Abstract
We report the first identification of a gene cluster involved in d-tagatose catabolism in Bacillus licheniformis. The pathway is closely related to the d-tagatose pathway of the Gram-negative bacterium Klebsiella oxytoca, in contrast to the d-tagatose 6-phosphate pathway described in the Gram-positive bacterium Staphylococcus aureus.
TEXT
D-Tagatose (Tag) is a ketohexose that is a C-4 epimer of d-fructose and a low-calorie natural sugar present in small amounts in fruits and milk products. d-Tagatose can easily replace the sucrose in food, since their taste, cooking, and texture properties are very similar. With a low glycemic index and an antihyperglycemic effect, d-tagatose seems to be an ideal sweetener (1, 2).
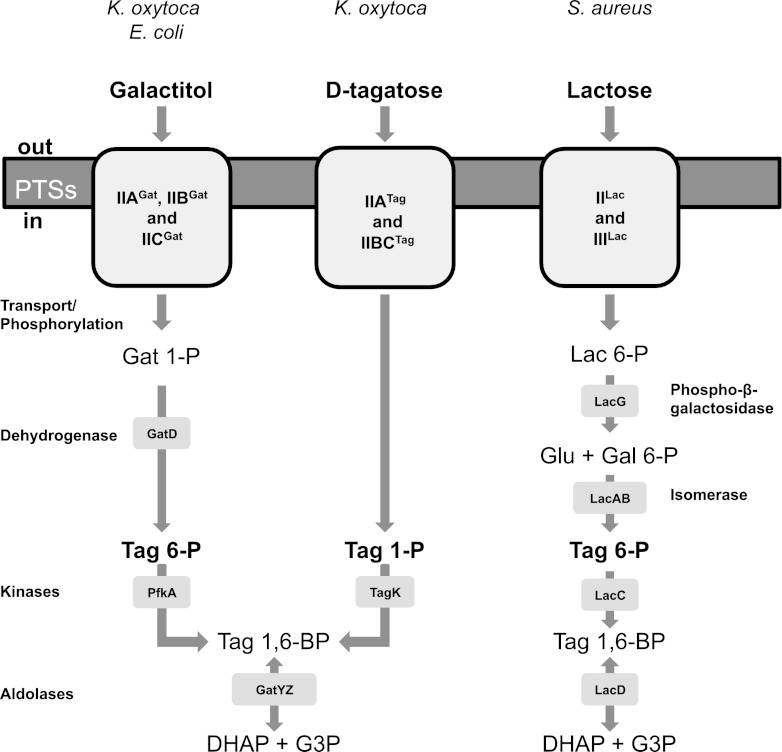
Depending on the bacterial species, d-tagatose is reported to be either a carbohydrate source, and thus transported into the cell by phosphotransferase uptake systems, or an intermediate in lactose, d-galactose, and galactitol catabolism (Fig. 1) (3, 4, 5, 6, 7, 8).
Fig 1.
Pathways for catabolism of galactitol (Gat), d-tagatose (Tag), and lactose (Lac) in different bacteria. In Gram-negative bacteria, such as E. coli or K. oxytoca, galactitol is used as a carbohydrate source and Tag 6-P is an intermediate of the pathway. In K. oxytoca, tagatose is also used as a carbohydrate source by being transported and phosphorylated to yield Tag 1-P. Tag 1,6-BP formed by the tagatose 1-phosphate kinase TagK is further cleaved into DHAP and G3P with the class II tagatose aldolase GatYZ (6). An example of the d-tagatose 6-P pathway of the Gram-positive bacterium S. aureus is shown on the right. In this case, lactose is used as the carbohydrate source and Tag 6-P is an intermediate of the pathway. Glu, d-glucose; Gal, d-galactose.
In the Gram-positive bacterium Staphylococcus aureus, lacABCD, the structural genes of the d-tagatose 6-phosphate pathway, are implicated in the catabolism of lactose and d-galactose. They are cotranscribed with genes lacFEG, which specify the proteins for transport, phosphorylation, and cleavage (3). LacF and lacE encode the lactose phosphoenolpyruvate phosphotransferase system (PEP-PTS) components commonly named enzymes IILac and IIILac, respectively. Phospho-β-galactosidase, responsible for the cleavage of lactose 6-phosphate (6-P), is encoded by lacG. d-Galactose 6-phosphate is further catabolized through d-tagatose derivatives as follows: d-galactose 6-phosphate → d-tagatose 6-phosphate (Tag 6-P) → d-tagatose 1,6-bisphosphate (Tag 1,6-BP) → dihydroxyacetone phosphate (DHAP) and d-glyceraldehyde 3-phosphate (G3P) (Fig. 1) (4). The gene lacD encodes a class I tagatose aldolase which catalyzes the reversible aldol condensation of DHAP and G3P to yield a mixture of the 1,6-bisphosphate derivatives of d-tagatose, d-fructose, d-sorbose, and d-psicose (5).
