Abstract
Infection with Leishmania braziliensis causes cutaneous or mucocutaneous leismaniasis in humans. Toll-like receptor 9 (TLR9) expression has been found in granulomas of lesions in L. braziliensis-infected individuals. L. braziliensis inoculation in mice induces very small lesions that are self-healing, whereas deficiency in the TLR adaptor molecule, MyD88, renders mice susceptible to infection. The TLR involved has not been identified, prompting us to investigate if TLR9 triggering by the parasite contributes to the strong resistance to infection observed in L. braziliensis-inoculated mice. The parasites activated wild-type (WT) dendritic cells (DCs) in vitro but not DCs derived from TLR9−/− mice. TLR9−/− mice inoculated with L. braziliensis exhibited a transient susceptibility characterized by increased lesion size and parasite burden compared to those of WT mice. Surprisingly, elevated levels of gamma interferon (IFN-γ) were measured at the site of infection and in draining lymph node T cells of TLR9−/− mice at the peak of susceptibility, suggesting that unlike observations in vitro, the parasite could induce DC activation leading to the development of Th1 cells in the absence of TLR9 expression. Taken together, these data show that TLR9 signaling is important for the early control of lesion development and parasite burden but is dispensable for the differentiation of Th1 cells secreting IFN-γ, and the high levels of this cytokine are not sufficient to control early parasite replication following L. braziliensis infection.
INTRODUCTION
Leishmania intracellular protozoan parasites cause a broad range of clinical manifestations from cutaneous lesions to fatal visceral disease. The outcome of disease depends largely on the Leishmania species involved and the immune status of the host. Leishmania braziliensis is the major etiologic agent for cutaneous leishmaniasis (CL) in Central and South America, which is predominantly characterized by lesions that self-heal after 6 to 15 months (1); however, 1 to 10% of the L. braziliensis patients develop mucocutaneous leishmaniasis (MCL), with severe tissue damage to the mucosal regions of the nose, throat, and pharynx, 1 to 5 years after the initial cutaneous lesions have healed (2, 3).
L. braziliensis infection in humans is associated with a potent cell-mediated immune response characterized by gamma interferon (IFN-γ) production at the site of infection and by circulating cells (4–7). Despite the presence of CD4+ T cells producing IFN-γ, many individuals still harbor small numbers of parasites in dermal lesions (2). In the murine model, most inbred mouse strains, including C57BL/6 and BALB/c mice, are resistant to L. braziliensis infection, with the development of nonulcerative lesions that rapidly heal within weeks (8–13). Similar to that in humans, parasite growth is controlled at the infection site, even though parasite persistence continues in the draining lymph nodes (dLNs) after the lesions have resolved.
Host resistance to L. braziliensis is thought to depend upon the development of Th1 cells, characterized by their secretion of IFN-γ and tumor necrosis factor alpha (TNF-α), and ablation of IFN-γ or TNF-α leads to increased susceptibility (9, 14, 15). In addition, control of L. braziliensis infection has been associated with the development of Th17 cells (16). Unlike other New World Leishmania species (L. amazonensis and L. mexicana), L. braziliensis or its secretory products can activate dendritic cells (DCs) to produce IL-12 and TNF-α, two cytokines involved in the activation of DCs and the development of the immune response (16–19).
Toll-like receptors (TLRs) have been shown to be important in controlling L. major infection (20–25). For example, C57BL/6 mice deficient in the TLR adaptor molecule, myeloid differentiation factor 88 (MyD88), inoculated with L. major developed transiently larger lesions than did wild-type (WT) mice and exhibited a Th2 response with increased levels of IL-4 in parallel with a decrease in the concurrent Th1 cell response characterized by lower levels of IFN-γ (26–29). Furthermore, recent evidence suggests that TLR9-dependent DC activation is critical for resistance against L. major infection (23, 30, 31). TLR9−/− mice inoculated with L. major displayed temporarily elevated Th2 cytokines; susceptibility in TLR9−/− mice was associated with either an intact or defective Th1 response, depending on the study (25, 30, 31). In addition, the presence of TLR9 was shown to be essential for the activation of classical DCs (cDCs), plasmacytoid DCs (pDCs), and NK cells in response to L. infantum as well as for the stimulation of cDCs and pDCs by L. braziliensis promastigotes (23).
Increasing evidence shows that the immune responses against distinct Leishmania species, and even within the same strains, can differ significantly (32). Given the role of TLR9 in resistance to other Leishmania infections, and the increased susceptibility seen in MyD88−/− mice infected with L. braziliensis (33), we wanted to examine the role of TLR9 following L. braziliensis infection. In this study, we showed that in vitro DC activation by L. braziliensis depends on TLR9. TLR9−/− mice inoculated with L. braziliensis exhibited transiently increased lesion sizes and parasite burdens compared to those of control mice, but the CD4+ Th1 response as assessed by secretion of IFN-γ was intact in TLR9−/− mice. Taken together, these data suggest that TLR9 plays an important role in the early events leading to resistance and parasite control during L. braziliensis infection by an IFN-γ-independent mechanism.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice were purchased from Charles River (Lyon, France). C57BL/6 TLR9−/− mice were given by P. Launois and originally obtained from S. Akira (Osaka University). TLR9−/− mice have been backcrossed 8 times to the C57BL/6 genetic background, and all mice were bred and housed under pathogen-free conditions in the animal facility of the Centre for Immunology and Infection (Epalinges, Switzerland) and used for experiments when they were between 6 and 8 weeks old. All animal experimental protocols were approved by the Swiss Federal Veterinary Office (authorization 1266.5 to F.T.-C.), and experiments were performed adhering to guidelines established by this office.
Parasites and infections.
L. braziliensis (MHOM/BR/01/BA788 strain) (34) parasites were a gift from Camila de Oliveira (FIOCRUZ, Salvador, Brazil). L. braziliensis and L. major (LV39 MRHO/Sv/59/P strain) parasites were maintained in vivo in BALB/c mice and grown in vitro in M199 medium (GIBCO, Paisley, United Kingdom) supplemented with 10% fetal calf serum (FCS; PAA Laboratories, Pasching, Austria), 4% HEPES (Amimed), and 2% antibiotics (penicillin, streptomycin, and neomycin) (GIBCO). For footpad infections, 3 × 106 stationary-phase promastigotes in 50 μl of incomplete Dulbecco modified Eagle medium (DMEM) medium (GIBCO) were injected subcutaneously into the hind right footpad. Footpad lesion size was monitored weekly and measured using a caliper. To determine parasite loads and cellular content, footpads were digested using 1 mg/ml of collagenase D (Roche, Rotkreuz, Switzerland) in Hanks balanced salt solution (HBSS; GIBCO) or dLNs were homogenized, and limiting dilution assays (LDAs) using 10-fold serial dilutions (plating 100 μl per well containing ∼1 mg of tissue or 5.0 × 105 cells in the first column of the dilution for footpads or dLN cells, respectively) were performed as previously described (35). Briefly, the number of viable parasites in each footpad was determined from the highest dilution of tissue at which parasites could be grown after 7 to 10 days of incubation at 26°C. The number of infected dLN cells containing at least one viable parasite was similarly determined by serial dilution of 5.0 × 105 dLN cells. The parasite frequency was calculated by the Taswell method using the program Estimfree (36).
