Abstract
Several molecular taxonomic studies have revealed that many natural (wild) Lactococcus lactis strains of dairy origin which are phenotypically representative of the L. lactis subspecies lactis cluster genotypically within subspecies cremoris and vice versa. Recently, we isolated two wild nisin-producing (Nis+) L. lactis strains, M78 and M104, of the lactis phenotype from Greek raw milk (J. Samelis, A. Lianou, A. Kakouri, C. Delbès, I. Rogelj, B. B. Matijašic, and M. C. Montel, J. Food Prot. 72:783–790, 2009); strain M78 possess a novel nisin A sequence (GenBank accession number HM219853). In this study, the actual subspecies identity of M78 and M104 isolates was elucidated, using 16S rRNA and acmA (encoding lactococcal N-acetylmuramidase) gene and histidine biosynthesis operon polymorphisms and 16S rRNA and ldh (encoding lactate dehydrogenase) gene phylogenies. Except the acmA gene analysis, molecular tools revealed that isolates M78 and M104 clustered with strains of the cremoris genotype, including the LMG 6897T strain, while they were distant from strains of the lactis genotype, including the LMG 6890T strain. The two wild isolates had identical repetitive sequence-based PCR (rep-PCR), randomly amplified polymorphic DNA (RAPD), plasmid, and whole-cell protein profiles and shared high 16S rRNA (99.9%) and ldh (100%) gene sequence homologies. In contrast, they exhibited identical sugar fermentation and enzymatic patterns which were similar to those of the subspecies lactis LMG 6890T strain. To our knowledge, this is the first complete identification report on a wild L. lactis subsp. cremoris genotype of the lactis phenotype which is capable of nisin A production and, thus, has strong potential for use as a novel dairy starter and/or protective culture.
INTRODUCTION
Lactococcus lactis is the most important and most extensively studied lactococcal species of high technological and safety significance for the dairy industry (1, 2). It currently includes three subspecies (L. lactis subsp. lactis, L. lactis subsp. cremoris, and L. lactis subsp. hordniae) plus a diacetyl-forming biovariety (L. lactis subsp. lactis biovar diacetylactis) (2, 3). Natural (wild) L. lactis strains are naturally present in raw cow, ewe, or goat milk (4, 5, 6) and commonly found in artisanal cheeses and other traditional dairy foods, where they often dominate over the microbiota of other lactic acid bacteria (LAB) (7, 8, 9, 10, 11). Therefore, L. lactis subsp. lactis and, to a lesser extent, L. lactis subsp. cremoris have long been extensively used in starter cultures for milk fermentation (cheeses, sour cream, and butter) composed of single or multiple lactose-fermenting strains with or without other LAB (1, 12, 13). Wild antipathogenic L. lactis strains are also naturally present or have been applied as bioprotective/costarter cultures in cheese and other foods to produce bacteriocins in situ, with nisin being the commonest and best characterized (14, 15, 16, 17). Nisin has been permitted to be used and applied as a natural generally recognized as safe (GRAS) preservative in many countries (17, 18, 19). In general, dairy lactococci, and L. lactis in particular, are considered safe for human health and consumption, and thus, their multiple applications in foods as well as probiotics are of continuously increasing scientific and industrial interest (2).
Based on classical phenotypic and biochemical criteria used in early identification keys, the important dairy subspecies of L. lactis, lactis and cremoris, are distinct and well differentiated from each other, including their type strains (20, 21). An early DNA homology study (22), plus several molecular studies in later years, have indicated, however, that these two subspecies are rather intermixed phylogenetically (5, 12, 23, 24). In particular, many wild L. lactis isolates from raw milk and cheese which are well identifiable as L. lactis subsp. lactis phenotypically actually cluster within subspecies cremoris genotypically (3, 4, 22) and vice versa (12, 25). Moreover, while the subspecies cremoris phenotype may have evolved in association with dairy-related practices and thus occurs in milk habitats only (12, 25, 26), wild cremoris genotypes having biochemical traits and capabilities similar to those of the subspecies lactis phenotype are frequent in dairy but also in nondairy niches (25, 27). This makes differentiation between the two subspecies problematic and often unreliable if based on phenotypic or genotypic criteria at the species level only (23, 28). Consequently, several, mainly molecular, approaches have been proposed and applied since early 1990s as specific taxonomic tools for analysis of the high heterogeneity of L. lactis intraspecies (23, 24, 28, 29, 30, 31, 32, 33, 34). A very recent polyphasic taxonomic study (3) confirmed the aforementioned diversity of wild dairy lactococci and further suggested that the lactis and cremoris genotypes of phenotypic L. lactis subsp. lactis may actually represent new true subspecies.
In accordance with those findings, in 2009, in the course of a research project on traditional European foods (www.truefood.eu), we reported the isolation from Greek raw milk of two wild L. lactis isolates, M78 and M104, typical of the subspecies lactis phenotype, which were of great interest due to their strong ability to produce nisin in synthetic media but also in milk and cheese; both isolates were genotypically confirmed to belong to L. lactis and to be genotypically distant from L. garvieae (35), whereas these first molecular results were not able to discriminate them on the subspecies level. Since then, the expression of nisin genes in L. lactis M78 in a cheese-like medium has been quantified by quantitative real-time PCR (qRT-PCR) (36), and its novel nisin A sequence has been published (GenBank accession number HM219853) and compared to other nisin sequences (37). L. lactis M104, which showed nearly the same phenotype as L. lactis M78 (35), has also been evaluated as a costarter and/or protective adjunct in pilot traditional Greek cheese trials (38, 39).
Reports of numerous food (including dairy) ecology studies on wild L. lactis strains which possess nisin-encoding genes, either singly or in copresence with lacticin 481, or on nisin characterization by chemical methods that have been published so far have identified the producer strains as L. lactis subsp. lactis (15, 16, 17, 40, 41, 42, 43). To the best of our knowledge, molecular identification studies specifically on wild nisin-producing (Nis+) L. lactis subsp. cremoris strains do not exist. All Nis+ strains of the cremoris genotype that have been reported so far are either genetically modified for this trait (14) or are natural (wild) isolates which, however, produce nisin Z (44), with the possible exception of one Italian strain claimed to produce nisin A in a very recent technological study by Dal Bello et al. (45). However, to claim detection of such a novel wild NisA+ L. lactis subsp. cremoris strain, a complete molecular identification study of the producer strain(s) would be necessary.
The present study was therefore undertaken to elucidate the subspecies identity of our wild L. lactis isolates M78 and M104, both possessing strong nisin-mediated antipathogenic activities. Identification was based on size polymorphisms of 16S rRNA (28), acmA (encoding lactococcal N-acetylmuramidase) (31, 34), and ldh (encoding lactate dehydrogenase) (33) genes and of a histidine biosynthesis operon (24, 32) previously reported in the literature to be effective for this purpose and on 16S rRNA and ldh gene phylogenies and was also accompanied by repetitive sequence-based PCR (rep-PCR) (46) plus randomly amplified polymorphic DNA (RAPD) (47), plasmid DNA, whole-cell protein (29), and phenotypic profiling of the strains. Based on the results, this report is the first complete identification study on natural (wild) strains of L. lactis subsp. cremoris which are capable of nisin A production.
MATERIALS AND METHODS
Lactococcal strains and culture conditions.
Strains of L. lactis used in this study are presented in Table 1. All lactococcal strains were maintained at −30°C in MRS broth (LAB M, Lancashire, United Kingdom) or M-17 broth (Merck, Darmstadt, Germany) with 20% glycerol added (Merck). Stock cultures were subcultured at least twice by transferring 0.1 ml of inoculum to 10 ml of fresh broth, and their purity was checked by streaking on the respective agar media, before use in the experiments. Unless otherwise stated, all lactococcal cultures for use in the biochemical and molecular tests of this study were grown in M-17 broth and agar media (Merck) at 30°C without shaking.
Table 1.