Gram-negative enteric bacteria, such as Klebsiella oxytoca, Klebsiella pneumoniae, and Salmonella enterica, catabolize external d-tagatose (Tag+ phenotype) through a PTS-dependent d-tagatose pathway encoded by the genes tagKTH and the genes gatYZ (Fig. 2). Galactitol is also catabolized by these bacteria via the products of the genes gatYZABCD. In this case, Tag 6-P is an intermediate of the galactitol degradation pathway (Fig. 1). The tag-gat genes are arranged in two operons and form a regulon controlled by the GatR repressor (6). GatYZ is a heterodimeric Zn2+-requiring class II tagatose aldolase with high and poor activities for Tag 1,6-BP and fructose 1,6-BP, respectively. GatZ is a protein required for the full activity and stability of the catalytic subunit GatY (7). This pathway differs from the d-tagatose 6-phosphate pathway of S. aureus because Tag 1-P, instead of Tag 6-P, is generated by the d-tagatose-PTS (transporters IIBC and IIA, encoded by tagT and tagH, respectively). Subsequently, Tag 1-P is phosphorylated by tagatose 1-phosphate kinase (TagK) to yield Tag 1,6-BP.
Fig 2.
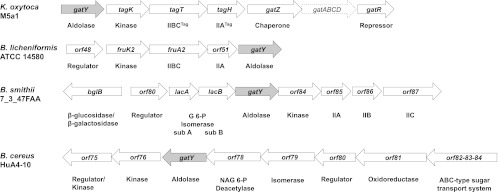
Genetic organization of the K. oxytoca gat-tag genes encoding a PTS-dependent d-tagatose pathway, of the B. licheniformis five-gene cluster encoding a d-tagatose pathway, and of B. smithii and B. cereus genes encoding hypothetical d-tagatose pathways. The regions enclosing the genes coding for class II tagatose-bisphosphate aldolase (gatY) and neighboring genes are shown. The dashed arrow shows genes not implicated in a d-tagatose pathway. orf80 to orf87 are locus tags HMPREF1015_01180 to HMPREF1015_01187 in the genome of B. smithii 7_3_47FAA. G 6-P isomerase sub A and sub B are the subunits of the galactose 6-P isomerase. IIA, IIB, and IIC are predicted products of the sugar-specific PTS system. orf75 to orf84 are locus tags IGC_03675 to IGC_03684 of the B. cereus HuA4_10 genome. Nag 6-P deacetylase is a putative N-acetylglucosamine 6-P deacetylase. orf75 encodes a putative transcriptional regulator/sugar kinase, NagC.
In Escherichia coli, the tagatose 1,6-BP aldolase GatY is temperature sensitive and involved only in galactitol catabolism (Fig. 1). Indeed, this bacterial species is unable to grow with d-tagatose as a source of carbohydrate (6, 7) because it lacks the tag genes.
In this short paper, we report the first identification of a five-gene cluster involved in d-tagatose catabolism in Bacillus licheniformis. The pathway is closely related to that described in the Gram-negative bacterium K. oxytoca. The functionality of the gene cluster has been demonstrated in E. coli and Bacillus subtilis by supplementation experiments.
The strains E. coli DH5α (ATCC PTA-1798), B. subtilis 168 (BGSC accession number 1A1; http://www.bgsc.org/), and Bacillus amyloliquefaciens FZB42 (BGSC accession number 10A6) were unable to catabolize d-tagatose as a carbohydrate source (Tag− phenotype). In contrast, we observed that B. licheniformis 749I (BGSC accession number 5A20) and B. licheniformis ATCC 14580 show a Tag+ phenotype. Following these observations, a search for a tagatose-bisphosphate aldolase-coding gene in the genome sequence of B. licheniformis ATCC 14580 (GenBank accession number NC-006322; http://www.ncbi.nlm.nih.gov/nuccore/) (see also Table S1 in the supplemental material) revealed a cluster of five coding sequences (locus tags Bli03548 to Bli03552) that we expected to be involved in d-tagatose catabolism (Fig. 2). The predicted gene products are annotated as follows: a helix-turn-helix (HTH)-type transcriptional regulator, 1-phosphofructokinase (fruK2 gene), the fructose-specific phosphotransferase system (PTS) IIC component FruA (fruA2 gene), a PTS IIA component, and tagatose-bisphosphate aldolase GatY (gatY gene), respectively.
