Abstract
We have recently reported the ability of Leptospira to capture plasminogen (PLG) and generate plasmin (PLA) bound on the microbial surface in the presence of exogenous activators. In this work, we examined the effects of leptospiral PLG binding for active penetration through the endothelial cell barrier and activation. The results indicate that leptospires with PLG association or PLA activation have enhanced migration activity through human umbilical vein endothelial cell (HUVEC) monolayers compared with untreated bacteria. Leptospira cells coated with PLG were capable of stimulating the expression of PLG activators by HUVECs. Moreover, leptospires endowed with PLG or PLA promoted transcriptional upregulation matrix metalloprotease 9 (MMP-9). Serum samples from patients with confirmed leptospirosis showed higher levels of PLG activators and total MMP-9 than serum samples from normal (healthy) subjects. The highest level of PLG activators and total MMP-9 was detected with microscopic agglutination test (MAT)-negative serum samples, suggesting that this proteolytic activity stimulation occurs at the early stage of the disease. Furthermore, a gelatin zymography profile obtained for MMPs with serum samples from patients with leptospirosis appears to be specific to leptospiral infection because serum samples from patients with unrelated infectious diseases produced no similar degradation bands. Altogether, the data suggest that the Leptospira-associated PLG or PLA might represent a mechanism that contributes to bacterial penetration of endothelial cells through an activation cascade of events that enhances the proteolytic capability of the organism. To our knowledge, this is the first proteolytic activity associated with leptospiral pathogenesis described to date.
INTRODUCTION
Leptospira interrogans is the causal agent of leptospirosis, a neglected zoonotic infectious disease of human and veterinary concern. The transmission of leptospirosis is associated with exposure of individuals to wild or farm animals (1). In urban settings, leptospirosis became a major health problem due to the lack of adequate sanitation measurements and the large population of urban rodent reservoirs that contaminate the environment through their urine (2). Although the leptospiral genome, functional genomics, and host-pathogen interactions are being actively investigated by many research groups, the molecular aspects of Leptospira virulence and pathogenesis and the origin of physiological leptospirosis symptoms and severity of disease remain to be understood (3–9).
L. interrogans has the capacity to disseminate rapidly, causing systemic infection after skin penetration. Indeed, the rapid transmigration of polarized MDCK cell monolayers by virulent leptospires has been reported (10), yet, the mechanisms involved in such transmigration and dissemination were not understood (10). The bacterial interaction with the host's fibrinolytic system by surface plasminogen (PLG) capture and/or PLG activation to plasmin (PLA) has been suggested to be a feature that significantly contributes to the virulence of several pathogens by facilitating penetration and invasion (11). It has been shown that the coating of Borrelia burgdorferi with PLA resulted in enhanced penetration of endothelial cell monolayers (12). We first described the interaction of Leptospira with the PLG/PLA system and some possible implications for pathogenesis (13). We demonstrated that leptospires interact with PLG and can acquire PLA activity associated with the surface without interfering in cell growth and viability, which occur through multiple receptors (13–17). Although nonvirulent leptospires can interact with PLG, there seems to be a correlation between the efficiency of PLG capture and virulence, suggesting a role in virulence and infection. In fact, PLG receptors seem to be differentially expressed in virulent strains.
In the present study, we focused on elucidating possible mechanisms involved when Leptospira cells associated with proteolytic activity are in contact with human cells. We show that PLA generation on the surface of leptospires facilitates the bacterial transmigration across the human endothelial cell barrier compared with leptospires lacking this proteolytic activity. We also show that the interaction with mammalian cells promoted an upregulation of PLG activators, thus increasing PLA generation on the bacterial surface. Furthermore, we describe how the interaction of PLA-associated Leptospira cells with human monolayer cells results in an upregulation of matrix metalloproteases (MMPs) from human umbilical vein endothelial cells (HUVECs). Moreover, we demonstrate that serum samples from patients with confirmed leptospirosis present different circulating amounts of PLG activators and MMPs than those from normal (healthy) individuals. Our data provide the first evidence of mechanisms involved during Leptospira-mammalian cell interactions that would contribute to tissue penetration and rapid bacterial dissemination.
MATERIALS AND METHODS
Bacterial isolates and culture conditions.
The virulent strain of L. interrogans serovar Kennewicki strain Pomona Fromm (strain LPF) was isolated from swine in the United States by the Salsbury/Solvay Laboratories. The strain is routinely cultured by iterative passages in Golden Syrian hamsters for maintenance of virulence. Recently weaned hamsters were intraperitoneally infected with 500 μl containing approximately 1.0 × 104 virulent leptospires. The animals were sacrificed after appearance of symptoms, such as loss of weight and mobility (approximately 5 days postinfection). The kidney and liver were removed and macerated, and the organ-derived leptospires were cultured at 28°C in modified Elinghausen-McCullough-Johnson-Harris (EMJH) medium supplemented with 10% Leptospira enrichment EMJH medium (BD, Difco).
Human serum sample collection.
Serum samples from patients with confirmed leptospirosis were obtained from the collection of Instituto Adolfo Lutz, São Paulo, Brazil; serum samples from patients with unrelated infectious diseases were obtained from the collections of the Laboratorio de Imunoepidemiologia, SUCEN, São Paulo, Brazil, the Laboratorio de Protozoologia, IMT/USP, São Paulo, Brazil (sera from patients with Chagas' disease), Laboratorio de Virologia, IMT/USP, São Paulo, Brazil (sera from patients with human immunodeficiency virus [HIV] infection and dengue), and the Nucleo de Estudos em Malária, SUCEN/IMT/USP, São Paulo, Brazil (sera from patients with malaria). Normal human sera were obtained from healthy donors with no reported leptospirosis history.
MAT.
The microscopic agglutination test (MAT) of the serum samples was performed according to Faine et al. (2). In brief, an array of serovars of Leptospira spp. as antigens were employed: Australis, Autumnalis, Bataviae, Canicola, Castellonis, Celledoni, Copenhageni, Cynopteri, Djasiman, Grippotyphosa, Hardjo, Hebdomadis, Icterohaemorrhagiae, Javanica, Panama, Patoc, Pomona, Pyrogenes, Sejroe, Shermani, Tarassovi, and Wolffi. All strains were maintained in EMJH liquid medium (Difco) supplemented with 10% rabbit serum at 28°C. A laboratory-confirmed case of leptospirosis was defined by the demonstration of a 4-fold microagglutination titer rise between paired serum samples. The probable predominant serovar was considered the titer that was the highest sample dilution with 50% agglutination. The MAT was considered negative when the titer was below 1:100. In addition to the MAT-negative and MAT-positive samples from individuals with laboratory and clinical confirmations of leptospirosis, we employed sera from normal, healthy donors without a known history of leptospirosis and a confirmed negative MAT.
Endothelial cell extraction and cultivation.
The HUVECs were obtained by collagenase digestion of umbilical veins as detailed by Jaffe et al. (18). The cells were cultured in RPMI 1640 medium (Cultilab) supplemented with 10% fetal bovine sera (FBS) (Cultilab), 45 μg/ml heparin (Roche), 1 mM sodium pyruvate (Spectrum Chemical), 2 mM l-glutamine (Sigma), 100 IU penicillin (Cultilab), 100 μg/ml streptomycin (Cultilab), 50 μM 2-mercaptoethanol (Pharmacia Biotech), 25 μg/ml endothelial growth factor (Sigma), and 0.75% mouse brain extract, as a supplement of growth factor (19). The latter was prepared by homogenization of approximately 5 g of mouse brain in 5 ml of RPMI medium, as described by Maciag et al. (20). Cells were maintained at 37°C in a humidified 5% CO2 incubator. Confluent HUVECs were passaged with 0.25% trypsin and 0.02% EDTA (Cultilab) and seeded in appropriate culture plates. First-passage confluent HUVECs were used in all experiments.
Leptospiral transmigration across HUVEC monolayers.
