Abstract
The laboratory mouse model of Lyme disease has revealed that Borrelia burgdorferi differentially expresses numerous outer surface proteins that influence different stages of infection (tick-borne transmission, tissue colonization, dissemination, persistence, and tick acquisition). Deletion of two such outer surface proteins, decorin-binding proteins A and B (DbpA/B), has been documented to decrease infectivity, impede early dissemination, and, possibly, prevent persistence. In this study, DbpA/B-deficient spirochetes were confirmed to exhibit an early dissemination defect in immunocompetent, but not immunodeficient, mice, and the defect was found to resolve with chronicity. Development of disease (arthritis and carditis) was attenuated only in the early stage of infection with DbpA/B-deficient spirochetes in both types of mice. Persistence of the DbpA/B-deficient spirochetes occurred in both immunocompetent and immunodeficient mice in a manner indistinguishable from that of wild-type spirochetes. Dissemination through the lymphatic system was evaluated as an underlying mechanism for the early dissemination defect. At 12 h, 3 days, 7 days, and 14 days postinoculation, DbpA/B-deficient spirochetes were significantly less prevalent and in lower numbers in lymph nodes than wild-type spirochetes. However, in immunodeficient mice, deficiency of DbpA/B did not significantly decrease the prevalence or spirochete numbers in lymph nodes. Complementation of DbpA/B restored a wild-type phenotype. Thus, the results indicated that deficiency of DbpA/B allows the acquired immune response to restrict early dissemination of spirochetes, which appears to be at least partially mediated through the lymphatic system.
INTRODUCTION
Borrelia burgdorferi, the etiologic agent of Lyme disease, utilizes a multitude of surface-exposed adhesins to bind to and interact with various components of the extracellular matrix in mammalian hosts. These adhesins include decorin-binding protein A (DbpA), DbpB, fibronectin-binding protein (Fbp), Borrelia glycosaminoglycan-binding protein (Bgp), RevA, Borrelia membrane proteins (Bmps), ErpX, and P66. Their respective ligands include decorin, fibronectin, various glycosaminoglycans, laminin, and αIIbβ3 integrin (1, 2, 3, 4, 5, 6, 7). This is by no means a completely inclusive list (8); for example, a yet-unidentified borrelial adhesion binds directly to native type I collagen (9), and thus far, ligands for BmpD and members of the OspF family have not been characterized (10). However, the interactions of adhesins and ligands, particularly DbpA and DbpB (DbpA/B) and decorin, appear to play an important role during all stages of infection.
DbpA and DbpB are encoded in a bicistronic operon (dbpBA) on plasmid lp54 of the prototype B. burgdorferi B31 strain (11) and were two of the first borrelial adhesins identified (6, 12, 13, 14). These 19-kDa and 20-kDa proteins, respectively, are encoded by and expressed within B. burgdorferi sensu stricto strains and also many B. burgdorferi sensu lato strains, albeit as heterogeneous homologs (12, 15, 16, 17). Expression is upregulated in the mammalian host after tick-borne infection (18), and DbpA and DbpB are highly antigenic during infection (14, 19, 20). Based on mRNA levels, DbpA and DbpB continue to be expressed throughout chronic infection (12, 14, 18, 19). In comparison to DbpB, DbpA has been established as the more crucial adhesin in the context of pathogenesis, eliciting stronger protective immunity (12, 14) and, on its own, restoring a wild-type phenotype to DbpA/B-deficient mutant B. burgdorferi (21, 22).
In the laboratory mouse model, DbpA and DbpB have been implicated in establishment of infection, dissemination, tissue colonization, persistence, and tick acquisition and transmission. Disruption of DbpA and DbpB, while nonessential to initial infection (23), will increase the infectious dose (21, 24, 25), decrease total spirochete tissue burdens (25), decrease recovery of spirochetes from tissues distant to the inoculation site (21, 23, 25), and decrease efficiency of tick acquisition and transmission (24). None of these cited studies addressed the influence of DbpA and DbpB disruption on disease development or persistence.
The early dissemination defect of DbpA/B-deficient mutants, represented by decreased recovery of spirochetes from tissues distant to the inoculation site (21, 23, 25), seems to be a key to understanding the role of decorin-binding proteins in Lyme borreliosis. With the genetic disruption or absence of these adhesins, spirochetes may be unable to travel by conventional routes or access important microenvironmental niches and thus manifest their altered dissemination phenotype. Although the extracellular matrix (ECM) is important in B. burgdorferi dissemination, as evidenced by direct dissemination through connective tissue (26, 27, 28, 29), spirochetes utilize alternate means to disseminate as well, including bacteremia (19, 29, 30, 31). In addition, a relatively unexplored means of dissemination is through lymphatics, as draining lymph nodes are often culture positive sooner than any other tissues proximal to the inoculation site (20, 25, 32). Few molecular mechanisms that enable the lymphatic route of dissemination have been proposed, but they probably involve the interaction between adhesins and ligands. For example, fibronectin-binding protein, glycosaminoglycans, and fibronectin facilitate microvascular interactions observed by intravital microscopy in infected mice (31), and both VlsE and OspC were implicated by phage display for in vivo adherence to vascular endothelium (10), which is likely to include lymphatic vessels as well.
The present study concurs with previous studies, in that decorin-binding proteins influence the early stages of infection (dissemination and tissue colonization). These early differences are unique to immunocompetent mice and are abolished in the chronic stage of infection. Results also demonstrate that decorin-binding proteins influence disease severity. We propose that the mechanism of influence pertains to the restricted routes by which spirochetes lacking dbpBA are able to disseminate, including lymphatic dissemination.
MATERIALS AND METHODS
Borrelial strains and mutagenesis.
B. burgdorferi sensu stricto strain B31-A3, a low-passage-number infectious clonal isolate of B31-MI, the prototype B31 strain utilized for genome sequencing (33, 34), was utilized as both the wild-type control and the parental strain for genetic manipulation (35). The dbpBA operon was disrupted by insertion of flgBp-aadA (36) by electroporation of competent B31-A3 as previously described (37) and selection in 50 μg/ml streptomycin, which yielded the B31-ΔdbpBA deletion mutant. All B. burgdorferi strains were cultivated in liquid modified Barbour-Stoenner-Kelly (BSKII) medium supplemented with 6% normal rabbit serum (38). For isolation of transformants, B. burgdorferi was cultured on semisolid gelatin-free BSKII medium supplemented with 1.7% dissolved agarose plus the appropriate antibiotic (37).
The dbpBA operon was genetically reconstituted in the B31-ΔdbpBA mutant by allelic exchange recombination, yielding the B31-dbpBA+ complement. The shuttle vector pBSV2G, containing a gentamicin resistance cassette (35), was utilized to create the construct in which the dbpBA operon was incorporated. One 1,649-bp fragment of B31 DNA, including the dbpBA operon, the promoter region from −266 to −1, and the terminator region after the stop codon from 1528 to 1649, was amplified by PCR with forward primer P1FBamHI (5′-TCGTGGGATCCCAAGCCAGATTGCATAGC-3′) and reverse primer P7RPstI (5′-TCGTGCTGTGATTATCGGGCGAAGAG-3′). Both pBSV2G and the amplicon were double digested with BamHI and PstI, ligated together, and sequenced to ensure the correct orientation of the dbpBA operon. The construct was electroporated into B31-ΔdbpBA mutants, and successful complements were selected with gentamicin (40 μg/ml). Six complemented mutants were obtained and confirmed by PCR for the presence of the dbpBA operon and gentamicin marker, as well as the absence of the streptomycin marker. Plasmid profiling confirmed that all six complemented mutants contained the plasmids lp25, lp28-1, lp54, cp26, and cp32, which are required for infectivity (39).
For construction of suicide vectors and general gene cloning, Escherichia coli strain TOP10F′ (Invitrogen, Inc., CA) was utilized and grown in lysogeny broth (LB) broth under aerobic conditions at 37°C. Transformed E. coli was cultured in LB medium with 50 μg/ml spectinomycin or 5 μg/ml gentamicin.
Mice and infections.
Specific-pathogen-free, 3- to 5-week-old female C3H/HeN (C3H), C3H.C-Prkdcscid/IcrSmnHsd (C3H-scid), and IcrTac:ICR-Prkdcscid (Swiss-scid) mice were acquired from Frederick Cancer Research Center (Frederick, MD), Harlan Sprague Dawley, Inc. (Indianapolis, IN), and Taconic Farms, Inc. (Hudson, NY), respectively. Pregnant outbred Crl:CD1 (ICR) mice were acquired from Charles River Laboratories (Hollister, CA). Mice were killed by carbon dioxide narcosis and cardiac exsanguination. Specific isolates of the borrelial mutants, B31-ΔdbpBA and B31-dbpBA+, were confirmed as infectious to infant ICR mice at all inoculation doses from 104 to 107 (data not shown). Any individual C3H, C3H-scid, or Swiss-scid mouse, in the experiments included herein, that could not be confirmed as infected (neither PCR positive nor culture positive) was excluded from data analysis.
