Abstract
Trichomonas vaginalis is an extracellular protozoan parasite that binds to the epithelium of the human urogenital tract during infection. In this study, we examined the propensities of 26 T. vaginalis strains to bind to and lyse prostate (BPH-1) and ectocervical (Ect1) epithelium and to lyse red blood cells (RBCs). We found that only three of the strains had a statistically significant preference for either BPH-1 (MSA1103) or Ect1 (LA1 and MSA1123). Overall, we observed that levels of adherence are highly variable among strains, with a 12-fold range of adherence on Ect1 cells and a 45-fold range on BPH-1 cells. Cytolysis levels displayed even greater variability, from no detectable cytolysis to 80% or 90% cytolysis of Ect1 and BPH-1, respectively. Levels of adherence and cytolysis correlate for weakly adherent/cytolytic strains, and a threshold of attachment was found to be necessary to trigger cytolysis; however, this threshold can be reached without inducing cytolysis. Furthermore, cytolysis was completely blocked when we prevented attachment of the parasites to host cells while allowing soluble factors complete access. We demonstrate that hemolysis was a rare trait, with only 4 of the 26 strains capable of lysing >20% RBCs with a 1:30 parasite/RBC ratio. Hemolysis also did not correlate with adherence to or cytolysis of either male (BPH-1)- or female (Ect1)-derived epithelial cell lines. Our results reveal that despite a broad range of pathogenic properties among different T. vaginalis strains, all strains show strict contact-dependent cytolysis.
INTRODUCTION
The exclusively human-infective flagellated parasitic protist Trichomonas vaginalis is the cause of the most common nonviral sexually transmitted disease, trichomoniasis. In women, the parasite resides in the vagina and colonizes the cervix; in men, it can be found in the urogenital tract and the prostate. In 2008 alone there were an estimated 276.4 million new cases in adults between 19 and 49 years of age, with a 10-fold-higher prevalence in females than in males (1). T. vaginalis in males often goes undetected, and thus the incidence is difficult to determine. However, it has been reported that in men with infected female partners, the incidence can range from 15 to 73% (2, 3, 4, 5). Additionally, men can clear infections 3 to 12 times faster than women whether the infection is treated or not (6, 7). The percentage of men who reportedly remain asymptomatic upon T. vaginalis infection is highly variable depending on the population examined and the diagnostic technique utilized. Regardless, it is thought that a higher proportion of men than women have asymptomatic infections (4, 8, 9).
T. vaginalis infection can cause symptoms ranging from irritation and swelling in the urogenital tract to severe complications such as cervical erosion and premature birth during pregnancy (10, 11, 12). Trichomoniasis is also associated with infertility in both men and women (13, 14). Moreover, infections are linked to cervical cancer (15, 16, 17, 18), benign prostate hyperplasia (19), advanced prostate cancer (20, 21, 22), and an increased risk of HIV infection (23, 24, 25, 26, 27). The increased recognition of the severe risks possible from a T. vaginalis infection along with reports of drug-resistant infections (28, 29, 30) underscores the need for a more complete understanding of host-pathogen interactions with a focus on differences between male and female infections.
Despite the severe consequences that can arise from trichomoniasis, the mechanisms underlying the establishment and propagation of an infection are poorly understood, even less so in the male than in the female host. T. vaginalis is an extracellular parasite that does not become internalized by the host cell. Instead, it adheres to host urogenital epithelium (31). Currently, only three T. vaginalis surface molecules have been shown to play a role in attachment of the parasite to host epithelial cells: an abundant lipoglycan called TvLG (32, 33, 34) and two related hypothetical proteins (35). Several other candidate proteins are also under investigation (36, 37). The only host cell receptor described for T. vaginalis is galectin-1, and it has been shown to bind to TvLG (32). Studies on galectin-1 indicate the presence of additional, yet-to-be-defined, host cell receptors.
So far, studies conducted using various mammalian cell lines aimed at addressing whether the cytolysis of the host cell by T. vaginalis is contact dependent have been inconclusive (38, 39, 40, 41, 42). Here we have selected 26 individual strains of T. vaginalis isolated from an array of geographic locations to examine the adherence to and cytolysis of host cells and to assess whether these phenotypes are correlative and whether cytolysis is contact dependent. We have used an immortalized ectocervical epithelial cell line (Ect1) (43) and a benign prostate hyperplasia cell line (BPH-1) (44) to study parasite interactions with cells derived from both the female and male urogenital tracts. The ability of T. vaginalis to lyse human red blood cells (RBCs), a property for which there are also conflicting data (6, 39, 40, 45), was also examined.
MATERIALS AND METHODS
Parasites, cell cultures, and media.
T. vaginalis strains (Table 1) were grown in TYM medium supplemented with 10% horse serum (Sigma), 10 U/ml penicillin (Invitrogen), 10 μg/ml streptomycin (Invitrogen), 180 μM ferrous ammonium sulfate, and 28 μM sulfosalicylic acid (46). Overnight cultures of parasites were placed on ice for 10 min and then vortexed for 15 to 30 s before daily passaging at a dilution of 1 × 106 cells into 15 ml fresh medium. While these experiments were conducted, parasites were not passaged for more than 2 weeks before new samples were thawed. Of the 26 strains examined, 3 were passaged long term (years) (T1, G3, and B7RC2) and 10 were passed for short periods of time (weeks) in culture (the 8 MSA strains, 2 LSU strains, the 2 SD strains, B268, and LA-1) before we obtained, expanded, and froze samples. The passage history of the remaining strains is unavailable. The loss of cytolytic properties of strains cultured long term in the laboratory may be a result of adaptation to culture. The human ectocervical cell line Ect1 E6/E7 (CRL 2614) was grown in keratinocyte-SFM (KSFM) (Invitrogen) as described previously (43). Ect1 cells were shown to have a similar cytokeratin profile and to be morphologically similar to the tissue of origin. They differ significantly from HeLa cells, an adenocarcinoma cervical cell line and the cell line most commonly used for study of lower genital tract cells. The human benign prostate hyperplasia (BPH-1) cells were cultured in RPMI–10 U/ml penicillin–10 μg/ml streptomycin with 10% fetal calf serum (all from Invitrogen) as previously described (44). BPH-1 cells were derived from transurethral resection and immortalized with simian virus 40 (SV40) large T antigen. Their cytokeratin profile was similar to that of the primary prostate epithelial cells from which they are derived.
Table 1.
Strain identification and year and location of isolation
| Strain | ATCC no. | Location | Yr | Reference or source |
|---|---|---|---|---|
| B7268 | Brisbane, Australia | 1992 | 69 | |
| B7RC2 | 50167 | Greenville, NC, USA | 1986 | 70 |
| CDC337 | 50144 | Columbus, OH, USA | 1983 | 71 |
| CDC570 | Columbus, OH, USA | 1983 | —a | |
| G3 | PRA98 | Beckenham, UK | 1973 | 72 |
| HsD:NIH | 50183 | Maryland, USA | 1964 | L. S. Diamond, depositor |
| LA1 | Los Angeles, CA, USA | 2009 | 73 | |
| LSU160 | New Orleans, LA, USA | 2008 | — | |
| LSU2048 | New Orleans, LA, USA | 2008 | — | |
| MSA881 | Toronto, Canada | 2000 | — | |
| MSA1042 | Augusta, GA, USA | 2005 | — | |
| MSA1043 | New Orleans, LA, USA | 2005 | — | |
| MSA1103 | St. Paul, MN, USA | 2004 | — | |
| MSA1106 | Columbus, OH, USA | 2004 | — | |
| MSA1123 | Richmond, VA, USA | 2008 | — | |
| MSA1124 | Rock Hill, SC, USA | 2008 | — | |
| MSA1132 | Mt. Dora, FL, USA | 2008 | — | |
| NYH209 | 50146 | New York, NY, USA | 1977 | 74 |
| NYH272 | 50147 | New York, NY, USA | 1977 | 74 |
| NYH286 | 50148 | New York, NY, USA | 1977 | 74 |
| RU382 | 50141 | Connecticut, USA | 1983 | 75 |
| RU384 | 50140 | Massachusetts, USA | 1983 | 75 |
| RU393 | 50142 | New York, USA | 1983 | 75 |
| SD7 | San Diego, CA, USA | 1999 | 35 | |
| SD10 | San Diego, CA, USA | 1999 | 35 | |
| T1 | Taiwan | 1993 | 76 |
—, available through the Centers for Disease Control and Prevention, Atlanta, GA.