Detection of LRV.
Parasites of L. guyanensis M5313 (WHI/BR/1978/M5313) and one of its derived nonmetastatic clones, Lg17, were used; these strains are known for their Leishmania RNA virus (LRV) content (37). LRV presence was assessed as described previously (37). Briefly, nucleic acids (containing mostly genomic DNA but also the LRV double-stranded RNA [dsRNA] genome if present) were extracted from stationary-phase promastigotes and quantified using a spectrophotometer. Using 1 μg of nucleic acid extract loaded on an 8% agarose gel, the amount and quality of the extracts were verified directly by electrophoresis before digestion of genomic DNA by DNase I (Roche) to visualize the viral genome.
Generation of BM-derived DCs and macrophages.
Fresh bone marrow (BM) cells were harvested and cultured in vitro at 37°C in RPMI 1640 medium (GIBCO) supplemented with 10% FCS, 1% HEPES, 1% antibiotics, and β-mercaptoethanol (Sigma, Steinheim, Germany) and expanded in 15% granulocyte-macrophage colony-stimulating factor (GM-CSF) for 6 to 7 days for the differentiation of conventional DCs (38). The culture at day 7 included ∼75% CD11c+ CD11b+ cells. Nonadherent cDCs were used for all experiments. To derive pDCs, 1% Flt3L, kindly provided by Hans Acha-Orbea (University of Lausanne), was added to BM cells for 9 days (39). Both adherent and nonadherent Flt3L-derived DCs were pooled and either used directly or further sorted with a fluorescence-activated cell sorter (FACS). pDCs were sorted as CD11c+ PDCA-1+ F4/80− to a purity of >96%. CD11c, CD11b, major histocompatibility complex class II (MHC-II), and B220 expression was confirmed by FACS analysis. BM-derived macrophages were cultured in DMEM supplemented with 5% FCS, 1% HEPES, 1% antibiotics, β-mercaptoethanol, and 10% supernatant from L929 cells as a source of M-CSF for 7 days.
In vitro cytokine production.
cDCs and Flt3L-derived DCs were plated in a 48-well plate (Costar, Corning, NY) at 2.5 × 105 cells per well in 500 μl of 10% FCS–RPMI complete medium. DCs were then stimulated or not with 1 μg/ml of lipopolysaccharide (LPS; Sigma, St. Louis, MO), 0.5 μg/ml of ODN1826 CpG (Invivogen, San Diego, CA), or 12.5 × 105 UV-treated L. braziliensis parasites (5:1) for 24 h. FACS-sorted pDCs were plated in a 96-well plate at 1 × 105 cells per well and stimulated 5:1 with UV-treated L. braziliensis parasites. Cell culture supernatants were collected and stored at −80°C until further use. Supernatants were analyzed for IL-12p40 by OptEIA enzyme-linked immunosorbent assay (ELISA) (BD Biosciences, San Jose, CA) according to the manufacturer's instructions.
Flow cytometry.
For examination of surface molecules, cells were incubated with monoclonal antibody (MAb) 24G2 to block Fc receptors (FcRs) and stained using anti-CD80-fluorescein isothiocyanate (anti-CD80-FITC), anti-CD86-phycoerythrin (anti-CD86-PE), and anti-B220-PE-Texas red (BD Pharmingen, San Diego, CA); anti-F4/80-biotin, anti-Ly6G-allophycocyanin (APC)-Cy7, and anti-MHC-II-Alexa Fluor 700 (BioLegend, San Diego, CA); and anti-CD40-biotin, anti-CD11b-eFluor 450, anti-CD11c-PE-Cy5, anti-pan-NK CD49b-PE, and streptavidin-PE-Cy7 (eBioscience, San Diego, CA). For intracellular cytokine staining, 1 × 106 dLN cells were restimulated with 50 ng/ml of phorbol myristate acetate (PMA) and 500 ng/ml of ionomycin for 4 h, washed, blocked, and stained on the surface using anti-CD4-Alexa-Fluor 700 and anti-CD8α-APC (BioLegend); they were then fixed and permeabilized with 0.5% saponin. Cells were stained using anti-IFN-γ-PE or the isotype control anti-rat IgG1-PE (eBioscience). All cell events were acquired on an LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo (Tree Star, Ashland, OR). For quantification, median fluorescent intensities (MFI) were averaged and plotted with the standard errors of the means (SEM).
Histology.
Footpads were taken at peak lesion development, fixed in 4% paraformaldehyde, washed 3 times in phosphate-buffered saline (PBS), decalcified and embedded in paraffin, sectioned, and subjected to hematoxylin and eosin staining. Three-micrometer-thick sections were examined by light microscopy, and images were acquired at magnifications of ×40 and ×400 using a Leica DM 2000 microscope with a camera and FireCam software, version 3.2 (Leica Microsystems, Heerbrug, Switzerland).
mRNA isolation and real-time PCR.
mRNA was extracted with an RNeasy Plus minikit (Qiagen, Hilden, Germany), and quantitative real-time PCR was carried out using random 9-mers, Moloney murine leukemia virus (MMLV) reverse transcriptase RNase H− (Promega), and SYBR green on a LightCycler system (Roche). The cytokine primer sequences have been previously published (40–42). The results were normalized to the hypoxanthine phosphoribosyltransferase (HPRT) housekeeping gene using the comparative threshold cycle method (ΔCT) for relative quantification (43).
Macrophage infection and killing in vitro.
A total of 5 × 105 bone marrow-derived macrophages (BMMϕ) were plated on coverslips (Thermo Scientific, Rochester, NY) in 24-well plates and allowed to adhere overnight in complete RPMI–10% FCS. Cells were stimulated for 24 h with 10 ng/ml of IFN-γ (BD Pharmingen) and 10 ng/ml of TNF-α (eBioscience). BMMϕ were exposed to 2.5 × 106 L. braziliensis or L. major parasites for 2 h; then, the extracellular parasites were removed by 3 rounds of washing, and BMMΦ were cultured for an additional 48 h in the presence or absence of IFN-γ and TNF-α. The coverslips were subjected to Diff-Quik staining (Medion Diagnostics, Dudingen, Switzerland) according to the manufacturer's instructions. Supernatant nitrite levels were measured by the Griess reaction as previously described (44). The percentage of infected macrophages and number of intracellular parasites per 100 infected macrophages were determined by 2 different individuals counting 100 macrophages in triplicate coverslips by microscopy.
Statistics.
Two-tailed Student's unpaired t test was carried out using GraphPad Prism 5 software (San Diego, CA).
RESULTS
DC activation by an LRV1-free L. braziliensis strain is TLR9 dependent in vitro.
