound healing, a pathophysiological process associated with inflammation and involving its release by keratinocytes into wound fluid. Psoriasin has been found to kill by sequestering trace elements, such as zinc, and by directly permeabilizing bacterial cell membranes (23). Gene S100A15 (koebnerisin) in mice, also known as the S100 calcium-binding protein A15 or A7A gene, is closely related to its human ortholog (24). The sequence of S100A15/koebnerisin is 95% identical with that of human psoriasin (25). Recently, expression of psoriasin not only in human skin but also in the lung and on the ocular surface and as a marker of cancerous tumorsin cancer has been described (26–28). Psoriasin expression is known to increase after viral infection of human skin or ocular surface (26, 29).
However, the antimicrobial activity and inducing effect of psoriasin in the CNS are still insufficiently understood. A recent study has found that psoriasin accumulates in the cerebrospinal fluid (CSF) in the course of Alzheimer's disease and in patients with early mild cognitive impairment (18, 30), which supports the hypothesis that psoriasin is associated with inflammatory processes in the CNS.
Some, but not all, members of the S100 family are described as ligands of the receptor for advanced glycation end products (RAGE) (31). Shubbar et al. (32) have described psoriasin as inducing cell proliferation in endothelial cells via RAGE, a member of the immunoglobulin superfamily that is highly expressed in and associated with inflammation-related disease (33). Within this context, this study investigated the expression and antimicrobial activity of psoriasin and the orthologous psoriasin (S100A15) in bacterial but also in viral infections of the CNS in rodents.
MATERIALS AND METHODS
CSF/serum sample preparation and psoriasin ELISA.
Samples of human cerebrospinal fluid (CSF) were provided by Oestern (Kiel, Germany). Ethical approval was granted by the Ethics Committee of Schleswig Holstein, Germany. CSF and serum samples were obtained from patients suffering from bacterial meningitis, cytomegalovirus meningitis, and herpes simplex encephalitis and from control patients. The CSF and serum samples were treated as described by Brandenburg et al. (13). The concentrations of psoriasin in the CSF and blood serum of patients with bacterial meningitis and those of healthy controls were measured with the enzyme-linked immunosorbent assay (ELISA) technique. An ELISA kit specific to human psoriasin was purchased from CircuLex.
Pneumococcal meningitis model.
The meningitis model was approved by the Animal Care and Experimentation Committee of the Canton of Bern, Bern, Switzerland. The animal studies followed National Institutes of Health guidelines for the performance of animal experiments. An established rat model of pneumococcal meningitis was used (3). Wistar rats were infected on postnatal day 11 by direct injection into the cisterna magna of a saline solution containing a defined inoculum of Streptococcus pneumoniae (serogroup 3). The animals were euthanized at 0, 12, or 24 h after infection. Uninfected control animals were injected with sterile saline solution. To document the existence of meningitis, CSF was obtained by puncturing the cisterna magna. For immunohistological studies, the animals were sacrificed and injected with 4% paraformaldehyde–phosphate-buffered saline (PBS) via the left cardiac ventricle.
Fluorescence microscopy.
Coronal brain sections, each 10 μm thick, were cut on a cryostat and collected on Superfrost slides (Merck, Germany). Slides were air-dried for 24 h and stored at −70°C. The sections were fixed with 4% formalin and permeabilized with 0.1% Triton X–PBS for 10 min at room temperature. Slices were incubated at 4°C with a pair of antibodies. The following antibodies were used: anti-S100A15 (sc34076; Santa Cruz), anti-GFAP (anti-glial fibrillary acidic protein) (ab10062; Abcam, Germany), anti-Thy-1 (anti-Thy-1 cell surface antigen) (MCA47R; AbD Serotec), anti-Iba-1 (anti-ionized calcium-binding adaptor molecule-1) (019-19741; Wako), and beta-actin (sc47778; Santa Cruz). Finally, the slices were incubated with anti-goat Alexa 488 (A11055; Life Technologies), anti-mouse Cy3 (115165044; Dianova, Germany), or anti-rabbit Cy3 (AP132C; Chemicon) for 1 h at room temperature. Nuclear counterstaining was performed with diamidino-2-phenylindole dihydrochloride (DAPI) (9542; Sigma, Germany). Cells were digitally photographed using a Zeiss Axio Z1 imager (Zeiss, Germany). Fluorescence images were analyzed and quantified using ImageJ, which determined the area, integrated density, and mean gray value. To calculate the fluorescence intensity, the corrected total cell fluorescence (CTCF) was used, following this formula: CTCF = integrated density − (area of selected cell × mean fluorescence of background readings). The ratio of CTCF-treated cells to untreated cells was calculated. The microglia cell area was calculated and analyzed as the ratio of the cell area of treated cells to that of untreated cells.
Reagents.
Bacterial supernatants were produced as described in detail by Brandenburg et al. (34). In these experiments, bacterial supernatants of Neisseria meningitidis (ATCC 13077), Pseudomonas aeruginosa (ATCC 11440), Streptococcus pneumoniae (ATCC 6303), and Staphylococcus aureus (ATCC 6538) were used, all at a dilution of 1:100. Lipopolysaccharide (LPS) (L9516; Sigma-Aldrich) from Salmonella enterica serovar Typhimurium was subsequently stimulated at a concentration of 100 ng/ml. Polyinosinic-poly(C) [poly(I·C)] (Invivogen) was used at a concentration of 25 μg/ml (35). Psoriasin was recombinantly expressed as described by Michalek et al. (23).
Cell culture.
For one experiment (n = 1), a primary cell culture of 12 to 15 2-day-old Wistar rat pups was cultivated. Isolated cerebral cortices and rostral mesencephali of the pups were stripped of the meninges. Cortices and mesencephali were minced and dissociated enzymatically with bovine pancreas trypsin (Sigma, Germany) in phosphate-buffered saline–1 mg/ml DNase (Roche Molecular Biochemicals) for 30 min at 37°C and crushed mechanically with scalpels. Astrocytes were cultivated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS; Sigma, Germany) and 1% penicillin and streptomycin (Sigma, Germany) according to McCarthy and Devellis (36). This method achieves a nearly (>97%) pure culture of astrocytes. Microglia were collected as free-floating cells from primary cultures of astrocytes and cultivated as described by Slowik et al. (37). This procedure raised the purity of the microglia preparation to >98%. To grow meningeal cells, pia mater and arachnoid were carefully removed from the cortices of 2-day-old Wistar rat pups. The tissue was dissociated, and the cells were plated onto poly-l-lysin-coated culture flasks. Meningeal cells were cultivated in DMEM supplemented with 10% FCS, 5% horse serum, and 1% penicillin and streptomycin. Cell numbers and viability of astrocytes, microglia, and meningeal cells were estimated by trypan blue exclusion. To test cell purity, cultures were stained with specific cell markers: for astrocytes, anti-GFAP; for microglia, anti-Iba-1; and for meningeal cells, anti-Thy-1.
Stimulation with bacterial supernatants, LPS, poly(I·C) and recombinant psoriasin.
Glial and meningeal cells were starved overnight by adding FCS-free medium (Opti-MEM, Gibco, United Kingdom). The cells were incubated for 0, 6, 12, or 24 h at 37°C.
HEK293 cells (American Type Culture Collection, Rockville, MD) were cultivated in DMEM supplemented with 10% FCS and 1% penicillin and streptomycin. The transfection and selection of HEK293 cells expressing FPRL1, hRAGE, or ΔRAGE were described previously (37, 38). The pcDNA3.1-RAGE plasmid containing a neomycin resistance gene was kindly provided by R. Donato (Perugia, Italy) (39). The insertion was subcloned into a pcDNA3.1 expression vector containing a Zeocin resistance gene (Invitrogen, Germany).
