Abstract
A UDP-glycosyltransferase from Bacillus licheniformis was exploited for the glycosylation of phloretin. The in vitro glycosylation reaction confirmed the production of five phloretin glucosides, including three novel glucosides. Consequently, we demonstrated the application of the same glycosyltransferase for the efficient whole-cell biocatalysis of phloretin in engineered Escherichia coli.
TEXT
Phloretin is a dihydrochalcone, an intermediate of the biosynthetic pathway of flavonoids in plants, which is abundantly present in the peel of apple (1, 2) and in strawberries (3). They occur in different glycosidic forms, such as naringin dihydrochalcone, phlorizin, and phloretin-4′-O-glucoside, in the different parts of the plants, contributing to various physiological properties of the plants, as well as to their color. Phloretin and its glycosides have been determined to have beneficial biological activities. Studies have uncovered that phloretin has inhibitory activity against glucose cotransporter 1 (4, 5), antioxidant activity (6), and activity to suppress the tumor necrosis factor alpha-induced inflammatory response, ameliorate inflammation of the colon, positively affect body weight loss (7), modulate Ca2+-activated K+ channels, and increase endothelial nitric oxide production, which might help to protect against atherosclerosis (8). Importantly, phloretin has other biological functions, like anticarcinogenic (9) and estrogenic activities (10) and inhibition of cardiovascular disease (11, 12).
Irrespective of their diverse physiological and pharmacological activities, the use of most of the natural polyphenols as drugs and food additives has been limited because of their water insolubility and low absorbability. Glycosylation enhances the bioavailability and pharmacological properties of compounds by increasing their solubility and stability (13, 14). Importantly, the sugar moieties of the glycosides often participate in the specific recognition of their biological targets and help to determine their efficacy in drug development (14, 15). According to the CAZy database (http://www.cazy.org/) (16, 17, 18), glycosyltransferase family 1 (GT1) proteins contain the UDP-glycosyltransferases that are common in all domains of life (19) and predominantly recognize small molecules as the sugar acceptors. A recent report showed that YjiC, a Bacillus licheniformis UDP-glycosyltransferase that falls in the GT1 family of proteins, can glycosylate at different hydroxyl positions of geldanamycin analogs (20). Here, we report the use of this glycosyltransferase for the biosynthesis of diverse phloretin glucosides in vitro and the subsequent application of YjiC for in vivo production of phloretin glucosides in an Escherichia coli mutant generating a cytoplasmic pool of UDP-glucose, since the YjiC-homologous glycosyltransferases from other Bacillus species were found to have flexible glycosyltransferase activities toward different flavonoid groups of compounds. Moreover, we found that by reversing the glycosylation reaction, the enzyme can deglycosylate the phloretin glucosides to form phloretin and UDP-glucose.