Adherence assay.
A modified version of a T. vaginalis cell binding assay was carried out (34). Briefly, Ect1 or BPH-1 cells were seeded on 12-mm coverslips in 24-well plates in appropriate culture medium and grown to confluence at 37°C and 5% CO2. T. vaginalis parasites were labeled with 10 μM Cell Tracker Blue CMAC (C2110; Invitrogen), and 5 × 104 labeled parasites in 0.5 ml of complete KSFM or RMPI were added to confluent Ect1 or BPH-1 cells. Cells were incubated together at 37°C and 5% CO2 for 30 min. Coverslips were subsequently washed in phosphate-buffered saline (PBS), fixed with 4% formaldehyde, and mounted on slides using Mowiol polyvinyl alcohol medium to prevent fading (Calbiochem). Fifteen fields were recorded per coverslip (at a magnification of ×10), and each experiment was carried out in triplicate for a total of 135 fields per strain. Fluorescent parasites that adhered to host cells were quantified using ImageJ software version 1.44p. Data are expressed as the percentage of parasites that adhere to host cells relative to the total number of parasites added per coverslip. Final mean values and standard deviations for each parasite/cell line combination are listed in Table S1 in the supplemental material.
Cytolysis assay.
T. vaginalis cytotoxicity assays were performed as previously described (34). Briefly, Ect1 or BPH-1 cells were seeded in 96-well plates in culture medium and grown to confluence at 37°C and 5% CO2, and then 5 × 104 log-phase parasites in 100 μl of culture medium were added to confluent Ect1 or BPH-1 cells. Parasites and human cells were incubated together at 37°C and 5% CO2 for 4 h. Ect1 and BPH-1 cell lysis was measured by determining lactate dehydrogenase (LDH) release using the CytoTox-One homogeneous membrane integrity assay per the manufacturer's instructions (Promega). Data were read on a Victor3 1420 plate reader (Perkin-Elmer). Data were normalized to background (LDH release from unexposed Ect1 or BPH-1 cells) and then expressed as a percentage of total lysis (LDH release after 0.18% Triton X-100 treatment). All experiments were repeated at least three times with triplicate samples. Final mean values and standard deviations are listed in Table S1 in the supplemental material.
Contact-dependent cytolysis.
To test for noncontact cytolysis of the epithelial cells by parasites, the Millicell-96 plate with a 0.4-μm porous membrane (PSHT004S5; Millipore) was used. This system consists of a 96-well culture plate (apical assist plate) that has a 0.4-μm porous membrane bottom and a 96-well receiver plate. Wells from both plates fit into each other and are separated by the membrane. The epithelial cells are seeded in the receiver plate, and the parasites are put in the culture plate. Ect1 or BPH-1 cells seeded in the 96-well receiver plate were grown to confluence. Parasites (5 × 104 in 75 μl of culture medium) were added to the apical assist plate. Observation by light microscopy confirmed that T. vaginalis cells (10 to 15 μm in diameter) did not transverse the membrane (data not shown). Plates were incubated at 37°C and 5% CO2 for 4 h. Ect1 and BPH-1 cell lysis was measured by determining LDH release as described above for the cytolysis assay. Strain MSA1132 was used as an internal control by placing the same number of parasites underneath the membrane in direct contact with the monolayer [MSA1132(*)]. All experiments were repeated at least three times in triplicate. Final mean values and standard deviations are listed in Table S1 in the supplemental material.
RBC lysis (hemolysis).
Overnight cultures of log-phase parasites were placed on ice for 10 min and vortexed for 15 to 30 s before being counted on a hemocytometer. A total of 3 × 106 cells per sample, at a concentration of 2 × 106 cells/ml, were centrifuged for 10 min at 2,000 × g and washed once in 15 mM maltose–PBS, pH 7.0 (M-PBS). Red blood cells (RBCs) were obtained from 4 donors provided by the Center for AIDS Research Core Virology laboratory at UCLA. The Research Core works with anonymous donors, and the blood group is not revealed. RBCs were separated from whole blood by washing 2 ml of freshly drawn blood in M-PBS and centrifuging for 5 min at 1,000 × g. Hemolysis was performed by combining 3 × 106 T. vaginalis cells with 9 × 107 RBCs (1:30 ratio) in 1.5 ml and incubating at 37°C with constant rotation for 1 h. Data were normalized to background (RBCs unexposed to parasites), and the values were calculated as the percentage of total lysis (RBCs treated with 0.1% Triton X-100). All experiments were repeated at least three times in triplicate. Absorbance was read using the Gen5 software on a Biotek Synergy2 plate reader at 404, 414, 540, 546, and 575 nm. The data for 404 nm are presented. Final mean values and standard deviations for individual parasite strains are listed in Table S1 in the supplemental material.
Fluorescence of parasite and host cells using a modified contact-dependent cytolysis assay.
Immunofluorescence experiments were performed on either Ect1 or BPH-1 cells plated and grown to confluence on coverslips in a 24-well plate. To label the monolayers, cells were incubated with 10 μM Cell Tracker Red CMTPX (C34552; Invitrogen) for 30 min in growth medium. Cells were washed three times with PBS, and fresh medium was added and left for 30 min. In parallel, 2 × 106 parasites from log-phase cultures were incubated with 10 μM Cell Tracker Blue CMAC for 30 min in TYM without serum. The parasites were allowed to recover in TYM complete growth medium for 30 min, after which they were transferred to the epithelial cell medium, counted on a hemocytometer, and adjusted to 1 × 106 cells/ml. Parasites (5 × 105 in 500 μl) were added to each sample, either directly on the monolayer or separated by a 0.4-μm porous membrane (catalog number 140620; Nunc), and left for 4 h as described above for the 96-well plate assay. The coverslips were then transferred into 4% formaldehyde for 15 min, washed twice in PBS and once in distilled water (dH2O), and mounted in Mowiol (Calbiochem). Fluorescent images were captured using a Zeiss Axio Imager.Z1 microscope with a 40× objective. All images were captured and analyzed using Axio Vision software.
Statistical analysis of adherence, cytolysis, and hemolysis correlations.
To estimate the degree of cross-correlation, we used a Pearson rank correlation coefficient, r:
The Pearson rank order correlation coefficient was applied to measure the correlation between attachment and cytolysis as well as that between cytolysis and hemolysis. The significance was calculated for a two-tailed distribution (47). The strains were ranked independently by adherence, cytolysis, and hemolysis (see Table S2 in the supplemental material).