Lactococcus lactis strains used in this study
| Strain | Comment(s) (reference) |
|---|---|
| L. lactis M78 | Strain under investigation; isolated from Greek raw milk (35) |
| L. lactis M104 | Strain under investigation; isolated from Greek raw milk (35) |
| L. lactis subsp. lactis LMG 6890T | Type strain |
| L. lactis subsp. cremoris LMG 6897T | Type strain |
| NCFB = NCDO 1402, L. lactis subsp. lactis | Nisin-producing (Nis+); nisin resistant; lactose and sucrose positive; proteinase negative; was among the Nis+ strains evaluated as starters in Cheddar cheese manufacture during the early 1990s (16); kindly provided by the Leatherhead Food Research Association (LFRA; Surrey, United Kingdom) microbiology laboratory for use as a bacteriocin-positive (Nis+) control strain indicator in our previous sakacin B studies (48) |
| F1 L. lactis subsp. lactis | Nis+, proteinase negative; sucrose-positive but lactose-negative strain, like NCFB 912, NIZO R5L0, and a few other natural Nis+ L. lactis subsp. lactis strains (15); recovered as a “contaminant” from a stock culture of NCFB 1402; thus, F1 is either a lactose-negative variant of NCFB 1402 or a strain like NCFB 912 since exptl Cheddar cheese trials with mixed Nis+ L. lactis/cremoris starter strains, such as those performed in the United States (14), were conducted also at the LFRA |
| CNRZ 125 L. lactis subsp. lactis | Strain of dairy origin provided by INRA, Jouy-en Josas, France |
| CNRZ 258 L. lactis subsp. lactis | Strain of dairy origin provided by INRA, Jouy-en Josas, France |
| CNRZ 301 L. lactis subsp. lactis | Strain of dairy origin provided by INRA, Jouy-en Josas, France |
| CNRZ 1075 L. lactis subsp. lactis | Strain of dairy origin provided by INRA, Jouy-en Josas, France |
| 1L8 L. lactis subsp. cremoris | Raw cow milk isolate provided by INRA, Poligny, France |
Biochemical characterization of the wild Nis+ lactococcal strains.
The phenotypic taxonomy within subspecies lactis of the Nis+ L. lactis isolates M78 and M104 (35) was reconfirmed by testing them for cell morphology, gas production from glucose, arginine hydrolysis, growth at 15°C and 45°C, growth at 4% and 6.5% NaCl, growth on kanamycin esculin azide agar, and survival at 60°C for 30 min under the same culture conditions as those used with the reference strains of L. lactis included in Table 1. The media and methods used were as described by Samelis et al. (35). Moreover, the entire sugar fermentation patterns and main enzymatic activities profiles of the wild strains compared to those of the L. lactis reference strains were determined by the use of API 50 CHL and API ZYM kits (bioMérieux, Marcy l'Etoile, Lyon, France), respectively. In addition, strains M78 and M104 along with strains LMG 6890T and LMG 6897T were subjected to SDS-PAGE of whole-cell crude protein extracts, as follows. Lactococcal cultures at an optical density of 0.5 to 0.6 grown in M-17 broth at 30°C were centrifuged at 12,000 × g for 10 min at room temperature, washed, and resuspended in 1 ml of distilled water (29). Cells were further disrupted in a mini Bead-Beater (Biospec Products, Bartlesville, OK) (using a total of 10 1-min vibrations performed using 0.1-mm-diameter zirconium beads). The cell debris homogenates were centrifuged at 12,000 × g for 15 min at 4°C, and the supernatants were used as protein extracts. Phenylmethylsulfonyl fluoride (PMSF) was added to the protein extracts at a final concentration of 1 mM immediately after centrifugation. The protein concentration was further determined by the Bradford method (49), and 20 μg of whole-cell proteins was loaded on the gels. Electrophoresis was carried out in a 12% (wt/vol) polyacrylamide gel at a constant current of 120 V until the dye front reached the bottom of the gel. Each gel was stained with a silver staining protocol as previously reported by Parapouli et al. (50). The profiles of the two wild and the two type strains were then compared macroscopically to detect the main differences in their protein bands.
Molecular characterization of the wild Nis+ lactococcal strains.
Identification of M78 and M104 isolates was verified by analysis of 16S rRNA and acmA (encoding lactococcal N-acetylmuramidase) gene and histidine biosynthesis operon polymorphisms and 16S rRNA and ldh (encoding lactate dehydrogenase) gene phylogenies as well as by rep-PCR, RAPD, and plasmid DNA analysis profile analyses. Strains LMG 6890T and LMG 6897T and a few additional strains (Table 1) served as controls. The genomic DNA used as the template for the amplification of 16S rRNA gene regions was extracted according to Pu et al. (28), whereas for acm and ldh gene amplifications as well as for RAPD analysis, DNA extraction was performed using a NucleoSpin tissue kit (Macherey-Nagel, Germany) according to the manufacturer's recommendations for Gram-positive bacteria. Plasmid DNA was purified using a NucleoSpin plasmid kit (Macherey-Nagel) according to the manufacturer's recommendations for Gram-positive bacteria. The heterogeneity of 16S rRNA gene sequences of the lactococcal species was studied using primers LacF, CreF, and LacreR according to the method of Pu et al. (28), whereas primers PALA 4 and PALA 14 (31) were employed for the detection of acm gene polymorphisms in comparisons of the different L. lactis subspecies as described by Garde et al. (34). For the amplification of the 16S rRNA gene, primers 8F and 1492R were used, while the PCR mixture was cycled using the following conditions: initial denaturation at 95°C for 5 min, 35 cycles consisting of 95°C for 1 min, 58°C for 2 min, and 72°C for 2 min, and a final extension step at 72°C for 10 min. The ldh gene sequences were amplified using primers LDHF1 and LDHR1 according to Urbach et al. (33). RAPD typing was performed with primer M13, as reported by Rossetti and Giraffa (47). All amplification reactions were carried out in a PTC-100 (version 7.0) thermocycler (MJ Research Inc.) using a Phusion High Fidelity DNA polymerase system (Finnzyme, Finland). The amplicons produced were purified using NucleoSpin Extract 2 in 1 (Macherey-Nagel). Molecular cloning was performed using a Zero Blunt kit (Invitrogen) according to the manufacturer's recommendations. Escherichia coli strain DH5α, grown aerobically in LB media at 37°C, served as the recombinant plasmid host (51). Amplified or extracted DNAs were separated by electrophoresis in 1% agarose gels, stained with ethidium bromide, and visualized using standard protocols (52). Plasmid DNA digestion by restriction enzymes and separation of fragments by agarose gel electrophoresis were also carried out using a standard methodology (52). Cloned 16S or ldh gene fragments were sequenced by VBC-Biotech (Austria). Taxonomic analysis was conducted by using the GenBank BLAST program (53). For phylogenetic and molecular evolutionary analysis, MEGA version 4.1 (54) was used. The 16S rRNA and ldh gene sequences of strains M78 and M104 were aligned with sequences from related taxa by using the CLUSTAL W program (55). The resultant tree topology was evaluated by bootstrap analysis by using the neighbor-joining method based on 1,000 resamplings. The size polymorphism of the histidine biosynthesis operon was checked using primers Lhis5F and Lhis6R, according to Beimfohr et al. (32). Rep-PCR analysis was performed using primers Rep-1R-Dt and REP2-D (46), as reported by Callon et al. (9). The dendrogram was derived from the unweighted-pair group method using average linkages (UPGMA) and based on Dice coefficients. Band position tolerance and optimization were set at 1.0 and 0.5%, respectively.
RESULTS
Phenotypic identification of the wild raw-milk isolates as L. lactis subsp. lactis.
All phenotypic/biochemical tests conducted (Table 2) confirmed previous results of the Samelis et al. (35) study which first reported that the wild Nis+ raw-milk isolates M78 and M104 were phenotypically identified as L. lactis subsp. lactis. Indeed, both strains are Gram-positive, catalase-negative, homofermentative, arginine-positive cocci which grow at 15°C and in 4% salt but not at 45°C and in 6.5% salt, while neither grows on kanamycin esculin azide agar or survives heating at 60°C for 30 min; thus, they are not enterococci. As expected, L. lactis subsp. lactis LMG 6890T and the Nis+ L. lactis subsp. lactis strains NCFB 1402 and F1 shared those phenotypic properties with strains M78 and M104, whereas L. lactis subsp. cremoris LMG 6897T differed in being arginine negative and unable to grow in 4% salt (Table 2). Moreover, strain LMG 6897T was confirmed to possess a very restricted sugar fermentation profile, since it gave positive results with glucose, fructose, mannose, lactose, and N-acetyloglucosamine only. In contrast, strains M78 and M104 had identical sugar fermentation profiles which were much more enriched than those of the cremoris type strain and similar to the profiles of the L. lactis subsp. lactis strains tested (Table 2). Specifically, compared with the lactis LMG 6890T strain, strains M78 and M104 were superior in fermenting mannitol and sucrose plus amygdalin, gentiobiose, and, with a delay, starch. In contrast, the main difference in sugar fermentation between our wild Nis+ strains M78 and M104 and Nis+ strains NCFB 1402 and F1 was the inability of the latter to ferment d-xylose and, of course, the known inability of strain F1 to ferment lactose (see Table 1) plus maltose (Table 2).
Table 2.