Polycarbonate membrane (5-μm) Transwell chambers of 6.5 mm (Corning) were prepared with 100 μl/well 4% gelatin. First-passage HUVECs (15,000/well) were seeded over the gelatin layer and incubated with 1.5 ml RPMI complete culture medium until total confluence of the monolayer was reached (approximately 24 h). L. interrogans serovar Pomona cells (5 × 108/sample) were treated with 50 μg PLG (Calbiochem) in 200 μl low-salt phosphate-buffered saline (lsPBS) (PBS with 50 mM NaCl) for 1.5 h, followed by addition of 5 U urokinase-type uroplasminogen activator (uPA) (Sigma) for 1 h at 37°C. As controls, samples were treated with only PLG or uPA or with no additions (PBS). After three washes with lsPBS, each sample was resuspended in 200 μl RPMI complete culture medium lacking FBS, and 100 μl of the bacterial suspension was added over the HUVECs together with 100 μl culture medium in the upper chamber. Four hundred microliters of culture medium was added to the lower chamber. After 4 h of incubation at 37°C in a 5% CO2 atmosphere, the lower chamber content was harvested and leptospires were separated by centrifugation at 10,000 × g. The bacterial cells were resuspended in 1 ml lsPBS and counted in a Petroff-Hausser chamber under dark-field microscopy. Three independent counts of all 25 squares were performed by sample, and the number of leptospires was determined as total counted cells × 50,000 × dilution.
Leptospiral HUVEC activation.
HUVECs were seeded (15,000 cells/well) onto cell culture microplates (96 wells) and incubated until they reached total adherence for 16 h at 37°C in a 5% CO2 atmosphere. L. interrogans serovar Pomona strain LPF (5 × 108/sample) was treated with 50 μg PLG (in 200 μl lsPBS) for 1.5 h, followed by addition of 5 U uPA and incubation for 1 h at 37°C. Controls were treated without uPA, PLG, or both PLG and uPA. After three washings with lsPBS, each sample was resuspended in 500 μl RPMI medium. The bacterial suspensions (100 μl/well plus 100 μl/well RPMI) were transferred to the microplates containing the HUVECs previously washed with PBS for the removal of the remaining FBS. As controls, HUVECs were incubated only with RPMI medium lacking the bacteria. After 6 or 24 h of incubation at 37°C, the media were collected, centrifuged for removal of cells and bacteria, and frozen in aliquots at −80°C until use.
Quantification of PLG activators.
Human serum samples were quantified as uPA and tPA (tissue-type plasminogen activator) by using commercial kits based on the capture enzyme-linked immunosorbent assay (ELISA) (AssayMax human urokinase and AssayMax human tissue-type plasminogen activator from Assaypro). The protocols were performed as recommended by the manufacturer.
Urokinase chromogenic activity assay.
Human serum samples were subjected to a uPA chromogenic activity direct assay (Assaypro) according to the instructions provided by the manufacturer. The samples were diluted 5 times prior to the analysis.
Zymography.
Eight percent SDS-PAGE gel was copolymerized with 0.1% (mass/vol) gelatin (Difco) or type I human collagen (Sigma). Nondenaturing 5× concentrated gel application solution (250 mM Tris-HCl [pH 6.8], 2% [wt/v] SDS, 0.1 g/ml bromophenol blue, 10% [vol/vol] glycerol) was added to the samples without a heating step. The samples were loaded, and after running, the gels were washed twice for 15 min with 2.5% (vol/vol) Triton X-100 in 50 mM Tris-HCl (pH 8.4), followed by two washes of 5 min in 50 mM Tris-HCl (pH 8.4). The gels were incubated overnight (∼18 h) at 37°C in activation solution (50 mM Tris-HCl [pH 8.4], 5 mM CaCl2, 1 μM ZnCl2) and then stained with Coomassie blue (0.25% Coomassie blue R, 45% [vol/vol] methanol, 10% [vol/vol] glacial acetic acid). The excess stain was removed by destaining solution (45% [vol/vol] methanol, 10% [vol/vol] glacial acetic acid) until adequate visualization of the degradation regions had been achieved. Control experiments were performed with activation solution lacking CaCl2 and ZnCl2 or with 25 mM EDTA added to the activation solution.
For experiments with human sera, samples were diluted 20 times, and 10 μl was applied per well. The sera were selected randomly from our collection, independently of the MAT titer and serovar(s) involved. For zymography of the cell culture supernatants, 20 μl of the samples was applied to each gel well. Culture supernatants from HT1080 cells (human fibrosarcoma cell line) were employed as the positive control for pro-MMP-2 and pro-MMP-9 degradation activity.
2D gels for zymography and MS.
For two-dimensional (2D) gel electrophoresis, immobilized pH gradient (IPG) strips (GE Healthcare) (7 cm; pH 3 to 10) were rehydrated with 8 μl human serum samples (normal, MAT negative, or MAT positive) diluted in 117 μl of solution containing 9 M urea, 4% (mass/vol) CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 0.05% (vol/vol) Triton X-100, and bromophenol blue. The strips were then subjected to isoelectric focalization, equilibration, 8% SDS-PAGE, and preparation of the selected spots for mass spectrometry (MS) as previously described (21). The only modification was the exclusion of dithiothreitol (DTT) and iodoacetamide (IAA) from the equilibration steps. The gels were performed in duplicates: one subjected to zymography, as described above, and the other subjected to Coomassie blue staining. The experiment was performed three times with similar results.
Metalloprotease activity test.
MMP-2 and MMP-9 were quantified by specific activity assay systems (GE Healthcare), as recommended by the manufacturer. When total (proenzymes and active enzymes) enzymes were quantified, p-aminophenylmercuric acetate (APMA) was employed with the samples. Serum samples were diluted 20 and 50 times for MMP-9 and MMP-2 determinations, respectively. Cell culture supernatants were analyzed without dilutions.
Real-time PCR.
Total RNA from the HUVECs after activation by leptospiral contact was extracted using the RNAspin minikit (GE Healthcare), as recommended by the manufacturer. The RNA was quantitated by spectrophotometry using a NanoDrop spectrophotometer (Thermo). The cDNA was synthesized using 1,000 ng total RNA, the SuperScript III reverse transcriptase kit (Invitrogen), and oligo(dT) primers, according to standard protocols. The resulting cDNA was used for quantitative PCR using TaqMan gene expression master mix (Applied Biosystems) and TaqMan gene expression assays (Applied Biosystems) in a 7300 real-time PCR system (Applied Biosystems). The genes evaluated were the human MMP-9, uPA, tPA, and β-actin (ACTB) genes (Applied Biosystems) (catalog no. hs00957555_m1, hs01547050, hs00263492, and 4333762T, respectively). ACTB was employed as constitutive control. Reaction conditions were as described in the TaqMan gene expression master mix with 50 ng of cDNA in a total volume of 20 μl, and the cycling protocol was as follows: 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 60 s. Each reaction was performed in triplicate, and the cycle threshold (CT) values were calculated using 7300 System SDS Relative Quantification Study software (Applied Biosystems). The relative expression ratio was calculated by the comparative 2−ΔΔCT method (22) using ACTB as a constitutive control, and with 6 h of incubation time of the RPMI sample (HUVECs) incubated without leptospires as the calibrator. The range for target genes' relative expression (maximum and minimum) was calculated by 2−ΔΔCT with ΔΔCT + s and ΔΔCT − s, where s is the standard deviation of the ΔΔCT value.
Ethics statement.
All animal studies were approved by the Ethics Committee of the Instituto Butantan, São Paulo, SP, Brazil, under protocol no. 798/11. The Committee in Animal Research in Instituto Butantan adopts the guidelines of the Brazilian College of Animal Experimentation. The use of HUVECs for experimentation is approved by the Ethics Committee of Universidade Federal de São Paulo (UNIFESP), under protocol no. 0664/05. Serum samples from patients with confirmed leptospirosis and serum samples from patients with unrelated infectious diseases were obtained from a serum collection, were rendered anonymous, and were donated to be used for research purposes only. Written informed consent was given by healthy blood donors.