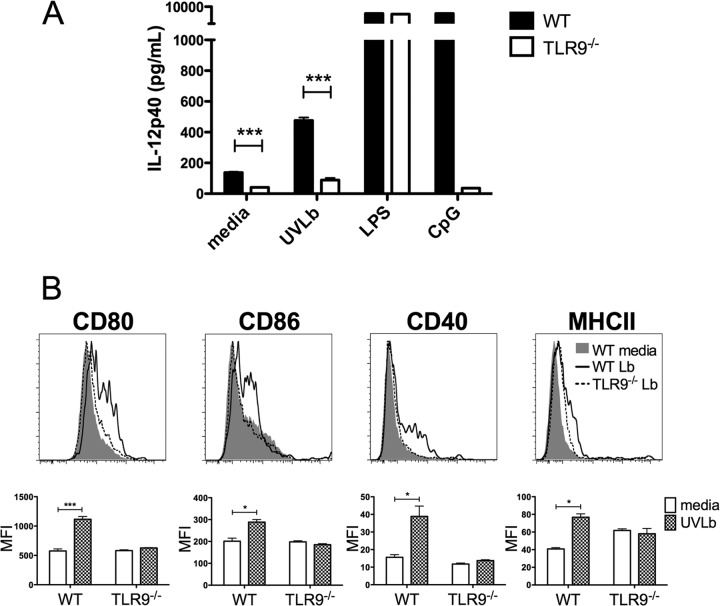
DC activation by different Leishmania species (L. major and L. infantum) was reported to be TLR9 dependent. L. braziliensis was also shown to induce IL-12 release by DCs in a TLR9-dependent manner (23, 25, 30, 31). However, L. braziliensis strains can harbor Leishmania RNA virus (LRV1) (45), and the presence of LRV1 could influence the immune response, as was shown in L. guyanensis infection (37). It was therefore important to first characterize whether the L. braziliensis strain used in this study was harboring any LRV1, a parameter which was not considered in previous studies. Nucleic extracts of the promastigote stage of the parasite were prepared, and genomic DNA was digested to visualize the presence of viral mRNA. No LRV1 mRNA was detectable in L. braziliensis strain BA-788, while it was detected in the LRV1-containing L. guyanensis positive control (see Fig. S1 in the supplemental material). Having excluded any possible role for LRV1, we could then examine if the L. braziliensis used in our in vivo experiments activated DCs in a TLR9-dependent manner. Bone marrow-derived conventional DCs stimulated with parasites produced significantly more IL-12p40 than did unstimulated DCs in vitro (P < 0.05). In contrast, cDCs from TLR9−/− mice displayed impaired IL-12p40 production compared to DCs from WT mice stimulated with L. braziliensis (Fig. 1A). The small increase in IL-12p40 detected in response to L. braziliensis in DCs from TLR9−/− mice was not statistically significant compared to the value for cells cultured in medium alone. LPS, which acts through TLR4-induced IL-12p40 production in cDCs from both WT and TLR9−/− mice, and CpG, a TLR9 ligand, induced IL-12p40 production selectively in cDCs from WT mice but not in DCs from TLR9-deficient mice. DC activation was further examined through the expression of costimulatory molecules. cDCs significantly upregulated the expression of CD80, CD86, CD40, and MHC-II upon stimulation with L. braziliensis parasites (Fig. 1B). In contrast, costimulatory molecule expression was not increased in cDCs from TLR9-deficient mice stimulated with L. braziliensis compared to that in unstimulated cells (Fig. 1B). Parallel experiments were carried out using bone marrow Flt3L-derived total DCs and FACS-sorted pDCs, and similar results were obtained (see Fig. S2 in the supplemental material). Collectively, these in vitro results demonstrate that L. braziliensis activated cDCs and pDCs in a TLR9-dependent manner.
Fig 1.
IL-12p40 production and surface expression of costimulatory molecules by cDCs in response to L. braziliensis are dependent on TLR9. (A) GM-CSF-derived cDCs from WT and TLR9−/− mice were stimulated or not with UV-treated L. braziliensis parasites (5:1) (UVLb), LPS, or CpG for 24 h. Supernatants were harvested and assessed for IL-12p40 production by ELISA. Experiments were completed in triplicate; results are means + SEM. ***, P < 0.0005 (Student's t test comparing TLR9−/− mice to control mice). Data shown are results from 1 experiment and representative of 4 independent experiments. (B) cDCs from WT and TLR9−/− mice were stimulated or not with UVLb (5:1) for 24 h. DCs were harvested and subjected to flow cytometric analysis. Representative histograms for CD40, CD80, CD86, and MHC-II expression are shown with corresponding quantification calculating the mean MFI + SEM of triplicates from 1 experiment representative of at least 3 independent experiments. Histograms show basal WT expression (gray), cells from WT mice stimulated by L. braziliensis (solid line), and cells from TLR9−/− mice stimulated by L. braziliensis (dotted line). ***, P < 0.0005; *, P < 0.05 (Student's t test comparing unstimulated DCs to L. braziliensis-stimulated DCs).
TLR9-deficient mice display transient increased lesion size and parasite burden after L. braziliensis infection.
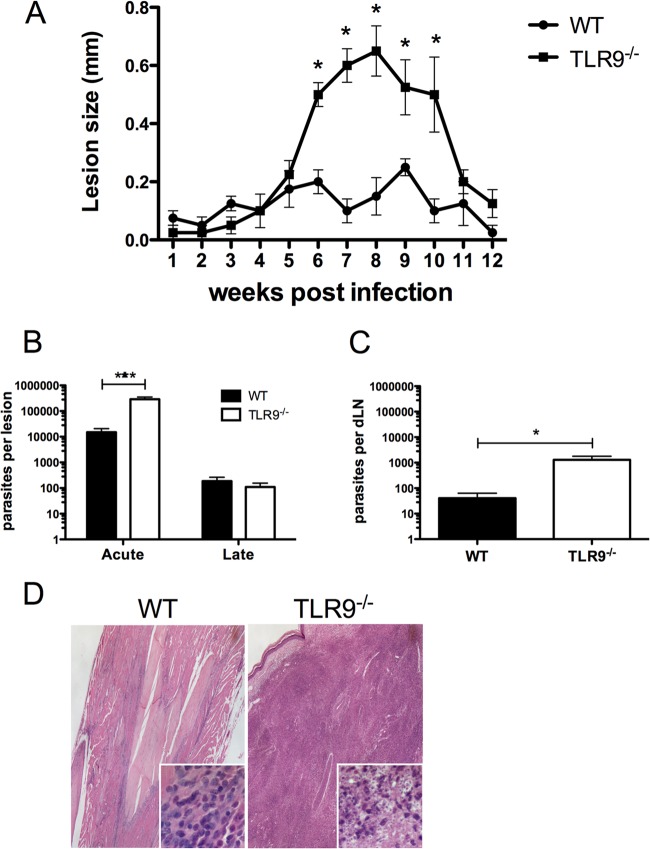
Since DCs induce T helper cell responses, we then determined if the absence of TLR9 expression would alter the course of infection and the development of the immune response to L. braziliensis inoculation. C57BL/6 WT and TLR9−/− mice were inoculated with L. braziliensis parasites, and lesion development was monitored. TLR9−/− mice developed significantly larger lesions than did WT mice even though lesions of TLR9−/− mice eventually healed later in infection (Fig. 2A). At the peak lesion development from 6 to 10 weeks postinoculation (p.i.), TLR9−/− mice also harbored significantly higher parasite numbers in the footpad than WT mice. The parasite load was eventually controlled correlating with the resolution of the lesion at 12 weeks p.i. (Fig. 2B). Since L. braziliensis has a specific tropism for the dLNs, parasite load in this organ was similarly analyzed. TLR9−/− mice exhibited a significantly higher parasite burden in the dLNs at peak lesion development than did WT mice (Fig. 2C). Only low numbers of parasites were detected at 12 weeks p.i. in the dLNs as determined by PCR, with no difference between TLR9−/− and WT mice (data not shown). At the peak lesion size, histology was carried out on the site of parasite inoculation to analyze the cellular infiltrate. Microscopy revealed that the TLR9−/− mice exhibited a severe inflammatory response with heavily parasitized macrophages, whereas the control group displayed a weak inflammatory response (Fig. 2D). Taken together, these findings demonstrate that TLR9 signaling is important in the early events controlling lesion development and parasite loads.