Differentiating phenotypic/biochemical identification reactions of the wild Nis+ Lactococcus lactis strains M78 and M104 in comparison with reference strainsa
| Phenotypic test |
Lactococcus lactis strain |
|||||
|---|---|---|---|---|---|---|
| M78 | M104 | LMG 6890T | LMG 6897T | NCFB 1402 | F1 | |
| NH3 from arginine | + | + | + | − | + | + |
| Growth in 4% salt | + | + | + | − | + | + |
| Fermentation of: | ||||||
| d-Ribose | + | + | + | − | + | + |
| d-Xylose | + | + | + | − | − | − |
| d-Galactose | + | + | + | − | + | + |
| d-Mannitol | + | + | − | − | + | + |
| Amygdalin | + | + | − | − | − | − |
| Arbutin | + | + | + | − | (+) | (+) |
| Esculin | + | + | + | − | + | + |
| Salicin | + | + | + | − | + | + |
| d-Cellobiose | + | + | + | − | + | + |
| d-Maltose | + | + | + | − | + | − |
| d-Lactose | + | + | + | + | + | − |
| d-Sucrose | + | + | − | − | + | + |
| d-Trehalose | + | + | + | − | − | − |
| Amidon (starch) | (+)d | (+)d | − | − | − | − |
| Gentiobiose | + | + | − | − | + | + |
All strains were Gram-positive, catalase-negative, homofermentative cocci which grew at 15°C. None grew at 45°C, in 6.5% salt, and on kanamycin esculin azide (KAA) agar. None survived heating at 60°C for 30 min. All strains fermented d-glucose, d-fructose, d-mannose, and N-acetylglucosamine. None fermented glycerol, erythritol, d-arabinose, l-arabinose, l-xylose, adonitol, α-methyl-d-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, d-sorbitol, α-methyl-d-mannopyranoside, α-methyl-d-glucopyranoside, d-melibiose, inulin, d-melezitose, d-raffinose, glycogen, xylitol, d-turanose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, gluconate, 2-ketogluconate, or 5-ketogluconate. +, positive reaction; −, negative reaction; (+), weak reaction; (+)d, delayed reaction.
Strains M78 and M104 had identical enzymatic activities profiles, which also were identical with the API ZYM profile of L. lactis subsp. lactis LMG 6890T (Table 3). A strong positive reaction with the substrate for α-glucosidase was actually the only major enzymatic difference between the three strains discussed above and the L. lactis subsp. cremoris LMG 6897T and Nis+ NCFB 1402 and F1 strains, which all gave negative results; all other differences were semiquantitative (based on the change in color intensity corresponding to how the strain utilized the same API ZYM substrates in the cupules) rather than qualitative (based on the ability of each strain to utilize a given substrate). On this basis, the cremoris type strain differed from the lactis type strain and the wild Nis+ strains M78 and M104 in displaying weaker valine and cystine arylamidase and alkaline phosphatase reactions and stronger esterase/lipase reactions. The API ZYM profiles of the Nis+ NCFB 1402 and F1 strains were intermediate overall but clearly were more similar to the subspecies lactis profile than to the subspecies cremoris profile with regard to their proteolytic reactions (Table 3).
Table 3.
Enzymatic activity reactions of the wild Nis+ Lactococcus lactis M78 and M104 in comparison with reference strains determined by the API ZYM methoda
| Enzyme assayed for |
Lactococcus lactis strain reaction color grade |
|||||
|---|---|---|---|---|---|---|
| M78 | M104 | LMG 6890T | LMG 6897T | NCFB 1402 | F1 | |
| Alkaline phosphatase | 5 | 5 | 5 | 3 | 4 | 4 |
| Esterase (C4) | 3 | 3 | 3 | 4 | 4 | 4 |
| Esterase lipase (C8) | 4 | 4 | 4 | 5 | 4 | 4 |
| Lipase (C14) | − | − | − | − | − | − |
| Leucine arylamidase | 5 | 5 | 5 | 5 | 5 | 5 |
| Valine arylamidase | 4 | 4 | 4 | 2 | 4 | 4 |
| Cystine arylamidase | 4 | 4 | 4 | 2 | 4 | 4 |
| Trypsin | − | − | − | − | − | − |
| α-Chymotrypsin | 3 | 3 | 2 | 3 | 3 | 3 |
| Acid phosphatase | 5 | 5 | 5 | 5 | 5 | 5 |
| Naphthol-AS-BI-phosphohydrolase | 5 | 5 | 5 | 5 | 5 | 5 |
| α-Galactosidase | − | − | − | − | − | − |
| β-Galactosidase | − | − | − | − | − | − |
| β-Glucuronidase | − | − | − | − | − | − |
| α-Glucosidase | 5 | 5 | 5 | − | − | − |
| β-Glucosidase | − | − | − | − | − | − |
| N-Acetyl-β-glucosaminidase | − | − | − | − | − | − |
| α-Mannosidase | − | − | − | − | − | − |
| α-Fucosidase | − | − | − | − | − | − |
Numbers indicate a positive reaction based on the change in color intensity in each API ZYM cupule. According to the manufacturer's instructions, the color change was graded using a scale from 1 (extremely weak reaction) to 5 (very strong positive reaction). −, negative reaction similar to that seen with the control. Reactions with a grade of 3 to 5 are considered as clearly positive.
Differentiation of L. lactis strains by SDS-PAGE.
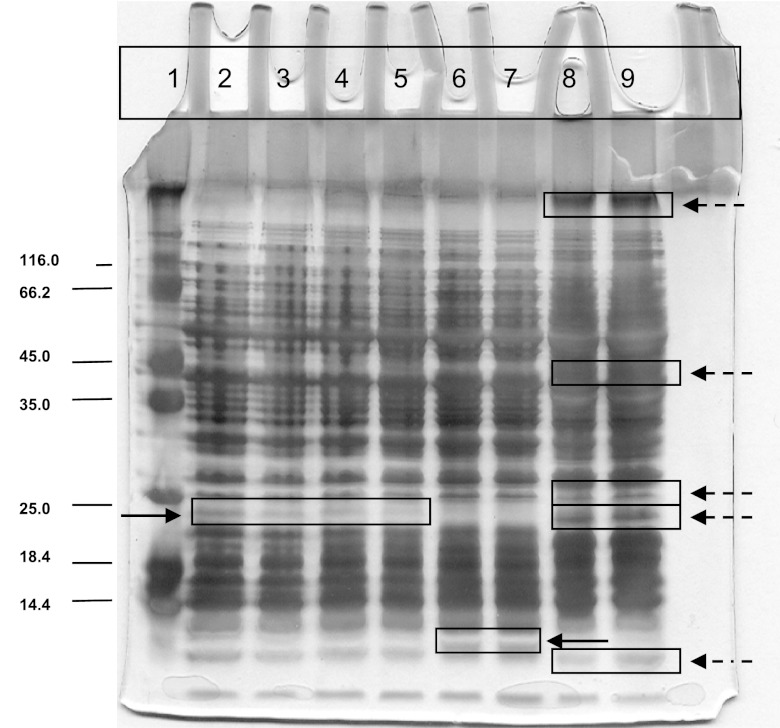
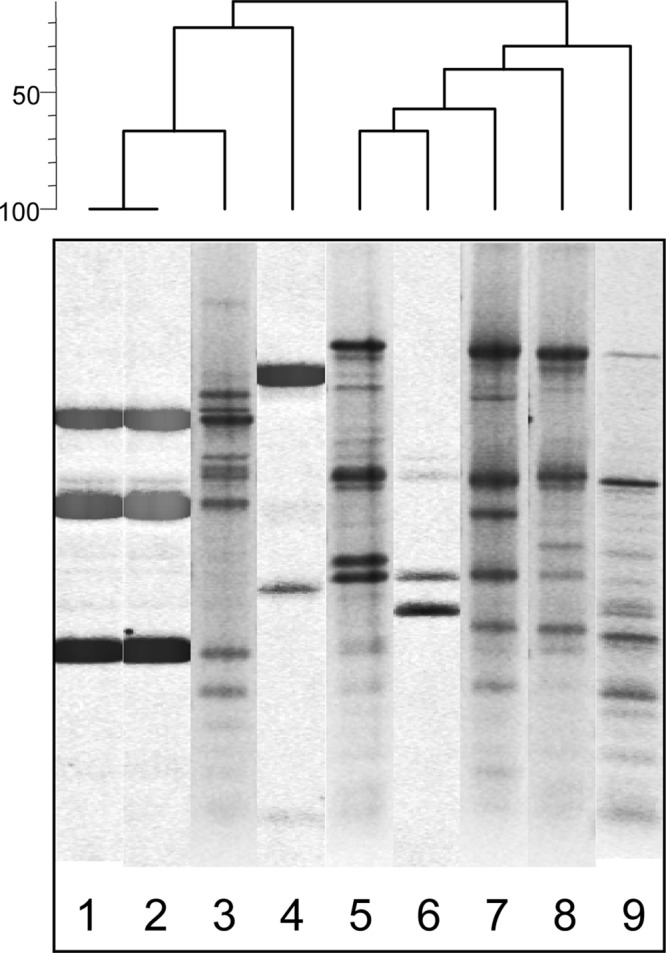
The wild Nis+ L. lactis M78 and M104 strains had identical whole-cell protein profiles, which macroscopically shared an overall higher similarity to the profile of the subspecies cremoris LMG 6897T strain than to that of the subspecies lactis LMG 6890T strain (Fig. 1). Indeed, while their profiles were clearly distinct macroscopically from the profile of the LMG 6890T strain, they showed only two differences in certain protein bands from the profiles of the LMG 6897T strain; in specific, whereas strains M78 and M104 exhibited a protein band of approximately 25 kDa that is not present in the protein profile of LMG 6897T strain, the latter possesses a protein below 14.4 kDa that strains M78 and M104 lack (Fig. 1).