RNA isolation and real-time RT-PCR.
Total RNA was isolated using an MN kit (Macherey and Nagel, Germany). The quality of the RNA was assessed with a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific) according to the manufacturer's instructions. Absorbance ratios of 1.8 to 2.0 for 260/280 or 260/230 nm indicated pure RNA samples. RNA samples were reverse transcribed (RT) according to cDNA protocols (Fermentas, Germany). The cDNA products were used immediately for Sybr green (Applied Biosystems) real-time RT-PCR and RT-PCR. Real-time RT-PCR was done using Step One Plus (Applied Biosystems) according to the manufacturer's protocol. The primer for S100A15 (QuantiTec Primer) was manufactured by Qiagen. The housekeeping reference primer was hypoxanthin-guanin-phosphoribosyltransferase (HPRT) (forward primer, TCAGTCCCAGCGTCGTGATTAGTG; reverse primer, CCTTCAGCACACAGAGGGCCAC; Eurofins MWG Operon, Germany). The relative quantities of target gene expression and amplification efficiencies were determined using a mathematical model previously described by Pfaffl (40). Target gene expression was normalized to expression of the housekeeping reference genes for Sybr green real-time RT-PCR. The experiments were performed in triplicate. The RT-PCR procedure was performed as previously described in detail by Brandenburg et al. (13). The S100A15 primer was also used for RT-PCR. As an internal control for the integrated cDNA, we used glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward primer, TCTACCCACGGCAAGTTCAAC; reverse primer, TCTCGCTCCTGGAAGATGGT; Eurofins, MWG Operon, Germany).
Western blotting.
Cells were harvested in a lysis buffer (50 mM Tris [pH 7.5], 100 mM NaCl, 5 mM EDTA, 1% Triton X, 2 mM sodium orthovanadate, 2.5 mM sodium pyrophosphate, 1 mM phenylmethylsulfonyl fluoride). Proteins were detected with a bicinchoninic acid (BCA) kit (Thermo Fisher Scientific). Proteins (5 μg) were resolved in SDS sample buffer, and a Western blotting procedure was performed. Membranes were incubated overnight at 4°C with polyclonal primary antibodies against phospho-extracellular signal-regulated kinase 1/2 (pERK1/2) (sc7383; Santa Cruz) or ERK2 (sc1647; Santa Cruz) and detected with peroxidase-labeled secondary antibodies. Antibody binding was detected using enhanced chemiluminescence (Millipore, Germany). The density of the bands was evaluated by Image J. The ratio of the protein to the corresponding loading control bands (ERK2) was calculated. The experiments were performed in triplicate.
CellTiter-Blue cell viability assay.
A CellTiter-Blue assay (Promega, Germany) was used to measure the viability of glial and meningeal cells after treatment with recombinant psoriasin. The cells were seeded in 96-well plates and starved overnight by adding FCS-free medium. The cells were treated with various concentrations of recombinant psoriasin for 24 h. A CellTiter-Blue assay was used according to the manufacturer's recommendations. Spectrophotometric evaluations were performed after 1, 2, and 4 h, and treated cells were compared with untreated cells. The results are displayed in Fig. S1 in the supplemental material.
Statistical analysis.
All measurements were performed at least in triplicate, and the values represent means ± standard errors of the means (SEM). The significance of the differences between test and control group results was analyzed using Student's t test procedures and analysis of variance (ANOVA), followed by Bonferroni's multiple-comparison test. All statistical data were analyzed using Graph Pad Prism 5.0 software.
RESULTS
Occurrence of psoriasin in the CSF of bacterial meningitis patients.
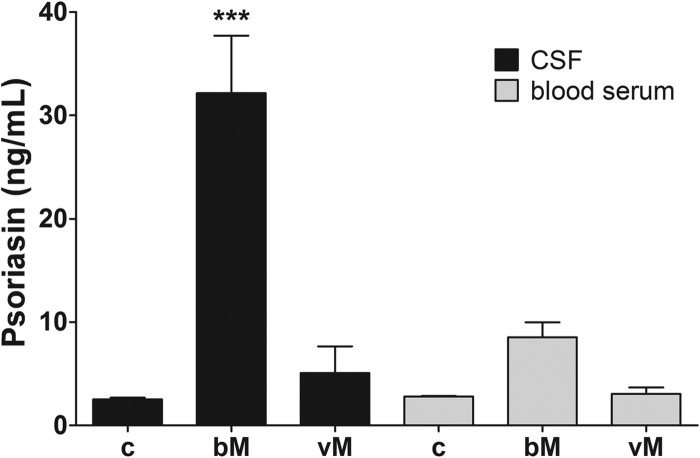
A first set of experiments investigated the presence of psoriasin in human cerebrospinal fluid. To that end, the psoriasin concentration in the CSF of patients suffering from bacterial meningitis was analyzed using high-performance liquid chromatography (HPLC) and ELISA techniques. Compared with the uninfected control psoriasin level of 3 ng/ml, we found much higher (up to 25 ng/ml) psoriasin concentrations associated with bacterial meningitis. To confirm these HPLC results, the concentration of psoriasin protein in CSF was analyzed with a commercial ELISA kit. The CSF of uninfected control patients showed a psoriasin concentration of 2.5 ng/ml. After bacterial meningitis, the psoriasin concentration increased significantly to 32.1 ± 5.6 ng/ml (Fig. 1). By comparison, CSF samples from patients who suffered from viral meningitis showed no significantly increased psoriasin concentration, and neither were any significant psoriasin concentrations detectable in blood serum samples.
Fig 1.
Increased psoriasin concentration in human cerebrospinal fluid from patients suffering from bacterial meningitis. Immunoreactive psoriasin concentrations were measured in cerebrospinal fluid (CSF) and blood serum from patients suffering from bacterial meningitis (bM) and viral meningitis (vM) and from uninfected controls (c) using an ELISA technique. The experiment was performed with five patients in triplicate. The graph represents means ± SEM. Statistical significance is marked as *** = P < 0.001 (ANOVA test followed by Bonferroni's multiple-comparison test).
S100A15 expression and cellular origin in an infant rat model of pneumococcal meningitis.
In the next step, an in vivo model was used to analyze the induction and cellular origin of S100A15 expression. S100A15 is the psoriasin ortholog found in rodents (24). To examine whether glial cells or meningeal cells or both are responsible for S100A15 expression, a pneumococcal meningitis model was used in conjunction with a cell-specific marker. To that end, juvenile rats were infected intracisternally with Streptococcus pneumoniae and killed 12 or 24 h after infection, respectively. To examine the localization of S100A15 by double-fluorescence microscopy, anti-GFAP, anti-Iba-1, and anti-Thy-1 were used as cell-specific antibodies for astrocyte, microglial, and meningeal cells, respectively (41–43). In uninfected rats, very little immunoreactivity to GFAP was measured, and no immunoreactivity to S100A15 was measured (Fig. 2A). Control brains showed very low levels of positive GFAP staining, because GFAP is a specific marker for activated astrocytes. In contrast, a strong increase in S100A15 expression was detected 12 h after infection with S. pneumoniae. We also observed colocalization of S100A15 and GFAP in these coronal brain sections. At 24 h after infection, coronal brain sections exhibited lower expression of S100A15. The number of GFAP-positive cells strongly increased 12 and 24 h after infection. In microglia cells, increased S100A15 expression was not found. Much as in the case of astrocytes, a strong increase in S100A15 expression was detected in meningeal cells 12 and 24 h after infection (Fig. 2C).