Fig 2.
Increased susceptibility and parasitemia of TLR9−/− mice infected with L. braziliensis. TLR9−/− mice and WT mice were injected in the hind footpad subcutaneously with stationary-phase L. braziliensis promastigotes. (A) Lesion sizes were monitored weekly with a caliper. (B) At 6 (acute) and 12 (late) weeks p.i., mice were sacrificed and footpad parasite loads were determined by LDA. Data shown are results pooled from 3 independent experiments and presented as means + SEM (n > 12 mice per group). (C) At the acute time point p.i., dLN parasite loads were determined by LDA; data are results pooled from two independent experiments (n > 7 mice per group). Data are presented as means + SEM. ***, P < 0.0005; *, P < 0.05 (Student's t test comparing TLR9−/− mice to control mice). (D) Histological analysis of footpad lesion by hematoxylin and eosin staining at a magnification of ×40, with insets at a magnification of ×400.
A high level of IFN-γ mRNA is expressed at the site of infection in TLR9-deficient mice.
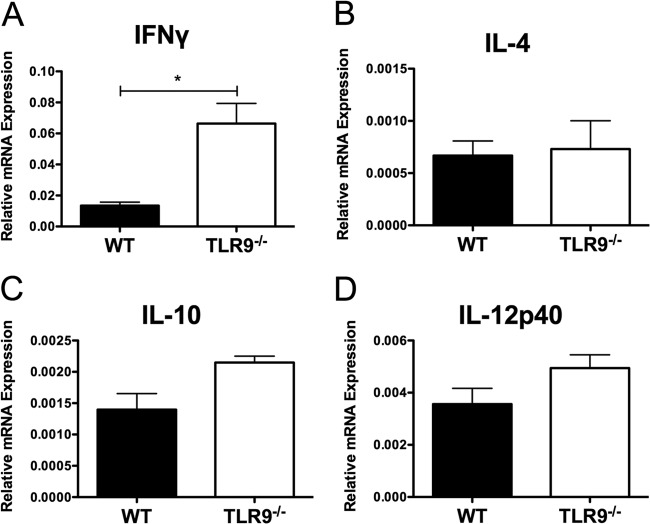
Given the increased lesion size and parasite burden in TLR9−/− mice, and the impaired IL-12 secretion by DCs from TLR9−/− mice in vitro, we hypothesized that L. braziliensis-inoculated TLR9−/− mice would have deficient levels of IL-12 at the site of infection. We first characterized the mRNA expression levels of various cytokines in footpads at peak lesion development by real-time PCR. Surprisingly, the levels of IL-12p40 mRNA were similar between TLR9−/− and WT mice (Fig. 3D). Moreover, TLR9-deficient animals had higher levels of IFN-γ mRNA at the site of infection than did control mice infected with L. braziliensis (Fig. 3A). In addition, the levels of IL-10 and IL-4 at the site of parasite inoculation were similarly low in WT and TLR9−/− mice (Fig. 3B and C). Fizz1 mRNA levels were not detectable, confirming the absence of Th2 cytokines and the corresponding lack of macrophage alternative activation (see Fig. S3 in the supplemental material). Thus, the major difference in the cytokines analyzed at the site of parasite inoculation is the increase in IFN-γ mRNA in TLR9−/− mice.
Fig 3.
L. braziliensis-infected mice exhibit higher levels of IFN-γ at the infection site. L. braziliensis parasites were injected in the footpads of WT and TLR9−/− mice and footpads were taken at peak lesion development and homogenized. RNA was isolated, cDNA was synthesized, and real-time PCR was carried out for IFN-γ (A), IL-4 (B), IL-10 (C), and IL-12p40 (D) expression. Relative mRNA expression normalized to the housekeeping HPRT gene is presented as the mean + SEM (n = at least 5 footpads per group) from 1 experiment representative of 2 independent experiment's. *, P < 0.05 (Student's t test comparing TLR9−/− mice to control mice).
L. braziliensis parasites are less susceptible to IFN-γ-mediated killing by BM-derived macrophages.
Surprisingly, the increased levels of IFN-γ mRNA in the footpads of infected TLR9−/− mice were associated with higher parasite burdens; therefore, we wanted to determine if macrophages from WT or TLR9−/− mice were able to kill Leishmania parasites with similar efficiencies in the presence or absence of IFN-γ and TNF-α. BMMϕ were activated in vitro with IFN-γ and TNF-α prior to infection with L. braziliensis; then parasites were removed and cells were continuously cultured with or without IFN-γ and TNF-α. L. major macrophage infection was used as a positive control. For BMMϕ from both WT and TLR9−/− mice, the presence of IFN-γ or TNF-α did not lead to a significant decrease in the percentage of L. braziliensis-infected macrophages after 48 h. In contrast, there was a significant reduction in the percentage of L. major-infected BMMϕ cultured with IFN-γ and TNF-α compared to L. major-infected BMMϕ alone, demonstrating induced killing of this parasite (Fig. 4A). In addition, activation of L. major-infected BMMø from WT mice with IFN-γ and TNF-α induced a significant reduction in the number of parasites per cell, while the activation of L. braziliensis-infected BMMϕ from WT mice did not lead to reduced number of parasites per cell. The presence or absence of TLR9 on BMMϕ did not change the number of parasites per macrophage (Fig. 4B). IFN-γ induces inducible nitric oxide synthase (iNOS), leading to the release of nitric oxide (NO). Similar levels of NO were measured in supernatants of IFN-γ-treated BMMϕ exposed to either L. major or L. braziliensis, suggesting that there was no defect in NO production by BMMϕ from WT or TLR9−/− mice exposed to L. braziliensis parasites that could explain defective parasite killing (Fig. 4C). Taken together, these data suggest that while L. major parasites are susceptible to IFN-γ-mediated killing by BMMϕ in vitro, L. braziliensis parasites are less susceptible to NO-induced killing.
Fig 4.