RESULTS
T. vaginalis exhibits wide variability in attachment to ectocervical and prostate epithelial cells.
For extracellular pathogens such as T. vaginalis, the ability of a parasite to adhere to its host is likely a determinant of pathogenesis. To examine the capacities of different T. vaginalis strains to adhere to host cells as well as to compare differences in attachment of T. vaginalis to epithelial cells derived from female and male urogenital tracts, we studied the attachment of 26 T. vaginalis strains to monolayers of ectocervical cells (Ect1) and benign prostate hyperplasia cells (BPH-1). The same number of parasites (5 × 104) was used for each strain, and the percentage of parasites attached to the human cell lines was determined after incubation at 37°C for 30 min and washing to remove unattached parasites. Attachment to Ect1 cells was highly strain dependent, with a 12-fold difference between the most weakly attaching strain, T1 (4% attached parasites), and the most strongly attaching strain, MSA1132 (50% attached parasites) (Fig. 1A and Fig. 2A, white bars). Of the 26 strains, 13 attached at low levels (<15% attachment), while the other 13 strains attached at higher levels (>15%) (Fig. 2A, white bars). While the strains showed a much broader range in attachment to BPH-1 cells than to Ect1 cells, with a 45-fold difference between the attachment of strains T1 (1.2% attached parasites) and SD7 (54% attached parasites) (Fig. 2A, gray bars), the range was comparable overall. Moreover, similar to the attachment to Ect1 cells, out of the 26 strains tested, 17 attached at low levels (<15%) and 9 attached at higher levels (>15%) to BPH-1 cells (Fig. 2A, gray bars). Only two strains showed preferential attachment to one cell type over the other; strain LA1 bound 3-fold more to Ect1 than to BPH-1 (P < 1 × 10−5), and MSA1103 bound 3-fold more to BPH-1 cells (P < 1 × 10−5).
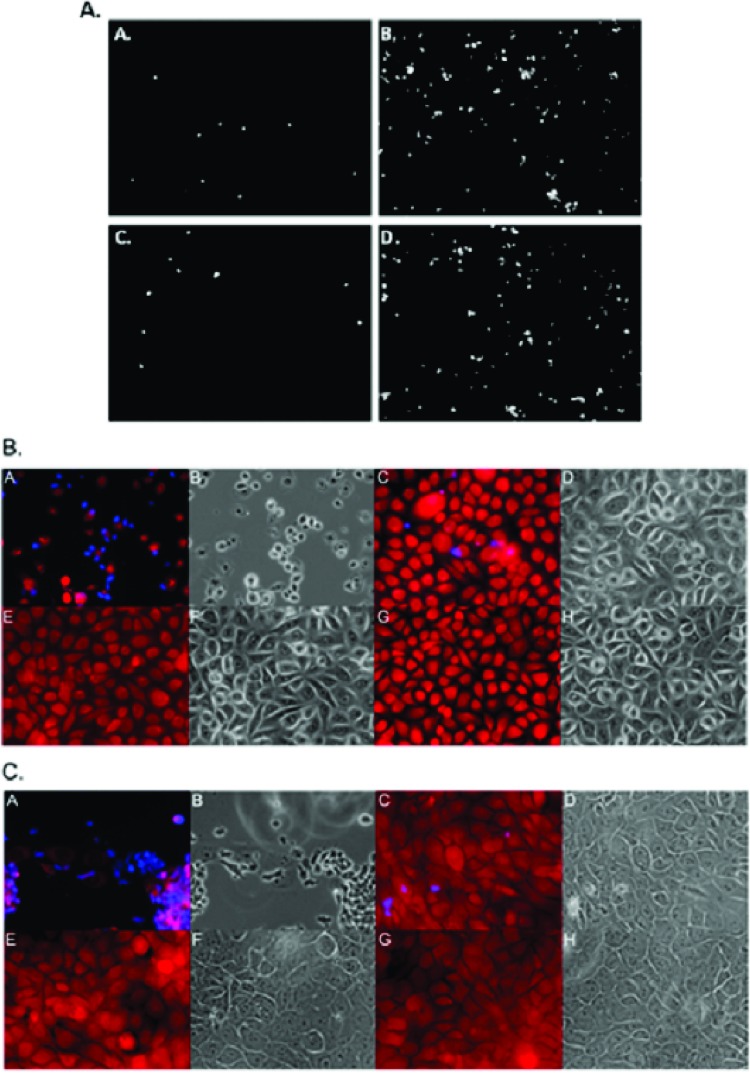
Fig 1.
Visualization of T. vaginalis adherence to and cytolysis of human ectocervical (Ect1) or benign hyperplasia prostate (BHP-1) cell monolayers. (A) Representative adherence fields of T. vaginalis strain T1 (panels A and C) or MSA1132 (panels B and D) parasites labeled with Cell Tracker Blue to monolayers of Ect1 (panels A and B) and BPH-1 (panels C and D) cells. Images were captured with a 10× objective. (B) Cell Tracker Blue-labeled MSA1132 (panels A, B, E, and F) or T1 (panels C, D, G, and H) parasites were exposed to Cell Tracker Red-labeled Ect1 monolayers (panels A to H) either directly (panels A to D) or with a 0.4-μm porous membrane barrier between them (panels E to H) for 4 h. (C) Cell Tracker Blue-labeled MSA1132 (panels A, B, E, and F) or T1 (panels C, D, G, and H) parasites were exposed to Cell Tracker Red-labeled BPH-1 (panels A to H) monolayers either directly (panels A to D) or with a 0.4-μm porous membrane barrier between them (panels E to H) for 4 h. All fluorescent images are of the red (epithelial cells) and blue (parasites) images merged; all the phase panels are the same field as the fluorescent image to the left. All images were taken with a 40× objective.
Fig 2.
Adherence to and cytolysis of human Ect-1 or BPH-1 cell monolayers by T. vaginalis. (A) Adherence of different strains of T. vaginalis to either Ect1 (white bars) or BPH-1 (gray bars) cells. Data are from three experiments performed in triplicate and show the average percentage of parasites attached per coverslip with standard deviation. Data are arranged in order of ability to attach to Ect1, and this order is maintained throughout the report. (B) Cytolysis of Ect1 (white bars) and BPH-1 (gray bars) cells by different T. vaginalis strains. Parasites were exposed to monolayers of the epithelial cells, and cytolysis was measured by determining LDH release. Data are from three experiments performed in triplicate, showing the average percentage of lysis with standard deviation. Lysis with Triton X-100 (+C) was used as a positive control for complete monolayer lysis, and the data set was fit from 0 to 100%.
Cytolysis of human epithelial cells by T. vaginalis.
We next measured T. vaginalis-mediated cytolysis of Ect1 and BPH-1 cells. Each of the 26 strains was added to monolayers of Ect1 or BPH-1 cells, and cytolysis was measured by LDH release (Fig. 2B). For 8 of the strains assayed on Ect1 (Fig. 2B, white bars), the LDH levels detected were below background levels (i.e., incubation of host cells under the same conditions but without the addition of parasites). This most likely results from very low or absent cytolysis, combined with the ability of T. vaginalis to scavenge and ingest proteins and other metabolites from the surrounding medium (48, 49, 50, 51), resulting in a lower LDH readout. A similar phenomenon of LDH values below background was observed in several previous studies (52, 53).