RESULTS
Leptospiral transmigration through HUVEC monolayers is enhanced by PLA.
To evaluate whether PLA generation on the surface of leptospires affects bacterial tissue penetration, virulent low-passage-number L. interrogans serovar Pomona strain LPF was used to infect primary cultures of HUVEC monolayers in Transwell chambers. The results show that when the organisms were treated with PLG or PLG plus uPA, the leptospires migrated to the lower chamber across the cell monolayers more efficiently than the control (PBS) leptospires (Fig. 1). The fastest migration of leptospires through the monolayer was achieved when the bacteria generated PLA on their surface compared to the ones treated only with PLG.
Fig 1.
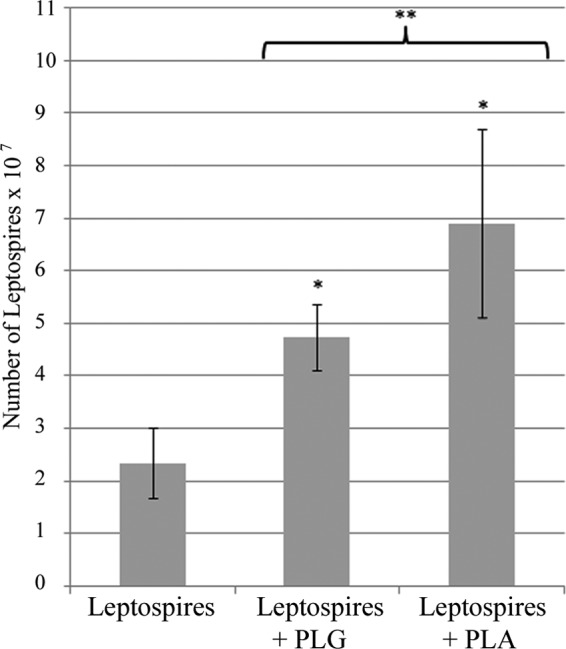
Penetration of HUVEC monolayers by leptospires. L. interrogans serovar Pomona strain LP was treated with PLG, PLG plus uPA, or only PBS. The HUVEC monolayers previously seeded above the Transwell chamber polycarbonate membrane were inoculated with the leptospires (2.5 × 108). After 4 h, the leptospires present in the lower chamber were recovered and counted by dark-field microscopy. The bars represent the medians ± standard deviations of two independent counts of each experimental duplicate. The data are representative of three independent experiments. *, P < 0.0025; **, P < 0.03.
HUVEC activation by leptospires.
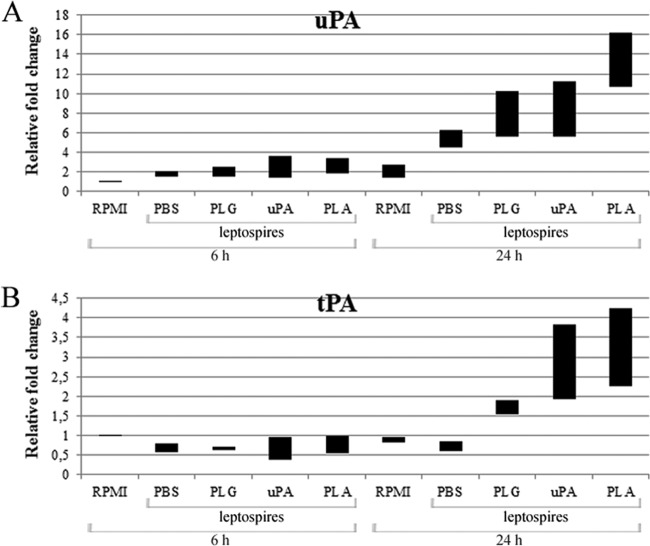
In our previous work, we have shown that leptospires do not have the capacity to convert PLG to active PLA and are dependent on the host's fibrinolytic activation system (13). As leptospires treated with PLG but lacking its activator showed higher migration activity across the cellular barrier compared to PBS-treated control bacteria (Fig. 1), we speculated whether leptospires could also promote upregulation of PLG activators when in contact with HUVECs. Endothelial cells were incubated with leptospires, and the total RNA of the cells was extracted, followed by cDNA synthesis and real-time PCR evaluation. HUVECs were tested for uPA and tPA PLG activator transcription; the ACTB gene was used as the endogenous constitutive control for the normalization of both activators levels. The data obtained indicate that leptospires in contact with HUVECs promoted on these cells an upregulation at the transcriptional level of both PLG activators compared to cells not exposed to the bacteria (treated with RPMI only) (Fig. 2A and B). The stimulation of the uPA transcription was clearly observed after 24 h of HUVEC-leptospire incubation; the transcription stimulation was higher when PLG was present, but the strongest induction of uPA and tPA transcriptional levels was achieved with PLA-associated leptospires (more than 10 times for uPA and 2 times for tPA) (Fig. 2A and B). A negligible increase in the transcription was observed upon 6 h of HUVEC-organism incubation for both activators. The control cells (endothelial cells incubated with RPMI only) produced insignificant differences in the levels of both PLG activators.
Fig 2.
L. interrogans upregulates the transcription of PLG activators from HUVECs. Primary cultures of HUVECs were incubated for 6 or 24 h with L. interrogans serovar Pomona without treatment (PBS) or were treated with PLG, uPA, or PLG plus uPA. As a negative control, HUVECs were incubated only with the culture medium, without bacteria (RPMI). The HUVECs' total RNA was extracted, and with the cDNAs as the template, real-time PCR was employed for CT analysis and fold change calculation relative to the HUVECs incubated in the absence of bacteria for 6 h and 24 h. In panels A and B are the data obtained for uPA and tPA, respectively. The bars represent the range of the relative fold changes from triplicates, calculated as described in Materials and Methods. The data are representative of two independent experiments.
MMP secretion by HUVECs incubated with leptospires.
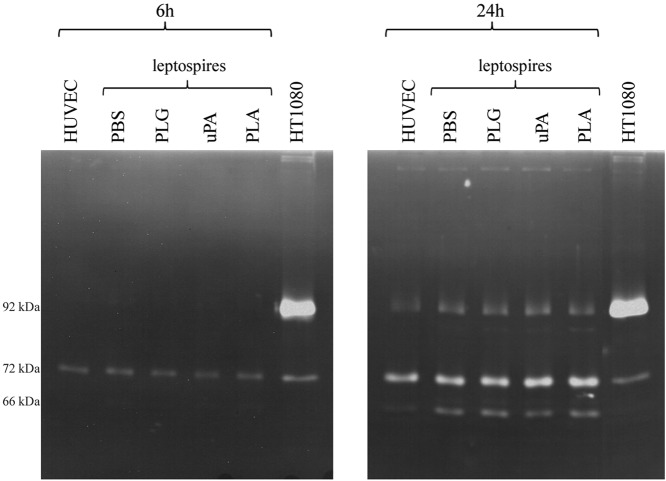
It has been proposed that PLA generated from PLG is an activator of pro-MMPs (23). The PLA generated in the spirochete B. burgdorferi was shown to activate pro-MMP-9 in human cells (24). We thus set up to investigate whether the PLA-generated activity on leptospires was capable of promoting the activation or release of MMPs by HUVECs coincubated in vitro with leptospires plus the PLG/PLA proteolytic system. Gelatin zymography assays of the cell culture supernatants were analyzed after incubation of HUVEC-PLA-associated leptospires and controls for 6 and 24 h. HT1080 cell culture medium was employed as a control for constitutive secretion of pro-MMP-2 (∼72 kDa) and pro-MMP-9 (∼92 kDa). As shown in Fig. 3, protein degradation bands with molecular masses similar to that of pro-MMP-2 activity upon gelatin substrate were visible on all samples, including both controls lacking leptospires and samples incubated with treated or untreated leptospires for 6 or 24 h (Fig. 3). Supernatants from 24 h of incubation show protein degradation bands with a molecular mass similar to that of pro-MMP-9 and active MMP-2 (∼66 kDa) in all samples. The white band with a molecular mass comparable to that of active MMP-9 (∼78 kDa) became visible, although weak, in the samples from HUVECs incubated for 24 h with leptospires, treated or untreated, and was absent in the control without bacteria (Fig. 3). Complementary control experiments were carried out by employing the activation solution lacking CaCl2 and ZnCl2 or with the addition of 25 mM EDTA. Under these conditions, no degradation bands were observed (data not shown), demonstrating that the proteolytic activity was due to metal-dependent activity of the MMPs.