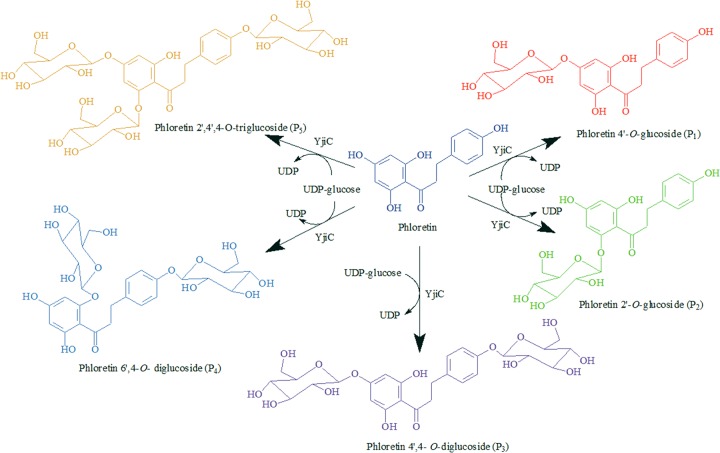
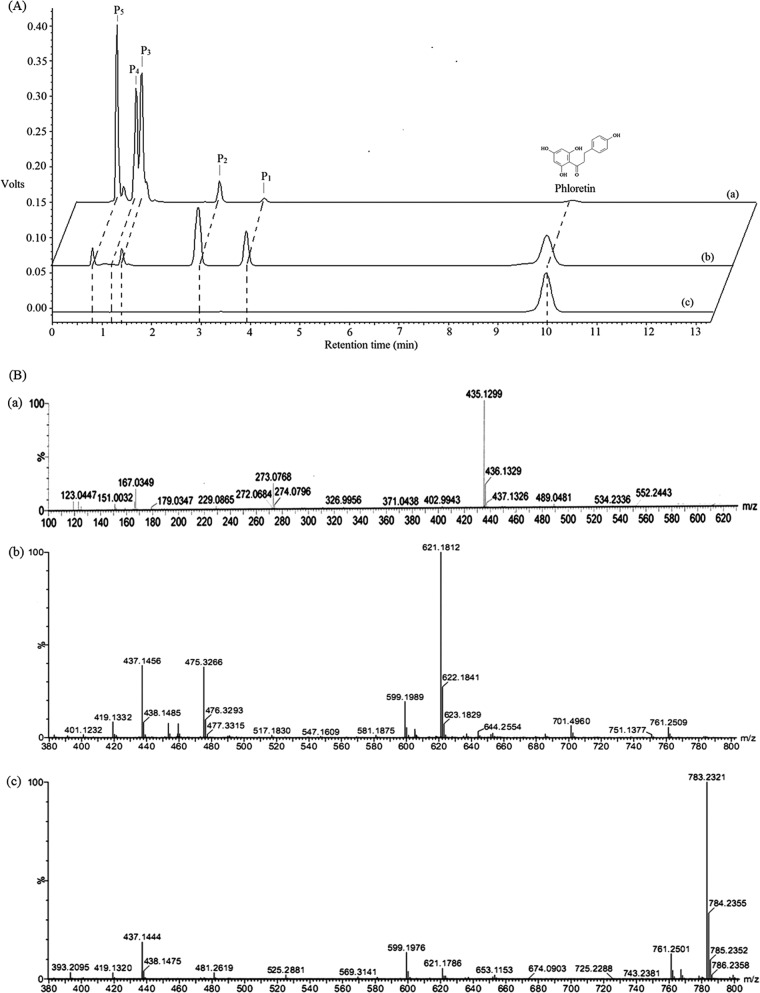
The functional YjiC protein (GenBank sequence accession no. AAU40842) was expressed using the pET28-YjiC expression vector (see materials and methods in the supplemental material). The approximately 46-kDa soluble protein was purified and used for the in vitro reactions. The reaction product analysis of phloretin and UDP-glucose with YjiC revealed the presence of five different glucosides in the high-pressure liquid chromatography–photo diode array (HPLC-PDA) assay (Fig. 1), with retention times of 0.8 min (P5), 1.2 min (P4), 1.4 min (P3), 2.9 min (P2), 3.9 min (P1), and the standard phloretin at 10 min. The liquid chromatography-quadrupole time of flight-electrospray ionization-tandem mass spectrometry (LC-QTOF-ESI-MS/MS) analysis of each peak found that the two products, P1 and P2, have exactly the same mass as monoglucosides of phloretin [M-H]− [m/z]− = 435.1302. Also, P3 and P4 have the same mass as phloretin diglucoside ([M + Na]+ [m/z]+ = 621.1812). Mass analysis of P5 determined it to be the triglucoside of phloretin ([M + Na]+ [m/z]+ = 783.2321) (Fig. 2). To further elucidate the structure of each product, an in vitro reaction was carried out in a large reaction mixture volume of 10 ml, as described in materials and methods in the supplemental material, and products were purified by preparative HPLC (prep)-HPLC. The purified compounds were subjected to various nuclear magnetic resonance (NMR) analyses, including 1H-NMR, 13C-NMR, and 2-dimensional NMR (homonuclear correlation spectroscopy [COSY], nuclear Overhauser effect [NOE] spectroscopy [NOESY], rotating-frame NOE spectroscopy [ROESY], heteronuclear multiple-bond correlation spectroscopy [HMBC], and heteronuclear multiple-quantum correlation spectroscopy [HMQC]). Products P1 (0.65 mg) and P2 (0.91 mg) were identified as phloretin 4′-O-glucoside and phloretin 2′-O-glucoside, respectively (see Fig. S7 to S11 in the supplemental material). The remaining products (P3 [4.48 mg], P4 [4.84 mg], and P5 [7.07 mg]) were analyzed by 1H NMR (500 MHz) and were found to be phloretin 4′,4-O-diglucoside, phloretin 4,6′-O-diglucoside, and phloretin 2′,4′,4-O-triglucoside (see Fig. S9 to S11 in the supplemental material). The last three glucosides were found to be novel compounds. The in vitro enzymatic conversion of phloretin to its glucosides under preparative conditions was found to be approximately 95%. Moreover, the enzyme has shown high flexibility toward glycosylation positions, producing five different glucosylated derivatives of phloretin. Previously, the enzyme has been shown to have glycosyltransferase activity toward geldanamycin analogs, and here, it has proved to accept phloretin efficiently. Thus, the enzyme might have broad substrate flexibility with other polyphenolic natural products and macrolides.