Fig 2.
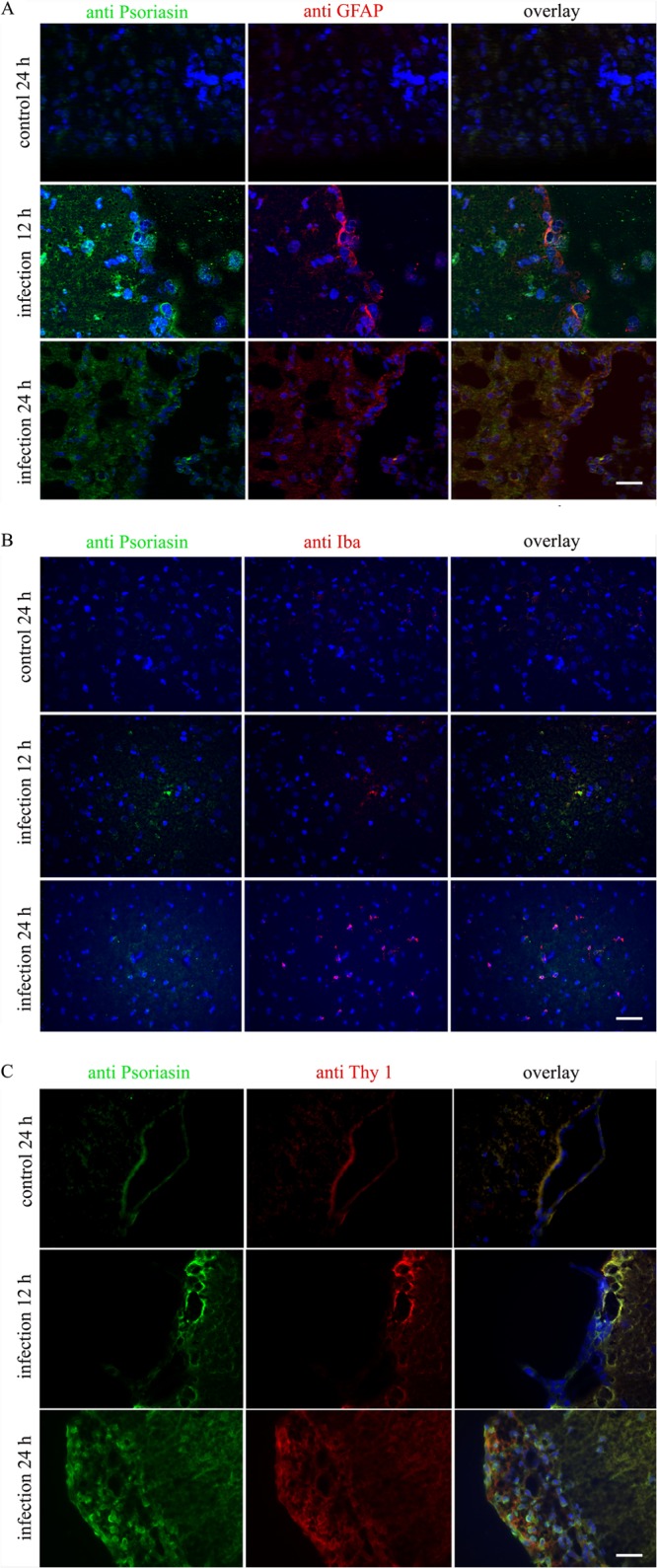
S100A15 expression and colocalization in an infant rat model of pneumococcal meningitis. Rat S100A15 expression in glial and meningeal cells was detected through immunohistochemistry in the infant rat model of pneumococcal meningitis. Juvenile rats were infected intracisternally with Streptococcus pneumoniae and sacrificed after 0, 12, or 24 h. The uninfected controls were injected with sterile saline. Coronal brain sections were fixed and immunolabeled with specific antibodies and examined with double-fluorescence microscopy. (A) Coronal brain sections were immunolabeled using anti-S100A15 (green) and anti-GFAP (red) to identify astrocytes and with DAPI for nuclear counterstaining (blue). (B) Coronal brain sections were immunolabeled using anti-S100A15 (green) and anti-Iba-1 (red) to identify microglia and with DAPI for nuclear counterstaining (blue). (C) Coronal brain sections were immunolabeled using anti-S100A15 (green) and anti-Thy-1 (red) to identify meningeal cells and with DAPI for nuclear counterstaining (blue). Coronal brain sections of three animals in triplicate were examined. Scale bar = 20 μm.
In vitro S100A15 protein expression in glial and meningeal cells after bacterial or LPS stimulation.
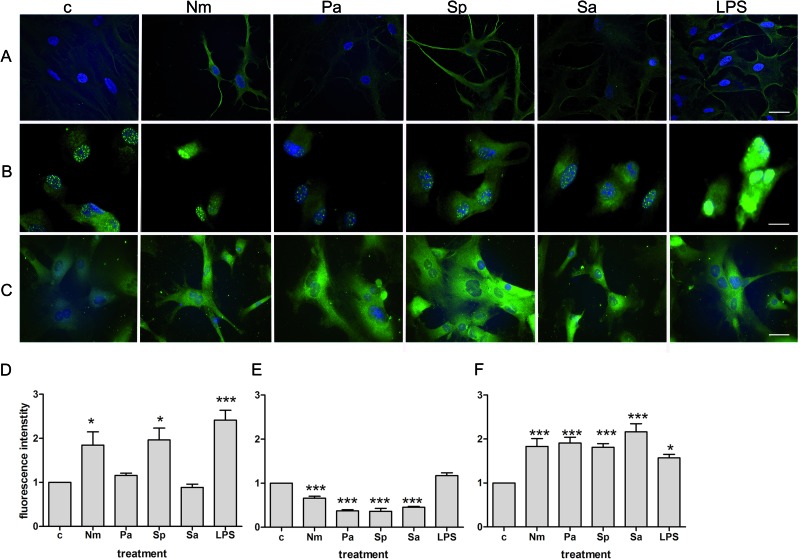
Next, an in vitro S100A15 protein expression experiment was performed to validate the previous in vivo findings. Primary rat astrocyte, microglia, and meningeal cells were seeded on cover glasses and stimulated for 24 h with the bacterial cell wall component lipopolysaccharide (LPS). In addition to LPS, we used bacterial supernatants of the Gram-negative bacteria Neisseria meningitidis and Pseudomonas aeruginosa and supernatants of the Gram-positive bacteria Streptococcus pneumoniae and Staphylococcus aureus. S100A15 expression was monitored using fluorescence microscopy. As shown in Fig. 3A, the astrocytes stimulated with N. meningitidis, S. pneumoniae, or LPS exhibited increased S100A15 expression. In contrast, microglia exhibited no increased S100A15 expression after bacterial stimulation (Fig. 3B). Increased S100A15 expression in meningeal cells was detectable for all bacterial supernatants and for LPS (Fig. 3C). To evaluate the increasing fluorescence, we calculated the relative fluorescence intensity as the ratio of the respective fluorescence intensities of the glial and meningeal cells compared to that of the untreated controls. Significant increases in S100A15 expression in astrocytes were induced after treatment with N. meningitidis (1.8- ± 0.3-fold increase in fluorescence intensity), S. pneumoniae (2.0 ± 0.3), and LPS (2.4 ± 0.2) (Fig. 3D). By the same token, we found significantly increased induction of S100A15 protein expression in meningeal cells after treatment with N. meningitidis (1.8- ± 0.2-fold increase in fluorescence intensity), P. aeruginosa (1.9 ± 0.1), S. pneumoniae (1.8 ± 0.1), S. aureus (2.2 ± 0.2), and LPS (1.6 ± 0.1) (Fig. 3F). In contrast to astrocytes and meningeal cells, microglia did not show an increase in S100A15 expression after bacterial or LPS treatment (Fig. 3E).