Fig 3.
Zymography analysis of MMPs released into the culture of HUVECs in response to Leptospira. Primary cultures of HUVECs were incubated for 6 or 24 h with L. interrogans serovar Pomona without treatment (PBS) or were treated with PLG, uPA, or PLG plus uPA. As a negative control, HUVECs were incubated only with the culture medium, without bacteria (HUVECs). HT1080 supernatants were employed as positive controls for pro-MMP-2 (∼72 kDa) and pro-MMP-9 (∼92 kDa) constitutive release. The samples were separated by 8% SDS-PAGE with gel copolymerized with 0.1% gelatin. After overnight incubation in activation solution, the gels were stained with Coomassie blue. The clear bands represent the regions where the gelatin substrate was degraded. The molecular masses of ∼92 kDa, ∼72 kDa, and ∼60 kDa, which correspond to pro-MMP-9, pro-MMP-2, and active MMP-2, respectively, are depicted.
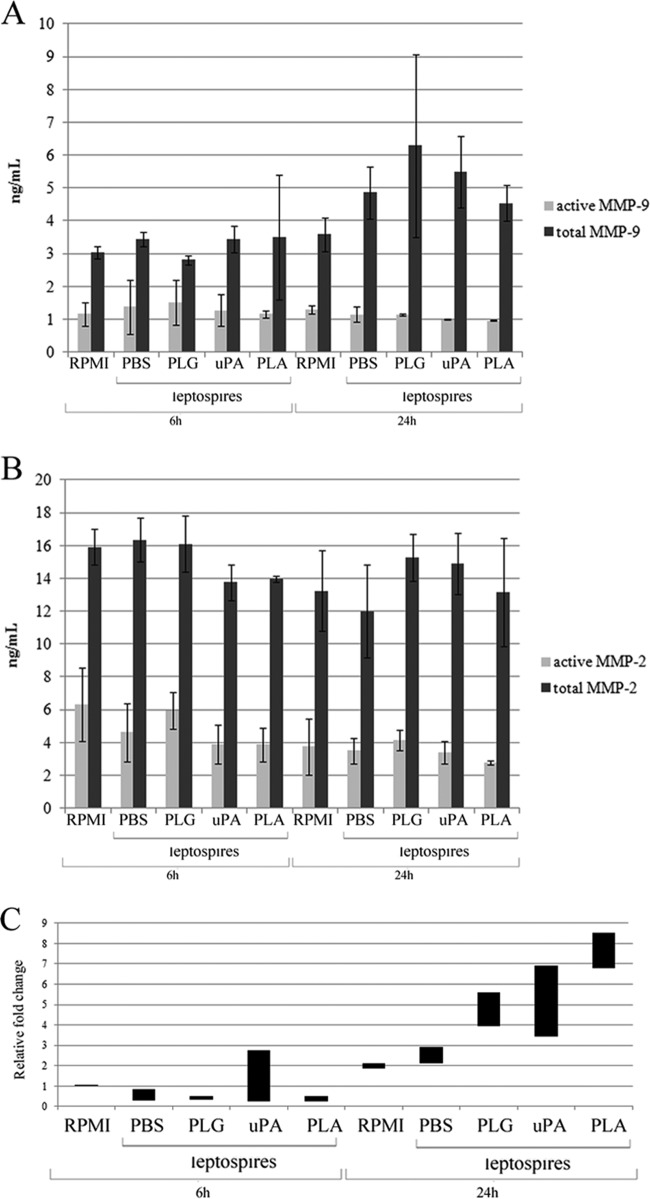
Thus, we decided to evaluate MMPs' activity on the cell culture supernatants, from the same systems described above, by using an ELISA based on capture. The results show that there was no increase in either active or total MMP-9 after 6 h of incubation. However, while there is a tendency toward an increase in total MMP-9 (Fig. 4A) with all of the supernatants from HUVECs incubated with leptospires, PLG treated for 24 h or not, the values were not statistically significant (P > 0.05). Active MMP-9 remained unaffected in the same supernatants after 24 h of incubation (P > 0.05) (Fig. 4A). Total or active MMP-2 showed no significant differences after 6 or 24 h of incubation in the presence or absence of leptospires, either PLG treated or not (P > 0.05) (Fig. 4B). To assess whether the higher release of total MMP-9 by HUVECs exposed to the bacteria seen in Fig. 3 was due to an upregulation of this enzyme, we performed real-time PCR using total HUVEC cDNA as the template and specific primers to MMP-9. The results indicate that PLA-associated Leptospira, bacteria plus PLG, or bacteria plus uPA promoted upregulation of MMP-9 from HUVECs, the highest transcriptional level being observed with PLA-coated leptospires incubated for 24 h (Fig. 4C).
Fig 4.
MMP-9 and MMP-2 levels released into the culture of HUVECs in response to Leptospira. Primary cultures of HUVECs were incubated for 6 or 24 h with L. interrogans serovar Pomona without treatment (PBS) or were treated with PLG, uPA, or PLG plus uPA. As a negative control, HUVECs were incubated only with the culture medium, without bacteria (RPMI). The cleared cell culture supernatants were evaluated for MMP-9 (A) or MMP-2 (B) activity by commercial ELISA kit. Total activities of MMP-9 and MMP-2 were achieved by APMA (Materials and Methods) activation of the pro-MMPs. The bars represent the medians of duplicates from two independent experiments ± standard deviations. (C) The cDNA obtained by extraction of the HUVECs' total RNA was subjected to real-time evaluation for MMP-9 gene transcription. The data were normalized according to the ACTB constitutive control. The bars represent the range of the relative fold changes from triplicates, calculated as described in Materials and Methods. The data are representative of two independent experiments.
Detection of PLG activators on serum samples from patients with confirmed leptospirosis.
L. interrogans organisms can incorporate PLG onto their surface, and PLA is generated in the presence of exogenous PLG activators (13). Therefore, we decided to investigate serum samples from patients with confirmed leptospirosis for the presence of uPA and tPA PLG activators using commercial kits based on capture ELISA. Serum samples at the beginning (MAT-negative) or convalescent (MAT positive) phase of leptospirosis were employed. Samples from healthy donors were employed for a comparative purpose. The results reveal that both uPA and tPA activators are present in higher quantities in serum samples from patients with confirmed leptospirosis than in samples from healthy donors (Fig. 5A and B). The highest levels of both activators were detected at the early phase of disease (MAT negative), suggesting that the bacteria induce the release of both uPA and tPA activators from the hosts during the first days of the illness. Very low levels of activators were detected in sera from healthy donors, particularly in the case of uPA (Fig. 5A). To investigate whether the increase of activators in these samples was not only at the concentration level but also was represented by activity, we measured the uPA specific activity of each serum sample employed. The results show that an increase in uPA activity was observed with leptospirosis patient serum samples compared to samples from normal, healthy donors (Fig. 5C). In all of the experiments, statistically significant values were calculated by comparing MAT-negative and MAT-positive samples with the control sera (normal) (*, P < 0.006; **, P < 0.130; ***, P < 0.06).
Fig 5.