Fig 1.
Reaction of YjiC with UDP-glucose and phloretin producing five different types of phloretin glucosides.
Fig 2.
Liquid chromatography and mass spectrometry analyses. (A) HPLC-PDA analysis. (a) In vitro reaction with phloretin (2 mM) and UDP-glucose (16 mM) for 12 h of incubation. (b) In vivo reaction [500 μg/ml of phloretin added to growing culture of E. coli BL21(DE3) Δpgi Δzwf ΔushA harboring pET28-YjiC] after 12 h of phloretin feeding at 20°C. (c) Standard phloretin used for reactions. (B) LC-QTOF-ESI-MS/MS analysis. Masses of monoglucosides (a), diglucosides (b), and triglucoside (c) of phloretin.
To determine the effects of UDP-glucose and time of incubation on product formation, a duplicate reaction was carried out at a microliter scale with various UDP-glucose concentrations (2.0 mM, 4.0 mM, 8.0 mM, and 16.0 mM) while keeping the phloretin concentration (2.0 mM) and other parameters (protein amount, MgCl2, and buffer) intact (see Table S3 in the supplemental material). Each set of reaction mixtures were incubated for 4 h and 12 h under identical conditions, and product formation was monitored by HPLC-PDA analysis at the end of the reaction. The final reaction product analysis showed surprising results at different donor substrate concentrations. When the donor substrate concentration was low, the formation of monoglucosides (P1 and P2) was preferred by the enzyme, but when the concentration of the donor substrate was increased to 2, 4, or 8 times that of the acceptor substrate, the same enzyme preferred the production of di- and triglucosides (P3, P4, and P5), and the concentration of monoglucoside products (P1 and P2) decreased throughout both incubation experiments (4 h and 12 h) (see Fig. S4 and S5 in the supplemental material). This information signifies that the monoglucosides produced initially might change the orientation of other available hydroxyl groups of phloretin to a more favorable orientation for glycosylation, which leads to the production of di- and triglucosides. More interestingly, throughout the 12 h of incubation, although there was a similar pattern of formation of reaction products, an additional peak (P4) was also observed at higher concentrations of UDP-glucose (Fig. 2A; see also Fig. S5). This result showed that YjiC can glycosylate phloretin at different hydroxyl positions, nonregiospecifically generating novel phloretin glucosides. P1 and P2 are favorably produced in the presence of limited UDP-glucose concentrations with a short incubation time. P3, P4, and P5 production is less preferred when UDP-glucose is limited. This demonstrates that the type of product formation depends on the concentration of donor substrate and the time of incubation of the reaction mixture.