Fig 1.
Polyacrylamide gel electrophoresis of crude whole-cell protein extracts of Lactococcus lactis spp. Lane 1, protein molecular mass (kDa) marker (Fermentas, Germany); lanes 2 and 3, M78 (wild Nis+ strain); lanes 4 and 5, M104 (wild Nis+ strain); lanes 6 and 7, L. lactis subsp. cremoris LMG 6897T; lanes 8 and 9, L. lactis subsp. lactis LMG 6890T. Solid/compact arrows indicate the positions of major protein differences between the wild Nis+ strains M78 and M104 and L. lactis subsp. cremoris LMG 6897T strain. Dashed arrows indicate the positions of major protein differences between the wild Nis+ strains M78 and M104 and L. lactis subsp. lactis LMG 6890T strain.
Molecular identification of the wild raw-milk isolates as L. lactis subsp. cremoris.
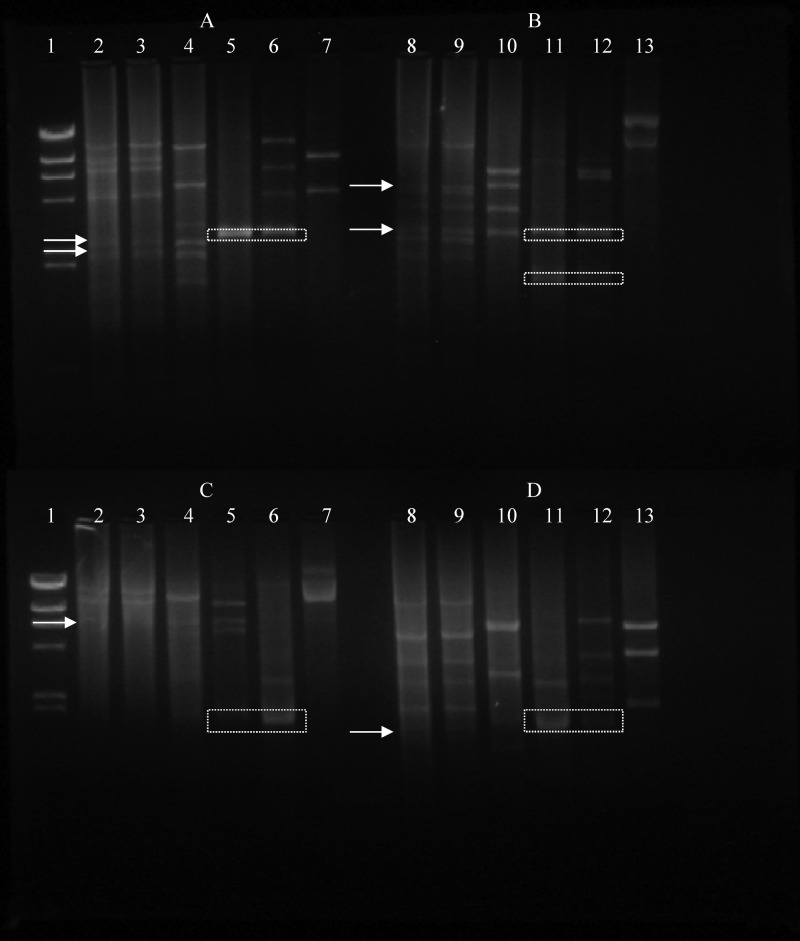
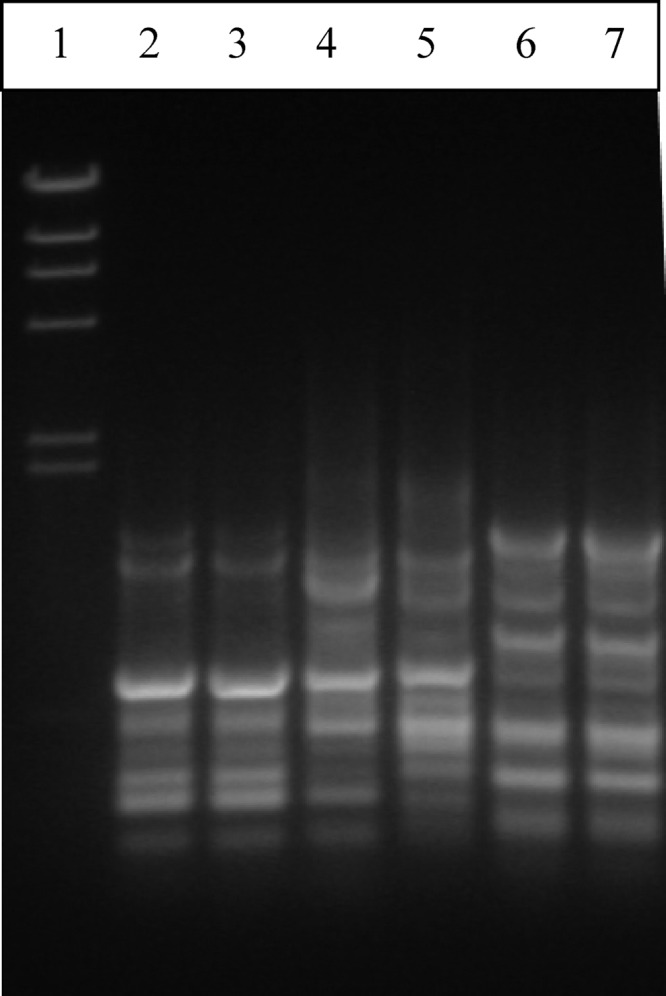
In contrast to the biochemical criteria (Tables 2 and 3) which confirmed that the Nis+ L. lactis M78 and M104 strains are phenotypically representative of subspecies lactis, specific molecular identification tools revealed that the two strains cluster genotypically within subspecies cremoris. Indeed, M78 and M104 strains produced the same rep-PCR (Fig. 2) and RAPD (Fig. 3) patterns; their rep-PCR pattern had a higher similarity with that of subspecies cremoris than with that of subspecies lactis (Fig. 2). Specifically, strains M78 and M104 clustered apart from all five representative L. lactis subsp. lactis strains, including the type strain LMG 6890T, while they clustered relatively closely with the L. lactis subsp. cremoris type strain LMG 6897T and together with subspecies cremoris strain 1L8 (Fig. 2). On the other hand, the RAPD profile of strains M78 and 104 was different from the RAPD profiles of the L. lactis subsp. lactis LMG 6890T and L. lactis subsp. cremoris LMG 6897T strains, which, as expected, were also different from each other, whereas it also was different from the RAPD profiles of Nis+ L. lactis subsp. lactis strains NCFB 1402 and F1, which were identical (Fig. 3).
Fig 2.
rep-PCR patterns of Lactococcus lactis wild and reference strains. Lane 1, M78 (wild Nis+ strain); lane 2, M104 (wild Nis+ strain); lane 3, L. lactis subsp. cremoris 1L8; lane 4, L. lactis subsp. cremoris LMG 6897T; lane 5, L. lactis subsp. lactis CNRZ 1075; lane 6, L. lactis subsp. lactis LMG 6890T; lane 7, L. lactis subsp. lactis CNRZ 301; lane 8, L. lactis subsp. lactis CNRZ 125; lane 9, L. lactis subsp. lactis CNRZ 258.
Fig 3.
RAPD profiles of Lactococcus lactis wild and reference strains determined using primer M13. Lane 1, molecular size marker λDNA/HindIII; lane 2, M78 (wild Nis+ strain); lane 3, M104 (wild Nis+ strain); lane 4, L. lactis subsp. cremoris LMG 6897T; lane 5, L. lactis subsp. lactis LMG 6890T; lane 6, L. lactis subsp. lactis F1 Nis+ strain; lane 7, L. lactis subsp. lactis NCFB 1402 Nis+ strain.
Plasmid DNA analysis results were, in general, consistent with the rep-PCR and RAPD analysis results reported above. As shown in Fig. 4, the plasmid restriction patterns of strains M78 and M104 were identical, exhibiting a unique profile in comparison with those of all the other L. lactis strains; existing bands in each profile were dependent on the restriction endonuclease used for the plasmid DNA digestion. However, with all four restriction enzymes used, strains M78 and M104 were found to share common bands with the L. lactis subsp. cremoris LMG 6897T strain whereas their plasmid profiles were greatly distinct from those of the subspecies lactis strains, including the LMG 6890T strain (Fig. 4).
Fig 4.