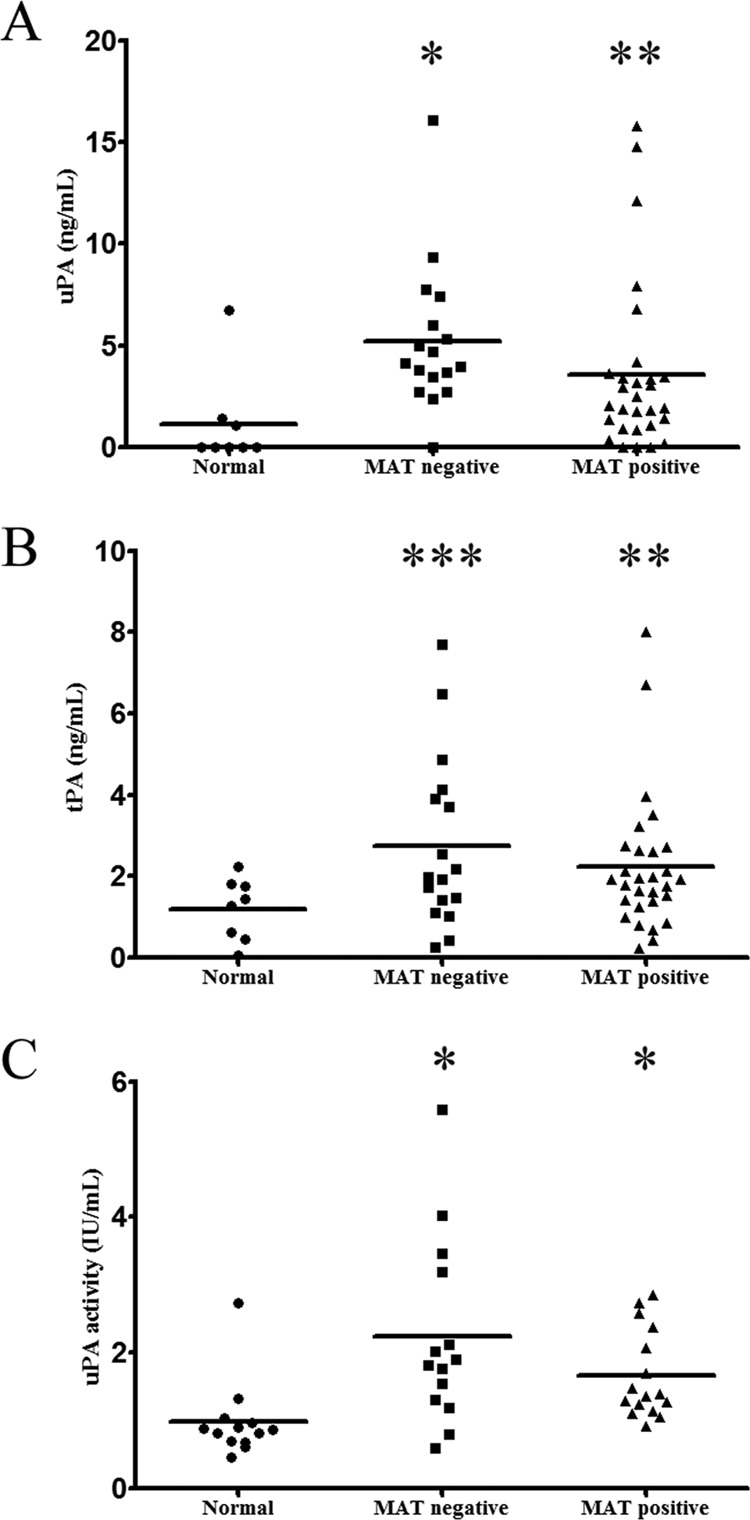
Measurement of PLG activators and uPA activity in serum samples from normal, healthy donors and patients with confirmed leptospirosis. Paired serum samples from individuals with confirmed leptospirosis in the initial (MAT-negative) or convalescent (MAT-positive) phase or serum samples from healthy donors (Normal) were quantified for the presence of uPA (A) or tPA (B) by specific capture ELISA kits. The quantification was based on uPA and tPA standard curves. The symbols represent the individual dosage of each sample, and the horizontal bars represent the medium. In panel C are shown the measurements of uPA activity by specific activity test. Plotted in the graphic is the activity determinate of each serum sample. The horizontal bars represent the means of the serum samples for each group. Statistically significant values were calculated by comparing MAT-negative and MAT-positive samples with the control sera (normal): *, P < 0.006; **, P < 0.130; ***, P < 0.06.
Zymography profile analysis of normal and leptospirosis human serum samples.
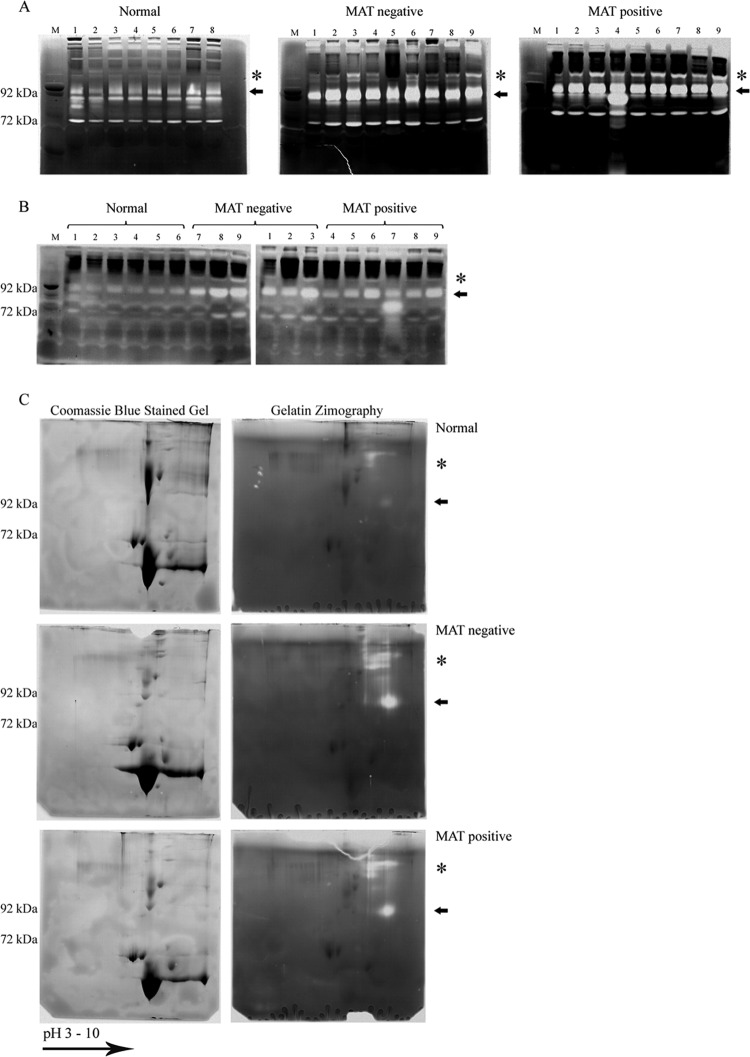
The interaction of leptospires with the PLG activation system, PLG or PLG plus uPA (25), has the capacity to promote upregulation of MMP-9 from human endothelial cells (Fig. 4). These results prompted us to investigate whether the MMPs' activity and expression are altered during natural human leptospirosis by performing zymography analysis. A human serum sample (0.5 μl) was applied to SDS-PAGE gel copolymerized with gelatin as a substrate for gelatinase (MMP) activity proteases. After incubation in activation solution, the gels were Coomassie blue stained; the white bands represent regions where the substrate was degraded. The gelatin zymogram profile obtained for MAT-negative and MAT-positive serum samples from patients with confirmed leptospirosis and from healthy individuals (normal) is presented in Fig. 6A. The arrows indicate the discrepancy in visual intensity between the serum samples from healthy subjects and those from patients with leptospirosis, corresponding to the molecular mass of approximately 90 kDa (Fig. 6A), probably representing pro-MMP-9, reflecting higher degradation activity during the disease. In addition, another region of substrate degradation is observed above the arrows in samples from patients with confirmed leptospirosis (Fig. 6A, indicated by asterisks).We further confirmed these activity data by performing the same experiment but with collagen type I zymography. The results depicted in Fig. 6B revealed elevated levels of degradation in the same region (∼90 kDa) with the serum samples from patients with leptospirosis compared with the serum samples from normal, healthy donors. Additional control experiments were performed using the activation solution lacking CaCl2 and ZnCl2 or with the addition of 25 mM EDTA. Under these conditions, no degradation bands were observed (data not shown), indicating that the proteolytic activity was due to metal-dependent activity of the MMPs.