In order to supplement E. coli DH5α and B. subtilis 168 with the five-gene cluster, a DNA sequence of 5,234 bp was amplified by PCR with oligonucleotide primers UP-TAG (forward primer) 5′-TGGTCTGATCGGATCCAGGGTACAACGAAGGGAAAACGAGG-3′ and RP-TAG (reverse primer) 5′-TAAGCTTCTAGGATCCAGGATAAGACAGTGCTCGTTTGGACGG-3′ (floating ends in boldface are complementary sequences of plasmid described below), using the genomic DNA of B. licheniformis ATCC 14580 strain as the template DNA. The amplified sequence corresponding to the 3393420-to-3398622 region was cloned into the BamHI restriction site of the pDG1730 plasmid, yielding pDGTAG by the In-Fusion method (In-Fusion HD Cloning kit, Clontech, United States). The pDG1730 plasmid (BGSC accession number ECE115 and GenBank sequence accession number U46199) harbors a replicative origin for E. coli and the 5′ and 3′ parts of the B. subtilis 168 α-amylase gene chromosome for integration by double recombination. The E. coli DH5α strain was transformed with the cloning reaction and with the pDG1730 plasmid for the negative control. The resulting construct, pDGTAG, was verified by DNA sequencing. Competent cells of B. subtilis 168 were transformed with the MluI-linearized pDGTAG plasmid or with the MluI-linearized pDG1730 vector (negative control). Spectinomycin-resistant (100 μg ml−1), erythromycin-sensitive (0.5 μg ml−1), and lincomycin-sensitive (12.5 μg ml−1) colonies indicated that a double crossover had occurred, rather than a simple Campbell-type insertion. Stable transformants of B. subtilis 168 obtained with the pDGTAG plasmid are designated B. subtilis 168 tag+ spc+ amyE, and those obtained with the pDG1730 vector are designated B. subtilis 168 spc+ amyE.
Supplemental E. coli ME4740 strain (ΔptsH-ptsI-crr::Cmr) and its parental strain MG1655 (NBRP E coli strain; http://www.shigen.nig.ac.jp/ecoli/strain/) harboring the pDGTAG vector were used to check whether the d-tagatose pathway, encoded by the five-gene cluster, is PTS dependent in E. coli. The soluble PTS proteins HPr and enzyme I (EI), encoded by the genes ptsH and ptsI, respectively, are common to all PTS carbohydrate pathways and funnel the phosphate groups from PEP to the membrane-bound, sugar-specific transporters called enzymes II (EIIs) (9, 10). The crr gene product is the soluble glucose transporter component EIIAGlc. Deletion of the ptsH-ptsI-crr operon in the ME4740 strain leads to a PTS sugar-negative phenotype (11).
Additionally, the E. coli RL257 strain (ΔpfkA ΔpfkB) (12) and its parental strain MQ (CGSC references 8247 and 8244, respectively; http://cgsc.biology.yale.edu/) (13) were chosen to test whether the products of the five-gene cluster of B. licheniformis metabolize Tag 6-P. Both strains are ΔfruK and ΔfruA; these genes encode 1-phosphofructokinase and the fructose-PTS enzyme IIA component, respectively. In contrast to its parental strain, the E. coli RL257 strain exhibiting no phosphofructokinase activity (ΔpfkA ΔpfkB) does not grow with galactitol as the sole carbon source and, consequently, accumulates Tag 6-P (Fig. 1).