L. braziliensis parasites are less susceptible to IFN-γ-mediated killing by macrophages. BM-derived macrophages from WT and TLR9−/− mice were stimulated for 24 h with IFN-γ and TNF-α, followed by infection for 2 h with L. braziliensis (Lb) or L. major (Lm) parasites. Extracellular parasites were washed away, and cells were cultured with or without IFN-γ and TNF-α for another 48 h. Coverslips were stained with Diff-Quik, and the percentage of infected macrophages (A) as well as the number of parasites per infected macrophage (B) was determined by microscopy. (C) Supernatants were collected and NO content was measured by the Griess reaction. Data shown are presented as the mean + SEM of triplicates. *, P < 0.05 (Student's t test comparing the percentages of BMMϕ infected with parasites in the presence and absence of IFN-γ and TNF-α). Data shown here are results from 1 representative experiment of 2 individual experiments.
IFN-γ production is higher in dLN cells from TLR9−/− mice following L. braziliensis inoculation.
Next, we determined the cytokines produced by dLN cells during infection. Consistent with the elevated levels of IFN-γ detected by real-time PCR at the site of infection, TLR9−/− dLN cells restimulated with L. braziliensis parasites secreted more IFN-γ at the peak lesion development (acute) as well as after the resolution of the lesion (late) compared to control mice (Fig. 5A). In addition, FACS analysis revealed significantly higher numbers of both CD4+ as well as CD8+ T cells producing IFN-γ in the dLNs of TLR9−/− mice than in those of WT mice (Fig. 5B). Upon restimulation, dLN cells secreted very low levels of IL-4 and IL-13, with no differences between TLR9−/− and WT mice (Fig. 5A). IL-10 was released at detectable levels by dLN cells after restimulation with parasites, but there was no difference between TLR9-deficient and control mice (Fig. 5A). Thus, higher levels of IFN-γ, indicative of a Th1 response, were detected in the dLNs of TLR9−/− mice than in those of WT mice, but there was no increase in the Th2 responses measured.
Fig 5.
dLN cells from L. braziliensis-infected TLR9−/− mice produce higher levels of IFN-γ. TLR9−/− and WT mice were infected in the footpad with L. braziliensis parasites. (A) dLN cells were taken at the time of lesion development (acute) and after lesion resolution (late) and exposed to UV-treated L. braziliensis (UVLb) parasites in vitro for 72 h. Supernatants were harvested and assessed for production of cytokines, including IFN-γ, IL-4, IL-13, and IL-10, by ELISA. Data shown are the means of triplicate measurements for each animal from at least 2 pooled experiments + SEM. (B) dLN cells were taken at the time of lesion development, and intracellular cytokine staining was performed as described in Materials and Methods. The number of IFN-γ-producing T cells was analyzed after gating on CD4+ or CD8+ T cells. Data presented here are means + SEM (n = at least 5 mice per group) representative of 2 independent experiments. *, P < 0.05 (Student's t test comparing TLR9−/− mice to control mice). nd, not detectable.
dLN hypertrophy is increased in TLR9−/− mice compared to controls.
In humans, L. braziliensis infection has been associated with the presence of enlarged dLNs that harbor parasites, and this lymphadenopathy occurs prior to lesion development (46, 47). The increased levels of IFN-γ at the site of infection and in the dLNs suggested that cell migration to the dLNs is normal in TLR9−/− mice infected with L. braziliensis, which is in contrast to findings for L. major-infected TLR9−/− mice, which exhibit dLN hypotrophy (31). We therefore determined if the increased susceptibility in L. braziliensis-inoculated TLR9−/− mice was associated with altered dLN expansion. Given that defects in dLN hypertrophy were seen as early as 3 days p.i. with L. major, we examined the number of dLN cells at early time points after L. braziliensis infection. After L. braziliensis inoculation, similar numbers of dLN cells were measured 3 and 7 days p.i. in TLR9−/− mice and WT mice, suggesting that TLR9 is not involved in the early ability of the dLNs to expand in response to L. braziliensis inoculation (Fig. 6A). These results contrast with the dLN hypotrophy measured in L. major-infected TLR9−/− mice (31). dLN expansion of TLR9−/− mice infected with L. braziliensis was intact, and there were significantly more dLN cells in TLR9−/− mice than in WT mice both at the peak of lesion development (acute) and after the resolution (late) of the lesion (Fig. 6B).
Fig 6.

dLN hypertrophy is intact in TLR9−/− mice infected with L. braziliensis. TLR9−/− and WT mice were infected in the footpad with L. braziliensis parasites. (A) dLN cells were taken at days 3 and 7 and counted. (B) dLN cells were taken at the time of lesion development (acute) and after lesion resolution (late) and counted. Data are presented as means + SEM (n = at least 3 mice per group). **, P < 0.005; *, P < 0.05 (Student's t test comparing TLR9−/− mice to control mice). Data shown here are results from 1 experiment representative of 2 independent experiments.
DISCUSSION
In this study, we showed that TLR9−/− mice are transiently susceptible to L. braziliensis infection, developing larger lesions and greater parasite loads, a phenotype similar to that observed following injection of L. major in TLR9−/− mice (25, 30, 31). However, the transient susceptibility observed in L. braziliensis-inoculated TLR9−/− mice was not associated with a failure to develop a CD4+ Th1 response, or an increase in the differentiation of Th2 cells, as observed in the L. major studies (25, 30). In contrast, higher levels of IFN-γ were measured in dLN T cells and at the site of parasite inoculation in TLR9−/− mice. In addition, dLN expansion in L. braziliensis-infected mice was comparable to that occurring in WT mice, further contrasting with what has been observed in L. major-infected TLR9−/− mice (31).
Infection with different strains of L. braziliensis in humans leads to different clinical outcomes. Similarly, significant differences in disease outcome in mice were observed following the injection of parasites isolated from patients presenting different manifestations of the disease (10, 48, 49). Several parasite-derived factors may contribute to this diversity, such as the strain of the parasite, the presence of the Leishmania virus, and ectonucleotidase activity (10, 37, 45, 48, 49). The presence of the Leishmania RNA virus may influence the immune response, as recently shown for the LRV1-carrying L. guyanensis strain (37). Some strains of L. braziliensis harbor LRV1 (45), but we show that the L. braziliensis strain used in this study does not host LRV1, excluding any viral effect on the course of infection.
Unlike other New World species such as L. mexicana or L. amazonensis, L. braziliensis can activate DCs to produce cytokines, including IL-12p40 and IFN-α/β, and upregulate costimulatory molecules (Fig. 1) (16, 18, 23). Furthermore, IL-12p40 induction has been shown to differ between promastigotes and amastigotes of New World Leishmania species (16, 50). The production of IL-12p40 by DCs in response to L. major or its DNA was shown to be dependent on TLR9 (25). Here, we show that the activation of DCs based on the production of IL-12p40 and upregulation of costimulatory expression in response to L. braziliensis in vitro is also TLR9 dependent, in accordance with a previous study (23). In contrast, TLR9−/− mice infected with L. braziliensis in vivo exhibited intact IL-12p40 mRNA levels at the site of infection. The difference in the requirements of TLR9 for IL-12p40 production observed in vitro and in vivo may be due to the parasite stage, as the presence of L. braziliensis amastigotes, which are strong inducers of DC activation, may bypass the need for TLR9 observed in response to L. braziliensis promastigotes (16). Alternatively, other mechanisms may compensate for the need for TLR9 signaling in DCs in vivo.