As seen for attachment, cytolysis was strain dependent, with values ranging from 1.5 to 78% of Ect1 cells and 1 to 96% of BPH-1 cells being lysed. In both target cell lines, 14 parasite strains caused low cytolysis (<20%) and 12 strains resulted in >20% lysis of Ect1 and BPH-1 cells (Fig. 1B and C). Overall, 10 out of 13 and 13 out of 17 of the weakly attaching strains exhibited low levels of cytolysis in Ect1 and BPH-1, respectively (compare Fig. 2A and B). Only two strains showed statistically significant preferential cytolysis of Ect1 cells over BPH-1 cells. MSA1123 lysed 3.6-fold more Ect1 than BPH-1 cells (P < 1 × 10−12), and LA1 lysed 4-fold more Ect1 cells (P < 1 × 10−10). In contrast, highly attaching strains did not necessarily lyse either cell type at a high level.
Relationship between adhesion and cytolysis of T. vaginalis.
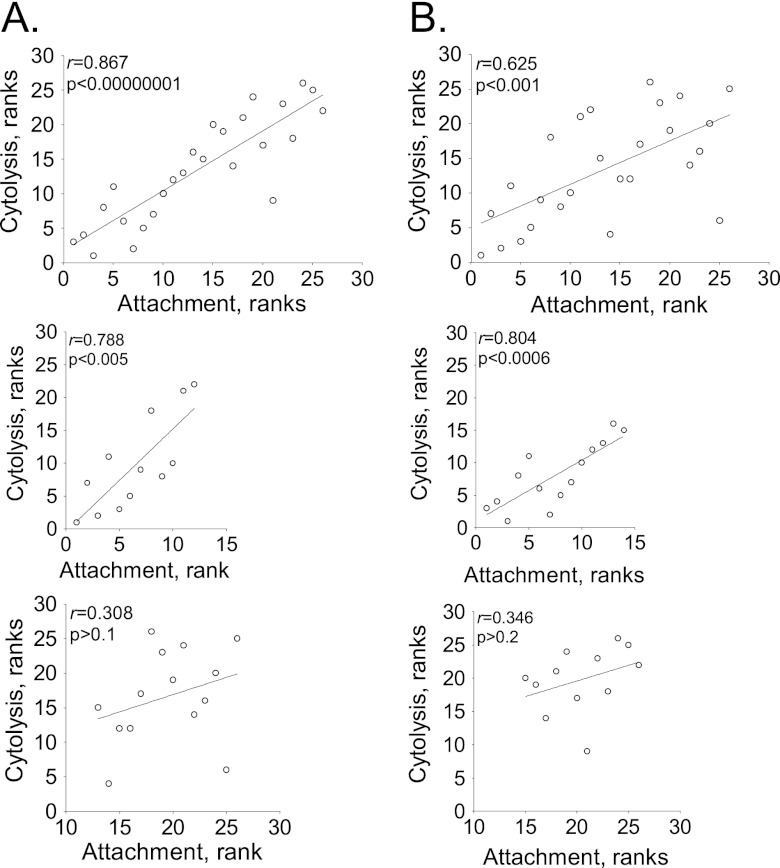
To verify the observed correlations between adhesion and cytolysis, we subjected the data to statistical analysis. We converted absolute values of adhesion and cytolysis to ranks to avoid excessive skewing by outliers (54). The strains were ranked in terms of cytotoxicity or adhesion from 1 to 26, and the Pearson correlation coefficient was calculated (Fig. 3A and B, top plots). We observed that attachment of T. vaginalis to Ect1 and BPH-1 correlates significantly with cytolysis (P < 0.001). Interestingly, when we divide the strains into low- and high-attaching groups for both cell lines, a different pattern emerges. Only the low-attaching strains show a very strong, significant correlation between attachment and cytolysis (Fig. 3A and B, middle plots). The high-attaching strains show a nonsignificant correlation (P > 0.1 for Ect1 cells and P > 0.5 for BPH-1 cells) (Fig. 3A and B, bottom plots). These data indicate that for cytolysis to be triggered, there needs to be a threshold level of attachment, but once that is achieved, the extent of cytolysis is not determined by the attachment.
Fig 3.
Correlation of attachment and cytolysis. The T. vaginalis strains were ordered according to increasing attachment and independently for increasing cytolysis, and ranked values were plotted for Ect-1 (A) and BHP-1 (B). The top scatter plot shows all strains assayed. Strains with low and high adherence were independently examined in the middle and bottom scatter plots, respectively. Lines are the best-fit trendlines. The Pearson coefficient (r) and the significance value (p) are on the upper left of each plot.
Cytolysis of Ect1 and BPH-1 cells by T. vaginalis is strictly contact dependent.
To establish whether the observed correlation between attachment and cytolysis in T. vaginalis is the result of the dependence of cytolysis on attachment, we exposed Ect1 and BPH-1 monolayers to the parasites using a 0.4-μm porous membrane to prevent direct contact of the parasites with the monolayers. This system allows for secreted factors and small vesicles to pass between the two cell types but keeps the parasites (∼10 to 15 μm in diameter) from contacting the monolayers. After incubation of the parasites and epithelial cells, cytolysis was measured by LDH release. None of the 26 strains, when separated by the membrane barrier from the monolayers, caused detectable cytolysis above background (Fig. 4). The inability of parasites to pass through the membrane was confirmed by fluorescent and light microscopy (Fig. 1B and C, panels A to H), and the monolayers were intact with no visible damage. These results indicate that T. vaginalis strictly uses a contact-dependent mechanism for cytolysis.
Fig 4.
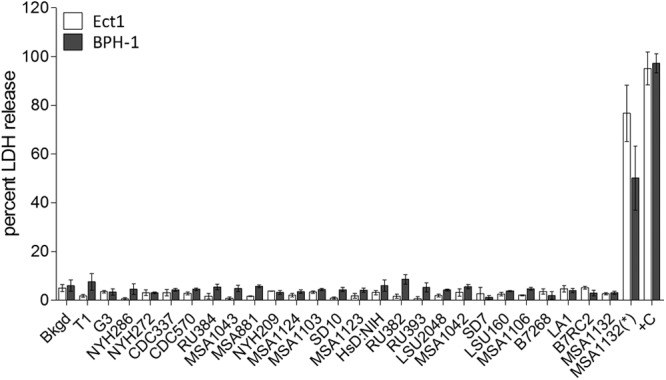
Contact-dependent cytolysis of Ect1 and BPH-1 cells by T. vaginalis, showing cytolysis of Ect1 and BPH-1 cells by T. vaginalis strains when contact was prevented by a 0.4-μm membrane. Parasites were exposed to monolayers of Ect1 (white bars) and BPH-1 (gray bars) cells with contact prevented by a 0.4-μm membrane. Cytolysis was measured by determining LDH release, and LDH background levels in the absence of parasites is shown (Bkgd). Data are from three experiments performed in triplicate, showing the average percentage of lysis with standard deviation. Complete lysis of the monolayer was achieved with Triton X-100 (+C), and the data set was fit from 0 to 100%. The control for direct monolayer lysis by T. vaginalis is shown as MSA1132(*), in which MSA1132 strain was placed under the membrane.
Hemolytic properties of T. vaginalis strains.