We were further interested to find the reason for the decreasing concentration of P1 and P2 over time, as the incubation progressed for more than a 4-h duration. We assumed that the enzyme can deglycosylate the glucosides to produce UDP-glucose and phloretin. We carried out the in vitro reaction of purified P1 with an excess amount of UDP and enzyme. The time-dependent HPLC-PDA analysis of the reaction products revealed that the concentration of P1 was decreasing with time. Meanwhile, the concentration of the phloretin standard was increasing (Fig. 3A). Moreover, other products (P2, P3, and P4) were also produced simultaneously. However, we could not detect the presence of P5 in the deglycosylation reaction mixture. This might be attributed to the lack of excess UDP-glucose available in the reaction mixture. This result shows that the enzyme deglycosylated the glucoside and, thus, produced UDP-glucose and phloretin, which were eventually utilized by the same enzyme to produce other products. In addition, we tried to determine the path by which di- and triglucosides form. To investigate this, we carried out a reaction with P1 and P2 independently with UDP-glucose and YjiC. The reaction with P1 and UDP-glucose showed that P1 changed to P3 and P5 rapidly, within 1 h of incubation in the presence of excess UDP-glucose. However, when the reaction was carried out for a longer period, the formation of P5 started to decline, whereas P1 and phloretin continued to be produced in the reaction mixture (Fig. 3B). Similarly, when the reaction was carried out with P2 under conditions identical to those used for P1, P2 changed to P5 only (Fig. 3C). With time, the concentration of P5 began to decrease and P3, P1, and phloretin began to increase. This experiment clarified that the triglucoside (P5) can be produced from both P1 and P2 via different intermediates. P5 is formed from P1 via P3, but we could not detect any diglucoside intermediate formed from P2. The time-dependent studies of P1 and P2 with UDP-glucose showed that P1 is rapidly converted to P3 and P5, within an hour. However, P2, another monoglucoside, converted to P5 very slowly relative to the time for conversion of P1. This evidence demonstrates that P2 is thermodynamically more stable than P1, which might favor easy further conversion of P1 to other products.