PCR.
DNA was extracted from tissue samples using DNeasy tissue kits according to the manufacturer's instructions (Qiagen, Valencia, CA). Samples were analyzed by quantitative PCR (qPCR) using optimized assays for flaB and dbpA, as previously described (18). Three oligonucleotides, two primers and an internal TaqMan probe, for the flaB (18) and the dbpA genes were used. Primers DbpAB31-247F (5′-GCGAGCTACTACAGTAGCGGAAA-3′) and DbpAB31-444R (5′-TTTCAAGCACTCCTTGAGCTGTA-3′) were created to amplify a 198-bp fragment of dbpA DNA. The internal probe DbpAB31-316P (5′-GTGAAACAGGTAGCAAGTATCAGAAAATTCAT-3′) contained 5′ 6-carboxyfluorescein reporter dye and 3′ 6-carboxytetramethyl rhodamine quencher dye. Quantification of gene copies was based on absolute standard curves prepared using plasmid standards (18). Target gene copy numbers were expressed as copy number per mg tissue or per μl blood. In addition, DNA extracted from positive cultures and DNA from tissue samples were used to verify B. burgdorferi genotypes recovered from infected mice.
Histology.
Tissues were fixed in 10% neutral buffered formalin, paraffin embedded, routinely processed, and stained with hematoxylin and eosin. Limbs were decalcified prior to processing. Tissue sections were blindly examined and graded for the presence of inflammation. The presence of arthritis in each mouse was determined by examination of knees and tibiotarsi. Sagittal sections through the heart, including sections of great vessels (aorta), were examined for the presence of carditis, as described previously (40, 41). Tibiotarsal arthritis severity was scored on a scale of 0 (no histologic evidence of inflammation) to 3 (severe), as described previously (42).
Enzyme-linked immunosorbent assay.
Ninety-six-well plates were coated with 1 μg/ml B. burgdorferi B31 whole-cell lysates in carbonate coating buffer (pH 9.6), as described previously (12). Antibody binding was recognized by a secondary alkaline phosphatase-conjugated goat anti-mouse Ig(H+L) antibody, diluted at 1:5,000 (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). Immunoreactivity was revealed using 1 mg/ml phosphate substrate (Sigma-Aldrich, St. Louis, MO) in diethanolamine buffer, and optical density values were measured at 405 nm on a kinetic microplate reader (Molecular Devices, Sunnyvale, CA), as described previously (41). Individual serum samples were titrated in 3-fold dilutions (starting at 1:300). Samples were tested in duplicate, and each assay included uninfected mouse serum as a negative control and 90-day B31-infected mouse serum as a positive control.
Infection, dissemination and colonization, and persistence experiments.
Mice were infected by subdermal inoculation of 105 to 106 mid-log-phase B. burgdorferi B31-A3, B31-ΔdbpBA, and/or B31-dbpBA+ spirochetes in 0.1 ml BSKII culture medium on the dorsal thoracic midline. Subsets from each group were necropsied at 14, 28, 42, 60, and/or 90 days postinoculation. Subinoculation site and urinary bladder tissues were aseptically collected for culture, as previously described (43). Tissues collected for DNA extraction and qPCR included skin, subinoculation site, heart base, ventricular muscle, quadriceps muscle, and left tibiotarsus. Tissues collected for histology included heart base, left knee, and right rear limb. Hearts were bisected along the longitudinal axis to provide samples for both DNA extraction and histology.
Lymphatic dissemination experiment.
Groups of C3H mice were infected by subdermal inoculation of 105 mid-log-phase B. burgdorferi B31-A3, B31-ΔdbpBA, and/or B31-dbpBA+ spirochetes in 0.1 ml BSKII culture medium in the skin of the right lateral thigh. Four mice from each group were necropsied at 12 h, 3 days, 7 days, and 14 days postinoculation. Right and left inguinal lymph nodes, spleen, and urinary bladder were aseptically collected for culture. Both right and left inguinal, popliteal, lumbar, and axial lymph nodes were collected for DNA extraction. Inguinal lymph nodes were bisected to provide samples for both culture and DNA extraction. Extralymphatic tissues, including skin at the inoculation site, heart base, and right tibiotarsus, were collected for DNA extraction. To evaluate lymphatic dissemination in the absence of acquired immunity, the experiment was repeated in Swiss-scid mice.
Statistics.
Analyses were performed using Fisher's exact test for differences, independent-sample t test, or two-way analysis of variance, followed by post hoc pairwise comparisons (Tukey's honestly significant difference [HSD] test) (PASW Statistics, version 18.0, and Prism, version 5, GraphPad software). Calculated P values of ≤0.05 were considered significant.
RESULTS
Borrelia burgdorferi deficient in DbpA and DbpB lacks an early dissemination defect in immunodeficient mice but exhibits attenuated disease development.
The dissemination and pathogenic capabilities of the B31-ΔdbpBA mutant compared to wild-type B31-A3 were initially evaluated in immunodeficient mice. Groups of 4 C3H-scid mice inoculated with 106 B31-ΔdbpBA or B31-A3 spirochetes were necropsied at 28 days postinoculation. The subinoculation sites and urinary bladders from all mice in both B31-ΔdbpBA- and B31-A3-inoculated groups were culture positive, and there were no statistical differences in tissue spirochete burdens by flaB qPCR between groups (data not shown). B31-ΔdbpBA-inoculated C3H-scid mice developed both arthritis and carditis (Table 1), but the severity of tibiotarsal inflammation was attenuated in the B31-ΔdbpBA infection (mean severity score ± standard error of the mean [SEM], 0.8 ± 0.2) compared to the wild-type B31-A3 infection (2.9 ± 0.1) (P = 0.03). Carditis was milder and in equal prevalence in the B31-ΔdbpBA-inoculated C3H-scid mice compared to mice infected with B31-A3. Therefore, when unrestricted by acquired immunity, B31-ΔdbpBA retained the ability to disseminate and colonize distant tissues and was pathogenic, but despite the presence of equal copy numbers of spirochetes in tissue compared to the wild type, B31-ΔdbpBA elicited less inflammation in both hearts and joints.
Table 1.
Inflammation associated with wild-type and B31-ΔdbpBA B. burgdorferi infectiona
| Mouse strain description | Isolate genotype | Day | Tibiotarsus |
Heart base |
||||
|---|---|---|---|---|---|---|---|---|
| No. of spirochetesb | Prevalence (no. of mice affected/total) | Arthritis severity score (mean ± SEM) | No. of spirochetes | Prevalence (no. of mice affected/total) | Carditis severity score (mean ± SEM) | |||
| T/B cell deficient | ΔdbpBA | 28 | 2.39E+04 | 8/9 | 0.8 ± 0.2c,d | 4.10E+04 | 6/9 | 0.4 ± 0.1 |
| 42 | ND | ND | ND | ND | ND | ND | ||
| 60 | 5.55E+03 | 4/4 | 2.8 ± 0.3e | 9.90E+05 | 4/4 | 1.0g | ||
| 90 | 3.19E+04 | 4/4 | 3.0f | 1.37E+06 | 4/4 | 1.0h | ||
| Wild type | 28 | 3.81E+04 | 8/8 | 2.9 ± 0.1c | 3.61E+04 | 8/8 | 0.8 ± 0.1 | |
| 42 | ND | ND | ND | ND | ND | ND | ||
| 60 | 5.68E+04 | 4/4 | 3.0 | 2.06E+06 | 4/4 | 1.0 | ||
| 90 | 3.00E+01 | 4/4 | 3.0 | 7.10E+06 | 4/4 | 1.0 | ||
| Immunocompetent | ΔdbpBA | 28 | 5.69E+02 | 0/4 | 0.0d | 2.28E+03 | 0/4 | 0.0i |
| 42 | 6.17E+04 | 2/4 | 0.4 ± 0.2 | 1.79E+03 | 1/4 | 0.1 ± 0.1 | ||
| 60 | ND | 3/4 | 0.4 ± 0.1e | 9.51E+02 | 1/4 | 0.1 ± 0.1g | ||
| 90 | 1.45E+02 | 3/4 | 0.8 ± 0.3f | 4.33E+03 | 1/4 | 0.1 ± 0.1h | ||
| Wild type | 28 | 2.59E+04 | 1/5 | 0.2 ± 0.2 | 3.92E+04 | 5/5 | 1.0i | |
| 42 | 1.08E+05 | 4/5 | 0.9 ± 0.3 | 6.43E+03 | 3/4 | 0.6 ± 0.2 | ||
| 60 | ND | 4/4 | 1.1 ± 0.3 | 2.27E+03 | 1/4 | 0.1 ± 0.1 | ||
| 90 | 3.45E+02 | 4/4 | 0.8 ± 0.1 | 2.30E+03 | 1/4 | 0.1 ± 0.1 | ||
Inflammation associated with B31-ΔdbpBA B. burgdorferi infection is not significantly different from inflammation associated with wild-type B. burgdorferi infection after day 28 postinoculation, in either immunodeficient or immunocompetent mice. More severe inflammation does not absolutely correspond with a significantly greater spirochete tissue burden. ND, not determined.