Fig 3.
In vitro S100A15 protein expression in rat glial and meningeal cells after bacterial stimulation. (A to C) Astrocytes (A), microglia cells (B), and meningeal cells (C) were incubated with supernatants of Neisseria meningitis (Nm), Pseudomonas aeruginosa (Pa), Streptococcus pneumoniae (Sp), or Staphylococcus aureus (Sa) or with lipopolysaccharide (LPS) for 24 h. After incubation, glial and meningeal cells were fixed and immunolabeled using anti-S100A15 antibody (green) and DAPI for nuclear counterstaining (blue) and examined with double-fluorescence microscopy. Panels A to C show representative results from one of three independent experiments. Scale bar = 20 μm for panels A and C; scale bar = 10 μm for panel B. (D to F) Fluorescence intensity was calculated as the ratio of untreated control cells (c) to the treated astrocytes (D), microglia cells (E), and meningeal cells (F). The experiment was performed in triplicate. These results were calculated from 20 separate cells. Statistical significance is marked as * = P < 0.05 and *** = P < 0.0005 (ANOVA test followed by Bonferroni's multiple-comparison test).
S100A15 mRNA expression in primary glial and meningeal cells after stimulation with bacterial supernatants or LPS.
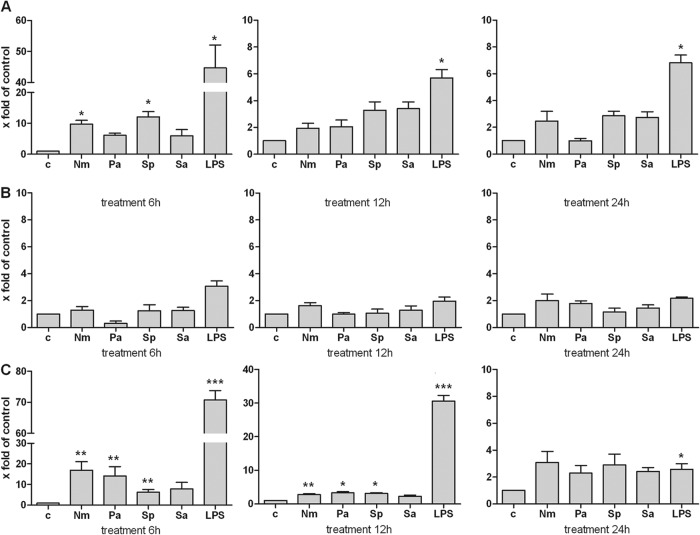
Based on the protein expression results of in vivo and in vitro experiments, real-time RT-PCR was used to investigate the level of S100A15 mRNA expression in glial and meningeal cells. The cells were incubated with different bacterial supernatants or LPS for 6, 12, or 24 h. In astrocytes, S100A15 mRNA levels were elevated after a treatment of 6 h (for N. meningitidis, a 9.8- ± 1.2-fold increase in the mRNA level; for S. pneumoniae, a 12.1- ± 1.7-fold increase; and for LPS, a 44.7- ± 7.4-fold increase). Significantly increased S100A15 mRNA levels were measured after 12 and 24 h of treatment with LPS (5.7- ± 0.6-fold and 6.8- ± 0.6-fold, respectively) (Fig. 4A). Compared with astrocytes, microglia did not exhibit significantly increased S100A15 mRNA levels after treatment with bacterial supernatants or LPS (Fig. 4B). As expected, meningeal cells showed a significant increase in S100A15 mRNA levels after 6 h of the respective treatments (for N. meningitidis, 19.9- ± 4.2-fold; for P. aeruginosa, 14.2- ± 4.5-fold; for S. pneumoniae, 6.2- ± 1.4-fold; and for LPS, 70.7- ± 3.0-fold) (Fig. 4C). After 12 h of treatment, significant increases in S100A15 mRNA levels were likewise observed for N. meningitidis (2.8- ± 0.2-fold), P. aeruginosa (3.3- ± 0.4-fold), S. pneumoniae (3.1- ± 0.2-fold), and LPS (30.6- ± 1.7-fold). After 24 h of treatment, the S100A15 mRNA level had declined (for LPS, 2.6- ± 0.4-fold).
Fig 4.
In vitro S100A15 mRNA expression in primary rat glial and meningeal cells after stimulation with bacterial supernatants or LPS. (A) Astrocytes; (B) microglia cells; (C) meningeal cells. Primary cells were stimulated with Neisseria meningitis (Nm), Pseudomonas aeruginosa (Pa), Streptococcus pneumoniae (Sp), or Staphylococcus aureus (Sa) or with lipopolysaccharide (LPS) for 6, 12, or 24 h. S100A15 mRNA expression was analyzed using SYBR green real-time RT-PCR, and results were compared to untreated sample results (c). The housekeeping gene hypoxanthin-guanin-phosphoribosyltransferase (HPRT) was used as an internal control. The experiment was performed with five animals in triplicate. Statistical significance is marked as * = P < 0.05, ** = P < 0.006, and *** = P < 0.0007 (representing the significance of the results of comparisons between control and corresponding bacterial supernatant or LPS determined using the t test).
S100A15 m RNA expression in primary glial and meningeal cells after stimulation with poly(I·C).
Polyiosinic-polycytidylic acid [poly(I·C)] was used to investigate the level of S100A15 mRNA expression after a viral infection. Poly(I·C) is a synthetic analog of double-stranded RNA, a molecular pattern associated with viral infection. Glial and meningeal cells were incubated with 25 μg/ml of poly(I·C) for 6, 12, or 24 h. Significant increases were detected in S100A15 mRNA levels in astrocytes after treatments of 6 h (60.8- ± 3.2-fold) and 12 h (37.9- ± 2.4-fold) (Fig. 5A). Meningeal cells also exhibited significant increases in S100A15 mRNA expression after 6 h of treatment (66.6 ± 3.4) and 12 h of treatment (42.8 ± 3.4) with poly(I·C) (Fig. 5C).
Fig 5.
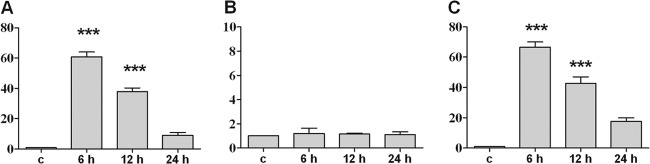
In vitro S100A15 mRNA expression in primary rat glial and meningeal cells after stimulation with poly(I·C). (A) Astrocytes; (B) microglia cells; (C) meningeal cells. Primary cells were stimulated with poly(I·C) for 6, 12, or 24 h. S100A15 mRNA expression was analyzed using SYBR green real-time RT-PCR, and results were compared to the untreated sample results (c). The housekeeping gene hypoxanthin-guanin-phosphoribosyltransferase (HPRT) was used as an internal control. The experiment was performed with five animals in triplicate. Statistical significance is marked as *** = P < 0.0001 [representing the significance of the results of comparisons between control and poly(I·C) treatment determined using the t test].