Fig 6.
Zymography profile of samples form healthy donors and patients with leptospirosis. Human serum samples from healthy donors (Normal) and paired serum samples from patients with leptospirosis at the early (MAT-negative) and convalescent (MAT-positive) phases of the disease were separated by 8% SDS-PAGE with gel copolymerized with 0.1% gelatin (A) or type I collagen (B). After incubation in activation solution, the gels were Coomassie blue stained. The white bands represent the regions where the substrate was degraded. The arrows and asterisks indicate the regions with the most pronounced differences in degradation between serum samples from patients with confirmed leptospirosis and normal human serum samples. Lanes: M, molecular mass marker; 1 to 9, individual serum samples. (C) 2D gel analysis (7-cm IPG strips [pH 3 to 10]) of normal, MAT-negative, and MAT-positive human serum samples. Extensive degradation regions are denoted by arrows and asterisks. Duplicates were Coomassie blue stained or subjected to gelatin zymography. The experiment was performed three times with similar results.
In an attempt to identity the MMPs responsible for the observed enhanced activity, we performed 2D gel analysis of human serum samples. 2D gel analyses were performed in duplicates, with one of the gels Coomassie blue stained and the other subjected to zymography (Fig. 6C). Similar to the data with the 1D gel, pronounced degradation activity was shown in gelatin 2D zymography in the same region (∼90 kDa) and above in confirmed-leptospirosis samples, both MAT negative and MAT positive, as denoted by arrows and asterisks in Fig. 6C. Almost no degradation activity was seen in normal serum samples (Fig. 6C). Coomassie blue protein spots were not visualized in the region where degradation activity was detected in zymography, possibly due to the higher sensitivity of the latter compared to protein-colored staining. Thus, by overlapping the gels, we excised 9 samples from each condition (in the regions corresponding to the white bands in the zymogram) from the Coomassie blue-stained gels and samples were prepared for MS. Although some peptides were detected after MS, protein identification was inconclusive, presumably as a result of the limited amounts of MMPs present or the interference of contaminant proteins in human serum.
To further validate the data obtained by zymography experiments, we also performed MMP activity assays and measured total and active MMP-9 in all serum samples. The results reveal an elevated level of total MMP-9 at the early stage of the disease (MAT-negative serum samples), while this level tends to reach normal values during the convalescent phase (MAT positive) (Fig. 7). In contrast, the amount of active MMP-9 was very low, and similar values were detected in normal and leptospirosis serum samples. The total MMP-9 concentration level observed among MAT-negative samples corresponds to approximately 2.5 times the normal samples' mean concentration (Fig. 7).
Fig 7.
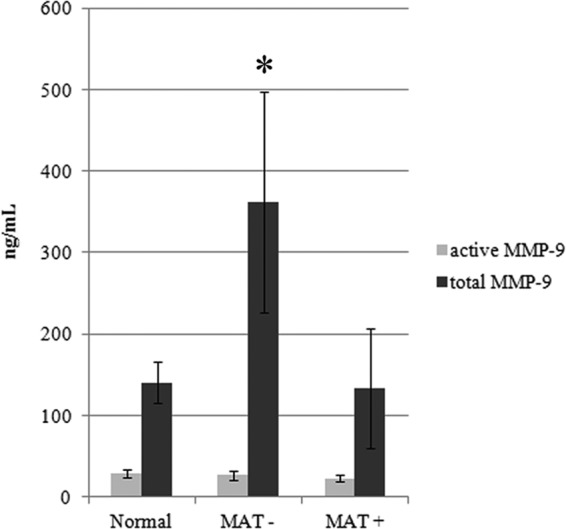
Total amount and enzymatic activity of MMP-9 from human serum samples. Human serum samples from healthy donors (Normal) and serum samples from patients with leptospirosis at the initial phase (MAT−) and convalescent phase (MAT+) were quantified for total contents and enzymatic activity of MMP-9. Total or enzymatic activity of MMP-9 was achieved by APMA activation of the pro-MMP. Bars represent the means of the samples (n = 8) quantified in duplicates ± standard deviations. *, P < 0.02.
Zymography analysis of serum samples from patients with leptospirosis and those with unrelated infectious diseases.
In view of the presence of gelatin/collagen-enhanced degradation activity in serum samples from patients with leptospirosis (Fig. 6) in concert with the elevated levels of total MMP-9 detected in MAT-negative serum samples (Fig. 7), we decided to investigate whether this elevated activity was restricted to leptospirosis or common to other infectious diseases. We performed gelatin zymography and compared both phases of leptospirosis with serum samples from patients with dengue, malaria, HIV, and Chagas disease (Fig. 8). Our data show a strong gelatinase activity present in serum samples from patients with both phases of leptospirosis, probably representing pro-MMP-9 (indicated by arrows), in contrasting to the absence of degradation bands in all serum samples from patients with the other infectious diseases tested. The zymography profiles obtained for dengue, malaria, and Chagas are similar to the ones observed with the normal human serum samples used as controls (Fig. 6). HIV samples resulted in a different profile where some other degradation bands lower than the 92-kDa molecular mass are presented.
Fig 8.
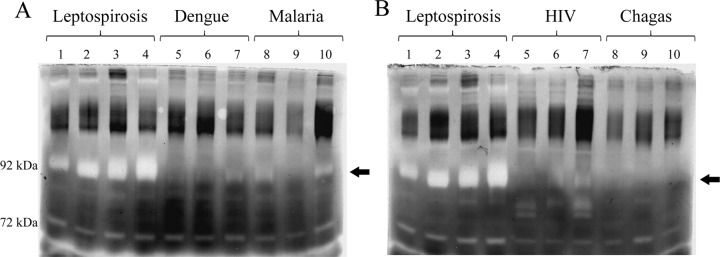
Comparison of zymography profiles from serum samples from patients with confirmed leptospirosis and other infectious diseases. In panel A are shown serum samples from patients with leptospirosis at the initial phase (lanes 1 and 2) or convalescent phase (lanes 3 and 4) and sera from patients with unrelated infectious diseases: dengue (lanes 5 to 7) and malaria (lanes 8 to 10). In panel B are shown results for samples from patients with HIV (lanes 5 to 7) and Chagas' disease (lanes 8 to 10). The samples were separated by 8% SDS-PAGE with gel copolymerized with 0.1% gelatin, incubated in activation solution, and stained by Coomassie blue. The clear bands represent the regions where the substrate was digested. The arrows indicate the regions with most pronounced difference in degradation between samples from patients with leptospirosis and those from patients with other diseases.
DISCUSSION
Previous work in our laboratory has described the ability of Leptospira to capture PLG on the surface and to generate PLA in the presence of exogenous uPA activator (13). Several outer membrane leptospiral proteins with expected roles in pathogenesis and virulence have been identified as PLG-binding receptors, suggesting that the interaction with the fibrinolytic system might be important during leptospirosis (14–17, 26). Indeed, the association of PLA with Leptospira renders the bacteria with proteolytic activity capable of degrading ECM components (13), which in turn, may help bacterial penetration and dissemination. Moreover, PLA-coated leptospires have also shown the ability to degrade IgG and C3b, which could facilitate bacterial immune evasion (27).