Plasmid DNA patterns of Lactococcus lactis strains determined using the restriction endonucleases EcoRI (A), EcoRV (B), BamHI (C), and HindIII (D). (A and C) Lanes 1, marker λDNA/HindIII; lanes 2, M78; lanes 3, M104; lanes 4, L. lactis subsp. cremoris LMG 6897T; lanes 5, L. lactis subsp. lactis NCFB 1402 Nis+ strain; lanes 6, L. lactis subsp. lactis F1 Nis+ strain; lanes 7, L. lactis subsp. lactis LMG 6890T. (B and D) Lanes 8, M78; lanes 9, M104; lanes 10, L. lactis subsp. cremoris LMG 6897T; lanes 11, L. lactis subsp. lactis NCFB 1402 Nis+ strain; lanes 12, L. lactis subsp. lactis F1 Nis+ strain; lanes 13, L. lactis subsp. lactis LMG 6890T. Arrows indicate common bands between the L. lactis subsp. cremoris LMG 6897T strain and the wild Nis+ M78 and M104 strains. Squares indicate common bands between the L. lactis subsp. lactis NCFB 1402 Nis+ strain and the L. lactis subsp. lactis F1 Nis+ strain.
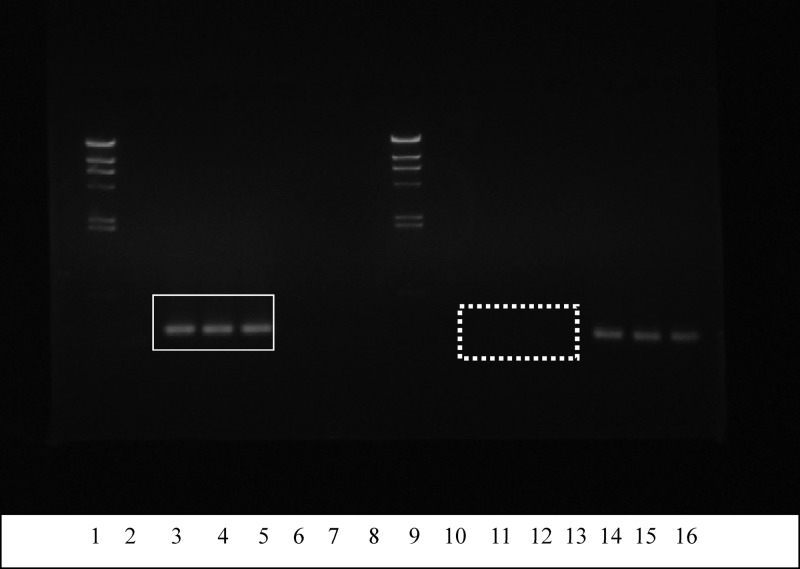
The differentiation of isolates M78 and M104 at the subspecies level was further evaluated by 16S rRNA gene polymorphism analyses employing primer pair CreF and LacreR and primer pair LacF and LacreR; both isolates were identified as L. lactis subsp. cremoris, exhibiting a 163-bp amplicon using primers CreF and LacreR (compact/solid square in Fig. 5), while no PCR product was amplified with primers LacF and LacreR (dashed square in Fig. 5). These results were confirmed by the size polymorphism of the histidine biosynthesis operon determined using primers Lhis5F and Lhis6R (32); the size of the latter PCR product was that expected for the L. lactis subsp. cremoris and not that expected for L. lactis subsp. lactis (Fig. 6). The expected sizes of amplification products for L. lactis subsp. cremoris and L. lactis subsp. lactis were 1,149 bp and 934 bp, respectively (32). In contrast, acm polymorphisms failed to discriminate between all tested L. lactis strains on the subspecies level (data not shown).
Fig 5.
PCR identification of Lactococcus lactis. PCR amplification products in lanes 2 to 8 were determined using primers CreF and LacreR. Lane 1, marker λDNA/HindIII; lane 2, no-DNA control; lane 3, L. lactis subsp. cremoris LMG 6897T; lane 4, M78; lane 5, M104; lane 6, L. lactis subsp. lactis LMG 6890T; lane 7, L. lactis subsp. lactis F1 Nis+ strain; lane 8, L. lactis subsp. lactis NCFB 1402 Nis+ strain. PCR amplification products in lanes 10 to 16 were determined using primers LacF and LacreR. Lane 9, marker λDNA/HindIII; lane 10, no-DNA control; lane 11, L. lactis subsp. cremoris LMG 6897T; lane 12, M78; lane 13, M104; lane 14, L. lactis subsp. lactis LMG 6890T; lane 15, L. lactis subsp. lactis F1 Nis+ strain; lane 16, L. lactis subsp. lactis NCFB 1402 Nis+ strain. The compact/solid square indicates the presence of amplified PCR product bands for the L. lactis subsp. cremoris LMG 6897T strain and the wild Nis+ M78 and M104 isolates when the cremoris-specific primer pair CreF and LacreR was used, whereas the dashed square indicates the absence of amplified PCR product bands for the same strains when the subspecies lactis-specific primer pair LacF and LacreR was used.
Fig 6.
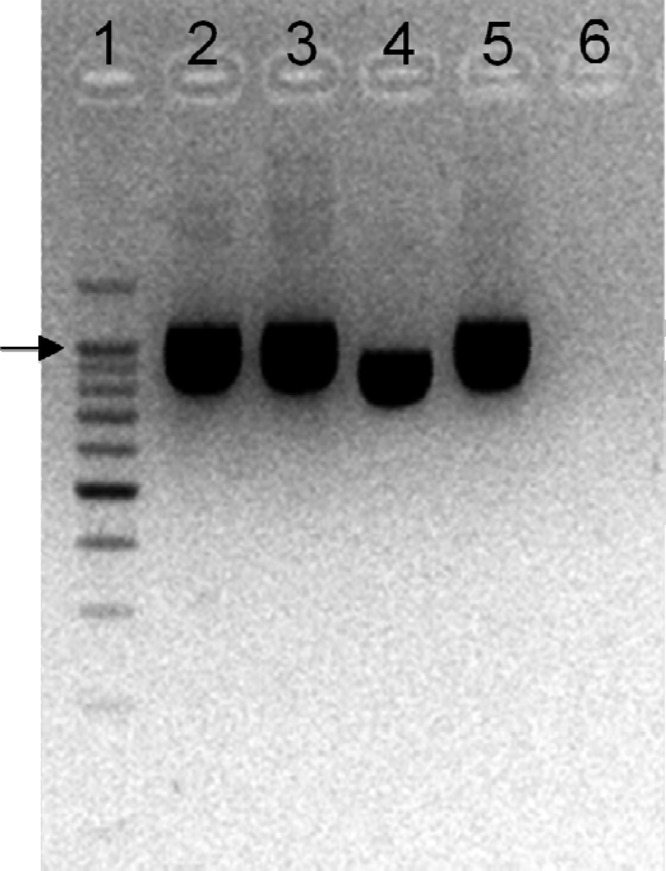
Size polymorphism in the histidine biosynthesis operon of Lactococcus lactis subsp. lactis and Lactococcus lactis subsp. cremoris. Lane 1, molecular size marker; lane 2, M78; lane 3, M104; lane 4, L. lactis subsp. lactis LMG 6890T; lane 5, L. lactis subsp. cremoris LMG 6897T; lane 6, no-DNA control. The arrow indicates the position of the 1,000-bp band in the molecular size marker.
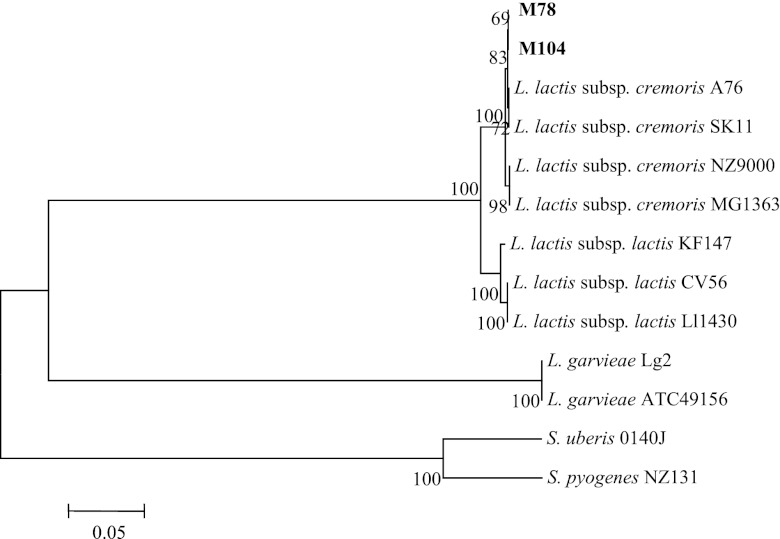
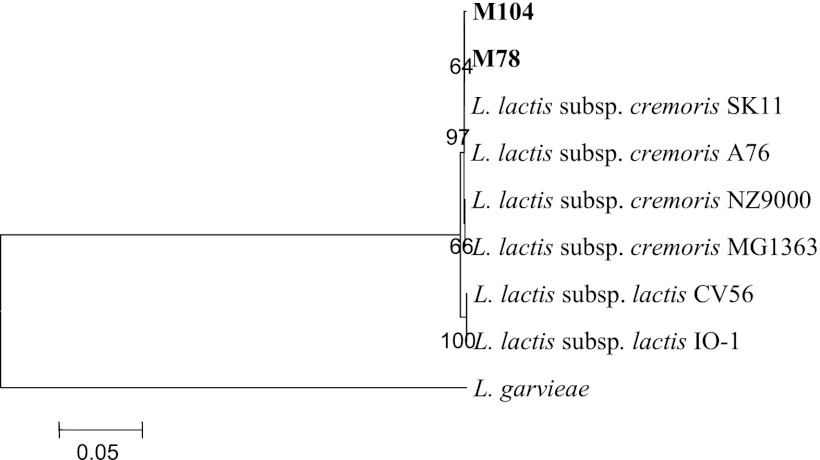
The ldh and 16S rRNA gene sequencing analyses further confirmed that strains M78 and M104 cluster genotypically with L. lactis subsp. cremoris whereas they are close relatives of L. lactis subsp. lactis and, of course, are of low genotypic relatedness to L. garviae (Fig. 7 and 8). The ldh sequences of isolates M78 and M104 share 100% homology (GenBank accession number JX402633), whereas their 16S rRNA sequences are nearly identical (99.9% homology), differing in two nucleotides only (GenBank accession numbers JX402634 and JX402635).