At the peak of lesion development from 6 to 10 weeks p.i., TLR9-deficient mice were more susceptible to L. braziliensis, as characterized by increased lesion sizes and parasite loads compared to those in WT mice. Surprisingly, at this stage of infection, the TLR9−/− mice had increased levels of IFN-γ at the inoculation site as well as in dLN cells compared to control mice. L. braziliensis inoculation induces a strong production of IFN-γ by dLN cells that has been shown to occur early (10, 15). Here we show CD4+ and CD8+ T cells are the major sources of IFN-γ in the dLNs. Various cells can produce IFN-γ in the tissue after Leishmania infection, including NK cells, CD4+ cells, and CD8+ cells. Increased numbers of NK cells have been found at the site of L. braziliensis infection in susceptible mice (10, 25), indicating that NK cells may also play an important role during L. braziliensis infection.
During Leishmania infection, IFN-γ-driven Th1 immune responses along with TNF-α lead to macrophage activation and the expression of iNOS, which catalyzes the synthesis of NO from arginine. NO has potent antimicrobial effects that lead to the killing of intracellular parasites. However, different Leishmania strains exhibit various susceptibilities to macrophage-mediated killing by NO (48, 51). Here we show that activation of L. major-infected macrophages with IFN-γ and TNF-α induced parasite killing in vitro, but L. braziliensis-infected macrophages exposed to the same levels of IFN-γ and TNF-α did not efficiently eliminate the parasites at the time points analyzed. Similar percentages of macrophages were infected with L. braziliensis or L. major after 2 h of infection in cells from both WT and TLR9-deficient mice, but within the time interval analyzed no evidence of parasite killing was detectable. The lower efficiency in parasite killing was not due to defective NO production by BMMϕ. These findings are consistent with other studies in which New World Leishmania parasites required larger amounts of NO for parasite killing than L. major, and lesion size was associated with the decreased susceptibility to NO toxicity (51–53). Furthermore, after isolation from patients, L. braziliensis strains with decreased susceptibility to NO were reported to multiply better in macrophages and could be linked to nonresponsiveness to antimony treatment (52, 54). The susceptibility to IFN-γ and TNF-α or IFN-γ and LPS to induce macrophage killing of L. braziliensis may vary between different L. braziliensis strains, as recently reported from studies performed in vitro (23, 48). Nevertheless, the killing of L. braziliensis observed in vitro in the present study did not differ between macrophages from WT and TLR9−/− mice, suggesting that other factors that remain to be determined contribute to the increased susceptibility observed in TLR9−/− infected mice.
We show here that unlike in previous studies with L. major (25, 30), the increased susceptibility in TLR9−/− mice infected with L. braziliensis is not due to an increased Th2 response, because only very low levels of IL-4 were detected at the site of infection and in the dLNs, which were similar to levels measured in WT mice. There were also no significant differences in IL-10 production, suggesting that the enhanced susceptibility in TLR9-deficient animals was not due to the presence of IL-10 and consequentially a down modulation of the immune response. The IL-12p40 mRNA expression detected at the infection site in TLR9-deficient mice was similar to that in controls, but the levels of IFN-γ were higher in TLR9−/− mice, suggesting that some of the IFN-γ is produced in an IL-12-independent manner, as reported in some cases (55–57). As with L. major (25, 30, 31), lesions of TLR9−/− mice infected with L. braziliensis do eventually heal, suggesting that other innate receptors (other TLRs) or other mechanisms may compensate for the inability of TLR9−/− mice to control lesion development and parasite burden early in infection. The role for other MyD88-dependent TLRs, such as TLR4, during L. braziliensis infection remains to be defined. However, TLR2−/− mice infected with L. braziliensis displayed parasite loads similar to those of control mice despite higher levels of IFN-γ in their dLNs, further showing that IFN-γ levels do not correlate with parasite killing in vivo in L. braziliensis infection (33).
In humans, a clinical feature of L. braziliensis infection is the development of lymphadenopathy (46, 47, 58). Similarly, in the murine model of L. braziliensis, dLNs from infected mice, independent of the strain used, increase in size and cell number over the course of infection, and the increase in dLN cell number does not correlate to the size or presence of the lesion at the inoculation site (10). Recently, it has been demonstrated that the activation of DCs through TLR9 is involved in dLN expansion via a CCR7-dependent mechanism in L. major (31). In addition, TLR9 triggering of pDCs contributed to the recruitment of innate cells after L. major stimulation (59). However, here we show that the dLNs isolated from TLR9-deficient mice infected with L. braziliensis expanded at a rate similar to that in control mice early in infection, and at later time points dLNs expanded markedly compared to the rate in WT mice, further revealing that the dependence on TLR9-triggered mechanisms during the infection varies between New World and Old World Leishmania parasites. The association between susceptibility and dLN hypertrophy during L. braziliensis infection appears to be independent of TLR9 signaling.
In conclusion, TLR9 signaling contributes to resistance to L. braziliensis infection, but interestingly, it is not required for the generation of an IFN-γ-driven Th1 immune response. Additionally, elevated levels of IFN-γ do not guarantee the elimination of L. braziliensis parasites from the site of infection or the dLNs. These data emphasize the crucial role that TLR9 plays in the early response to L. braziliensis infection and that IFN-γ is not a surrogate marker of protection in this infection. These results contribute to the understanding of the innate mechanisms controlling parasite elimination in leishmaniasis. Such studies will help define the immunologic mechanisms required for protective immunity that could be exploited for therapeutic intervention and vaccine development.
Supplementary Material
ACKNOWLEDGMENTS
We thank Pascal Launois and Camila Indiani de Oliveira (FIOCRUZ, Salvador, Brazil) for critical reading of the manuscript, as well as Catherine Ronet, Devika Ashok, Manuel Coutaz, and Benjamin Hurrell for discussion and Corentin Pasche for technical support (UNIL, Lausanne, Switzerland).
This work was supported by grants from the Swiss National Foundation (3100030-129852 and Candoc 129700/1) and the Brazilian-Swiss Research Program (BSJRP) to F.T.-C. Work in the N. Fasel lab was supported by the Swiss National Foundation (3100A0-116665/1 and IZ70Z0-131421) and aIAR.
Footnotes
Published ahead of print 25 February 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01401-12.