We focused the present study on the analysis of the effects of leptospiral PLG binding and PLA generation on the dissemination and activation of endothelial cells. We show that Leptospira cells with surface-associated PLG or an active PLA coating have an enhanced transmigration activity through endothelial cell barriers compared to the leptospires' basal proteolytic activity (Fig. 1). In fact, proteinase/gelatinase activity has been reported in many strains of Leptospira (28, 29). It has been shown that cells of the Lyme disease spirochete B. burgdorferi coated with PLA migrate faster through cell monolayers than the bacteria without the proteolytic activity (12). Although B. burgdorferi enters the host after tick bite, Coleman and colleagues (12) discussed that PLG could be bound just before bacterial penetration and converted to PLA by activators present in the inflammatory skin focus. As mentioned before, PLA-coated Leptospira cells have the capacity to degrade ECM components in vitro (13). The faster transmigration of cellular barrier observed with Leptospira cells endowed with the PLG/PLA system is most probably due to the PLA proteolytic activity conferred to the bacteria. Moreover, the doubling time for pathogenic leptospires is 14 to 18 h (30) and the presence of PLG/PLA reagents does not impair cell division because there is no modification in culture growth monitored for 48 h (M. L. Vieira and A. L. T. O. Nascimento, unpublished results). The fact that we have employed a PLG concentration close to the physiological condition (31) suggests that our data might have implications during infection. Indeed, the PLG/PLA binding has been described to facilitate the keratinocyte invasion by Streptococcus pyogenes (32). Furthermore, it has been reported that the fungal pathogen Cryptococcus neoformans coated with PLG promotes extracellular matrix (ECM) invasion (33). Surprisingly, the cellular transmigration efficiency of leptospires treated only with PLG and lacking uPA was higher than that in the untreated control. Previous studies in our laboratory have indicated that Leptospira cells do not have an endogenous mechanism for PLG activation (13). We therefore hypothesized that the bacterial contact could stimulate the expression and/or secretion of PLG activators by the HUVECs, transforming the bound PLG into enzymatically active PLA. The increased level of uPA and tPA transcripts revealed that the expression of both activators is, in fact, upregulated when the HUVECs are coincubated with the leptospires. Intriguingly, the upregulation of the activators was negligible after 6 h of incubation (Fig. 2), while the enhanced penetration was already detected after 4 h of treatment (Fig. 1). Thus, it is possible that Leptospira-associated PLG was activated in the early hours of incubation by releasing PLG activators from the endothelial cells. In fact, it is documented that endothelial cells contain several distinct populations of regulated secretory organelles, including the tPA organelle (34), and that the secretion of this activator is induced during injury or the inflammation process (35). Dengue virus infection was also reported to stimulate the release of tPA from human endothelial cells (36). Consequently, the higher availability of PLG activators could elicit the activation of the leptospiral-bound PLG to active enzyme, triggering PLA-mediated ECM proteolysis and promoting an enhanced transmigration activity.
Interestingly, serum samples from patients with confirmed leptospirosis have larger amounts of both PLG activators available in circulation compared to normal serum samples. We were able to confirm that in the case of uPA, the activity in serum samples from patients with leptospirosis is also elevated, suggesting that there is no concomitant increase in uPA inhibitors, like PLG activator inhibitors PAI-1 and PAI-2. The fact that higher PLG activator levels were detected in MAT-negative serum samples suggests that this activation is elicited at the beginning of the invasion process, thus increasing the PLA proteolytic activity generation that would be important during the leptospiral invasion.
MMPs are well known to be directly and actively involved in the origin of pathology in some infections, as MMP expression can be stimulated by many pathogens (24, 37, 38). Furthermore, PLA can induce directly or indirectly MMP expression and is able to activate MMPs by the proteolytic cleavage of the propeptide domains that confer latency to the secreted pro-MMPs (24, 39–41). It has been suggested that the spirochete B. burgdorferi promoted an upregulation of MMPs mediating an activation cascade that is started by PLA bound to the bacterial surface (24). The incubation of leptospires with HUVECs stimulates an upregulation of MMP-9 by endothelial cells, an activity that could contribute to the disruption of ECM and endothelial tissues, favoring the penetration and invasion of the bacteria. It is possible that this cascade of events occurs during human leptospirosis infection because elevated levels of circulating total MMP-9 were detected in serum samples from patients with confirmed leptospirosis. Interestingly, similar to the levels of PLG activators detected, the MMP-9 concentration was higher with MAT-negative serum samples, suggesting that the cascade of proteolytic activity generation takes place at the early onset of the illness. PLA generated on the surface of bacteria seems to be associated with spirochete infection, such as Lyme disease, relapsing fever, and leptospirosis, because unrelated infectious diseases appear to lack this activity. Further studies are needed to understand the effects of the high levels of MMPs during human leptospirosis in addition to the facilitation of penetration and invasion of tissues.
In conclusion, the present work shows that the interaction of L. interrogans with the PLG and PLA fibrinolytic systems enhances the migration of the bacteria through endothelial monolayer cells. Moreover, Leptospira-bound PLG has the capacity to stimulate the release and expression of PLG activators from endothelial cells, thus promoting increased generation of PLA-coated leptospires. Furthermore, this proteolytic system is capable of eliciting an upregulation of MMP-9 from HUVECs. Altogether, our results suggest that leptospires associated with the fibrinolytic system can trigger a cascade of proteolytic activity initiated by PLA generation after the binding of PLG to the leptospiral surface. MMPs together with PLA-associated Leptospira may represent one possible mechanism that contributes to the invasion and the rapid dissemination of this organism.
ACKNOWLEDGMENT
We are deeply indebted to Henrique Krambeck Rofatto (Centro de Biotecnologia, Instituto Butantan, São Paulo, Brazil) for the use of real-time PCR and helpful discussion.