Fig 3.
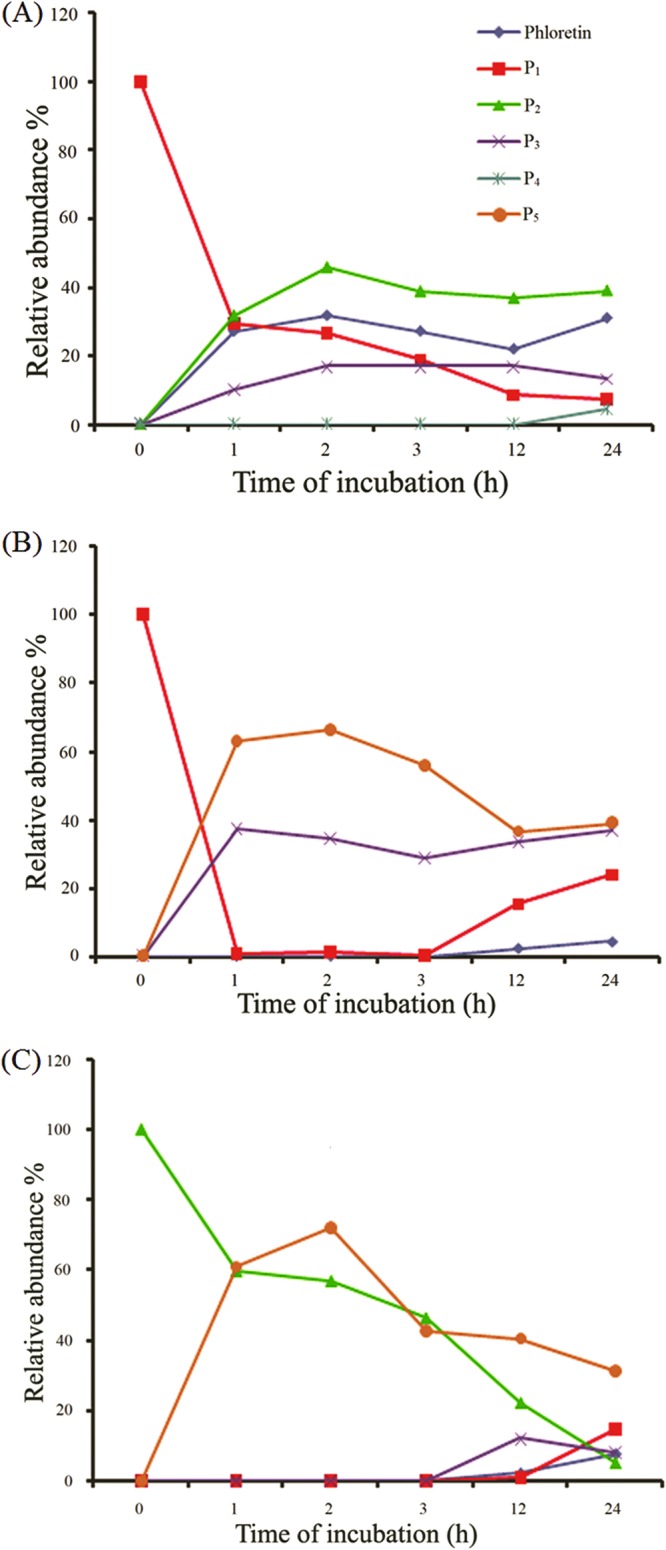
Deglycosylation by YjiC. (A) Reaction of P1 with UDP and YjiC. (B) Reaction of P1 with UDP-glucose and YjiC. (C) Reaction of P2 with UDP-glucose and YjiC.
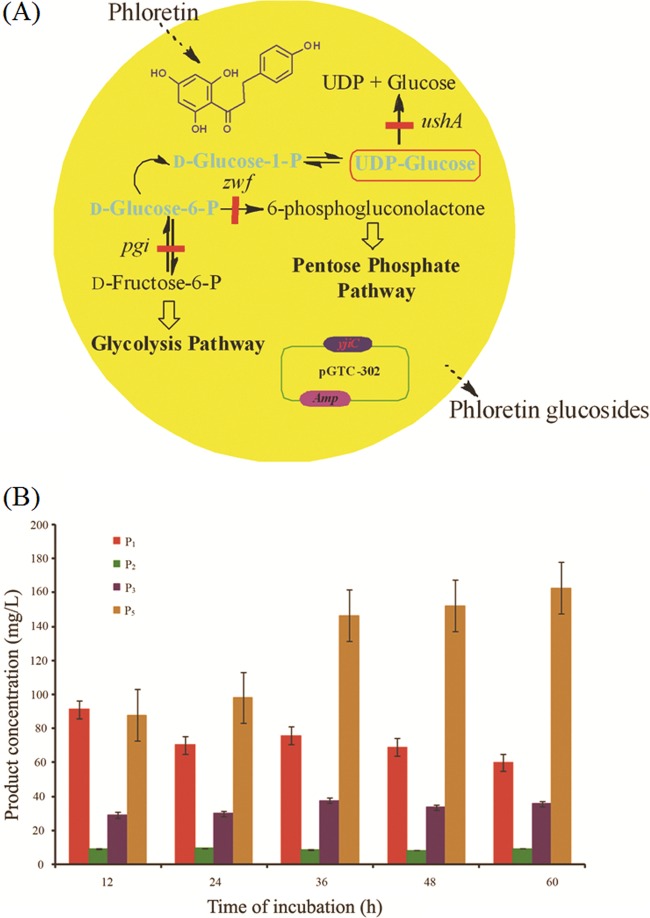
Since commercially available UDP-glucose is relatively expensive for the large-scale production of phloretin glucosides, we applied the YjiC glycosyltransferase for in vivo production of phloretin glucosides. E. coli BL21(DE3) Δpgi Δzwf ΔushA, which is expected to produce an intracellular pool of UDP-glucose (21), was transformed with pET28-YjiC recombinant vector and tested for the production of different phloretin glucosides (Fig. 4A), as described in materials and methods in the supplemental material. The optimal growth conditions described in our previous report (21) were used for the bioconversion. The HPLC-PDA analysis of in vivo reactions showed the production of four phloretin glucosides with the same retention time as in in vitro reactions (Fig. 2A). However, we were unable to detect P4 identified from the in vitro reaction. During in vivo biotransformation, there might not be sufficient amounts of freely available UDP-glucose molecules in the cell cytoplasm as there are in the in vitro reaction, which could limit the formation of P4 during in vivo biotransformation. The in vivo reaction at different concentrations of phloretin (100 μg/ml [0.37 mM], 250 μg/ml [0.925 mM] and 500 μg/ml [1.85 mM]) was also found to have patterns of product formation similar to those of in vitro reactions. The products P1, P2, and P3 were gradually reduced with incubation time, whereas the product P5 was produced until 60 h of incubation (Fig. 4B; see also Fig. S6 in the supplemental material). The time- and substrate-dependent study showed that the highest concentrations of products were obtained at 60 h when 500 μg/ml of phloretin was supplemented. Under these optimal product formation conditions, 60.1 mg/liter (137.7 μM) of P1, 9.59 mg/liter (21.98 μM) of P2, 35.96 mg/liter (60.0 μM) of P3, and 162.8 mg/liter (214 μM) of P5 were produced without supplementation of extracellular UDP-glucose, showing overall 23.3% bioconversion of phloretin to its glucosides at the