Previous work has shown that T. vaginalis is capable of lysing red blood cells (RBCs) (55, 56). To determine if RBC lysis is widespread among T. vaginalis strains, we examined whether there is a correlation between the abilities to lyse epithelial cells and RBCs. The 26 strains of T. vaginalis were exposed to RBCs at a T. vaginalis/RBC ratio of 1:30 for 1 h at 37°C. Contrary to what was observed for cytolysis of epithelial cells, the strains showed a narrower range of hemolysis, 0 to 40% (Fig. 5). Most of the strains (22 strains) lysed fewer than 20% (low), and the remaining 4 lysed 20 to 40% (high), of the RBCs. None of the strains were capable of lysing more than 40% of the RBCs. Analysis of all strains revealed no significant correlation between lyses of epithelial cells and RBCs (Fig. 6A and B). While lyses of RBCs and epithelial cells did not correlate, the cytolysis between ectocervical and prostate epithelial cells showed a strong correlation (P < 1 × 10−11) (Fig. 6C) as did the attachment between the two cell lines (Fig. 6C inset). These data suggest that hemolysis proceeds through mechanisms that differ from those that drive cytolysis. They also suggest that hemolysis does not play an important, generalized role in T. vaginalis nutrient acquisition, as it does not appear to be a widespread property.
Fig 5.
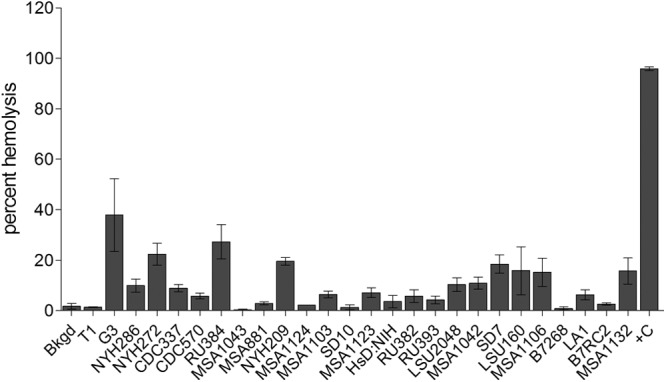
Hemolysis by T. vaginalis strains. Hemolysis was carried out for 1 h with a parasite/RBC ratio of 1:30 with constant rotation. Hemolysis was measured by hemoglobin release by absorbance at 404 nm. Data are from three experiments performed at least in triplicate, showing the average percentage of lysis with standard deviation. Complete RBC lysis was with Triton X-100, and the data set was fit from 0 to 100%.
Fig 6.
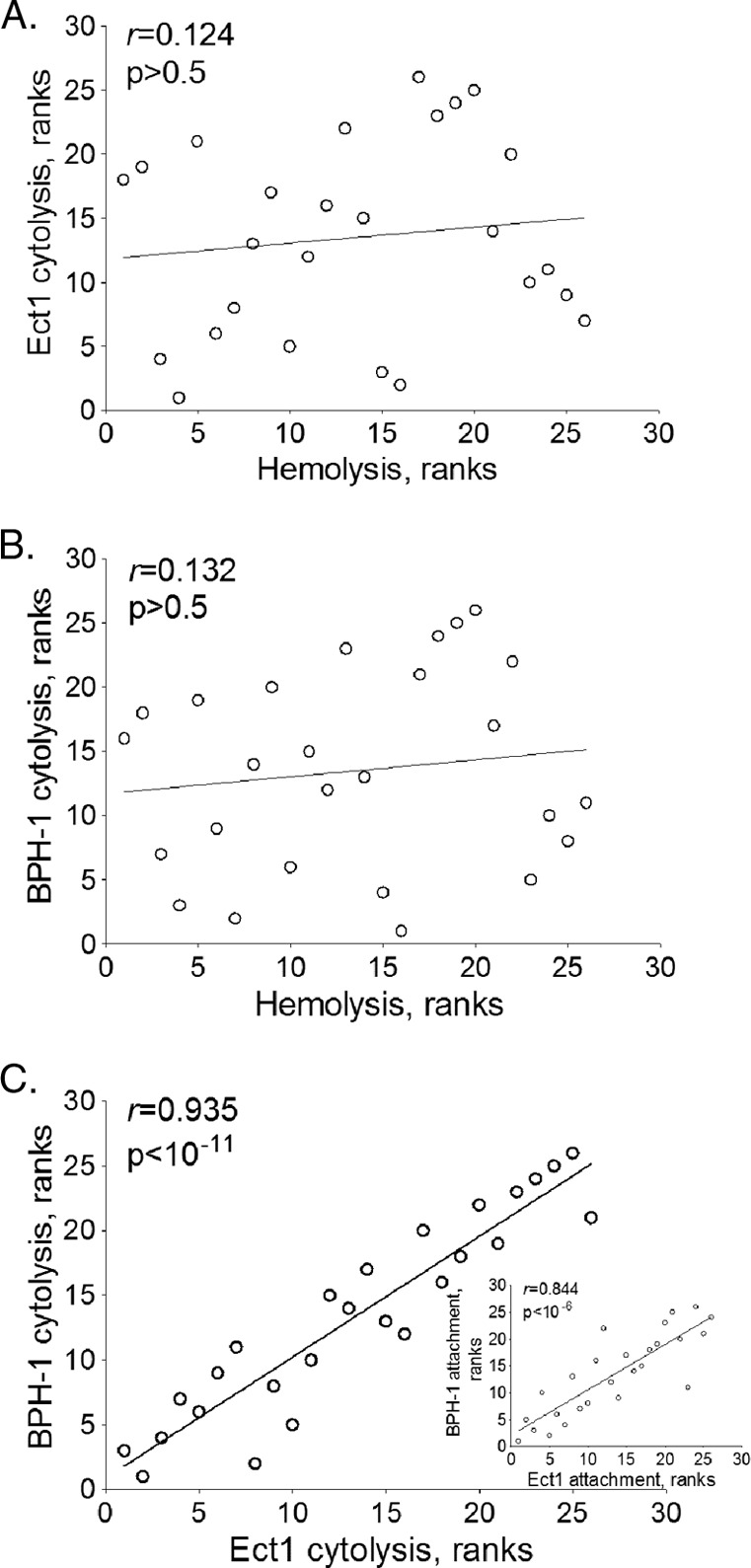
Correlation of hemolysis and cytolysis. The T. vaginalis strains were independently ordered according to increasing cytolysis and hemolysis (see Table S2 in the supplemental material). Ranked values were plotted for Ect-1 and hemolysis (A), BPH-1 and hemolysis (B), and Ect1 and BPH-1 cytolysis (C). The inset in panel C shows the correlation of Ect1 and BPH-1 attachment data. Lines are the best-fit trendlines. The Pearson coefficient (r) and the significance value (p) are in the upper left of each plot.
DISCUSSION
As an extracellular parasite, T. vaginalis binds to human host epithelial cells to establish and maintain an infection. The differences in binding abilities of T. vaginalis strains are therefore likely to play an important role in determining the outcome of an infection. Variations in the pathogenesis of T. vaginalis strains are well established (35, 56, 57, 58, 59, 60, 61).
To our knowledge, prior to this study, the adherence to and cytolysis of cells originating from the male urogenital tract by T. vaginalis had not been examined, notwithstanding that as early as 1936, Drummond reported that T. vaginalis can infect the prostate (62). Recent reports link trichomoniasis to prostate cancer (20, 21, 55, 63), benign hyperplastic prostatic tissue (19), and, at a high prevalence, male nongonococcal urethritis (NGU) (3).
We have compared the attachment and cytolytic properties of 26 T. vaginalis stains on an ectocervical cell line, Ect1, and a benign prostate cell line, BPH-1. A high degree of variability in attachment to and cytolysis of both cell lines was observed, with no general trend associating higher levels of attachment or cytolysis for either cell line. However, adherence to and cytolysis of both prostate and ectocervical cells by T. vaginalis strains were found to be highly correlated. Interestingly, adherence correlated with cytolysis in weakly adhering strains but not in highly adhering strains. This indicates that while attachment is necessary for T. vaginalis cytolysis, greater levels of attachment do not necessarily lead to higher levels of cytolysis. We also demonstrated that cytolysis is contact dependent for all 26 strains. Finally, we found that cytolysis of epithelial cells did not correlate with lysis of red blood cells.