Footnotes
Published ahead of print 11 March 2013
REFERENCES
- 1. Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM, Peru-United States Leptospirosis Consortium 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3:757–771 [DOI] [PubMed] [Google Scholar]
- 2. Faine S, Adler B, Bolin C, Perolat P. 1999. Leptospira and leptospirosis, 2nd ed, p 259 MediSci, Melbourne, Australia [Google Scholar]
- 3. Ren SX, Fu G, Jiang XG, Zeng R, Miao YG, Xu H, Zhang YX, Xiong H, Lu G, Lu LF, Jiang HQ, Jia J, Tu YF, Jiang JX, Gu WY, Zhang YQ, Cai Z, Sheng HH, Yin HF, Zhang Y, Zhu GF, Wan M, Huang HL, Qian Z, Wang SY, Ma W, Yao ZJ, Shen Y, Qiang BQ, Xia QC, Guo XK, Danchin A, Saint Girons I, Somerville RL, Wen YM, Shi MH, Chen Z, Xu JG, Zhao GP. 2003. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature 422:888–893 [DOI] [PubMed] [Google Scholar]
- 4. Nascimento AL, Verjovski-Almeida S, Van Sluys MA, Monteiro-Vitorello CB, Camargo LE, Digiampietri LA, Harstkeerl RA, Ho PL, Marques MV, Oliveira MC, Setubal JC, Haake DA, Martins EA. 2004. Genome features of Leptospira interrogans serovar Copenhageni. Braz. J. Med. Biol. Res. 37:459–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bulach DM, Zuerner RL, Wilson P, Seemann T, McGrath A, Cullen PA, Davis J, Johnson M, Kuczek E, Alt DP, Peterson-Burch B, Coppel RL, Rood JI, Davies JK, Adler B. 2006. Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc. Natl. Acad. Sci. U. S. A. 103:14560–14565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Picardeau M, Bulach DM, Bouchier C, Zuerner RL, Zidane N, Wilson PJ, Creno S, Kuczek ES, Bommezzadri S, Davis JC, McGrath A, Johnson MJ, Boursaux-Eude C, Seemann T, Rouy Z, Coppel RL, Rood JI, Lajus A, Davies JK, Medigue C, Adler B. 2008. Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS One 3:e1607 doi:10.1371/journal.pone.0001607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adler B, Lo M, Seemann T, Murray GL. 2011. Pathogenesis of leptospirosis: the influence of genomics. Vet. Microbiol. 153:73–81 [DOI] [PubMed] [Google Scholar]
- 8. Cinco M. 2010. New insights into the pathogenicity of leptospires: evasion of host defences. New Microbiol. 33:283–292 [PubMed] [Google Scholar]
- 9. Evangelista KV, Coburn J. 2010. Leptospira as an emerging pathogen: a review of its biology, pathogenesis and host immune responses. Future Microbiol. 5:1413–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barocchi MA, Ko AI, Reis MG, McDonald KL, Riley LW. 2002. Rapid translocation of polarized MDCK cell monolayers by Leptospira interrogans, an invasive but nonintracellular pathogen. Infect. Immun. 70:6926–6932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lahteenmaki K, Kuusela P, Korhonen TK. 2001. Bacterial plasminogen activators and receptors. FEMS Microbiol. Rev. 25:531–552 [DOI] [PubMed] [Google Scholar]
- 12. Coleman JL, Sellati TJ, Testa JE, Kew RR, Furie MB, Benach JL. 1995. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect. Immun. 63:2478–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vieira ML, Vasconcellos SA, Goncales AP, de Morais ZM, Nascimento AL. 2009. Plasminogen acquisition and activation at the surface of Leptospira species lead to fibronectin degradation. Infect. Immun. 77:4092–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vieira ML, Atzingen MV, Oliveira TR, Oliveira R, Andrade DM, Vasconcellos SA, Nascimento AL. 2010. In vitro identification of novel plasminogen-binding receptors of the pathogen Leptospira interrogans. PLoS One 5:e11259 doi:10.1371/journal.pone.0011259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oliveira R, de Morais ZM, Goncales AP, Romero EC, Vasconcellos SA, Nascimento AL. 2011. Characterization of novel OmpA-like protein of Leptospira interrogans that binds extracellular matrix molecules and plasminogen. PLoS One 6:e21962 doi:10.1371/journal.pone.0021962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mendes RS, Von Atzingen M, de Morais ZM, Goncales AP, Serrano SM, Asega AF, Romero EC, Vasconcellos SA, Nascimento AL. 2011. The novel leptospiral surface adhesin Lsa20 binds laminin and human plasminogen and is probably expressed during infection. Infect. Immun. 79:4657–4667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Domingos RF, Vieira ML, Romero EC, Goncales AP, Morais ZM, Vasconcellos SA, Nascimento AL. 2012. Features of two proteins of Leptospira interrogans with potential role in host-pathogen interactions. BMC Microbiol. 12:50 doi:10.1186/1471-2180-12-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jaffe EA, Nachman RL, Becker CG, Minick CR. 1973. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest. 52:2745–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoshi H, McKeehan WL. 1984. Brain- and liver cell-derived factors are required for growth of human endothelial cells in serum-free culture. Proc. Natl. Acad. Sci. U. S. A. 81:6413–6417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maciag T, Cerundolo J, Ilsley S, Kelley PR, Forand R. 1979. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc. Natl. Acad. Sci. U. S. A. 76:5674–5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vieira ML, Pimenta DC, de Morais ZM, Vasconcellos SA, Nascimento ALTO. 2009. Proteome analysis of Leptospira interrogans virulent strain. Open Microbiol. J. 3:69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 23. Carmeliet P, Moons L, Lijnen R, Baes M, Lemaitre V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, Collen D. 1997. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat. Genet. 17:439–444 [DOI] [PubMed] [Google Scholar]
- 24. Gebbia JA, Coleman JL, Benach JL. 2001. Borrelia spirochetes upregulate release and activation of matrix metalloproteinase gelatinase B (MMP-9) and collagenase 1 (MMP-1) in human cells. Infect. Immun. 69:456–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Plank R, Dean D. 2000. Overview of the epidemiology, microbiology, and pathogenesis of Leptospira spp. in humans. Microbes Infect. 2:1265–1276 [DOI] [PubMed] [Google Scholar]
- 26. Verma A, Brissette CA, Bowman AA, Shah ST, Zipfel PF, Stevenson B. 2010. Leptospiral endostatin-like protein A is a bacterial cell surface receptor for human plasminogen. Infect. Immun. 78:2053–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vieira ML, de Morais ZM, Vasconcellos SA, Romero EC, Nascimento AL. 2011. In vitro evidence for immune evasion activity by human plasmin associated to pathogenic Leptospira interrogans. Microb. Pathog. 51:360–365 [DOI] [PubMed] [Google Scholar]
- 28. Madathiparambil MG, Cattavarayane S, Manickam GD, Singh K, Perumana SR, Sehgal SC. 2011. A zymography analysis of proteinase activity present in Leptospira. Curr. Microbiol. 62:917–922 [DOI] [PubMed] [Google Scholar]
- 29. Madathiparambil MG, Cattavarayane S, Perumana SR, Manickam GD, Sehgal SC. 2011. Presence of 46 kDa gelatinase on the outer membrane of Leptospira. Curr. Microbiol. 62:1478–1482 [DOI] [PubMed] [Google Scholar]
- 30. Zuerner RL. 1 October2005. Laboratory maintenance of pathogenic Leptospira. Curr. Protoc. Microbiol. Chapter 12:12E.1.1-12E1.13. doi:10.1002/9780471729259.mc12e01s00 [DOI] [PubMed] [Google Scholar]
- 31. Collen D, Tytgat G, Claeys H, Verstraete M, Wallen P. 1972. Metabolism of plasminogen in healthy subjects: effect of tranexamic acid. J. Clin. Invest. 51:1310–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Siemens N, Patenge N, Otto J, Fiedler T, Kreikemeyer B. 2011. Streptococcus pyogenes M49 plasminogen/plasmin binding facilitates keratinocyte invasion via integrin-integrin-linked kinase (ILK) pathways and protects from macrophage killing. J. Biol. Chem. 286:21612–21622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stie J, Bruni G, Fox D. 2009. Surface-associated plasminogen binding of Cryptococcus neoformans promotes extracellular matrix invasion. PLoS One 4:e5780 doi:10.1371/journal.pone.0005780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Knipe L, Meli A, Hewlett L, Bierings R, Dempster J, Skehel P, Hannah MJ, Carter T. 2010. A revised model for the secretion of tPA and cytokines from cultured endothelial cells. Blood 116:2183–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oliver JJ, Webb DJ, Newby DE. 2005. Stimulated tissue plasminogen activator release as a marker of endothelial function in humans. Arterioscler. Thromb. Vasc. Biol. 25:2470–2479 [DOI] [PubMed] [Google Scholar]
- 36. Huang YH, Lei HY, Liu HS, Lin YS, Chen SH, Liu CC, Yeh TM. 2003. Tissue plasminogen activator induced by dengue virus infection of human endothelial cells. J. Med. Virol. 70:610–616 [DOI] [PubMed] [Google Scholar]
- 37. Vanlaere I, Libert C. 2009. Matrix metalloproteinases as drug targets in infections caused by Gram-negative bacteria and in septic shock. Clin. Microbiol. Rev. 22:224–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elkington PT, O'Kane CM, Friedland JS. 2005. The paradox of matrix metalloproteinases in infectious disease. Clin. Exp. Immunol. 142:12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liotta LA, Goldfarb RH, Brundage R, Siegal GP, Terranova V, Garbisa S. 1981. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 41:4629–4636 [PubMed] [Google Scholar]
- 40. Liotta LA, Goldfarb RH, Terranova VP. 1981. Cleavage of laminin by thrombin and plasmin: alpha thrombin selectively cleaves the beta chain of laminin. Thromb. Res. 21:663–673 [DOI] [PubMed] [Google Scholar]
- 41. Lahteenmaki K, Edelman S, Korhonen TK. 2005. Bacterial metastasis: the host plasminogen system in bacterial invasion. Trends Microbiol. 13:79–85 [DOI] [PubMed] [Google Scholar]