microscale level (500 μl) in a 96-well plate. If the bioconversion reaction is carried out at fermentor scale under optimized conditions of medium, temperature, pH, aeration, etc., additional supplementation of glucose could lead to higher conversion of the substrate. This showed the feasibility of the production of phloretin glucosides from in vivo systems, too, as the simplest and cheapest way of producing different glucosides, like other glucosides that are natural products (22).
Fig 4.
(A) Schematic representation of in vivo glycosylation of phloretin using YjiC in E. coli BL21(DE3) Δpgi Δzwf ΔushA. P, phosphate. (B) In vivo production of P1, P2, P3, and P5 by E. coli BL21(DE3) Δpgi Δzwf ΔushA/pET28-YjiC supplemented with 500 μg/ml of phloretin. mg/L, mg/liter. Error bars show standard deviations.
To our knowledge, phloretin 4′, 4-O-diglucoside, phloretin 4,6′-O-diglucoside, and phloretin 2′,4′,4-O-triglucoside have not been reported from a natural source. Such new compounds might have novel biological activities. Though the biological activities of these compounds have not yet been determined, they might have some medicinally, cosmetically, and pharmacologically important properties, as these have been previously identified for phloretin and its glucosides. Potential health-benefiting properties of these compounds could help to develop future therapeutic agents. Naringin dihydrochalcone (diglucoside of phloretin) has been used as an artificial sweetener, and other studies have found that phloretin aglycone itself is medicinally important in different aspects. We have biosynthesized five different phloretin glucosides by using YjiC; however, to produce the target phloretin glucoside, we need to understand the structure of the protein and its mechanism. The mutagenesis and crystallographic studies will be helpful to understand the flexibility of this enzyme with phloretin.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Next-Generation BioGreen 21 Program (SSAC, grant no. PJ00948302) of the Rural Development Administration and from the Converging Research Center Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant 20090082333), Republic of Korea.
Footnotes
Published ahead of print 29 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00409-13.