Fig 7.
Phylogenetic location of the wild Nis+ Lactococcus lactis strains M78 and M104 among closely related species of bacteria based on the lactate dehydrogenase (ldh) gene sequence. The scale bar indicates 0.05 substitutions per nucleotide position. S. uberis, Streptococcus uberis; S. pyogenes, Streptococcus pyogenes.
Fig 8.
Phylogenetic location of the wild Nis+ Lactococcus lactis strains M78 and M104 among closely related species of bacteria based on the 16S rRNA gene sequence. The scale bar indicates 0.05 substitutions per nucleotide position.
DISCUSSION
Based on the results of the analysis of polymorphisms in a combination of selected genes previously reported as specific for the differentiation of L. lactis subsp. lactis from subsp. cremoris (24, 28, 32, 33), this study showed that the wild Nis+ L. lactis M78 and M104 isolates from Greek raw-milk cluster genotypically within subspecies cremoris although their phenotypical-biochemical properties are highly representative of subspecies lactis. In fact, according to this study, M78 and M104 may represent duplicate isolates of a novel L. lactis subsp. cremoris NisA+ strain given that isolate M78 was originally picked from M-17 agar plates incubated at 42°C whereas isolate M104 was from the respective MRS agar plates at 30°C of the same sample of raw bulk milk (35). In that study (35), the ability of isolate M104 to grow in MRS broth at 45°C, and in MRS broth with added 6.5% NaCl at 30°C, was the only phenotypic difference between the two isolates. Growth of isolate M104 at 45°C and in 6.5% salt, generally considered atypical phenotypic properties in L. lactis (8), was not confirmed in this study (Table 2). This discrepancy observed in these physiological tests/assays at the growth boundaries of L. lactis leads us to the logical conclusion that those initial results were a technical artifact. Conversely, the same basic biochemical—sugar fermentation—reactions of isolates M78 and M104 in miniplates reported by Samelis et al. (35) were fully confirmed in this study by the API 50CHL method (Table 2). Results further showed that the two isolates share their entire sugar profiles, including a delayed-positive reaction with starch; the later is quite surprising and, in our opinion, worthy of noting because L. lactis is typically starch negative (20, 21). In fact, very few species among the total number in all LAB genera ferment starch, and those are mostly plant-associated lactobacilli. So, the delayed, even weak starch fermentation, along with the pronounced ability of isolates M78 and M104 to ferment, all together, xylose, maltose, sucrose, mannitol, and amygdalin plus its derivative gentiobiose, unlike the LMG 6890T strain and other L. lactis strains (Table 2), suggests that these wild raw-milk isolates may actually have a plant-based origin. That several L. lactis strains were isolated directly from plants, or from milk and cheese while originating from plants, has been well established in recent studies (27, 56), supporting the concept of the plant-milk transition for wild Lactococcus strains (12, 25).
In addition to their sugar fermentation patterns, this study showed that isolates M78 and M104 exhibit identical enzymatic, whole-cell protein, rep-PCR, RAPD, and plasmid restriction profiles, facts that further support the hypothesis that M78 and M104 may actually represent isolates of a single novel wild Nis+ L. lactis strain/genotype, given that, as previously reported (35), they were randomly selected colonies grown on different agar media of a raw-milk sample. In regard to their enzymatic activities, the negative reactions of both isolates and all other L. lactis strains for lipase (C14), trypsin, α-galactosidase, β-glucuronidase, N-acetyl-β-glucosaminidase, α-mannosidase, and α-fucosidase are in complete agreement with the API ZYM results of Nomura et al. (27), who tested 41 natural L. lactis strains from milk and plants. The remaining enzymatic reactions in Table 3 also are in general agreement with those of the Japanese L. lactis subsp. lactis biovar diacetylactis or L. lactis subsp. cremoris strains mentioned above (27). The most prominent differences are that several Japanese strains were positive for β-galactosidase and β-glucosidase (27) whereas, in this study, none of the six L. lactis strains tested positive (Table 3). Nomura et al. (27) showed that 63% to 100% of the subsp. lactis biovar diacetylactis plant or milk strains compared to 38% of the subspecies cremoris milk strains were positive for α-glucosidase, which was the most clearly differentiating API ZYM reaction for our strains, as shown in Table 3. This further supports the notion that M78 and M104 isolates belong to less-frequent cremoris genotypes which possess enzymatic and biochemical properties that are highly representative of subspecies lactis.
Interestingly, the SDS-PAGE of whole-cell proteins was the only biochemical analysis of this study which indicated a closer genotypic relatedness of M78 and M104 isolates with subspecies cremoris than with subspecies lactis. Considering, however, that several species or subspecies of lactic acid bacteria are known to be very diverse in their whole-cell protein profiles at the strain level (30), it is necessary to apply SDS-PAGE analysis of isolates M78 and M104 in comparison with analyses of large numbers of reference or wild L. lactis subsp. lactis or cremoris strains. Nevertheless, the different protein profile of isolates M78 and M104 compared to the subspecies lactis LMG 6890T strain plus the distinct differences from the subspecies cremoris LMG 6897T strain (Fig. 1) are the first results underlining on the protein level the uniqueness of L. lactis strains presenting genotypic subspecies cremoris and phenotypic subspecies lactis properties. However, a future comparative proteomic study would draw useful conclusions on the technological and bioprotective potential of these strains.
The uniqueness of M78 and M104 and their closer relationship to subspecies cremoris above discussed were further confirmed by the results of rep-PCR analysis (Fig. 2). In addition, RAPD (Fig. 3) and plasmid DNA (Fig. 4) profiling clearly showed the genetic relationship of the two isolates under study but failed to discriminate them on the subspecies level. At this point, it should be noted that the two Nis+ NCFB 1402 and F1 strains share the same RAPD profile (Fig. 3), whereas they also share certain plasmid DNA restriction bands (Fig. 4). Thus, strain F1 may indeed represent a lactose-negative variant of strain NCFB 1402 (see Table 1).
The first molecular identification tools that clearly delineated the taxonomy of isolates M78 and M104 on the subspecies level were 16S rRNA gene and histidine biosynthesis operon size polymorphism analyses. As presented in Fig. 5, both isolates exhibited a 163-bp amplicon using primers CreF and LacreR (compact/solid square) whereas no PCR product was amplified with primers LacF and LacreR (dashed square). As reported by Pu et al. (28), primer pair CreF and LacreR and primer pair LacF and LacreR can differentiate representatives of species L. lactis on the subspecies level: with primers CreF and LacreR, a 163-bp amplicon is obtained for strains L. lactis subsp. cremoris, while a 163-bp product is obtained for L. lactis subsp. lactis strains using primers LacF and LacreR. Regarding histidine biosynthesis operon size polymorphisms, in analyses performed using primer pair Lhis5F and Lhis6R, strains M78 and M104 exhibited the PCR product expected for subspecies cremoris representatives (Fig. 6), as reported by Beimfohr et al. (32). On the subspecies level, in contrast, acm polymorphisms failed to discriminate the isolates under study. According to Garde et al. (34), with primer pair PALA 4 and PALA 14, a 1,131-bp product was obtained with a L. lactis subsp. cremoris template whereas an additional product of 700 bp was amplified for a L. lactis subsp. lactis template. In this study, however, all L. lactis strains exhibited a one-band profile (data not shown), a finding contradictory to that of the Garde et al. (34) approach that has also been previously reported by Prodelalová et al. (57).