No. of spirochetes in respective tissues represented as mean copy no. of flaB per mg tissue.
Differences in arthritis severity are statistically significantly different (all P values < 0.05), but differences in spirochete tissue burdens are not statistically significant.
Arthritis severity is significantly different (P < 0.05) and corresponds with significantly greater tissue spirochete burden (P = 0.007).
Arthritis severity is significantly different (P < 0.05).
Differences in arthritis severity are significantly different (P < 0.05), but differences to spirochete tissue brudens are not statistically significant.
Carditis severity is significantly different (P < 0.05) and corresponds with significantly greater tissue spirochete burden (P = 0.0005).
Carditis severity is significantly different (P < 0.05) and corresponds with significantly greater tissue spirochete burden (P = 0.002).
Carditis severity is significantly different (P < 0.05) and corresponds with significantly greater tissue spirochete burden (P = 0.003).
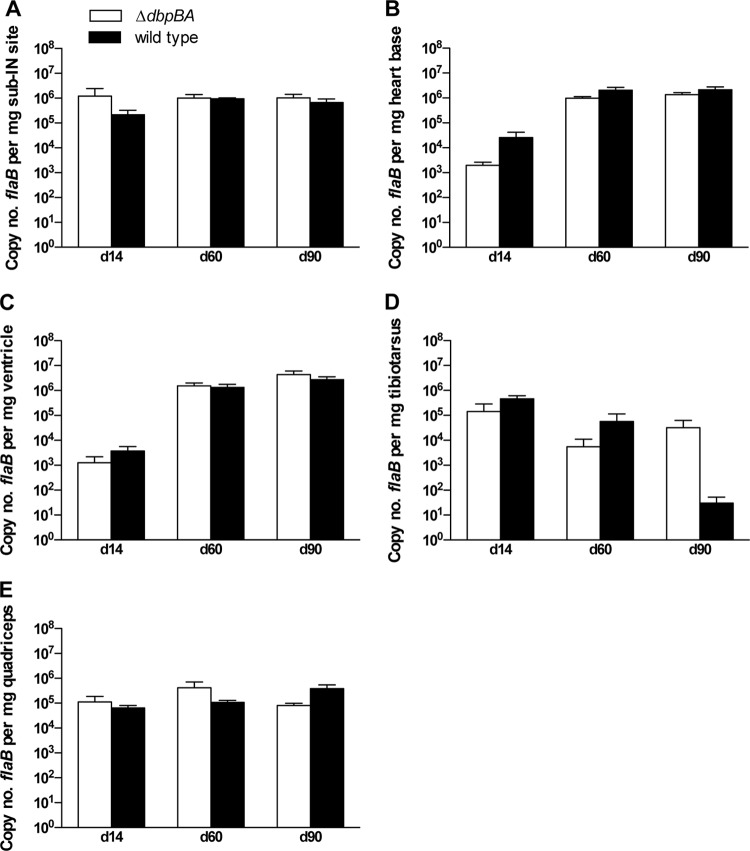
In the above-described experiment and similar studies by others with immunodeficient mice (24, 25), 1 month (28 to 30 days) postinoculation was the maximum experiment duration for evaluating infections utilizing DbpA/B-deficient spirochetes. In order to evaluate the capability of B31-ΔdbpBA to persist in immunodeficient mice, we extended the duration to 90 days. Groups of 12 C3H-scid mice were inoculated with 106 B31-ΔdbpBA or B31-A3 spirochetes, and subsets of 4 mice per group were necropsied at 14 days, 60 days, and 90 days postinoculation. The subinoculation sites and urinary bladders from all mice were culture positive at all intervals and in both groups. Copy numbers of flaB DNA in the subinoculation site, heart base, ventricle, quadriceps muscle, and tibiotarsal tissues were not significantly different between B31-ΔdbpBA- and wild-type B31-A3-inoculated mice at any interval (Fig. 1). The severities of tibiotarsal arthritis and carditis similarly were indistinguishable between B31-ΔdbpBA- and wild-type B31-A3-inoculated mice at 60 and 90 days postinoculation (Table 1). The qPCR and histology data confirmed that in immunodeficient mice, B31-ΔdbpBA spirochetes can disseminate to distant tissues, proliferate therein to an equal degree, incite inflammation, and persist in a manner similar to that of wild-type spirochetes.
Fig 1.
DbpA/B are not essential for dissemination, colonization, or persistence in immunodeficient mice. Shown are copy numbers of B. burgdorferi flaB DNA per mg tissue (mean ± SEM) in the subinoculation (sub-IN) site (A), heart base (B), ventricle (C), tibiotarsus (D), and quadriceps muscle (E) from C3H-scid mice inoculated with B31-ΔdbpBA (white bars) compared to wild-type B31-A3 (black bars) at 14 days, 60 days, and 90 days postinoculation. No significant differences were observed.
The early dissemination defect of dbpBA-deficient spirochetes in immunocompetent mice is abolished in the chronic stage of infection and is rescued by complementation.
To evaluate whether similar spirochete tissue dissemination, persistence, and disease development would occur with B31-ΔdbpBA infection in immunocompetent mice, groups of 15 C3H mice were inoculated with 105 B31-ΔdbpBA or B31-A3 spirochetes. Five mice from each group were necropsied at 14, 28, and 42 days postinoculation. Fewer culture-positive tissues, and fewer positive mice, were identified for the B31-ΔdbpBA-inoculated mice than for wild-type-inoculated mice at day 14 and day 28, but by day 42, numbers of culture-positive tissues and numbers of culture-positive mice increased until differences between B31-ΔdbpBA and B31-A3 infections were diminished (Table 2).
Table 2.
Viable, cultivable spirochetes lacking dbpBA are recovered from tissue in increasing frequency over time in immunocompetent C3H mice
| Isolate genotype | Day | No. of positive cultures/total |
No. of positive mice/total | |
|---|---|---|---|---|
| Subinoculation site | Bladder | |||
| ΔdbpBA | 14 | 2/5 | 0/5 | 2/5 |
| 28 | 4/5 | 0/5 | 4/5 | |
| 42 | 4/5 | 4/5a | 4/5 | |
| Wild type | 14 | 5/5 | 2/4 | 5/5 |
| 28 | 5/5 | 5/5 | 5/5 | |
| 42 | 5/5 | 1/5 | 5/5 | |
In 3 of the 4 positive cultures, spirochetes were observed only rarely.
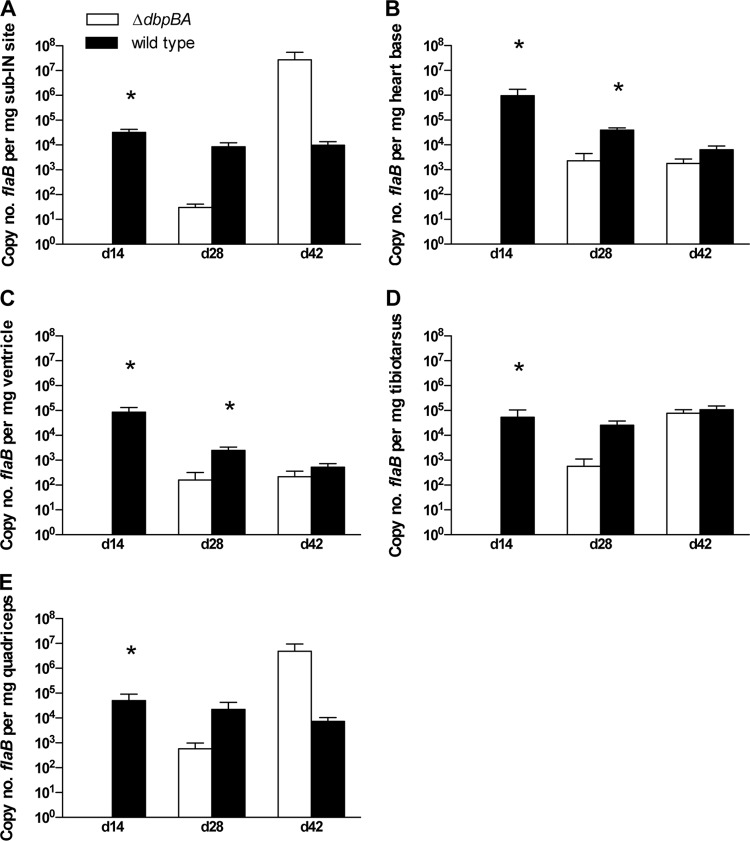
Similarly, at day 14, tissue spirochete burdens were undetectable in multiple tissues, including the subinoculation site, heart base, ventricular muscle, quadriceps muscle, and tibiotarsus (all P = 0.0079), in B31-ΔdbpBA-infected mice compared to wild-type-infected mice (Fig. 2). At day 28, spirochete tissue burdens in the heart base (P = 0.034) and ventricular muscle (P = 0.033) were significantly lower in B31-ΔdbpBA-infected mice than in those infected with the wild type. However, by day 42 postinoculation, qPCR tissue burdens were equivalent in both groups. No inflammation was observed on day 28, and only minimal carditis (0.1 ± 0.1; 1 out of 4 mice) and mild arthritis (0.4 ± 0.2; 2 out of 4 mice) were observed at day 42 in B31-ΔdbpBA-inoculated mice (Table 1). In contrast, in the wild-type-inoculated mice at day 28, there was statistically significantly greater carditis (1.0 ± 0.0; 5 out of 5 mice; P < 0.05) and a mild arthritis (0.2 ± 0.2; 1 out of 5 mice). At day 42, there was a trend toward slightly more severe and more prevalent disease, with mild carditis (0.6 ± 0.2) and mild to moderate arthritis (0.9 ± 0.3) in 4 out of 5 mice. Results demonstrated that B31-ΔdbpBA spirochetes retained the capacity to infect, disseminate, and persist in immunocompetent mice, and eventually attain equal levels of tissue burdens and disease, but were delayed and initially only able to induce attenuated disease.