REFERENCES
- 1. Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. 2007. Cutaneous leishmaniasis. Lancet Infect. Dis. 7:581–596 [DOI] [PubMed] [Google Scholar]
- 2. Bittencourt AL, Barral A. 1991. Evaluation of the histopathological classifications of American cutaneous and mucocutaneous leishmaniasis. Mem. Inst. Oswaldo Cruz 86:51–56 [DOI] [PubMed] [Google Scholar]
- 3. Marsden PD. 1985. Clinical presentations of Leishmania braziliensis braziliensis. Parasitol. Today 1:129–133 [DOI] [PubMed] [Google Scholar]
- 4. Carvalho EM, Johnson WD, Barreto E, Marsden PD, Costa JL, Reed S, Rocha H. 1985. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J. Immunol. 135:4144–4148 [PubMed] [Google Scholar]
- 5. Marsden PD. 1986. Mucosal leishmaniasis (“espundia” Escomel, 1911). Trans. R. Soc. Trop. Med. Hyg. 80:859–876 [DOI] [PubMed] [Google Scholar]
- 6. Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, Gollob KJ, Carvalho EM. 2002. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect. Immun. 70:6734–6740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pirmez C, Yamamura M, Uyemura K, Paes-Oliveira M, Conceicao-Silva F, Modlin RL. 1993. Cytokine patterns in the pathogenesis of human leishmaniasis. J. Clin. Invest. 91:1390–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Childs GE, Lightner LK, McKinney L, Groves MG, Price EE, Hendricks LD. 1984. Inbred mice as model hosts for cutaneous leishmaniasis. I. Resistance and susceptibility to infection with Leishmania braziliensis, L. mexicana, and L. aethiopica. Ann. Trop. Med. Parasitol. 78:25–34 [DOI] [PubMed] [Google Scholar]
- 9. DeKrey GK, Lima HC, Titus RG. 1998. Analysis of the immune responses of mice to infection with Leishmania braziliensis. Infect. Immun. 66:827–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Indiani de Oliveira C, Teixeira MJ, Teixeira CR, Ramos de Jesus J, Bomura Rosato A, Santa da Silva J, Brodskyn C, Barral-Netto M, Barral A. 2004. Leishmania braziliensis isolates differing at the genome level display distinctive features in BALB/c mice. Microbes Infect. 6:977–984 [DOI] [PubMed] [Google Scholar]
- 11. Maioli TU, Takane E, Arantes RM, Fietto JL, Afonso LC. 2004. Immune response induced by New World Leishmania species in C57BL/6 mice. Parasitology Res. 94:207–212 [DOI] [PubMed] [Google Scholar]
- 12. Neal RA, Hale C. 1983. A comparative study of susceptibility of inbred and outbred mouse strains compared with hamsters to infection with New World cutaneous leishmaniases. Parasitology 87(Part 1):7–13 [DOI] [PubMed] [Google Scholar]
- 13. Samuelson J, Lerner E, Tesh R, Titus R. 1991. A mouse model of Leishmania braziliensis braziliensis infection produced by coinjection with sand fly saliva. J. Exp. Med. 173:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Souza-Neto SM, Carneiro CM, Vieira LQ, Afonso LC. 2004. Leishmania braziliensis: partial control of experimental infection by interleukin-12 p40 deficient mice. Mem. Inst. Oswaldo Cruz 99:289–294 [DOI] [PubMed] [Google Scholar]
- 15. Rocha FJ, Schleicher U, Mattner J, Alber G, Bogdan C. 2007. Cytokines, signaling pathways, and effector molecules required for the control of Leishmania (Viannia) braziliensis in mice. Infect. Immun. 75:3823–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vargas-Inchaustegui DA, Xin L, Soong L. 2008. Leishmania braziliensis infection induces dendritic cell activation, ISG15 transcription, and the generation of protective immune responses. J. Immunol. 180:7537–7545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bennett CL, Misslitz A, Colledge L, Aebischer T, Blackburn CC. 2001. Silent infection of bone marrow-derived dendritic cells by Leishmania mexicana amastigotes. Eur. J. Immunol. 31:876–883 [DOI] [PubMed] [Google Scholar]
- 18. Carvalho LP, Pearce EJ, Scott P. 2008. Functional dichotomy of dendritic cells following interaction with Leishmania braziliensis: infected cells produce high levels of TNF-alpha, whereas bystander dendritic cells are activated to promote T cell responses. J. Immunol. 181:6473–6480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soong L. 2008. Modulation of dendritic cell function by Leishmania parasites. J. Immunol. 180:4355–4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Müller I, Freudenberg M, Kropf P, Kiderlen AF, Galanos C. 1997. Leishmania major infection in C57BL/10 mice differing at the Lps locus: a new non-healing phenotype. Med. Microbiol. Immunol. 186:75–81 [DOI] [PubMed] [Google Scholar]
- 21. Kropf P, Freudenberg MA, Modolell M, Price HP, Herath S, Antoniazi S, Galanos C, Smith DF, Muller I. 2004. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect. Immun. 72:1920–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kropf P, Freudenberg N, Kalis C, Modolell M, Herath S, Galanos C, Freudenberg M, Muller I. 2004. Infection of C57BL/10ScCr and C57BL/10ScNCr mice with Leishmania major reveals a role for Toll-like receptor 4 in the control of parasite replication. J. Leukoc. Biol. 76:48–57 [DOI] [PubMed] [Google Scholar]
- 23. Schleicher U, Liese J, Knippertz I, Kurzmann C, Hesse A, Heit A, Fischer JA, Weiss S, Kalinke U, Kunz S, Bogdan C. 2007. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J. Exp. Med. 204:893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tuon FF, Amato VS, Bacha HA, Almusawi T, Duarte MI, Amato Neto V. 2008. Toll-like receptors and leishmaniasis. Infect. Immun. 76:866–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liese J, Schleicher U, Bogdan C. 2007. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur. J. Immunol. 37:3424–3434 [DOI] [PubMed] [Google Scholar]
- 26. de Veer MJ, Curtis JM, Baldwin TM, DiDonato JA, Sexton A, McConville MJ, Handman E, Schofield L. 2003. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 33:2822–2831 [DOI] [PubMed] [Google Scholar]
- 27. Debus A, Glasner J, Rollinghoff M, Gessner A. 2003. High levels of susceptibility and T helper 2 response in MyD88-deficient mice infected with Leishmania major are interleukin-4 dependent. Infect. Immun. 71:7215–7218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muraille E, De Trez C, Brait M, De Baetselier P, Leo O, Carlier Y. 2003. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J. Immunol. 170:4237–4241 [DOI] [PubMed] [Google Scholar]
- 29. Revaz-Breton M, Ronet C, Ives A, Torre YH, Masina S, Tacchini-Cottier F, Launois P. 2010. The MyD88 protein 88 pathway is differently involved in immune responses induced by distinct substrains of Leishmania major. Eur. J. Immunol. 40:1697–1707 [DOI] [PubMed] [Google Scholar]
- 30. Abou Fakher FH, Rachinel N, Klimczak M, Louis J, Doyen N. 2009. TLR9-dependent activation of dendritic cells by DNA from Leishmania major favors Th1 cell development and the resolution of lesions. J. Immunol. 182:1386–1396 [DOI] [PubMed] [Google Scholar]