As T. vaginalis is not internalized by the host cell and remains attached to its surface, the variation in attachment of different strains observed might be predicted to play a role in the variable documented infection outcomes (9, 20, 64). Overall, the strains do not exhibit a preference for binding to ectocervical cells over prostate cells. This suggests that attachment to the urogenital tract of men is not the limiting factor in the decreased pathogenesis observed in the male host. However, as the single epithelial cell monolayers used in our assays are far simpler than the complex milieu of cells in the urogenital tract, these data may not fully represent the adherence and cytolysis levels that occur in situ during an infection. Moreover, variations in adherence and cytolysis among strains indicate that different biological mechanisms may be employed by different T. vaginalis strains for interacting with different host epithelial cells. Alternatively, common mechanisms may be used and the proteins or surface molecules mediating these interactions would vary greatly, either in abundance or type, between strains.
The observation that adherence and cytolysis correlate for weakly adhering strains led us to examine whether cytolysis is contact dependent by placing a 0.4-μm filter between parasites and host cells. This prevented contact while allowing secreted proteins and vesicles to diffuse freely. We found no cytolysis when parasite attachment to host cells was blocked. Previous analyses aimed at determining whether T. vaginalis cytolysis is contact dependent have yielded mixed results (39, 40, 41, 42). Unlike the simple physical barrier used here, these studies used a variety of methods to prepare “cell-free filtrates” without controls to eliminate contamination with T. vaginalis plasma membrane debris. Our finding that 26/26 T. vaginalis strains required contact with host cells to induce cytolysis strongly argues this is a general property of this parasite.
T. vaginalis hemolytic activity has been widely reported (11, 56, 65); however, there are conflicting data on whether hemolysis correlates with virulence (45) or cytopathology (56). The levels of hemolysis reported also vary, which may be in part due to differences in methods and conditions used for measuring hemolysis. Analyses of the 26 strains studied here revealed that only 4 are capable of lysing >20% of RBCs and that the majority of strains (15) lyse fewer than 10% in assays using a 1:30 parasite/RBC ratio. Notably, 2 of the 3 more hemolytic strains exhibit low levels of adherence to and cytolysis of epithelial cells. Thus, no correlation between T. vaginalis adherence to and cytolysis of epithelial cells and its hemolytic activity was observed. This is in agreement with the observations of Krieger et al. (41) that the ability of T. vaginalis strains to cause hemolysis correlates poorly with virulence. Finding that so few strains are capable of lysing RBCs raises the question of whether T. vaginalis hemolysis is important in vivo. T. vaginalis allegedly lyses RBCs to gain nutrients and iron present during menses (40, 63). However, the nutrient-rich components of the menstrual flow are endometrial tissue, squamous epithelium debris, white blood cells, and carbohydrate-rich mucus, not RBCs (66, 67). Furthermore, menses happens only once a month and does not occur at all in postmenopausal women, who have a higher prevalence of T. vaginalis infection than younger women (68). Our results suggest that while some strains of T. vaginalis can lyse RBCs, this is not a common attribute.
In summary, we show that T. vaginalis cytolysis of human epithelial cells is contact dependent. We found that to initiate cytolysis, attachment must reach a threshold, but higher levels of attachment do not necessarily induce greater cytolysis. We conclude that while the trigger for cytolysis is attachment, cytolysis mechanisms are independent of attachment. Adherence is likely to serve as a trigger for activation and/or secretion of additional critical factors immediately upon contact. It will be of interest to determine whether differences in expressed proteins and/or surface lipoglycans correlate with levels of adherence and cytolysis. Similarly, identifying differences between the three strains that displayed gender preference in adherence and cytolysis may be a first step toward dissecting the causes of gender-specific outcomes of infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank our colleagues in the laboratory for helpful discussions and useful comments on the manuscript.
This work was supported by National Institutes of Health grant R01AI069058 (to P.J.J.) and by a postdoctoral fellowship from the American Cancer Society (to C.M.R.).
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 19 February 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01244-12.
REFERENCES
- 1. Rowley R, Toskin I, Ndowa F. 2012. Global incidence and prevalence of selected curable sexually transmitted infections—2008. 978 92 4 150383 9. WHO, Geneva, Switzerland [Google Scholar]
- 2. Borchardt KA, al-Haraci S, Maida N. 1995. Prevalence of Trichomonas vaginalis in a male sexually transmitted disease clinic population by interview, wet mount microscopy, and the InPouch TV test. Genitourin. Med. 71:405–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwebke JR, Hook EW., III 2003. High rates of Trichomonas vaginalis among men attending a sexually transmitted diseases clinic: implications for screening and urethritis management. J. Infect. Dis. 188:465–468 [DOI] [PubMed] [Google Scholar]
- 4. Sena AC, Miller WC, Hobbs MM, Schwebke JR, Leone PA, Swygard H, Atashili J, Cohen MS. 2007. Trichomonas vaginalis infection in male sexual partners: implications for diagnosis, treatment, and prevention. Clin. Infect. Dis. 44:13–22 [DOI] [PubMed] [Google Scholar]
- 5. Wendel KA, Erbelding EJ, Gaydos CA, Rompalo AM. 2003. Use of urine polymerase chain reaction to define the prevalence and clinical presentation of Trichomonas vaginalis in men attending an STD clinic. Sex. Transm. Infect. 79:151–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krieger JN, Verdon M, Siegel N, Holmes KK. 1993. Natural history of urogenital trichomoniasis in men. J. Urol. 149:1455–1458 [DOI] [PubMed] [Google Scholar]
- 7. Schmid GS, Rowley J. 2011. Prevalence and incidence of selected sexually transmitted infections, Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis: methods and results used by WHO to generate 2005 estimates. 978 92 4 150245 0. WHO, Geneva, Switzerland [Google Scholar]
- 8. Fouts AC, Kraus SJ. 1980. Trichomonas vaginalis: reevaluation of its clinical presentation and laboratory diagnosis. J. Infect. Dis. 141:137–143 [DOI] [PubMed] [Google Scholar]
- 9. Krieger JN. 1995. Trichomoniasis in men: old issues and new data. Sex. Transm. Dis. 22:83–96 [PubMed] [Google Scholar]
- 10. Cotch MF, Pastorek JG, II, Nugent RP, Hillier SL, Gibbs RS, Martin DH, Eschenbach DA, Edelman R, Carey JC, Regan JA, Krohn MA, Klebanoff MA, Rao AV, Rhoads GG. 1997. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex. Transm. Dis. 24:353–360 [DOI] [PubMed] [Google Scholar]