Fig 2.
Early defects in dissemination and colonization, attributed to the disruption of DbpA/B, are not observed in the chronic stages of Lyme borreliosis in immunocompetent mice. Shown are copy numbers of B. burgdorferi flaB DNA per mg tissue (mean ± SEM) in tissues from C3H mice inoculated with B31-ΔdbpBA (white bars) compared to wild-type B31-A3 (black bars) at 14 days, 28 days, and 42 days postinoculation. *, all P values ≤ 0.034.
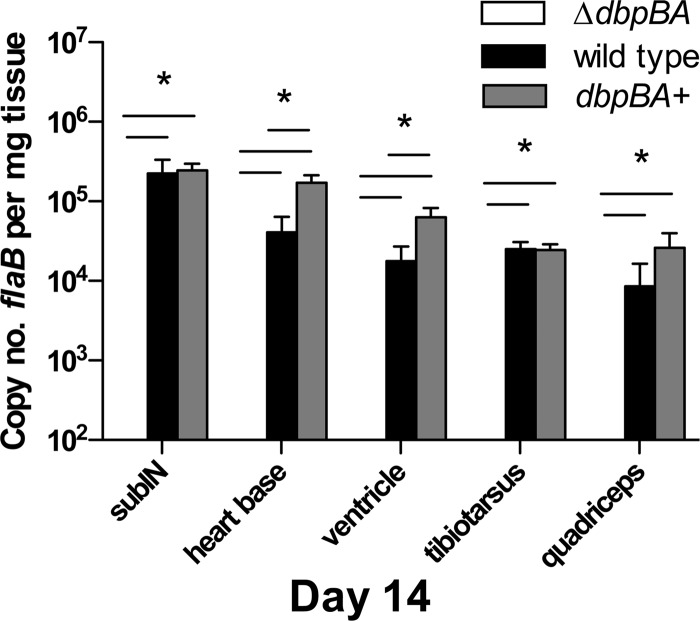
The duration of infection in immunocompetent mice was next extended to 90 days postinoculation in order to fully evaluate the capability of the DbpA/B-deficient mutant to persist. The complemented mutant B31-dbpBA+ was included in the experiment to evaluate whether genetic complementation could rescue the phenotype of the DbpA/B-deficient mutant. Groups of 12 C3H mice were inoculated with 106 B31-ΔdbpBA, B31-dbpBA+, or B31-A3 spirochetes. Subsets of 4 mice were necropsied at 14 days, 60 days, and 90 days postinoculation. Among mice inoculated with B31-ΔdbpBA, there were notably fewer culture- and/or qPCR-positive mice (1/4) and minimal or no detectable spirochete tissue burdens in B31-ΔdbpBA-infected mice at day 14 compared to both wild-type B31- and B31-dbpBA+-infected mice (Fig. 3). At subsequent intervals (day 60 and 90), 3/4 and 4/4 B31-ΔdbpBA-inoculated mice were culture and/or qPCR positive, and the level of spirochete tissue burden (Fig. 4) and severity of arthritis and carditis (Table 1) were not significantly different from those of B31-A3-inoculated mice. All B31-A3- and B31-dbpBA+-inoculated mice were positive at 14, 60, and 90 days, and tissue spirochete burdens in B31-dbpBA+-inoculated mice either were not statistically different or were not significantly lower than in mice inoculated with wild-type B31-A3 (day 14 shown in Fig. 3). Similarly, the severities of arthritis and carditis were not significantly different between B31-A3- and B31-dbpBA+-inoculated mice on days 60 and 90 (data not shown). The appropriate infecting B. burgdorferi genotypes (wild type, mutant, and complemented mutant) were confirmed among isolates from each mouse group at necropsy. Thus, DbpA/B-deficient spirochetes, despite their early dissemination defect, were capable of persistence and inducing disease in immunocompetent C3H mice, and complementation of the mutant restored the early dissemination phenotype.
Fig 3.
Complementation of the dbpBA-deficient mutant restores a wild-type phenotype. Shown are copy numbers of B. burgdorferi flaB DNA per mg tissue (mean ± SEM) in tissues from C3H mice inoculated with B31-ΔdbpBA (white bars) compared to the complemented mutant B31-dbpBA+ (gray bars) and wild-type B31-A3 (black bars). *, P ≤ 0.03.
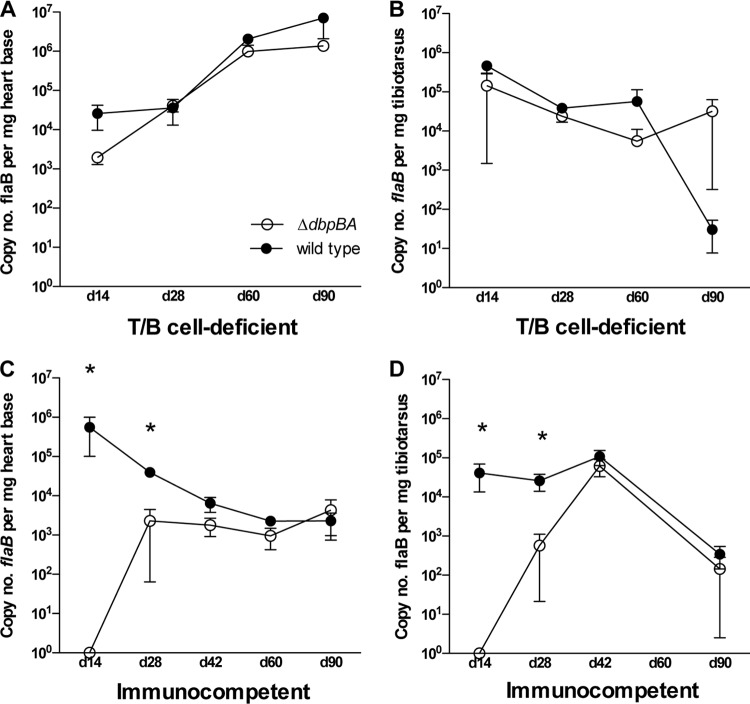
Fig 4.
The early dissemination defect is dependent on an acquired immune response. Shown are copy numbers of B. burgdorferi flaB DNA per mg tissue (mean ± SEM) in heart base (A) and tibiotarsus (B) from C3H-scid mice and heart base (C) and tibiotarsus (D) from C3H mice at days 14, 28, 42, 60, and 90 postinoculation. Mice were inoculated with B31-ΔdbpBA (white circles) or wild-type B31-A3 (black circles). Each data point represents 4 to 9 mice from 2 separate experiments. *, P ≤ 0.035.
The early dissemination defect is dependent on the presence of an acquired immune response.