For supplementation experiments, the growth of E. coli DH5α and B. subtilis 168 was tested in a defined medium (DM) supplemented with 20 mM d-glucose (positive control of growth), d-tagatose, d-galactose, or d-fructose, or 10 mM lactose. For E. coli MG1655 and ME4740, only d-tagatose and d-glucose were used. For E. coli MQ and RL257, d-tagatose and galactitol (20 mM) were used. The composition of DM is a 1× stock solution of salt, vitamins, and minerals. The 20× salt mixture (1 liter) was prepared with KH2PO4 (54.4 g), K2HPO4 (208.8 g), and NH4Cl (12 g). The 100× vitamin mix (50 ml) was composed of 5 mg of thiamine-HCl, nicotinic acid, folic acid, d-l-pantothenic acid, d-biotin, and riboflavin and 10 mg of pyridoxal-HCl. The 1,000× mineral mix (50 ml) contained MgCl2·6H2O (1 g), CaCl2·2H2O (0.25 g), FeCl2·4H2O (25 mg), ZnSO4·7H2O (25 mg), CoCl2·H2O (12.5 mg), CuSO4·5H2O (0.5 mg), and MnSO4·H2O (0.14 g). l-Casamino Acids were added to the DM at 0.1% (wt/vol). For E. coli MG1655 and ME4740, 2 mM cyclic AMP (cAMP) was also added to the DM composition. For the growth of B. subtilis 168, l-tryptophan was added to the DM at 0.05 mg ml−1, and for E. coli, 100 μg ml−1 of spectinomycin was added to the culture flask. Cultures were performed in 50 ml of DM with 0.5 ml of preculture under agitation (250 rpm), at 28°C for around 48 h for E. coli MQ and RL257, because of the galactitol temperature-sensitive phenotype of E. coli K-12 (14), at 42°C for around 8 h for B. subtilis, and at 28°C for 17 h for E. coli DH5α, MG1655, and ME4740, followed by an incubation at 37°C for around 7 h. Growth curves were monitored by measuring the absorbance at 600 nm of the culture incubated at 28°C for E. coli MQ and RL257, at 37°C for E. coli DH5α, MG1655, and ME4740 and at 42°C for B. subtilis.
As shown by the results in Figure 3a, E. coli DH5α catabolized d-glucose and d-fructose independently of the plasmid (pDG1730 or pDGTAG) and used d-galactose in the Leloir pathway (15), but not lactose, since E. coli DH5α is Δ(lacZYA). As expected, transformants of E. coli DH5α carrying the pDGTAG plasmid were able to grow in the DM supplemented with d-tagatose, in contrast to E. coli DH5α carrying the pDG1730 vector.
Fig 3.
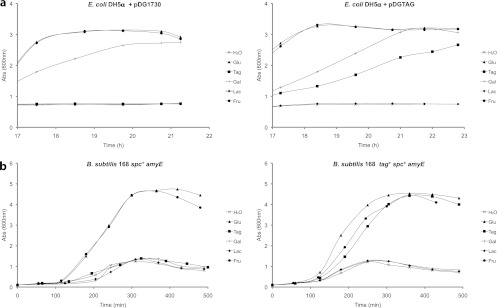
Growth curves of E. coli DH5α harboring the pDG1730 or pDGTAG plasmid (a) and of B. subtilis 168 spc+ amyE and tag+ spc+ amyE (b). Growth was performed in the DM supplemented with a carbohydrate source (H2O, control; Glu, d-glucose; Tag, d-tagatose; Gal, d-galactose; Lac, lactose; Fru, d-fructose). Abs, absorbance.
As shown by the results in Figure 3b, B. subtilis 168 catabolized d-glucose and d-fructose at similar rates. Lactose and d-galactose were not catabolized by B. subtilis 168, since it lacks the lactose-specific (16) and galactose-specific (17) transporters. As observed with E. coli, B. subtilis 168 tag+ spc+ amyE was able to grow in the DM supplemented with d-tagatose, while B. subtilis 168 spc+ amyE was unable to.
Interestingly, the growth rates of B. subtilis 168 tag+ spc+ amyE in the presence of d-tagatose or the PTS sugars d-glucose and d-fructose are similar (Fig. 3b). This confirms that B. licheniformis has conserved an efficient pathway for the use of d-tagatose. In contrast, the growth rate of E. coli DH5α in the DM supplemented with d-tagatose is rather similar to that observed with the non-PTS sugar d-galactose (Fig. 3a).
To explore these observations, E. coli mutant ME4740 (ΔptsH-ptsI-crr::Cmr) and its parental strain MG1655 were used. In E. coli mutant ME4740, growth in the DM supplemented with d-glucose is strongly affected compared to the growth of its parental strain MG1655, consistent with the ptsH-ptsI-crr deletion (see Fig. S2 in the supplemental material). In both strains (parental and mutant) harboring the pDGTAG plasmid, the growth rates observed in the DM supplemented with d-tagatose are similar to those obtained with d-glucose in the mutant. This suggests that in E. coli, the PTS encoded by the gene cluster of B. licheniformis could be nonfunctional.