In consistency with the findings presented above, ldh and 16S rRNA gene phylogenies also successfully taxonomized both isolates as representatives of the L. lactis subsp. cremoris genotype (Fig. 7 and 8). Their high sequence homologies, 100% for the ldh gene and 99.9% for the 16S rRNA gene, further suggest that isolates M78 and M104 may actually represent different raw-milk isolates of the same wild Nis+ strain/genotype.
In conclusion, the wild isolates M78 and M104 from Greek raw milk should be considered to represent a novel L. lactis subsp. cremoris strain or genotype. Their actual novelty is not supported by their genotypic subspecies identification, which contradicts their phenotypic identification, since many previous dairy ecology studies have shown similar results for several wild L. lactis strains (3, 5, 12, 23, 24, 25). L. lactis subsp. cremoris M78 and M104 are novel because they are strong nisin (Nis+) producers (35, 36) capable of inactivating or inhibiting growth of Listeria monocytogenes and Staphylococcus aureus in vitro as well as in situ in various dairy foods (35, 38, 39). Other wild Nis+ L. lactis subsp. cremoris strains that have been reported so far are raw-milk or cheese isolates which, however, produce nisin Z and not nisin A (44). Regardless of genotype, however, NisZ+ L. lactis strains generally are of lower inhibitory efficacy against pathogens than typical NisA+ L. lactis subsp. lactis strains (45), including strain ATCC 11454 used to create the nisin-positive transconjugant L. lactis subsp. cremoris JS102 strain (14).
To our knowledge, there is only one very recent technological study, by Dal Bello et al. (45), which described the effective use of 40FEL3, an Italian wild NisA+ L. lactis subsp. cremoris strain, to control L. monocytogenes in cottage cheese. This strain, originally isolated and described by Dal Bello et al. (44) as the producer of a nonidentified bacteriocin, was later found to be most inhibitory against L. monocytogenes in vitro and in cottage cheese (45). Likewise, our wild NisA+ L. lactis subsp. cremoris M78 and M104 isolates (37) have been shown to show high nisin production rates (36) and strong nisin-mediated antilisterial and/or antistaphylococcal activity in vitro in culture broth, skim milk, and/or model cheese, as well as in situ in thermized milk and traditional Greek fresh whey cheese or fermented hard cheese curds; their activity was higher than that of L. lactis subsp. lactis NCFB 1402 and other Nis+ L. lactis strains (35, 38, 39). Additional studies are therefore required to genotypically and technologically compare our wild NisA+ M78 and M104 isolates with other L. lactis raw-milk or cheese strains, including the Italian strain 40FEL3 and several nisin-negative Greek lactococcal isolates available in our laboratory (13, 35). Such comparisons have been out of the scope of this study, which focused on elucidating the subspecies identity of the M78 and M104 isolates. This is the first complete report on wild L. lactis subsp. cremoris strains of the subspecies lactis phenotype which produce nisin A and thus have high potential for use as a novel dairy starter, costarter, and/or protective adjunct cultures.
ACKNOWLEDGMENT
This work was mainly supported by the Hellenic General Secretariat for Research and Development through the national funding contribution allocated to TRUEFOOD (Traditional United Europe Food), which has been an integrated project financed by the European Commission under the 6th RTD Framework (2006 to 2010; contract FOOD-CT-2006-016264).
Footnotes
Published ahead of print 29 March 2013
REFERENCES
- 1. Beresford TR, Fitzsimons NA, Brennan NL, Cogan TM. 2001. Recent advances in cheese microbiology. Int. Dairy J. 11: 259–274 [Google Scholar]
- 2. Casalta E, Montel MC. 2008. Safety assessment of dairy microorganisms: the Lactococcus genus. Int. J. Food Microbiol. 126: 271–273 [DOI] [PubMed] [Google Scholar]
- 3. Fernández E, Alegría A, Delgado S, Cruz Martin M, Mayo B. 2011. Comparative phenotypic and molecular genetic profiling of wild Lactococcus lactis subsp. lactis strains of the L. lactis subsp. lactis and L. lactis subsp. cremoris genotypes, isolated from starter-free cheeses made of raw milk. Appl. Environ. Microbiol. 77: 5324–5335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weerkamp AH, Klijn N, Neeter R, Smit G. 1996. Properties of mesophilic lactic acid bacteria from raw milk and natually fermented raw milk products. Neth. Milk Dairy J. 50: 319–332 [Google Scholar]
- 5. Gaya P, Babin M, Medina M, Nunez M. 1999. Diversity among lactococci isolated from ewes' raw milk and cheese. J. Appl. Microbiol. 87: 849–855 [DOI] [PubMed] [Google Scholar]
- 6. Callon C, Duthoit F, Delbès C, Ferrand M, Le Frileux Y, De Crémoux R, Montel MC. 2007. Stability of microbial communities of goat milk during a lactation year: molecular approaches. Syst. Appl. Microbiol. 30: 547–560 [DOI] [PubMed] [Google Scholar]
- 7. Cogan TM, Barbosa M, Beuvier E, Bianchi-Salvadori B, Cocconcelli PS, Fernandes I, Gomez J, Gomez R, Kalantzopoulos G, Ledda A, Medina M, Rea MC, Rodriguez E. 1997. Characterisation of the lactic acid bacteria in artisanal dairy products. J. Dairy Res. 64: 409–421 [Google Scholar]
- 8. Mannu L, Paba A, Pes M, Scintu MF. 2000. Genotypic and phenotypic heterogeneity among lactococci isolated from traditional Pecorino Sardo cheese. J. Appl. Microbiol. 89: 191–197 [DOI] [PubMed] [Google Scholar]
- 9. Callon C, Millet L, Montel MC. 2004. Diversity of lactic acid bacteria isolated from AOC Salers cheese. J. Dairy Res. 71: 231–244 [DOI] [PubMed] [Google Scholar]
- 10. Psoni L, Kotzamanidis C, Yiangou M, Tzanetakis N, Litopoulou-Tzanetaki E. 2007. Genotypic and phenotypic diversity of Lactococcus lactis isolates from Batzos, a Greek PDO raw goat milk cheese. Int. J. Food Microbiol. 114: 211–220 [DOI] [PubMed] [Google Scholar]
- 11. Dolci P, Alessandria V, Rantsiou K, Rolle L, Zeppa G, Cocolin L. 2008. Microbial dynamics of Castelmagno PDO, a traditional Italian cheese, with a focus on lactic acid bacteria ecology. Int. J. Food Microbiol. 122: 302–311 [DOI] [PubMed] [Google Scholar]
- 12. Wouters JTM, Ayad EHE, Hugenholtz J, Smit G. 2002. Microbes from raw milk for fermented dairy products. Int. Dairy J. 12: 91–109 [Google Scholar]
- 13. Samelis J, Kakouri A, Pappa EC, Matijasić BB, Georgalaki MD, Tsakalidou E, Rogelj A. 2010. Microbial stability and safety of traditional Greek Graviera cheese: characterization of the lactic acid bacterial flora and culture-independent detection of bacteriocin genes in the ripened cheeses and their microbial consortia. J. Food Prot. 73: 1294–1303 [DOI] [PubMed] [Google Scholar]
- 14. Roberts RF, Zottola EA, McKay LL. 1992. Use of a nisin-producing starter culture suitable for Cheddar cheese manufacture. J. Dairy Sci. 75: 2353–2363 [DOI] [PubMed] [Google Scholar]
- 15. De Vuyst L. 1994. Nisin production variability between natural Lactococcus lactis subsp. lactis strains. Biotechnol. Lett. 16: 287–292 [Google Scholar]
- 16. Rodriguez E, Gonzalez B, Gaya P, Nunez M, Medina M. 2000. Diversity of bacteriocins produced by lactic acid bacteria isolated from raw milk. Int. Dairy J. 10: 7–15 [Google Scholar]
- 17. Galvez A, Lopez RL, Abriouel H, Valdivia E, Ben Omar N. 2008. Application of bacteriocins in the control of foodborne pathogenic and spoilage bacteria. Crit. Rev. Biotechnol. 28: 125–152 [DOI] [PubMed] [Google Scholar]
- 18. Delves-Broughton J, Blackburn P, Evans RJ, Hugenholtz J. 1996. Applications of the bacteriocin nisin. Antonie Van Leeuwenhoek 69: 193–202 [DOI] [PubMed] [Google Scholar]
- 19. Samelis J, Kakouri A, Rogga KJ, Savvaidis IN, Kontoninas MG. 2003. Nisin treatments to control Listeria monocytogenes post-processing contamination on Anthotyros, a traditional Greek whey cheese, stored at 4°C in vacuum packages. Food Microbiol. 20: 661–669 [Google Scholar]
- 20. Schleifer KH, Kraus J, Dvorak C, Kilpper-Bälz R, Collins MD, Fischer W. 1985. Transfer of Streptococcus lactis and related streptococci to the genus Lactococcus gen. nov. Syst. Appl. Microbiol. 6: 183–195 [Google Scholar]