No significant S100A15 mRNA expression was observed in microglia cells after treatment with poly(I·C) (Fig. 5B).
Recombinant psoriasin induced ERK1/2 phosphorylation in glial and meningeal cells.
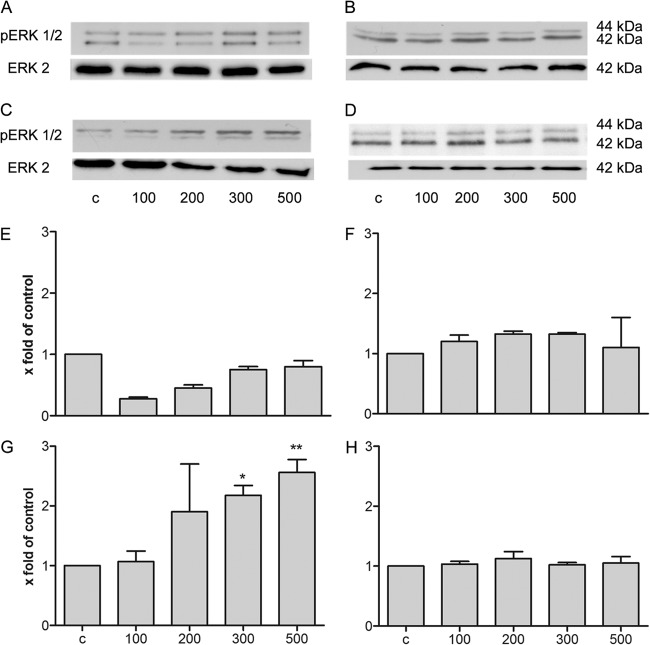
Our first results demonstrated an induction of expression of S100A15 in astrocytes and meningeal cells after bacterial meningitis or bacterial/viral stimulation. Besides their antimicrobial activity, AMPs have immunomodulatory functions (44). Hence, we studied the immunomodulatory effect of psoriasin as a signal protein for glial and meningeal cells. Glial cells and meningeal cells were stimulated with various concentrations of recombinant psoriasin ranging from 25 to 500 ng/ml. To determine the cytotoxicity of recombinant psoriasin, we used a cell viability assay which revealed that glia and meningeal cells treated with recombinant psoriasin are viable cells (see Fig. S1 in the supplemental material). First, the induction of the ERK (extracellular signal-related kinase) signal transduction pathway was analyzed using Western blot analysis. The ERK1/2 signal transduction pathway is involved in cell proliferation and differentiation (45). Data from one of our three independent experiments are displayed in Fig. 6C. The results from the Western blot analysis were analyzed by densitometric quantification (Fig. 6D to F). Astrocytes showed no increase of ERK1/2 phosphorylation after treatment with psoriasin at the various concentrations (Fig. 6A and D). In microglia, significant ERK1/2 phosphorylation (a 8.0- ± 0.9-fold increase), caused by psoriasin treatment in the range of 100 to 500 ng/ml, was observed (Fig. 6B and E). As shown in Fig. 6C and F, psoriasin treatment at up to 100 ng/ml activated the ERK1/2 signal transduction pathway in meningeal cells (resulting in a 6.5- ± 0.9-fold increase in ERK1/2 phosphorylation).
Fig 6.
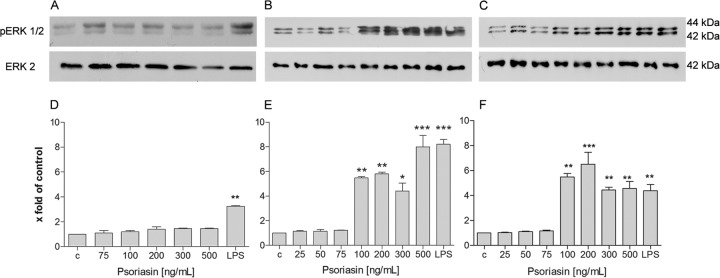
Psoriasin-induced ERK1/2 phosphorylation in rat glial and meningeal cells. (A to C) For analysis of ERK1/2 phosphorylation, astrocytes (A), microglia cells (B), and meningeal cells (C) were treated with recombinant psoriasin at concentrations ranging from 25 to 500 ng/μl or with LPS. Cells were lysed in buffer, and 5 μg of protein was separated by SDS-PAGE followed by immunoblotting of ERK1/2. The results of a Western blot analysis of three independent experiments are displayed. (D to F) The means ± SD of the results of three independent experiments were evaluated by densitometric quantification. (D) Astrocytes; (E) microglia cells; (F) meningeal cells. The ratio of the protein to the corresponding loading control bands (ERK2) was calculated. The experiment was performed with eight animals in triplicate. Statistical significance is marked as * = P < 0.05, ** = P < 0.003, and *** = P < 0.0006 (ANOVA test followed by Bonferroni's multiple-comparison test).
Treatment with recombinant psoriasin induced a phenotype change in primary rat microglia.
When activated, microglia rapidly change their phenotype and contribute to pathophysiological situations, including inflammation, tissue remodeling, and neurogenesis (46). We therefore tested the change in microglia phenotype after treatment with various concentrations of recombinant psoriasin. Microglia were seeded on cover glasses and stimulated with psoriasin concentrations of between 25 and 500 ng/ml for 24 h. To examine the morphology of the treated microglia compared with untreated controls, we performed double-fluorescence microscopy (Fig. 7A). Microglia seemed to be enlarged after psoriasin treatment at 100 and 200 ng/ml. Subsequently, the phenotype change was quantified by measuring and calculating the microglial cell area (Fig. 7B). We calculated significantly larger areas for microglia treated with 100 and 200 ng/ml of psoriasin (2.1- ± 0.1-fold and 1.6- ± 0.1-fold increases in cell area) compared to untreated cells. Treatment with 300 and 500 ng/ml of psoriasin, respectively, showed no significant effect.
Fig 7.
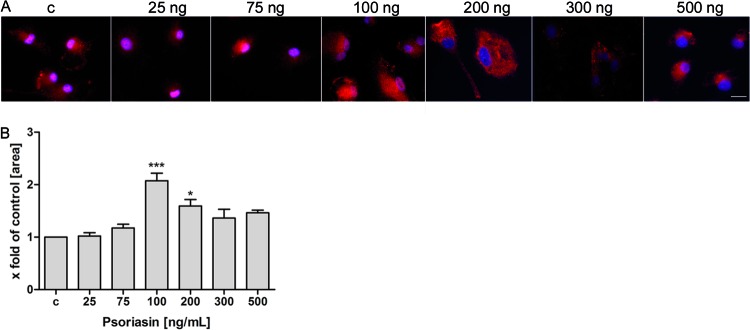
Treatment with recombinant psoriasin induced a phenotype change in primary rat microglia. Microglia were incubated with recombinant psoriasin for 24 h. The concentrations of psoriasin ranged from 25 ng to 500 ng/ml. After incubation, microglia were fixed and immunolabeled using beta-actin antibody (red) and DAPI for nuclear counterstaining (blue) and examined with double-fluorescence microscopy. (A) Representative results from one of three independent experiments. Scale bar = 10 μm. (B) Cell area was measured and calculated as the ratio of untreated control cells (c) to treated cells. The experiment was performed with 10 animals in triplicate. These results were calculated for 20 separate cells. Statistical significance is marked as * = P < 0.05 and *** = P < 0.0001 (ANOVA test followed by Bonferroni's multiple-comparison test).