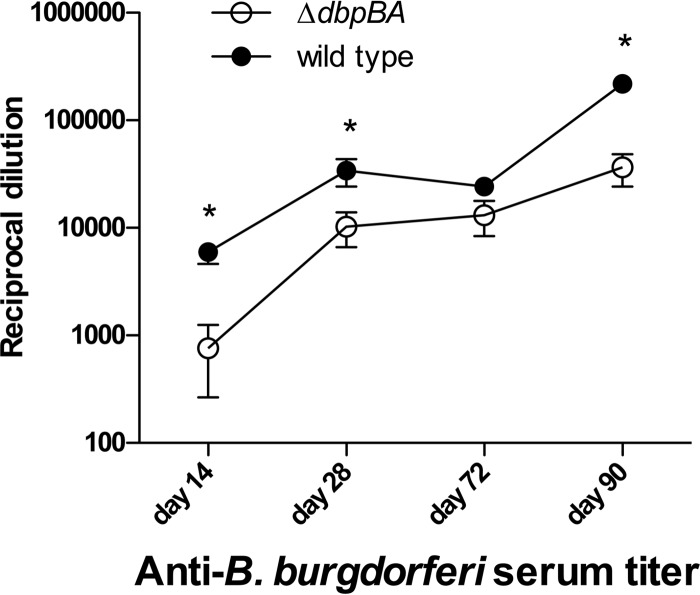
The flaB qPCR data from the above-described experiments were combined to evaluate spirochete dissemination and colonization kinetics from day 14 to day 90 postinoculation in immunocompetent C3H mice compared to immunodeficient C3H-scid mice (Fig. 4). Heart base and tibiotarsal results were focused upon because these two tissues are distant from the inoculation site and are often poorly colonized by DbpA/B-deficient spirochetes, due to and representative of the dissemination defect (21, 25). Serology from the above-described immunocompetent C3H mouse experiments was also combined to evaluate the acquired immune response between mice inoculated with DbpA/B-deficient and wild-type strains. In C3H-scid mice, no significant differences were observed in tissue spirochete burdens in the heart base (Fig. 4A) or tibiotarsus (Fig. 4B) between the B31-ΔdbpBA mutant and wild-type B31-A3. In contrast, B31-ΔdbpBA tissue spirochete burdens in C3H mice were markedly lower than those obtained with the wild type (or were absent) at early time points (day 14 and day 28), but these differences were abolished by day 42 postinoculation. Despite a continuous rise in B. burgdorferi-specific antibody titer in mice inoculated with both genotypes, differences between the titers in B31-ΔdbpBA and wild-type infections were not abolished after day 42 and remained statistically significantly greater in the wild-type-inoculated mice and in the B31-ΔdbpBA-inoculated mice (Fig. 5).
Fig 5.
Borrelia burgdorferi-specific antibody titers steadily rise over time, regardless of borrelial genotype, but remain significantly greater in mice inoculated with wild-type spirochetes than in mice inoculated with DbpA/B-deficient spirochetes. Mice were inoculated with B31-ΔdbpBA (white circles) or wild-type B31-A3 (black circles). Each data point represents the mean reciprocal dilution ± SEM of 4 to 5 mice from 2 separate experiments. *, P = 0.006, P = 0.05, and P < 0.001, in order of appearance.
dbpBA deficiency prevents early dissemination though the lymphatic system.
Regional lymph nodes have been reported to become rapidly culture positive following infection (by needle inoculation, tick transmission, and tissue graft) during infection with wild-type as well as DbpA/B-deficient B. burgdorferi (20, 25, 32). One study reported that distant lymph nodes in mice infected with wild-type B. burgdorferi became progressively culture positive over time, in the order of their proximity to the inoculation site (32). The same study concluded that spirochetes were in fact within lymph nodes, rather than in the surrounding connective tissue, by identifying morphologically intact spirochetes in subcapsular sinuses (32). In another study, in mice inoculated with DbpA/B-deficient spirochetes, spirochetes were frequently cultured from lymph nodes at 12 h and 2 and 3 weeks postinoculation (25). Based on these observations, both wild-type and DbpA/B-deficient spirochetes appeared to be able to enter into, survive within, and potentially migrate through the lymphatic system. This is in contrast to the observed dissemination defect in DbpA/B-deficient spirochetes in which heart and joint tissues (tissues that should be accessible by hematogenous or direct routes of dissemination) are less frequently colonized by DbpA/B-deficient spirochetes (21, 25) than by wild-type spirochetes. Based on these observations, we postulated that the lymphatic dissemination route might be utilized by spirochetes lacking DbpA/B more readily than other routes.
To investigate this possibility, we determined the prevalence of wild-type B31-A3, B31-ΔdbpBA mutant, and B31-dbpBA+ complemented spirochetes within lymph nodes, both proximal and distal to the inoculation site, and at multiple intervals (0.5, 3, 7, and 14 days) during early infection by culture and qPCR for flaB DNA. Any animal that was neither culture nor flaB qPCR positive was considered uninfected and dropped from the data set. Both right and left sides from each pair of lymph nodes (popliteal, inguinal, lumbar, and axillary) were evaluated, and if either one or both sides were qPCR or culture positive, then the pair of lymph nodes was considered positive (Table 3). Initially, we inoculated mice asymmetrically in the right hind limb to evaluate any influence of proximity, but the effect of side (right versus left) was negligible; therefore, each pair of lymph nodes was combined as a unit of evaluation.
Table 3.
Dbp deficiency in samples from mice and recovery of spirochetes from the lymphatic systema
| Isolate genotype | Day | No. of samples positive for flaB by PCR/total (no. of samples positive by culture/total) |
||||
|---|---|---|---|---|---|---|
| Poplitealb | Inguinal | Lumbar | Axillary | Extralymphatic tissuesc | ||
| Wild type | 0.5 | 2/4 | 3/4 (0/4) | 4/4 | 1/4 | 2/12 (0/4) |
| 3 | 1/3 | 0/3 (0/3) | 0/3 | 0/3 | 2/9 (0/4) | |
| 7d,f | 4/4 | 4/4 (4/4) | 4/4 | 4/4 | 12/12 (4/4) | |
| 14c,g | 4/4 | 4/4 (4/4) | 4/4 | 4/4 | 12/12 (4/4) | |
| ΔdbpBA | 0.5 | 4/4 | 2/4 (0/4) | 1/4 | 1/4 | 6/12 (0/4) |
| 3 | 0/3 | 0/3 (0/3) | 0/3 | 0/3 | 4/9 (0/4) | |
| 7d,f | 2/4 | 1/4 (0/4) | 0/4 | 2/4 | 3/12 (0/4) | |
| 14d,e,g | 1/3 | 0/3 (0/3) | 0/3 | 0/3 | 4/9 (0/4) | |
| dbpBA+ | 0.5 | 1/4 | 2/4 (0/4) | 1/4 | 0/4 | 4/12 (0/4) |
| 3 | 0/2 | 0/2 (0/2) | 0/2 | 0/2 | 2/6 (0/4) | |
| 7 | 1/1 | 1/1 (0/1) | 1/1 | 1/1 | 2/3 (0/4) | |
| 14e | 3/4 | 3/4 (3/4) | 3/4 | 3/4 | 9/12 (1/4) | |
Dbp deficiency prevents the recovery of spirochetes from the lymphatic system in the early stage of infection in immunocompetent laboratory mice. Complementation of dbpBA recovers the wild-type phenotype.
Includes both right- and left-sided nodes.
Extralymphatic tissues collected for PCR included skin, heart base, and tibiotarsus. Extralymphatic tissues collected for culture included spleen and urinary bladder.
Prevalence of flaB DNA in lymph nodes from mice infected with the ΔdbpBA strain is significantly lower (P < 0.0001 by Fisher's exact test) than in wild-type-infected mice.
Prevalence of flaB DNA in lymph nodes from mice infected with the ΔdbpBA strain is significantly lower (P < 0.0001) than in mice infected with the dbpBA+ (complemented) strain.
The number of PCR-positive extralymphatic tissues from mice infected with the ΔdbpBA strain is significantly lower (P < 0.0001) than in wild-type-infected mice.
The number of PCR-positive extralymphatic tissues from mice infected with the ΔdbpBA strain is significantly lower (P = 0.0062) than in wild-type-infected mice.
At the earliest time points, qPCR-positive lymph nodes were identified in mice infected with all three B. burgdorferi genotypes within hours after inoculation (day 0.5), but the same lymph nodes were universally negative at the following time point (day 3), suggesting drainage of DNA, but not viable spirochetes, from the inoculum. At day 7, the number of positive lymph nodes from B31-ΔdbpBA-inoculated mice was significantly lower (P < 0.0001) than the number of positive lymph nodes in wild-type-inoculated mice. At day 14, the number of positive lymph nodes from B31-ΔdbpBA-inoculated mice was significantly lower (P < 0.0001) than from mice infected with the wild type and the complemented mutant. Similarly, at day 7 and day 14, spirochete tissue burdens in lymph nodes from B31-ΔdbpBA-inoculated mice (copy number of flaB DNA per mg tissue [mean ± SEM], 10,572 ± 10,536 and 225 ± 0.0) were lower, though not significantly, than those of mice infected with the wild type (45,904 ± 19,596 and 38,995 ± 12,279).