- 31. Carvalho LP, Petritus PM, Trochtenberg AL, Zaph C, Hill DA, Artis D, Scott P. 2012. Lymph node hypertrophy following Leishmania major infection is dependent on TLR9. J. Immunol. 188:1394–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tacchini-Cottier F, Weinkopff T, Launois P. 2012. Does T helper differentiation correlate with resistance or susceptibility to infection with L. major? Some insights from the murine model. Front. Immunol. 3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vargas-Inchaustegui DA, Tai W, Xin L, Hogg AE, Corry DB, Soong L. 2009. Distinct roles for MyD88 and Toll-like receptor 2 during Leishmania braziliensis infection in mice. Infect. Immun. 77:2948–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Moura TR, Novais FO, Oliveira F, Clarencio J, Noronha A, Barral A, Brodskyn C, de Oliveira CI. 2005. Toward a novel experimental model of infection to study American cutaneous leishmaniasis caused by Leishmania braziliensis. Infect. Immun. 73:5827–5834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Titus RG, Marchand M, Boon T, Louis JA. 1985. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 7:545–555 [DOI] [PubMed] [Google Scholar]
- 36. Taswell C. 1981. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J. Immunol. 126:1614–1619 [PubMed] [Google Scholar]
- 37. Ives A, Ronet C, Prevel F, Ruzzante G, Fuertes-Marraco S, Schutz F, Zangger H, Revaz-Breton M, Lye LF, Hickerson SM, Beverley SM, Acha-Orbea H, Launois P, Fasel N, Masina S. 2011. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science 331:775–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brasel K, De Smedt T, Smith JL, Maliszewski CR. 2000. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood 96:3029–3039 [PubMed] [Google Scholar]
- 40. Charmoy M, Megnekou R, Allenbach C, Zweifel C, Perez C, Monnat K, Breton M, Ronet C, Launois P, Tacchini-Cottier F. 2007. Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. J. Leukoc. Biol. 82:288–299 [DOI] [PubMed] [Google Scholar]
- 41. Otten LA, Tacchini-Cottier F, Lohoff M, Annunziato F, Cosmi L, Scarpellino L, Louis J, Steimle V, Reith W, Acha-Orbea H. 2003. Deregulated MHC class II transactivator expression leads to a strong Th2 bias in CD4+ T lymphocytes. J. Immunol. 170:1150–1157 [DOI] [PubMed] [Google Scholar]
- 42. Ronet C, Hauyon-La Torre Y, Revaz-Breton M, Mastelic B, Tacchini-Cottier F, Louis J, Launois P. 2010. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. J. Immunol. 184:886–894 [DOI] [PubMed] [Google Scholar]
- 43. Charmoy M, Brunner-Agten S, Aebischer D, Auderset F, Launois P, Milon G, Proudfoot AE, Tacchini-Cottier F. 2010. Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice. PLoS Pathog. 6:e1000755 doi:10.1371/journal.ppat.1000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chakour R, Allenbach C, Desgranges F, Charmoy M, Mauel J, Garcia I, Launois P, Louis J, Tacchini-Cottier F. 2009. A new function of the Fas-FasL pathway in macrophage activation. J. Leukoc. Biol. 86:81–90 [DOI] [PubMed] [Google Scholar]
- 45. Salinas G, Zamora M, Stuart K, Saravia N. 1996. Leishmania RNA viruses in Leishmania of the Viannia subgenus. Am. J. Trop. Med. Hyg. 54:425–429 [DOI] [PubMed] [Google Scholar]
- 46. Barral A, Barral-Netto M, Almeida R, de Jesus AR, Grimaldi Junior G, Netto EM, Santos I, Bacellar O, Carvalho EM. 1992. Lymphadenopathy associated with Leishmania braziliensis cutaneous infection. Am. J. Trop. Med. Hyg. 47:587–592 [DOI] [PubMed] [Google Scholar]
- 47. Barral A, Guerreiro J, Bomfim G, Correia D, Barral-Netto M, Carvalho EM. 1995. Lymphadenopathy as the first sign of human cutaneous infection by Leishmania braziliensis. Am. J. Trop. Med. Hyg. 53:256–259 [DOI] [PubMed] [Google Scholar]
- 48. Leite PM, Gomes RS, Figueiredo AB, Serafim TD, Tafuri WL, de Souza CC, Moura SA, Fietto JL, Melo MN, Ribeiro-Dias F, Oliveira MA, Rabello A, Afonso LC. 2012. Ecto-nucleotidase activities of promastigotes from Leishmania (Viannia) braziliensis relates to parasite infectivity and disease clinical outcome. PLoS Negl. Trop. Dis. 6:e1850 doi:10.1371/journal.pntd.0001850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Teixeira MJ, Fernandes JD, Teixeira CR, Andrade BB, Pompeu ML, Santana da Silva J, Brodskyn CI, Barral-Netto M, Barral A. 2005. Distinct Leishmania braziliensis isolates induce different paces of chemokine expression patterns. Infect. Immun. 73:1191–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xin L, Li K, Soong L. 2008. Down-regulation of dendritic cell signaling pathways by Leishmania amazonensis amastigotes. Mol. Immunol. 45:3371–3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gomes IN, Calabrich AF, Tavares Rda S, Wietzerbin J, de Freitas LA, Veras PS. 2003. Differential properties of CBA/J mononuclear phagocytes recovered from an inflammatory site and probed with two different species of Leishmania. Microbes Infect. 5:251–260 [DOI] [PubMed] [Google Scholar]
- 52. Giudice A, Camada I, Leopoldo PT, Pereira JM, Riley LW, Wilson ME, Ho JL, de Jesus AR, Carvalho EM, Almeida RP. 2007. Resistance of Leishmania (Leishmania) amazonensis and Leishmania (Viannia) braziliensis to nitric oxide correlates with disease severity in tegumentary leishmaniasis. BMC Infect. Dis. 7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mukbel RM, Patten C, Jr, Gibson K, Ghosh M, Petersen C, Jones DE. 2007. Macrophage killing of Leishmania amazonensis amastigotes requires both nitric oxide and superoxide. Am. J. Trop. Med. Hyg. 76:669–675 [PubMed] [Google Scholar]
- 54. Souza AS, Giudice A, Pereira JM, Guimaraes LH, de Jesus AR, de Moura TR, Wilson ME, Carvalho EM, Almeida RP. 2010. Resistance of Leishmania (Viannia) braziliensis to nitric oxide: correlation with antimony therapy and TNF-alpha production. BMC Infect. Dis. 10:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Skokos D, Nussenzweig MC. 2007. CD8− DCs induce IL-12-independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. J. Exp. Med. 204:1525–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, Dudziak D, Nussenzweig MC, Steinman RM. 2007. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J. Exp. Med. 204:1095–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Müller U, Kohler G, Mossmann H, Schaub GA, Alber G, Di Santo JP, Brombacher F, Holscher C. 2001. IL-12-independent IFN-gamma production by T cells in experimental Chagas' disease is mediated by IL-18. J. Immunol. 167:3346–3353 [DOI] [PubMed] [Google Scholar]
- 58. Sousa Ade Q, Parise ME, Pompeu MM, Coehlo Filho JM, Vasconcelos IA, Lima JW, Oliveira EG, Vasconcelos AW, David JR, Maguire JH. 1995. Bubonic leishmaniasis: a common manifestation of Leishmania (Viannia) braziliensis infection in Ceara, Brazil. Am. J. Trop. Med. Hyg. 53:380–385 [DOI] [PubMed] [Google Scholar]
- 59. Guillerey C, Mouries J, Polo G, Doyen N, Law HK, Chan S, Kastner P, Leclerc C, Dadaglio G. 2012. Pivotal role of plasmacytoid dendritic cells in inflammation and NK-cell responses after TLR9 triggering in mice. Blood 120:90–99 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.