RAGE-mediated psoriasin-induced ERK1/2 phosphorylation in transfected HEK293 cells.
Previous studies suggested that the pattern recognition receptor (PRR) RAGE mediates the cellular effects of members of the S100 family (31). To confirm these results, psoriasin-induced ERK1/2 phosphorylation was tested in different HEK293 cells, including untransfected HEK293 cells, HEK/FPRL1 cells (cells transfected with formyl-peptide-receptor-like-1 [FPLR1]), HEK/RAGE cells (transfected with RAGE), and HEK/ΔRAGE cells (the inactive RAGE receptor). HEK/ΔRAGE cells served as a control. FPRL1 is a chemotactic G protein-coupled receptor that recognizes pathogen-associated molecular patterns (47). Our previous results showed that FPRL1 is involved in bacterial meningitis (12), and we used it as a receptor control. Additionally, we used transfected HEKΔRAGE cells. This is a transfected cell line with an inactive RAGE receptor. Untransfected HEK293 cells, transfected HEK/FPRL1, HEK/RAGE cells, and HEK/ΔRAGE cells were treated with various concentrations of recombinant psoriasin for 5 min. The results of one of our three independent Western blot experiments are shown in Fig. 8A to D. The Western blot results were analyzed by densitometric quantification (Fig. 8E to H). In untransfected HEK293 cells, none of the treatments led to an increase in ERK1/2 phosphorylation. HEK/FPRL1 cells also showed no increase in ERK1/2 phosphorylation after treatment with psoriasin (Fig. 8B and F). The HEK/RAGE cell group was the only one to show a significant increase in ERK1/2 phosphorylation, found after treatment with 300 and 500 ng/ml of psoriasin (2.2- ± 0.2-fold and 2.6- ± 0.2-fold increases in ERK1/2 phosphorylation, respectively) (Fig. 8C and G). The control group using HEK/ΔRAGE cells showed no phosphorylation of ERK (Fig. 8D and H). These results clearly show that the receptor RAGE mediates the activity of psoriasin. In addition, the expression of RAGE in untreated glial and meningeal cells was monitored by RT-PCR (see Fig. S2 in the supplemental material). We were able to detect a RAGE cDNA amplification product in astrocytes that represented expression at a level that was weaker than that in microglia or meningeal cells.
Fig 8.
RAGE-mediated psoriasin-induced ERK1/2 phosphorylation in transfected HEK293 cells. (A to D) For analysis of ERK1/2 phosphorylation, untransfected HEK293 cells (A), transfected HEK/hFPRL1 cells (B), HEK/RAGE cells (C), and HEK/Δ RAGE cells (D) were treated with recombinant psoriasin at concentrations ranging from 100 to 500 ng/ml. Cells were lysed in buffer, and 5 μg of protein was separated by SDS-PAGE, followed by immunoblotting of ERK1/2. The results of a Western blot analysis of three independent experiments are shown. (E to H) The means ± SD of the results of three independent experiments were evaluated by densitometric quantification. (E) HEK293 cells; (F) transfected HEK/hFPRL1 cells; (G) HEK/RAGE cells; (H) HEK/Δ RAGE cells. The ratio of the protein to the corresponding loading control bands (ERK2) was calculated. The experiments were performed in triplicate. One representative Western blot is shown. Statistical significance is marked as * = P < 0.05 and ** = P < 0.005 (ANOVA test followed by Bonferroni's multiple-comparison test).
DISCUSSION
Astrocytes are likely the major contributor to bacterial or viral responses within the CNS (7). They are heavily involved in the development and integrity of the blood brain barrier.
Brandenburg et al. (13) have demonstrated that astrocytes express the rat cathelicidin rCRAMP, a homologue of human LL-37, after bacterial treatment. In addition, recent studies have demonstrated that defensins expressed in astrocytes are linked to antiviral and or antimicrobial defense (15, 48, 49). One study suggested that released AMPs may also modify the host inflammatory astrocyte response (50). In the present study, we found an increased S100A15 mRNA level in astrocytes after bacterial and viral stimulation. Our CSF samples showed no increased psoriasin level after viral stimulation. Unfortunately, we did not know exactly when the liquor samples were harvested. Liquor harvesting is highly variable and is determined by the stage of the disease, so it is possible that the CSF samples show a decreased psoriasin level.
In addition, our results demonstrated that S100A15 is induced in meningeal cells after both bacterial and viral stimulation. Meningeal cells treated with different bacterial components, including Streptococcus pneumoniae or Neisseria meningitis, induce secretion of various inflammatory mediators such as interleukin-1 (IL-1) or interleukin-6 (IL-6) as well as of tumor necrosis factor alpha (TNF-α) (8). Furthermore, meningeal cells exhibit an increase in rCRAMP expression in vitro and in vivo after bacterial infection (34). The study demonstrated that meningeal cells incubated with bacterial or viral components exhibit antimicrobial activity (34). This shows that meningeal cells have direct antibacterial activity after inflammation, e.g., meningitis.
Interestingly, microglia show no increase in S100A15 expression after bacterial or viral treatment. Our previous findings demonstrated that the level of rCRAMP is greatly increased in primary rat microglial cells in an early phase after bacterial treatment (50). Such early induction is consistent with their functioning in the innate immune system, and this early AMP expression might activate other brain or immune cells in the CNS to modulate the immune response.
To confirm the hypothesis that psoriasin is involved in immunomodulatory functions, we analyzed the ERK signal pathway. A large variety of cellular activities, such as proliferation, cellular differentiation, and cellular activation, have been shown to involve ERK activation (45, 51). In neutrophils, psoriasin induces phosphorylation of ERK1/2 (52). Our results reveal an activating of ERK1/2 in microglia and meningeal cells after psoriasin treatment.
In the presence of LPS and psoriasin, the phenotype of the microglia cells is enlarged. Recent studies have reported that microglia rapidly change their phenotype and contribute to various processes, including inflammation and neurogenesis (46). This appearance of rod-shaped cells is a classic example of microglia activation after a neurological infection (53). Block et al. reported that microglia change their phenotype into an enlarged phagocytotic microglial subtype after a neurodegenerative disease (54). For this reason, a treatment with LPS led to a change of phenotype to a large, circular flat shape in microglia (55). Microglia activation implicated a release of cytokines such as TNF-α and IL-1 (56).
Interestingly, our results show that microglia change their phenotype into a large, flat shape after treatment with LPS or psoriasin. We assume that treatment of microglia with psoriasin effects a change in their phenotype into an active shape. Further studies must determine the cytokine expression.
One previous study has shown that RAGE mediates cellular effects in some members of the S100 family. RAGE is a member of the immunoglobulin superfamily, is one of the pattern recognition receptors (PRR), and has a broad range of ligands (57). We demonstrated in this study that psoriasin is a ligand of RAGE. Its binding to RAGE leads to phosphorylation of ERK1/2. In contrast, the cell line transfected with an inactive RAGE receptor showed no phosphorylation. Therefore, RAGE must mediate the psoriasin-induced cellular effects in glial cells. These findings indicate that psoriasin and its receptor, RAGE, probably play an important role in the innate immune system in the CNS. RAGE transduces inflammatory responses and is involved in the pathogenesis of several diseases, including neurodegeneration and inflammation (58). Yan et al. (33) demonstrated that the S100-RAGE interaction leads to an infiltration of the CNS by immune and inflammatory cells. Further studies must clarify the importance of RAGE in the course of infection (31, 59).