Though there was a trend toward greater numbers of PCR-positive tissues in B31-ΔdbpBA extralymphatic tissues (skin, tibiotarsus, and heart base) than in lymph nodes, only on day 3 was the difference significant (P = 0.0211). Otherwise, there were significantly fewer PCR-positive extralymphatic tissues from B31-ΔdbpBA-inoculated mice than from wild type-infected mice at the later time points (day 7 P < 0.0001; day 14 P = 0.0062) (Table 3). At day 7 and day 14, spirochete tissue burdens in extralymphatic tissues from B31-ΔdbpBA-inoculated mice (25 ± 4 and 54,037 ± 49,271) were lower, though not significantly, than in tissues from wild-type-infected mice (7,381,000 ± 6,459,000 versus 103,140 ± 60,179). Based on culture, viable spirochetes could be recovered from the lymphatic system and extralymphatic tissue (urinary bladder) earliest in B31-A3-inoculated mice (day 7), followed by B31-dbpBA+-inoculated mice (day 14), but were not recovered from B31-ΔdbpBA-inoculated mice at any interval (Table 3). Therefore, the early dissemination defect of DbpA/B-deficient spirochetes in immunocompetent C3H mice was characterized by (i) minimal presence in lymph nodes, (ii) greater presence in extralymphatic tissues, and (iii) an overall lower spirochete tissue burden in lymph nodes and extralymphatic tissues compared to those in wild-type-infected mice. These data demonstrate that the lymphatic route is not a dominant means of dissemination or migration utilized by DbpA/B-deficient spirochetes.
Early exclusion of dbpBA-deficient spirochetes from the lymphatic system requires an acquired immune response.
Results indicated that the early dissemination defect of B31-ΔdbpBA spirochetes occurs only in C3H and not C3H-scid mice. Therefore, we next sought to determine if an acquired immune response is necessary to exclude B31-ΔdbpBA spirochetes from lymphatic dissemination. To investigate this possibility, we intended to repeat the previous experiment in congenic C3H-scid mice; however, C3H-scid mice became unavailable due to elimination of this mouse strain by the vendor. Therefore, the prevalence and tissue burdens of wild-type, mutant, and complemented spirochetes within lymph nodes and extralymphatic tissues during the early stage of infection were assessed in equally susceptible Swiss-scid mice.
Culture- and PCR-positive lymph nodes were identified in B31-ΔdbpBA-inoculated scid mice within hours after inoculation (day 0.5) (Table 4). By day 7, the number of positive lymph nodes from B31-ΔdbpBA-inoculated scid mice was significantly lower (P < 0.0001) than the number of positive lymph nodes in wild-type- and B31-dbpBA+-inoculated scid mice. However, by day 14, significant differences between the numbers of positive lymph nodes in wild-type-, B31-ΔdbpBA-, and B31-dbpBA+-inoculated scid mice were no longer apparent, and spirochete tissue burdens in lymph nodes from B31-ΔdbpBA-inoculated scid mice (2,352 ± 701) were not significantly different than those in mice inoculated with the wild type (33,497 ± 11,578) and B31-dbpBA+ (35,938 ± 10,355). At the same time point, the number of positive lymph nodes was significantly greater in scid mice inoculated with B31-ΔdbpBA (P < 0.0001) than in similarly inoculated C3H mice. No significant differences were observed between the number of positive lymph nodes and extralymphatic tissues in B31-ΔdbpBA-inoculated scid mice. Viable spirochetes could be recovered from the lymphatic system and extralymphatic tissues earliest in B31-A3-inoculated scid mice (day 3), followed by B31-dbpBA+-inoculated scid mice (day 7) and B31-ΔdbpBA-inoculated scid mice (day 14) (Table 4). In summary, DbpA/B-deficient spirochetes in immunodeficient Swiss-scid mice were not excluded from the lymphatic route of dissemination.
Table 4.
Dbp deficiency in samples from mice and utilization of the lymphatic system early in infection in immunodeficient micea
| Isolate genotype | Day | No. of samples positive for flaB by PCR/total (no. of samples positive by culture/total) |
||||
|---|---|---|---|---|---|---|
| Poplitealb | Inguinal | Lumbar | Axillary | Extralymphatic tissuesc | ||
| Wild type | 0.5 | 0/4 | 0/4 (0/4) | 0/4 | 1/4 | 5/12 (0/4) |
| 3 | 2/4 | 1/4 (1/4) | 0/4 | 1/4 | 5/12 (0/4) | |
| 7d,g | 4/4 | 4/4 (4/4) | 4/4 | 4/4 | 12/12 (3/4) | |
| 14f | 4/4 | 4/4 (4/4) | 4/4 | 4/4 | 12/12 (4/4) | |
| ΔdbpBA | 0.5 | 4/4 | 0/4 (4/4) | 1/4 | 4/4 | 7/12 (0/4) |
| 3 | NA | NA | NA | NA | NA | |
| 7d,e,g | 0/4 | 0/4 (0/4) | 2/4 | 0/4 | 5/12 (0/4) | |
| 14f | 3/3 | 3/3 (3/3) | 3/3 | 3/3 | 9/9 (3/3) | |
| dbpBA+ | 0.5 | 1/4 | 0/4 (0/4) | 0/4 | 0/4 | 4/12 (0/4) |
| 3 | 3/4 | 1/4 (0/4) | 0/4 | 0/4 | 3/12 (0/4) | |
| 7e,g | 4/4 | 3/4 (4/4) | 4/4 | 4/4 | 12/12 (0/4) | |
| 14f | 4/4 | 4/4 (4/4) | 4/4 | 4/4 | 12/12 (4/4) | |
Dbp deficiency decreases but does not prevent spirochetes from utilizing the lymphatic system in the early stage of infection in immunodeficient laboratory mice. NA, not applicable.
Includes both right- and left-sided nodes.
Extralymphatic tissues collected for PCR included skin, heart base, and tibiotarsus. Extralymphatic tissues collected for culture included spleen and urinary bladder.
Prevalence of flaB DNA in lymph nodes from mice infected with the ΔdbpBA strain is significantly lower (P < 0.0001 by Fisher's exact test) than in wild-type-infected mice.
Prevalence of flaB DNA in lymph nodes from mice infected with the ΔdbpBA strain is significantly lower (P < 0.0001 by Fisher's exact test) than in mice infected with the dbpBA+ strain.
All lymph nodes from mice infected with the wild-type, ΔdbpBA, and dbpBA+(complemented) strains are positive for flaB DNA and therefore could not be analyzed by Fisher's exact test.
The number of PCR-positive extralymphatic tissues from mice infected with the ΔdbpBA strain is significantly lower (all P = 0.0046) than in mice infected with the wild-type and dbpBA+ strains.
DISCUSSION
The role of individual borrelial ECM adhesins is a common theme of investigation, given the importance of ECM to the life cycle and pathogenesis of B. burgdorferi (44). Though adhesins may be necessary to a specific stage in borreliosis, no single adhesin has been shown to be absolutely essential. For instance, several studies have independently documented that deletion of dbpBA attenuates but does not abolish infectivity of B. burgdorferi (21, 23, 24). Similarly, deletion of other adhesins has not been sufficient to alter the course of initial infection. Disruption of Bgp led to an uninterrupted infectious phenotype in immunodeficient mice after 2 weeks postinoculation (45), and deletion of fibronectin-binding protein did not alter infection in immunocompetent mice at 3 weeks (46), although the median infectious dose was increased (47). Deletion of another adhesin, P66, resulted in loss of in vitro spirochetal attachment to the ligand integrin αvβ3 (48) and loss of infectivity in both immunocompetent and immunodeficient mice, with retention of the ability to infect ticks and survive in in vivo dialysis membrane chambers (49). Therefore, lack of any single adhesin may not be essential but, as we and others have demonstrated, may influence pathogenicity by altering the course of infection, by changing the ability to disseminate, colonize, cause disease, or persist.
While not necessary to establish infection in immunocompetent mice (23), deletion of dbpBA was reported to decrease infectivity (21, 24), display a dissemination defect (21, 23, 25), and, potentially, alter the ability to persist (25). In this study, we confirmed that DbpA/B-deficient spirochetes manifested an early dissemination defect, but we demonstrated that the defect resolved with chronicity (after day 28 postinoculation) and that persistence occurred in a manner indistinguishable from that with wild-type spirochetes. Furthermore, we demonstrated, for the first time, that deletion of DbpA/B resulted in early attenuation of disease development and prevented early dissemination and colonization within the lymphatic system. We propose that one mechanism by which the early dissemination defect of DbpA/B-deficient spirochetes occurs is restriction of lymphatic dissemination through which, by comparison, wild-type spirochetes can rapidly migrate.