- 21. Holt JG. 1994. Gram-positive cocci, p 527–558 In Holt G, Krieg NR, Sneath PHA, Staley JT, Williams ST. (ed), Bergey's manual of determinative bacteriology, 9th ed. Williams & Wilkins, Baltimore, MD [Google Scholar]
- 22. Jarvis AW, Jarvis BDW. 1981. Deoxyribonucleic acid homology among lactic streptococci. Appl. Environ. Microbiol. 41: 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Godon JJ, Delorme C, Ehrlich SD, Renault P. 1992. Divergence of genomic sequences between Lactococcus lactis subsp. lactis and Lactococcus lactis subsp. cremoris. Appl. Environ. Microbiol. 58: 4045–4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Corroler D, Desmasures N, Gueguen M. 1999. Correlation between polymerase chain reaction analysis of the histidine biosynthesis operon, randomly amplified polymorphic DNA analysis and phenotypic characterization of dairy Lactococcus isolates. Appl. Microbiol. Biotechnol. 51: 91–99 [DOI] [PubMed] [Google Scholar]
- 25. Kelly WJ, Ward LJH, Leahy SC. 2010. Chromosomal diversity in Lactococcus lactis and the origin of dairy starter cultures. Genome Biol. Evol. 2: 729–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salama M, Sandine W, Giovannoni S. 1991. Development and application of oligonucleotide probes for identification of Lactococcus lactis subsp. cremoris. Appl. Environ. Microbiol. 57: 1313–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nomura M, Kobayashi M, Narita T, Kimoto-Nira H, Okamoto T. 2006. Phenotypic and molecular characterization of Lactococcus lactis from milk and plants. J. Appl. Microbiol. 101: 396–405 [DOI] [PubMed] [Google Scholar]
- 28. Pu ZY, Dobos M, Limsowtin GKY, Powell IB. 2002. Integrated polymerase chain reaction-based procedures for the detection and identification of species and subspecies of the gram-positive bacterial genus Lactococcus. J. Appl. Microbiol. 93: 353–361 [DOI] [PubMed] [Google Scholar]
- 29. Elliott JA, Collins MD, Pigott NE, Facklam RR. 1991. Differentiation of Lactococcus lactis and Lactococcus garvieae from humans by comparison of whole-cell protein patterns. J. Clin. Microbiol. 29: 2731–2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pot B, Devriese LA, Ursi D, Vandamme P, Haesebrouck F, Kersters K. 1996. Phenotypic identification and differentiation of Lactococcus strains isolated from animals. Syst. Appl. Microbiol. 19: 213–222 [Google Scholar]
- 31. Buist G, Kok J, Leenhouts KJ, Dabrowska M, Venema G, Haandrikman AJ. 1995. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J. Bacteriol. 177: 1554–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beimfohr C, Ludwig W, Schleifer KH. 1997. Rapid genotypic differentiation of Lactococcus lactis subspecies and biovar. Syst. Appl. Microbiol. 20: 216–221 [Google Scholar]
- 33. Urbach E, Daniels B, Salama MS, Sandine WE, Giovannoni SJ. 1997. The ldh phylogeny for environmental isolates of Lactococcus lactis is consistent with rRNA genotypes but not with phenotypes. Appl. Environ. Microbiol. 63: 694–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garde S, Babin M, Gaya P, Nunez M, Medina M. 1999. PCR amplification of the gene acmA differentiates Lactococcus lactis subsp. lactis and L. lactis subsp. cremoris. Appl. Environ. Microbiol. 65: 5151–5153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Samelis J, Lianou A, Kakouri A, Delbès C, Rogelj I, Bogovic-Matijasić B, Montel MC. 2009. Changes in the microbial composition of raw milk induced by thermization treatments applied prior to traditional Greek hard cheese processing. J. Food Prot. 72: 783–790 [DOI] [PubMed] [Google Scholar]
- 36. Trmčić A, Monnet C, Rogelj I, Bogovič Matijašić B. 2011. Expression of nisin genes in cheese—a quantitative real-time polymerase chain reaction approach. J. Dairy Sci. 94: 77–85 [DOI] [PubMed] [Google Scholar]
- 37. Trmčić A, Samelis J, Monnet C, Rogelj I, Matijašic BB. 2011. Complete nisin A gene cluster from Lactococcus lactis M78 (HM219853): obtaining the nucleic acid sequence and comparing it to other published nisin sequences. Genes Genomics 33: 217–221 [Google Scholar]
- 38. Samelis J, Giannou E, Matijašic BB, Rogelj I. 2009. Fate of Staphylococcus aureus in traditional Greek Graviera cheese manufactured with or without Lactococcus lactis M104, a nisin-producing raw milk isolate. Abstr. EFFoST 2009 Conf. New Chall. Food Preserv., Budapest, Hungary, abstr P-329, p P298 [Google Scholar]
- 39. Kakouri A, Lianou A, Samelis J. 2012. Antilisterial activity of wild, novel nisin-producing Lactococcus lactis subsp. cremoris strains in synthetic culture media and different dairy foods. Abstr. 23rd Intl. ICFMH Symp. (FoodMicro 2012), Istanbul, Turkey, abstr P-573, p 761 [Google Scholar]
- 40. Ayad EHE, Verheul A, Wouters JTM, Smit G. 2002. Antimicrobial-producing wild lactococci isolated from artisanal and non-dairy origins. Int. Dairy J. 12: 145–150 [Google Scholar]
- 41. Bravo D, Rodriguez E, Medina M. 2009. Nisin and lacticin 481 coproduction by Lactococcus lactis strains isolated from raw ewes' milk. J. Dairy Sci. 92: 4805–4811 [DOI] [PubMed] [Google Scholar]
- 42. Alegría A, Delgado S, Roces C, Lopez B, Mayo B. 2010. Bacteriocins produced by wild Lactococcus lactis strains isolated from traditional, starter-free cheeses made of raw milk. Int. J. Food Microbiol. 143: 61–66 [DOI] [PubMed] [Google Scholar]
- 43. Perin LM, Moraes PM, Vicosa GN, Junior AS, Nero LA. 2012. Identification of bacteriocinogenic Lactococcus isolates from raw milk and cheese capable of producing nisin A and nisin Z. Int. Dairy J. 25: 46–51 [Google Scholar]
- 44. Dal Bello B, Rantsiou K, Bellio A, Zeppa G, Ambrosoli R, Civera T, Cocolin L. 2010. Microbial ecology of artisanal products from North West of Italy and antimicrobial activity of the autochthonous populations. LWT—Food Sci. Technol. 43: 1151–1159 [Google Scholar]
- 45. Dal Bello B, Cocolin L, Zeppa G, Field D, Cotter PD, Hill C. 2012. Technological characterization of bacteriocin producing Lactococcus lactis strains employed to control Listeria monocytogenes in cottage cheese. Int. J. Food Microbiol. 153: 58–65 [DOI] [PubMed] [Google Scholar]
- 46. Versalovic J, Koeuth T, Lupski JR. 1991. Distribution of repetitive DNA-sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19: 6823–6831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rossetti L, Giraffa G. 2005. Rapid identification of dairy lactic acid bacteria by M13-generated, RAPD-PCR fingerprint databases. J. Microbiol. Methods 63: 135–144 [DOI] [PubMed] [Google Scholar]
- 48. Samelis J, Roller S, Metaxopoulos J. 1994. Sakacin B, a bacteriocin produced by Lactobacillus sake isolated from Greek dry fermented sausages. J. Appl. Bacteriol. 76: 475–486 [Google Scholar]
- 49. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254 [DOI] [PubMed] [Google Scholar]
- 50. Parapouli M, Fragkos-Livanios L, Samiotaki M, Koukkou AI, Perisynakis A, Hatziloukas E, Panagiotou G, Drainas C. 2010. Comparative proteomic analysis of alcoholic fermentation employing a new environmental strain of Saccharomyces cerevisiae. Proc. Biochem. 45: 1094–1102 [Google Scholar]
- 51. Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166: 557–580 [DOI] [PubMed] [Google Scholar]
- 52. Sambrook J, Russell D. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 53. Altschul SF, Madden TL, Schäver AA, Zhang J, Zhang Z, Miller W, Lipman D. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- 55. Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fallico V, McAuliffe O, Fitzgerald GF, Ross RP. 2011. Plasmids of raw milk cheese isolate Lactococcus lactis subsp. lactis biovar diacetylactis DPC3901 suggest a plant-based origin for the strain. Appl. Environ. Microbiol. 77: 6451–6462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Prodelalová J, Spanová A, Rittich B. 2005. Application of PCR, rep-PCR and RAPD techniques for typing of Lactococcus lactis strains. Folia Microbiol. (Praha) 50: 150–154 [DOI] [PubMed] [Google Scholar]