These results provide initial evidence that psoriasin/S100A15 may have a dual function in the brain in inflammatory situations such as meningitis or viral infections. It may be that psoriasin is involved as an antimicrobial protein that modulates the immune system after a bacterial or viral infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Susanne Echterhagen, Lian Shen, Michaela Nicolau, and Marie Pradella for excellent technical assistance. We thank Jens-Michael Schröder (Department of Dermatology, University Hospital Schleswig-Holstein, Kiel, Germany) for providing the HPLC data.
This study was supported by the Else Kröner-Fresenius-Stiftung (L.-O.B.), START-Program of the RWTH Aachen University (L.-O.B.), and the Swiss National Science Foundation (grant no. 138094; S.L.L.).
Footnotes
Published ahead of print 11 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01265-12.
REFERENCES
- 1. Fauci AS. 2001. Infectious diseases: considerations for the 21st century. Clin. Infect. Dis. 32:675–685 [DOI] [PubMed] [Google Scholar]
- 2. Schuchat A, Robinson K, Wenger D, Harrison LH, Farley MF, Reingold AL, Lefkowitz L, Perkins B. 1997. Bacterial meningitis in the United States in 1995. N. Engl. J. Med. 337:970–976 [DOI] [PubMed] [Google Scholar]
- 3. Leib S, Leppert D, Clements J, Täuber M. 2000. Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect. Immun. 68:615–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holm TH, Draeby D, Owens T. 2012. Microglia are required for astroglial toll-like receptor 4 response and for optimal TLR2 and TLR3 response. Glia 60:630–638 [DOI] [PubMed] [Google Scholar]
- 5. Saijo K, Glass CK. 2011. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 11:775–787 [DOI] [PubMed] [Google Scholar]
- 6. Hanisch UK, Kettenmann H. 2007. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10:1387–1394 [DOI] [PubMed] [Google Scholar]
- 7. Konat GW, Kielian T, Marriott I. 2006. The role of Toll-like receptors in CNS response to microbial challenge. J. Neurochem. 99:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fowler MI, Weller RO, Heckels JE, Christodoulides M. 2004. Different meningitis-causing bacteria induce distinct inflammatory responses on interaction with cells of the human meninges. Cell. Microbiol. 6:555–567 [DOI] [PubMed] [Google Scholar]
- 9. Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395 [DOI] [PubMed] [Google Scholar]
- 10. Bulet P. 2004. Anti-microbial peptides: from invertebrates to vertebrates. Immunol. Rev. 198:169–184 [DOI] [PubMed] [Google Scholar]
- 11. Bergman P, Termen S, Johansson L, Nystrom L, Arenas E, Jonsson AB, Hokfelt T, Gudmundsson GH, Agerberth B. 2005. The antimicrobial peptide rCRAMP is present in the central nervous system of the rat. J. Neurochem. 93:1132–1140 [DOI] [PubMed] [Google Scholar]
- 12. Braun JB, Slowik A, Leib SL, Lucius R, Varoga D, Wruck C, Jansen S, Podschun R, Pufe T, Brandenburg LO. 2011. The formyl peptide receptor like-1 and scavenger receptor MARCO are involved in glial cell activation in bacterial meningitis. J. Neuroinflammation 8:11 doi:10.1186/1742-2094-8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brandenburg LO, Varoga D, Nicolaeva N, Leib S, Wilms H, Podschun R, Wruck C, Schroder JM, Pufe T, Lucius R. 2008. Role of glial cells in the functional expression of LL-37/rat cathelin-related antimicrobial peptide in meningitis. J. Neuropathol. Exp. Neurol. 67:1041–1054 [DOI] [PubMed] [Google Scholar]
- 14. Brandenburg L-O, Merres J, Albrecht L-J, Varoga D, Pufe T. 2012. Antimicrobial peptides: multifunctional drugs for different applications. Polymers 4:539–560 [Google Scholar]
- 15. Su Y, Zhang K, Schluesener HJ. 2010. Antimicrobial peptides in the brain. Arch. Immunol. Ther. Exp. (Warsz) 58:365–377 [DOI] [PubMed] [Google Scholar]
- 16. De Smet K, Contreras R. 2005. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol. Lett. 27:1337–1347 [DOI] [PubMed] [Google Scholar]
- 17. Gläser R, Harder J, Lange H, Bartels J, Christophers E, Schroder JM. 2005. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat. Immunol. 6:57–64 [DOI] [PubMed] [Google Scholar]
- 18. Qin W, Ho L, Wang J, Peskind E, Pasinetti GM. 2009. S100A7, a novel Alzheimer's disease biomarker with non-amyloidogenic alpha-secretase activity acts via selective promotion of ADAM-10. PLoS One 4:e4183 doi:10.1371/journal.pone.0004183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. 2006. Calcium-dependent and -independent interactions of the S100 protein family. Biochem. J. 396:201–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heizmann CW, Fritz G, Schafer BW. 2002. S100 proteins: structure, functions and pathology. Front. Biosci. 7:d1356–d1368 [DOI] [PubMed] [Google Scholar]
- 21. Madsen P, Rasmussen HH, Leffers H, Honore B, Dejgaard K, Olsen E, Kiil J, Walbum E, Andersen AH, Basse B, Lauridsen JB, Ratz GP, Celis A, Vandekerckhove J, Celis JE. 1991. Molecular cloning, occurrence, and expression of a novel partially secreted protein psoriasin that is highly up-regulated in psoriatic skin. J. Investig. Dermatol. 97:701–712 [DOI] [PubMed] [Google Scholar]
- 22. Moubayed N, Weichenthal M, Harder J, Wandel E, Sticherling M, Gläser R. 2007. Psoriasin (S100A7) is significantly up-regulated in human epithelial skin tumours. J. Cancer Res. Clin. Oncol. 133:253–261 [DOI] [PubMed] [Google Scholar]
- 23. Michalek M, Gelhaus C, Hecht O, Podschun R, Schroder JM, Leippe M, Grotzinger J. 2009. The human antimicrobial protein psoriasin acts by permeabilization of bacterial membranes. Dev. Comp. Immunol. 33:740–746 [DOI] [PubMed] [Google Scholar]
- 24. Wolf R, Voscopoulos CJ, FitzGerald PC, Goldsmith P, Cataisson C, Gunsior M, Walz M, Ruzicka T, Yuspa SH. 2006. The mouse S100A15 ortholog parallels genomic organization, structure, gene expression, and protein-processing pattern of the human S100A7/A15 subfamily during epidermal maturation. J. Investig. Dermatol. 126:1600–1608 [DOI] [PubMed] [Google Scholar]
- 25. Kulski JK, Lim CP, Dunn DS, Bellgard M. 2003. Genomic and phylogenetic analysis of the S100A7 (Psoriasin) gene duplications within the region of the S100 gene cluster on human chromosome 1q21. J. Mol. Evol. 56:397–406 [DOI] [PubMed] [Google Scholar]
- 26. Garreis F, Gottschalt M, Schlorf T, Glaser R, Harder J, Worlitzsch D, Paulsen FP. 2011. Expression and regulation of antimicrobial peptide psoriasin (S100A7) at the ocular surface and in the lacrimal apparatus. Invest. Ophthalmol. Vis. Sci. 52:4914–4922 [DOI] [PubMed] [Google Scholar]
- 27. West NR, Watson PH. 2010. S100A7 (psoriasin) is induced by the proinflammatory cytokines oncostatin-M and interleukin-6 in human breast cancer. Oncogene 29:2083–2092 [DOI] [PubMed] [Google Scholar]