As unlikely as it may seem for an organism dedicated to immune evasion and persistence, there is abundant evidence that B. burgdorferi spirochetes actively migrate within the lymphatic system. Lymph nodes are rapidly and consistently culture positive in both acute and chronic stages of infection (20, 25) and become progressively culture positive in order of proximity to the inoculation site (20), and morphologically intact spirochetes have been identified in subcapsular sinuses of regional lymph nodes (20). Indeed, a recent study found that the direct presence of viable (in contrast to nonviable) spirochetes in lymph nodes deceptively stimulates an atypical immune response that may actually favor survival of spirochetes during early infection (50). In the current study, we provide additional evidence for migration of wild-type spirochetes through the lymphatic system and demonstrate the diminished ability of DbpA/B-deficient spirochetes to do likewise. Taken together, all the evidence shows that the lymphatic system appears to be a route of dissemination for B. burgdorferi, and DbpA and DbpB may be important for that behavior.
Based on data presented in this study and by Weening et al. (25), DbpA/B-deficient spirochetes can gain initial and sporadic access to the lymphatic system, but we postulate that the inability to maintain access and migrate therein essentially results in exclusion that coincides with the repeatedly documented early dissemination defect. Involvement of the acquired immune response is strongly implicated, as only in immunocompetent mice has the dissemination defect been observed (21, 23, 25) and, notably, only in immunocompetent mice have we observed exclusion from the lymphatic system.
The importance of the acquired immune response, B cell- and antibody-mediated immunity in particular, to disease resolution and spirochete reduction in the host is well established (41, 51, 52, 53). How this clears or prevents access of DbpA/B-deficient spirochetes to lymphatics is perplexing because these genetically manipulated spirochetes lack one of the more immunogenic antigens, DbpA (12, 14). Without a vulnerable target, one might expect DbpA/B-deficient spirochetes to escape immune pressure; however, based on our observations, this is incorrect. We showed that the acquired immune response to DbpA/B-deficient spirochetes (by B. burgdorferi-specific serum titer) remains significantly lower than the immune response to the wild type (Fig. 5), despite equilibration of tissue spirochete burdens to a wild-type level (Fig. 4C and D). This reduced immune response remains capable of excluding DbpA/B-deficient spirochetes from the lymphatics, at least within the early stages of infection.
Several mechanisms that would prevent lymphatic dissemination of DbpA/B-deficient spirochetes in immunocompetent mice are possible: (i) DbpA/B-deficient spirochetes have increased vulnerability to antibody clearance within lymphatics, (ii) DbpA/B-deficient spirochetes have increased vulnerability to non-antibody-mediated clearance within lymphatics, or (iii) lymphatics become inaccessible to DbpA/B-deficient spirochetes after the initial establishment of infection. Our observations are more consistent with the first two possibilities, since involvement of the acquired immune response is implicated. If DbpA/B-deficient spirochetes are more vulnerable to antibody clearance, then increased exposure to IgM could account for the greater susceptibility. IgM dominates the antiborrelial immune response (50), and though it may be too large and unwieldy to penetrate collagenous tissues, it is present in blood and lymph (54). The caveat remains that evidence exists to refute the hypothesis that steric hindrance alone prevents the antibody response from targeting spirochetes embedded in collagen (55, 56). As for non-antibody-mediated clearance, recent investigations into invariant natural killer T (iNKT) cells are reminders that there are alternate immune mechanisms to consider (57, 58). For instance, disruption of the phagocyte (macrophage or Kupffer cell)-iNKT cell interaction results in diminished gamma interferon (IFN-γ) production, decreased phagocytic clearance, and increased bacterial loads (57) and dissemination (58).
Similarly, the exact mechanism by which the DbpB/A-deficient spirochetes maintain the capability to incite inflammation despite the absence of a strongly immunogenic antigen is speculative at best. Only during the earlier stage of infection (day 28) was there a statistically significant difference in severity of arthritis (in C3H-scid mice) or carditis (in C3H mice) between B31-ΔdbpBA- and wild-type-inoculated mice. However, in C3H mice, there was a slight attenuation in disease severity in B31-ΔdbpBA extending to day 60. Relative tissue spirochete burdens are not sufficient to explain the difference in disease severity, since attenuation of disease in B31-ΔdbpBA-inoculated mice extends past the point (day 42) of equilibration between genotypes (Fig. 4C and D). Rapidity of dissemination to and colonization of a site of predilection for inflammation (heart base or tibiotarsus) may be an alternate possible explanation for the initially attenuated inflammation associated with B31-ΔdbpBA spirochetes. For example, at the earlier time points (<14 days), histologically evident inflammation often lags behind the wave of directly disseminating wild-type spirochetes in immunodeficient C3H-scid mice (D. M. Imai, unpublished data).
In summary, we demonstrated and confirmed that disruption of dbpBA results in an early dissemination defect that is dependent on the presence of acquired immunity, resolves with chronicity of infection, and appears to reflect restricted migration through the lymphatic system. We confirmed that deficiency in dbpBA does not diminish the ability to infect, to cause disease, or to persist. The counterintuitive dispensability of DbpA and DbpB, immunodominant (12, 19, 20) but potentially protective (12, 19, 59) outer surface proteins that afford the ability to disseminate in the face of acquired immunity, is only one indication of the complexity of the borrelial pathogen-host relationship.
ACKNOWLEDGMENTS
We thank Patricia Rosa for providing the B. burgdorferi B31-A3 strain and Kevin Holden, Beth Todd, and Edlin Escobar for technical assistance.
This work was supported by NIAID grants R01-AI26815 (S.W.B.), T32-AI06055 and T32-OD011147 (D.M.I.), and R01-AI051486 (D.S.S.).
Footnotes
Published ahead of print 4 March 2013
REFERENCES
- 1. Brissette CA, Verma A, Bowman A, Cooley AE, Stevenson B. 2009. The Borrelia burgdorferi outer-surface protein ErpX binds mammalian laminin. Microbiology 155:863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brissette CA, Bykowski T, Cooley AE, Bowman A, Stevenson B. 2009. Borrelia burgdorferi RevA antigen binds host fibronectin. Infect. Immun. 77:2802–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coburn J, Chege W, Magoun L, Bodary SC, Leong JM. 1999. Characterization of the candidate Borrelia burgdorferi β3-chain integrin ligand identified using a phage display library. Mol. Microbiol. 34:926–940 [DOI] [PubMed] [Google Scholar]
- 4. Fischer JR, Parveen N, Magoun L, Leong JM. 2003. Decorin-binding proteins A and B confer distinct mammalian cell type-specific attachment by Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. U. S. A. 100:7307–7312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fischer JR, LeBlanc KT, Leong JM. 2006. Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect. Immun. 74:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo BP, Norris SJ, Rosenberg LC, Höök M. 1995. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect. Immun. 63:3467–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parveen N, Leong JM. 2000. Identification of a candidate glycosaminoglycan-binding adhesin of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:1220–1234 [DOI] [PubMed] [Google Scholar]
- 8. Antonara S, Ristow L, Coburn J. 2011. Adhesion mechanisms of Borrelia burgdorferi. Adv. Exp. Med. Biol. 715:35–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zambrano MC, Beklemisheva AA, Bryksin AV, Newman SA, Cabello FC. 2004. Borrelia burgdorferi binds to, invades, and colonizes native type I collagen lattices. Infect. Immun. 72:3138–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Antonara S, Chafel RM, LaFrance M, Coburn J. 2007. Borrelia burgdorferi adhesins identified using in vivo phage display. Mol. Microbiol. 66:262–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hagman KE, Lahdenne P, Popova TG, Porcella SF, Akins DR, Radolf JD, Norgard MV. 1998. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect. Immun. 66:2674–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng S, Hodzic E, Stevenson B, Barthold SW. 1998. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection in laboratory mice. Infect. Immun. 66:2827–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo BP, Brown EL, Dorward DW, Rosenberg LC, Höök M. 1998. Decorin-binding adhesins from Borrelia burgdorferi. Mol. Microbiol. 30:711–723 [DOI] [PubMed] [Google Scholar]