- 11. Petrin D, Delgaty K, Bhatt R, Garber G. 1998. Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 11:300–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soper D. 2004. Trichomoniasis: under control or undercontrolled? Am. J. Obstet. Gynecol. 190:281–290 [DOI] [PubMed] [Google Scholar]
- 13. Grodstein F, Goldman MB, Cramer DW. 1993. Relation of tubal infertility to history of sexually transmitted diseases. Am. J. Epidemiol. 137:577–584 [DOI] [PubMed] [Google Scholar]
- 14. Lloyd GL, Case JR, De Frias D, Brannigan RE. 2003. Trichomonas vaginalis orchitis with associated severe oligoasthenoteratospermia and hypogonadism. J. Urol. 170:924. [DOI] [PubMed] [Google Scholar]
- 15. Misra JS, Das K, Chandrawati 1998. Results of clinically downstaging cervical cancer in a cytological screening programme. Diagn. Cytopathol. 19:344–348 [DOI] [PubMed] [Google Scholar]
- 16. Sayed el-Ahl SA, el-Wakil HS, Kamel NM, Mahmoud MS. 2002. A preliminary study on the relationship between Trichomonas vaginalis and cervical cancer in Egyptian women. J. Egypt Soc. Parasitol. 32:167–178 [PubMed] [Google Scholar]
- 17. Zhang ZF, Begg CB. 1994. Is Trichomonas vaginalis a cause of cervical neoplasia? Results from a combined analysis of 24 studies. Int. J. Epidemiol. 23:682–690 [DOI] [PubMed] [Google Scholar]
- 18. Zhang ZF, Graham S, Yu SZ, Marshall J, Zielezny M, Chen YX, Sun M, Tang SL, Liao CS, Xu JL, et al. 1995. Trichomonas vaginalis and cervical cancer. A prospective study in China. Ann. Epidemiol. 5:325–332 [DOI] [PubMed] [Google Scholar]
- 19. Mitteregger D, Aberle SW, Makristathis A, Walochnik J, Brozek W, Marberger M, Kramer G. 2012. High detection rate of Trichomonas vaginalis in benign hyperplastic prostatic tissue. Med. Microbiol. Immunol. 201:113–116 [DOI] [PubMed] [Google Scholar]
- 20. Stark JR, Judson G, Alderete JF, Mundodi V, Kucknoor AS, Giovannucci EL, Platz EA, Sutcliffe S, Fall K, Kurth T, Ma J, Stampfer MJ, Mucci LA. 2009. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians' Health Study. J. Natl. Cancer Inst. 101:1406–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sutcliffe S, Alderete JF, Till C, Goodman PJ, Hsing AW, Zenilman JM, De Marzo AM, Platz EA. 2009. Trichomonosis and subsequent risk of prostate cancer in the Prostate Cancer Prevention Trial. Int. J. Cancer 124:2082–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sutcliffe S, Giovannucci E, Alderete JF, Chang TH, Gaydos CA, Zenilman JM, De Marzo AM, Willett WC, Platz EA. 2006. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev. 15:939–945 [DOI] [PubMed] [Google Scholar]
- 23. McClelland RS, Sangare L, Hassan WM, Lavreys L, Mandaliya K, Kiarie J, Ndinya-Achola J, Jaoko W, Baeten JM. 2007. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J. Infect. Dis. 195:698–702 [DOI] [PubMed] [Google Scholar]
- 24. Van Der Pol B, Kwok C, Pierre-Louis B, Rinaldi A, Salata RA, Chen PL, van de Wijgert J, Mmiro F, Mugerwa R, Chipato T, Morrison CS. 2008. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J. Infect. Dis. 197:548–554 [DOI] [PubMed] [Google Scholar]
- 25. Wasserheit JN. 1992. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex. Transm. Dis. 19:61–77 [PubMed] [Google Scholar]
- 26. Mason PR, Fiori PL, Cappuccinelli P, Rappelli P, Gregson S. 2005. Seroepidemiology of Trichomonas vaginalis in rural women in Zimbabwe and patterns of association with HIV infection. Epidemiol. Infect. 133:315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mavedzenge SN, Pol BV, Cheng H, Montgomery ET, Blanchard K, de Bruyn G, Ramjee G, Straten A. 2010. Epidemiological synergy of Trichomonas vaginalis and HIV in Zimbabwean and South African women. Sex. Transm. Dis. 37:460–466 [DOI] [PubMed] [Google Scholar]
- 28. Kulda J, Vojtechovska M, Tachezy J, Demes P, Kunzova E. 1982. Metronidazole resistance of Trichomonas vaginalis as a cause of treatment failure in trichomoniasis—a case report. Br. J. Vener. Dis. 58:394–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmid G, Narcisi E, Mosure D, Secor WE, Higgins J, Moreno H. 2001. Prevalence of metronidazole-resistant Trichomonas vaginalis in a gynecology clinic. J. Reprod. Med. 46:545–549 [PubMed] [Google Scholar]
- 30. Workowski KA, Berman SM. 2006. Sexually transmitted diseases treatment guidelines, 2006. MMWR. Recommend. Rep. 55:1–94 [PubMed] [Google Scholar]
- 31. Ryan CM, de Miguel N, Johnson PJ. 2011. Trichomonas vaginalis: current understanding of host-parasite interactions. Essays Biochem. 51:161–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okumura CY, Baum LG, Johnson PJ. 2008. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell. Microbiol. 10:2078–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ryan CM, Mehlert A, Richardson JM, Ferguson MA, Johnson PJ. 2011. Chemical structure of Trichomonas vaginalis surface lipoglycan: a role for short galactose (beta1-4/3) N-acetylglucosamine repeats in host cell interaction. J. Biol. Chem. 286:40494–40508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bastida-Corcuera FD, Okumura CY, Colocoussi A, Johnson PJ. 2005. Trichomonas vaginalis lipophosphoglycan mutants have reduced adherence and cytotoxicity to human ectocervical cells. Eukaryot. Cell 4:1951–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Miguel N, Lustig G, Twu O, Chattopadhyay A, Wohlschlegel JA, Johnson PJ. 2010. Proteome analysis of the surface of Trichomonas vaginalis reveals novel proteins and strain-dependent differential expression. Mol. Cell. Proteomics 9:1554–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hirt RP, de Miguel N, Nakjang S, Dessi D, Liu YC, Diaz N, Rappelli P, Acosta-Serrano A, Fiori PL, Mottram JC. 2011. Trichomonas vaginalis pathobiology new insights from the genome sequence. Adv. Parasitol. 77:87–140 [DOI] [PubMed] [Google Scholar]
- 37. Noel CJ, Diaz N, Sicheritz-Ponten T, Safarikova L, Tachezy J, Tang P, Fiori PL, Hirt RP. 2010. Trichomonas vaginalis vast BspA-like gene family: evidence for functional diversity from structural organisation and transcriptomics. BMC Genomics 11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Christian RT, Miller NF, Ludovici PP, Riley GM. 1963. A study of Trichomonas vaginalis in human cell culture. Am. J. Obstet. Gynecol. 85:947–954 [DOI] [PubMed] [Google Scholar]
- 39. Fiori PL, Rappelli P, Addis MF, Mannu F, Cappuccinelli P. 1997. Contact-dependent disruption of the host cell membrane skeleton induced by Trichomonas vaginalis. Infect. Immun. 65:5142–5148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fiori PL, Rappelli P, Addis MF, Sechi A, Cappuccinelli P. 1996. Trichomonas vaginalis haemolysis: pH regulates a contact-independent mechanism based on pore-forming proteins. Microb. Pathog. 20:109–118 [DOI] [PubMed] [Google Scholar]