- 28. Andresen E, Lange C, Strodthoff D, Goldmann T, Fischer N, Sahly H, Branscheid D, Heine H. 2011. S100A7/psoriasin expression in the human lung: unchanged in patients with COPD, but upregulated upon positive S. aureus detection. BMC Pulm. Med. 11:10 doi:10.1186/1471-2466-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Erhart W, Alkasi O, Brunke G, Wegener F, Maass N, Arnold N, Arlt A, Meinhold-Heerlein I. 2011. Induction of human beta-defensins and psoriasin in vulvovaginal human papillomavirus-associated lesions. J. Infect. Dis. 204:391–399 [DOI] [PubMed] [Google Scholar]
- 30. Ho L, Gineste C, Pompl PN, Dang A, Schall M, Pasinetti GM. 2002. Expression of psoriasin and cystatin c in the csf of early Alzheimer's disease dementia cases correlates with abeta1-40 content and severity of dementia. Abstr. Soc. Neurosci., abstr 686.682. [Google Scholar]
- 31. Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ, Lotze MT. 2009. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J. Transl. Med. 7:17 doi:10.1186/1479-5876-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shubbar E, Vegfors J, Carlstrom M, Petersson S, Enerback C. 2012. Psoriasin (S100A7) increases the expression of ROS and VEGF and acts through RAGE to promote endothelial cell proliferation. Breast Cancer Res. Treat. 134:71–80 [DOI] [PubMed] [Google Scholar]
- 33. Yan SS, Wu ZY, Zhang HP, Furtado G, Chen X, Yan SF, Schmidt AM, Brown C, Stern A, LaFaille J, Chess L, Stern DM, Jiang H. 2003. Suppression of experimental autoimmune encephalomyelitis by selective blockade of encephalitogenic T-cell infiltration of the central nervous system. Nat. Med. 9:287–293 [DOI] [PubMed] [Google Scholar]
- 34. Brandenburg LO, Varoga D, Nicolaeva N, Leib SL, Podschun R, Wruck CJ, Wilms H, Lucius R, Pufe T. 2009. Expression and regulation of antimicrobial peptide rCRAMP after bacterial infection in primary rat meningeal cells. J. Neuroimmunol. 217:55–64 [DOI] [PubMed] [Google Scholar]
- 35. De Miranda J, Yaddanapudi K, Hornig M, Lipkin WI. 2009. Astrocytes recognize intracellular polyinosinic-polycytidylic acid via MDA-5. FASEB J. 23:1064–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCarthy KD, Devellis J. 1980. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 85:890–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Slowik A, Merres J, Elfgen A, Jansen S, Mohr F, Wruck C, Pufe T, Brandenburg LO. 2012. Involvement of formyl peptide receptors in receptor for advanced glycation end products (RAGE)– and amyloid beta 1-42-induced signal transduction in glial cells. Mol. Neurodeg. 7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brandenburg LO, Konrad M, Wruck CJ, Koch T, Lucius R, Pufe T. 2010. Functional and physical interactions between formyl-peptide-receptors and scavenger receptor MARCO and their involvement in amyloid beta 1-42-induced signal transduction in glial cells. J. Neurochem. 113:749–760 [DOI] [PubMed] [Google Scholar]
- 39. Bianchi R, Giambanco I, Donato R. 2010. S100B/RAGE-dependent activation of microglia via NF-κB and AP-1: co-regulation of COX-2 expression by S100B, IL-1β and TNF-α. Neurobiol. Aging 31:665–677 [DOI] [PubMed] [Google Scholar]
- 40. Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45 doi:10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Campbell DG, Gagnon J, Reid KBM, Williams AF. 1981. Rat-brain Thy-1 glycoprotein. The amino acid sequence, disulphide bonds and an unusual hydrophobic region. Biochem. J. 195:15–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. 1996. A novel gene iba1in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem. Biophys. Res. Commun. 224:855–862 [DOI] [PubMed] [Google Scholar]
- 43. Bramanti V, Tomassoni D, Avitabile M, Amenta F, Avola R. 2010. Biomarkers of glial cell proliferation and differentiation in culture. Front. Biosci. (Schol Ed) 2:558–570 [DOI] [PubMed] [Google Scholar]
- 44. Easton DM, Nijnik A, Mayer ML, Hancock REW. 2009. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 27:582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang LF, Karin M. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37–40 [DOI] [PubMed] [Google Scholar]
- 46. Olah M, Biber K, Vinet J, Boddeke H. 2011. Microglia phenotype diversity. CNS Neurol. Disord. Drug Targets 10:108–118 [DOI] [PubMed] [Google Scholar]
- 47. Le YY, Hu JY, Gong WH, Shen WP, Li BQ, Dunlop NM, Halverson DO, Blair DG, Wang JM. 2000. Expression of functional formyl peptide receptors by human astrocytoma cell lines. J. Neuroimmunol. 111:102–108 [DOI] [PubMed] [Google Scholar]
- 48. Hao H-N, Zhao J, Lotoczky G, Grever WE, Lyman WD. 2001. Induction of human β-defensin-2 expression in human astrocytes by lipopolysaccharide and cytokines. J. Neurochem. 77:1027–1035 [DOI] [PubMed] [Google Scholar]
- 49. Williams WM, Castellani RJ, Weinberg A, Perry G, Smith MA. 2012. Do beta-defensins and other antimicrobial peptides play a role in neuroimmune function and neurodegeneration? ScientificWorldJournal 2012:905785 doi:10.1100/2012/905785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brandenburg LO, Jansen S, Wruck CJ, Lucius R, Pufe T. 2010. Antimicrobial peptide rCRAMP induced glial cell activation through P2Y receptor signalling pathways. Mol. Immunol. 47:1905–1913 [DOI] [PubMed] [Google Scholar]
- 51. Dong C, Davis RJ, Flavell RA. 2002. MAP kinases in the immune response. Annu. Rev. Immunol. 20:55–72 [DOI] [PubMed] [Google Scholar]
- 52. Zheng Y, Niyonsaba F, Ushio H, Ikeda S, Nagaoka I, Okumura K, Ogawa H. 2008. Microbicidal protein psoriasin is a multifunctional modulator of neutrophil activation. Immunology 124:357–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kreutzberg GW. 1996. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19:312–318 [DOI] [PubMed] [Google Scholar]
- 54. Block ML, Zecca L, Hong J-S. 2007. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 8:57–69 [DOI] [PubMed] [Google Scholar]
- 55. abd-el-Basset E, Fedoroff S. 1995. Effect of bacterial wall lipopolysaccharide (LPS) on morphology, motility, and cytoskeletal organization of microglia in cultures. J. Neurosci. Res. 41:222–237 [DOI] [PubMed] [Google Scholar]
- 56. Nakamura Y. 2002. Regulating factors for microglial activation. Biol. Pharm. Bull. 25:945–953 [DOI] [PubMed] [Google Scholar]
- 57. Xie J, Reverdatto S, Frolov A, Hoffmann R, Burz DS, Shekhtman A. 2008. Structural basis for pattern recognition by the receptor for advanced glycation end products (RAGE). J. Biol. Chem. 283:27255–27269 [DOI] [PubMed] [Google Scholar]
- 58. Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. 2005. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 15:16R–28R [DOI] [PubMed] [Google Scholar]
- 59. Yan SF, Ramasamy R, Schmidt AM. 2009. Receptor for AGE (RAGE) and its ligands—cast into leading roles in diabetes and the inflammatory response. J. Mol. Med. (Berl) 87:235–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.