Moreover, our results demonstrate that the five-gene cluster identified in B. licheniformis encodes a catabolic pathway for d-tagatose that is independent of the catabolism of lactose and d-galactose, in contrast to the d-tagatose 6-P pathway described in other Gram-positive bacteria, such as S. aureus and Streptococcus gordonii (3, 4, 5, 8).
Surprisingly, we found that the d-tagatose pathway of B. licheniformis is closely related to that described in the Gram-negative bacterium K. oxytoca (6) (Fig. 2). Indeed, high amino acid sequence identity was found using the BLAST algorithm (http://www.ncbi.nlm.nih.gov/BLAST/) to compare homologous enzymes. Sequence alignments show that the fructose-specific PTS component IIC (fruA2 gene product) also contains a domain B and exhibits 52% identity with the IIBCTag component of K. oxytoca. For these reasons, we propose to rename it IIBC (Fig. 2). The tagatose-bisphosphate aldolase GatY of B. licheniformis ATCC 14580 exhibits 58% identity with GatY of K. oxytoca. The predicted product 1-phosphofructokinase encoded by the fruK2 gene has no activity on Tag 6-P, otherwise E. coli RL257 (ΔpfkA ΔpfkB) harboring the pDGTAG plasmid would have grown in the DM supplemented with galactitol (see Fig. S3 in the supplemental material). In addition, the protein encoded by the fruK2 gene has 39% identity with TagK of K. oxytoca, which is a tagatose 1-P kinase. This may suggest that this protein would be active on Tag 1-P.
In enteric bacteria, some authors have proposed that GatZ could have a chaperone-like function to explain the full activity and the stabilizing effect on GatY (7). No GatZ was found in the B. licheniformis genome. Either the B. licheniformis GatY does not require GatZ or the chaperone function is played by an as-yet-unidentified protein.
Although our results describe the first identification of a d-tagatose pathway in Bacillus, a BLAST search of the tagatose aldolase GatY of B. licheniformis ATCC 14580 (GenBank sequence accession number YP_006714845) against the Bacillus protein sequences revealed a high similarity with tagatose aldolase of Bacillus smithii (GenBank sequence accession number ZP_09351531, 74% identity) and that of Bacillus cereus (GenBank sequence accession number ZP_17500767, 60% identity). This means that a d-tagatose pathway could exist in other Bacillus species (Fig. 2). As expected, no tagatose aldolase was found in B. subtilis 168 and B. amyloliquefaciens FZB42. By an in silico search, we found that in the sequence of the B. smithii genome (GenBank sequence accession number NZ_JH414740), the class II tagatose-bisphosphate aldolase-coding gene (gatY) is located near several genes that could form a cluster encoding a d-tagatose pathway. Indeed, this region contains genes coding for the following putative enzymes: β-glucosidase/β-galactosidase BglB, an HTH-type transcriptional regulator, galactose 6-P isomerase subunits LacA and LacB, tagatose-bisphosphate aldolase, tagatose 6-P kinase, and sugar-specific PTS IIA, IIB, and IIC (GenBank sequence accession numbers ZP_09351527 to ZP_09351535, respectively). In the genome of B. cereus (GenBank accession number AHEA01000026), the tagatose-bisphosphate aldolase-coding gene is surrounded by genes encoding the following putative enzymes: a sugar kinase, 1-phosphofructokinase, N-acetylglucosamine 6-P deacetylase, tagatose-6-P ketose/hexose isomerase, a transcriptional regulator, an oxidoreductase, and an ABC-type sugar transport system (GenBank sequence accession numbers EJQ77376 to EJQ77385, respectively).
More investigations should be undertaken to study the regulation and activity of each gene encoding the new d-tagatose pathway found in B. licheniformis and to explore the potential d-tagatose pathways in other Bacillus species.
Supplementary Material
ACKNOWLEDGMENTS
Edwige Van der Heiden was the recipient of a FRIA (Fonds de la Recherche pour l'Industrie et l'Agriculture) fellowship.
We thank Ana Amoroso for providing the composition of the defined medium (DM) used for growth curves, Patricia Simon for contributing to the growth curve monitoring, Olivier Verlaine for improving figures in EPS (encapsulated PostScript) format, and David Thorn for proofreading the manuscript.
Footnotes
Published ahead of print 22 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03918-12.