- 41. Krieger JN, Ravdin JI, Rein MF. 1985. Contact-dependent cytopathogenic mechanisms of Trichomonas vaginalis. Infect. Immun. 50:778–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pindak FF, Mora de Pindak M, Gardner WA., Jr 1993. Contact-independent cytotoxicity of Trichomonas vaginalis. Genitourin. Med. 69:35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fichorova RN, Rheinwald JG, Anderson DJ. 1997. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. 57:847–855 [DOI] [PubMed] [Google Scholar]
- 44. Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P. 1995. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell. Dev. Biol. Animal 31:14–24 [DOI] [PubMed] [Google Scholar]
- 45. Krieger JN, Poisson MA, Rein MF. 1983. Beta-hemolytic activity of Trichomonas vaginalis correlates with virulence. Infect. Immun. 41:1291–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Clark CG, Diamond LS. 2002. Methods for cultivation of luminal parasitic protists of clinical importance. Clin. Microbiol. Rev. 15:329–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cohen J. 2003. Applied multiple regression/correlation analysis for the behavioral sciences, 3rd ed L. Erlbaum Associates, Mahwah, NJ [Google Scholar]
- 48. Harris DI, Beechey RB, Linstead D, Barrett J. 1988. Nucleoside uptake by Trichomonas vaginalis. Mol. Biochem. Parasitol. 29:105–116 [DOI] [PubMed] [Google Scholar]
- 49. Peterson KM, Alderete JF. 1984. Selective acquisition of plasma proteins by Trichomonas vaginalis and human lipoproteins as a growth requirement for this species. Mol. Biochem. Parasitol. 12:37–48 [DOI] [PubMed] [Google Scholar]
- 50. ter Kuile BH. 1994. Adaptation of the carbon metabolism of Trichomonas vaginalis to the nature and availability of the carbon source. Microbiology 140:2503–2510 [DOI] [PubMed] [Google Scholar]
- 51. Zuo X, Lockwood BC, Coombs GH. 1995. Uptake of amino acids by the parasitic, flagellated protist Trichomonas vaginalis. Microbiology 141:2637–2642 [DOI] [PubMed] [Google Scholar]
- 52. Bopp SK, Lettieri T. 2008. Comparison of four different colorimetric and fluorometric cytotoxicity assays in a zebrafish liver cell line. BMC Pharmacol. 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kendig DM, Tarloff JB. 2007. Inactivation of lactate dehydrogenase by several chemicals: implications for in vitro toxicology studies. Toxicol. In Vitro 21:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sigal A, Milo R, Cohen A, Geva-Zatorsky N, Klein Y, Liron Y, Rosenfeld N, Danon T, Perzov N, Alon U. 2006. Variability and memory of protein levels in human cells. Nature 444:643–646 [DOI] [PubMed] [Google Scholar]
- 55. Fiori PL, Rappelli P, Rocchigiani AM, Cappuccinelli P. 1993. Trichomonas vaginalis haemolysis: evidence of functional pores formation on red cell membranes. FEMS Microbiol. Lett. 109:13–18 [DOI] [PubMed] [Google Scholar]
- 56. Krieger JN, Wolner-Hanssen P, Stevens C, Holmes KK. 1990. Characteristics of Trichomonas vaginalis isolates from women with and without colpitis macularis. J. Infect. Dis. 161:307–311 [DOI] [PubMed] [Google Scholar]
- 57. Honigberg BM. 1961. Comparative pathogenicity of Trichomonas vaginalis and Trichomonas gallinae to mice. I. Gross pathology, quantitative evaluation of virulence, and some factors affecting pathogenicity. J. Parasitol. 47:545–571 [PubMed] [Google Scholar]
- 58. Kuczynska K, Choromanski L, Honigberg BM. 1984. Comparison of virulence of clones of two Trichomonas vaginalis strains by the subcutaneous mouse assay. Z Parasitenkd. 70:141–146 [DOI] [PubMed] [Google Scholar]
- 59. Reardon LV, Ashburn LL, Jacobs L. 1961. Differences in strains of Trichomonas vaginalis as revealed by intraperitoneal injections into mice. J. Parasitol. 47:527–532 [PubMed] [Google Scholar]
- 60. Warton A, Honigberg BM. 1980. Lectin analysis of surface saccharides in two Trichomonas vaginalis strains differing in pathogenicity. J. Protozool. 27:410–419 [DOI] [PubMed] [Google Scholar]
- 61. Warton A, Honigberg BM. 1983. Analysis of surface saccharides in Trichomonas vaginalis strains with various pathogenicity levels by fluorescein-conjugated plant lectins. Z Parasitenkd. 69:149–159 [DOI] [PubMed] [Google Scholar]
- 62. Drummond AC. 1936. Trichomonas infestation of the prostate gland. Am. J. Surg. 31:98–103 [Google Scholar]
- 63. Honigberg BM. 1990. Trichomonads parasitic in humans. Springer-Verlag, New York, NY [Google Scholar]
- 64. Swygard H, Sena AC, Hobbs MM, Cohen MS. 2004. Trichomoniasis: clinical manifestations, diagnosis and management. Sex. Transm. Infect. 80:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fiori PL, Rappelli P, Addis MF. 1999. The flagellated parasite Trichomonas vaginalis: new insights into cytopathogenicity mechanisms. Microbes Infect. 1:149–156 [DOI] [PubMed] [Google Scholar]
- 66. Tabibzadeh S. 1996. The signals and molecular pathways involved in human menstruation, a unique process of tissue destruction and remodelling. Mol. Hum. Reprod. 2:77–92 [DOI] [PubMed] [Google Scholar]
- 67. Wolf DP, Sokoloski JE, Litt M. 1980. Composition and function of human cervical mucus. Biochim. Biophys. Acta 630:545–558 [DOI] [PubMed] [Google Scholar]
- 68. Spinillo A, Bernuzzi AM, Cevini C, Gulminetti R, Luzi S, De Santolo A. 1997. The relationship of bacterial vaginosis, Candida and Trichomonas infection to symptomatic vaginitis in postmenopausal women attending a vaginitis clinic. Maturitas 27:253–260 [DOI] [PubMed] [Google Scholar]
- 69. Upcroft JA, Upcroft P. 2001. Drug susceptibility testing of anaerobic protozoa. Antimicrob. Agents Chemother. 45:1810–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hollander DH, Tysor JS. 1987. Isolation of a stable clone of the ameboid-adherent (AA) variant of Trichomonas vaginalis. J. Parasitol. 73:1074–1075 [PubMed] [Google Scholar]
- 71. Muller M, Lossick JG, Gorrell TE. 1988. In vitro susceptibility of Trichomonas vaginalis to metronidazole and treatment outcome in vaginal trichomoniasis. Sex. Transm. Dis. 15:17–24 [DOI] [PubMed] [Google Scholar]
- 72. Van den Bossche H. (ed). 1976. Biochemistry of parasites and host-parasite relationships: proceedings of the second International Symposium on the Biochemistry of Parasites and Host-Parasite Relationships, Beerse, Belgium, 28 June-1 July, 1976 North Holland Publishing Co., New York, NY [Google Scholar]
- 73. Goldman LM, Upcroft JA, Workowski K, Rapkin A. 2009. Treatment of metronidazole-resistant Trichomonas vaginalis. Sex. Health 6:345–347 [DOI] [PubMed] [Google Scholar]
- 74. Muller M, Meingassner JG, Miller WA, Ledger WJ. 1980. Three metronidazole-resistant strains of Trichomonas vaginalis from the United States. Am. J. Obstet. Gynecol. 138:808–812 [DOI] [PubMed] [Google Scholar]
- 75. Lossick JG, Muller M, Gorrell TE. 1986. In vitro drug susceptibility and doses of metronidazole required for cure in cases of refractory vaginal trichomoniasis. J. Infect. Dis. 153:948–955 [DOI] [PubMed] [Google Scholar]
- 76. Tai JH, Su HM, Tsai J, Shaio MF, Wang CC. 1993. The divergence of Trichomonas vaginalis virus RNAs among various isolates of Trichomonas vaginalis. Exp. Parasitol. 76:278–286 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.