- 14. Hanson MS, Cassatt DR, Guo BP, Patel NK, McCarthy MP, Dorward DW, Höök M. 1998. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect. Immun. 66:2143–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benoit VM, Fischer JR, Lin Y, Parveen N, Leong JM. 2011. Allelic variation of the Lyme disease spirochete adhesin DbpA influences spirochetal binding to decorin, dermatan sulfate, and mammalian cells. Infect. Immun. 79:3501–3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roberts WC, Mullikin BA, Lathigra R, Hanson MS. 1998. Molecular analysis of sequence heterogeneity among genes encoding decorin binding proteins A and B of Borrelia burgdorferi sensu lato. Infect. Immun. 66:5275–5285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salo J, Loimaranta V, Lahdenne P, Viljanen MK, Hytönen J. 2011. Decorin binding by DbpA and B of Borrelia garinii, Borrelia afzelii and Borrelia burgdorferi sensu stricto. J. Infect. Dis. 204:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hodzic E, Feng S, Freet KJ, Barthold SW. 2003. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect. Immun. 71:5042–5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cassatt DR, Patel NK, Ulbrandt ND, Hanson MS. 1998. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect. Immun. 66:5379–5387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tunev SS, Hastey CJ, Hodzic E, Feng S, Barthold SW, Baumgarth N. 2011. Lymphadenopathy during Lyme borreliosis is caused by spirochete migration-induced specific B cell activation. PLoS Pathog. 7:e1002066 doi:10.1371/journal.ppat.1002066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi Y, Xu Q, McShan K, Liang FT. 2008. Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect. Immun. 76:1239–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi Y, Xu Q, Seemanaplli SV, McShan K, Liang FT. 2008. Common and unique contributions of decorin-binding proteins A and B to the overall virulence of Borrelia burgdorferi. PLoS One 3:e3340 doi:10.1371/journal.pone.0003340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi Y, Xu Q, Seemanaplli SV, McShan K, Liang FT. 2006. The dbpBA locus of Borrelia burgdorferi is not essential for infection of mice. Infect. Immun. 74:6509–6512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blevins JS, Hagman KE, Norgard MV. 2008. Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol. 8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weening EH, Parveen N, Trzeciakowski JP, Leong JM, Höök M, Skare JT. 2008. Borrelia burgdorferi lacking DbpBA exhibits an early survival defect during experimental infection. Infect. Immun. 76:5694–5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Motameni AT, Bates TC, Juncadella IJ, Petty C, Hedrick MN, Anguita J. 2005. Distinct bacterial dissemination and disease outcome in mice subcutaneously infected with Borrelia burgdorferi in the midline of the back and the footpad. FEMS Immunol. Med. Microbiol. 45:279–284 [DOI] [PubMed] [Google Scholar]
- 27. Shih CM, Pollack RJ, Telford SR, Spielman A. 1992. Delayed dissemination of Lyme disease spirochetes from the site of deposition in the skin of mice. J. Infect. Dis. 4:827–831 [DOI] [PubMed] [Google Scholar]
- 28. Shih CM, Telford SR, Pollack RJ, Spielman A. 1993. Rapid dissemination by the agent of Lyme disease in hosts that permit fulminating infection. Infect. Immun. 61:2396–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wormser GP. 2006. Hematogenous dissemination in early Lyme disease. Wien. Klin. Wochenschr. 118:634–637 [DOI] [PubMed] [Google Scholar]
- 30. Barthold SW, Persing DH, Armstrong AL, Peeples RA. 1991. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am. J. Pathol. 139:263–273 [PMC free article] [PubMed] [Google Scholar]
- 31. Norman UM, Moriarty TJ, Dresser AR, Millen B, Kubes P, Chaconas G. 2008. Molecular mechanisms involved in vascular interactions of the Lyme disease pathogen in a living host. PLoS Pathog. 4:e1000169 doi:10.1371/journal.ppat.1000169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Straubinger RK, Straubinger AF, Härter L, Jacobson RH, Chang Y, Summers BA, Erb HN, Appel MJG. 1997. Borrelia burgdorferi migrates into joint capsules and causes up-regulation of interleukin-8 in synovial membranes of dogs experimentally infected with ticks. Infect. Immun. 65:1273–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson RJ, Haft D, Hickey E, Gwinn M, White O, Fraser CM. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490–516 [DOI] [PubMed] [Google Scholar]
- 34. Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb J, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quakenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580–586 [DOI] [PubMed] [Google Scholar]
- 35. Elias AF, Stewart PE, Grimm D, Caimano MJ, Eggers CH, Tilly K, Bono JL, Akins DR, Radolf JD, Schwan TG, Rosa P. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70:2139–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frank KL, Bundle SF, Kresge ME, Eggers CH, Samuels DS. 2003. aadA confers streptomycin-resistance in Borrelia burgdorferi. J. Bacteriol. 185:6723–6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Samuels DS. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol. Biol. 47:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barbour AG. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521–525 [PMC free article] [PubMed] [Google Scholar]
- 39. Casjens SR, Mongodin EF, Qui WG, Luft BJ, Schutzer SE, Gilcrease EB, Huang WM, Vujadinovic M, Aron JK, Vargas LC, Freeman S, Radune D, Weidman JF, Dimitrov GI, Khouri HM, Sosa JE, Halpin RA, Dunn JJ, Fraser CM. 2012. Genome stability of Lyme disease spirochetes: comparative genomics of Borrelia burgdorferi plasmids. PLoS One 7:e33280 doi:10.1371/journal.pone.0033280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Armstrong AL, Barthold SW, Persing DH, Beck DS. 1992. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 47:249–258 [DOI] [PubMed] [Google Scholar]
- 41. Barthold SW, Hodzic E, Tunev S, Feng S. 2006. Antibody-mediated disease remission in the mouse model of Lyme borreliosis. Infect. Immun. 74:4817–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barthold SW. 1991. Infectivity of Borrelia burgdorferi relative to route of inoculation and genotype in laboratory mice. J. Infect. Dis. 163:419–420 [DOI] [PubMed] [Google Scholar]
- 43. Barthold SW, de Souza MS, Janotka JL, Smith AL, Persing DH. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:959–971 [PMC free article] [PubMed] [Google Scholar]
- 44. Cabello FC, Godfrey HP, Newman SA. 2007. Hidden in plain sight: Borrelia burgdorferi and the extracellular matrix. Trends Microbiol. 15:350–354 [DOI] [PubMed] [Google Scholar]
- 45. Parveen N, Cornell KA, Bono JL, Chamberland C, Rosa P, Leong JM. 2006. Bgp, a secreted glycosaminoglycan-binding protein of Borrelia burgdorferi strain N40, displays nucleosidase activity and is not essential for infection of immunodeficient mice. Infect. Immun. 74:3016–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li X, Liu X, Beck DS, Kantor FS, Fikrig E. 2006. Borrelia burgdorferi lacking BBK32, a fibronectin-binding protein, retains full pathogenicity. Infect. Immun. 74:3305–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Seshu J, Esteve-Gassent MD, Labandeira-Rey M, Kim JH, Trzeciakowski JP, Höök M, Skare JT. 2006. Inactivation of the fibronectin-binding adhesion gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol. Microbiol. 59:1591–1601 [DOI] [PubMed] [Google Scholar]
- 48. Coburn J, Cugini C. 2003. Targeted mutation of the outer membrane protein P66 disrupts attachment of the Lyme disease agent, Borrelia burgdorferi, to integrin αvβ3. Proc. Natl. Acad. Sci. U. S. A. 100:7301–7306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ristow LC, Miller HE, Padmore LJ, Chettri R, Salzman N, Caimano MJ, Rosa PA, Coburn J. 2012. The β3-integrin ligand of Borrelia burgdorferi is critical for infection of mice but not ticks. Mol. Microbiol. 85:1105–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hastey CJ, Elsner RA, Barthold SW, Baumgarth N. 2012. Delays and diversions mark the development of B cell responses to Borrelia burgdorferi infection. J. Immunol. 188:5612–5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barthold SW, deSouza M, Feng S. 1996. Serum-mediated resolution of Lyme arthritis in mice. Lab. Invest. 74:57–67 [PubMed] [Google Scholar]
- 52. McKisic MD, Redmond WL, Barthold SW. 2000. T cell-mediated pathology in murine Lyme borreliosis. J. Immunol. 164:6096–6099 [DOI] [PubMed] [Google Scholar]
- 53. Schaible UE, Wallich R, Kramer MD, Nerz G, Stehle T, Museteanu C, Simon MM. 1994. Protection against Borrelia burgdorferi infection in SCID mice is conferred by presensitized spleen cells and partially by B but not T cells alone. Int. Immunol. 6:671–681 [DOI] [PubMed] [Google Scholar]
- 54. Murphy K, Travers P, Walport M. 2008. Janeway's immunobiology, 7th ed, p 400–401 Garland Science, New York, NY [Google Scholar]
- 55. Liang FT, Brown EL, Wang T, Iozzo RV, Fikrig E. 2004. Protective niche for Borrelia burgdorferi to evade humoral immunity. Am. J. Pathol. 165:977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Strother KO, Hodzic E, Barthold SW, de Silva AM. 2007. Infection of mice with Lyme disease spirochetes constitutively producing outer surface protein A and B. Infect. Immun. 75:2786–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hawley K, Navasa N, Olson CM, Jr, Bates TC, Garg R, Hedrick MN, Conze D, Rincon M, Anguita J. 2012. Macrophage p38 mitogen-activated protein kinase activity regulates invariant natural killer T-cell responses during Borrelia burgdorferi infection. J. Infect. Dis. 206:283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee WY, Moriarty TJ, Wong CH, Zhou H, Strieter RM, van Rooijen N, Chaconas G, Kubes P. 2010. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat. Immunol. 11:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu Q, McShan K, Liang FT. 2008. Essential protective role attributed to the surface lipoproteins of Borrelia burgdorferi against innate defences. Mol. Microbiol. 69:15–29 [DOI] [PMC free article] [PubMed] [Google Scholar]