REFERENCES
- 1. Levin GV. 2002. Tagatose, the new GRAS sweetener and health product. J. Med. Food 5: 23– 36 [DOI] [PubMed] [Google Scholar]
- 2. Oh DK. 2007. Tagatose: properties, applications, and biotechnological processes. Appl. Microbiol. Biotechnol. 76: 1– 8 [DOI] [PubMed] [Google Scholar]
- 3. Rosey EL, Oskouian B, Stewart GC. 1991. Lactose metabolism by Staphylococcus aureus: characterization of lacABCD, the structural genes of the tagatose 6-phosphate pathway. J. Bacteriol. 173: 5992– 5998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bissett DL, Anderson RL. 1974. Genetic evidence for the physiological significance of the d-tagatose 6-phosphate pathway of lactose and d-galactose degradation in Staphylococcus aureus. J. Bacteriol. 119: 698– 704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bissett DL, Anderson RL. 1980. Lactose and d-galactose metabolism in Staphylococcus aureus. IV. Isolation and properties of a class I d-ketohexose-1,6-diphosphate aldolase that catalyzes the cleavage of d-tagatose 1,6-diphosphate. J. Biol. Chem. 255: 8750– 8755 [PubMed] [Google Scholar]
- 6. Shakeri-Garakani A, Brinkkötter A, Schmid K, Turgut S, Lengeler JW. 2004. The genes and enzymes for the catabolism of galactitol, d-tagatose, and related carbohydrates in Klebsiella oxytoca M5a1 and other enteric bacteria display convergent evolution. Mol. Gen. Genomics 271: 717– 728 [DOI] [PubMed] [Google Scholar]
- 7. Brinkkötter A, Shakeri-Garakani A, Lengeler JW. 2002. Two class II d-tagatose-bisphosphate aldolases from enteric bacteria. Arch. Microbiol. 177: 410– 419 [DOI] [PubMed] [Google Scholar]
- 8. Zeng L, Martino NC, Burne RA. 2012. Two gene clusters coordinate galactose and lactose metabolism in Streptococcus gordonii. Appl. Environ. Microbiol. 78: 5597– 5605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Reuse H, Lévy S, Zeng G, Danchin A. 1989. Genetics of the PTS components in Escherichia coli K-12. FEMS Microbiol. Rev. 63: 61– 68 [DOI] [PubMed] [Google Scholar]
- 10. Plumbridge J. 1999. Expression of the phosphotransferase system both mediates and is mediated by Mlc regulation in Escherichia coli. Mol. Microbiol. 33:260–273. [DOI] [PubMed] [Google Scholar]
- 11. Hernandez-Montalvo V, Martinez A, Hernandez-Chavez G, Bolivar F, Valle F, Gosset G. 2003. Expression of galP and glk in a Escherichia coli PTS mutant restores glucose transport and increases glycolytic flux to fermentation products. Biotechnol. Bioeng. 83: 687– 694 [DOI] [PubMed] [Google Scholar]
- 12. Lovingshimer MR, Siegele D, Reinhart GD. 2006. Construction of an inducible, pfkA and pfkB deficient strain of Escherichia coli for the expression and purification of phosphofructokinase from bacterial sources. Prot. Expr. Purif. 46: 475– 482 [DOI] [PubMed] [Google Scholar]
- 13. Peters JE, Thate TE, Craig NL. 2003. Definition of the Escherichia coli MC4100 genome by use of DNA array. J. Bacteriol. 185: 2017– 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brinkkötter A, Klöb H, Alpert CA, Lengeler JW. 2000. Pathways for the utilization of N-acetyl-galactosamine and galactosamine in Escherichia coli. Mol. Microbiol. 37: 125– 135 [DOI] [PubMed] [Google Scholar]
- 15. Holden HM, Rayment I, Thoden JB. 2003. Structure and function of enzymes of the Leloir pathway for galactose metabolism. J. Biol. Chem. 278: 43885– 43888 [DOI] [PubMed] [Google Scholar]
- 16. Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, Morgenstern B, Voss B, Hess WR, Reva O, Junge H, Voigt B, Jungblut PR, Vater J, Sussmuth R, Liesegang H, Strittmatter A, Gottschalk G, Borriss R. 2007. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 25: 1007– 1014 [DOI] [PubMed] [Google Scholar]
- 17. Krispin O, Allmansberger R. 1998. The Bacillus subtilis galE gene is essential in the presence of glucose and galactose. J. Bacteriol. 180: 2265– 2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.