REFERENCES
- 1. Escarpa A, Gonzalez MC. 1998. High-performance liquid chromatography with diode-array detection for the determination of phenolic compounds in peel and pulp from different apple varieties. J. Chromatogr. A 823: 331– 337 [DOI] [PubMed] [Google Scholar]
- 2. Tsao R, Yang R, Young JC, Zhu H. 2003. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 51: 6347– 6353 [DOI] [PubMed] [Google Scholar]
- 3. Hilt P, Schieber A, Yildirim C, Arnold G, Klaiber I, Conrad J, Beifuss U, Carle R. 2003. Detection of phloridzin in strawberries (Fragaria x ananassa Duch.) by HPLC-PDA-MS/MS and NMR spectroscopy. J. Agric. Food Chem. 51: 2896– 2899 [DOI] [PubMed] [Google Scholar]
- 4. Raja MM, Tyagi NK, Kinne RKH. 2003. Phlorizin recognition in a C-terminal fragment of SGLT1 studied by tryptophan scanning and affinity labeling. J. Biol. Chem. 278: 49154– 49163 [DOI] [PubMed] [Google Scholar]
- 5. Chao EC, Henry RR. 2010. SGLT2 inhibition—a novel strategy for diabetes treatment. Nat. Rev. Drug Discov. 7: 551– 559 [DOI] [PubMed] [Google Scholar]
- 6. Rezk BM, Haenen GRMM, Van der Vijgh WJF, Bast A. 2002. The antioxidant activity of phloretin: the disclosure of a new antioxidant pharmacophore in flavonoids. Biochem. Biophys. Res. Commun. 295: 9– 13 [DOI] [PubMed] [Google Scholar]
- 7. Lee JH, Regmi SC, Kim JA, Cho MH, Yun H, Lee CS, Lee J. 2011. Apple flavonoid phloretin inhibits Escherichia coli O157:H7 biofilm formation and ameliorates colon inflammation in rats. Infect. Immun. 79: 4819– 4827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Munz BM, Bauer GM, Schaefer CA, Erdogan A, Tillmanns H, Waldecker B, Kuhlmann CRW, Wiecha J. 2005. Dietary flavonoid phloretin modulates Ca2+-activated K+ channels resulting in an increase of endothelial nitric oxide production. Int. J. Pharmacol. 1: 38– 43 [Google Scholar]
- 9. Yang CS, Landau JM, Huang MT, Newmark HL. 2001. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 21: 381– 406 [DOI] [PubMed] [Google Scholar]
- 10. Calliste CA, Bail JCL, Trouillas P, Pouget C, Habrioux G, Chulia AJ, Duroux JL. 2001. Chalcones: structural requirements for antioxidant, estrogenic and antiproliferative activities. Anticancer Res. 21: 3949– 3956 [PubMed] [Google Scholar]
- 11. Figtree GA, Griffiths H, Lu YQ, Webb CM, MacLeod K, Collins P. 2000. Plant-derived estrogens relax coronary arteries in vitro by a calcium antagonistic mechanism. J. Am. Coll. Cardiol. 35: 1977– 1985 [DOI] [PubMed] [Google Scholar]
- 12. Stangl V, Lorenz M, Ludwig A, Grimbo N, Guether C, Sanad W, Ziemer S, Martus P, Baumann G, Stangl K. 2005. The flavonoid phloretin suppresses stimulated expression of endothelial adhesion molecules and reduces activation of human platelets. J. Nutr. 135: 172– 178 [DOI] [PubMed] [Google Scholar]
- 13. Shimoda K, Otsuka T, Morimoto Y, Hamada H, Hamada H. 2007. Glycosylation and malonylation of quercetin, epicatechin, and catechin by cultured plant cells. Chem. Lett. 36: 1292– 1293 [Google Scholar]
- 14. Weymouth-Wilson AC. 1997. The role of carbohydrates in biologically active natural products. Nat. Prod. Rep. 14: 99– 110 [DOI] [PubMed] [Google Scholar]
- 15. Griffith BR, Langenhan JM, Thorson JS. 2005. ‘Sweetening’ natural products via glycorandomization. Curr. Opin. Biotechnol. 16: 622– 630 [DOI] [PubMed] [Google Scholar]
- 16. Campbell JA, Davies GJ, Bulone V, Henrissat B. 1997. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem. J. 326: 929– 939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. 2009. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res. 37: 233– 238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coutinho PM, Deleury E, Davies GJ, Henrissat B. 2003. An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 328: 307– 317 [DOI] [PubMed] [Google Scholar]
- 19. Vogt T, Jones P. 2000. Glycosyltransferases in plant natural product synthesis: characterization of a supergene family. Trends Plant Sci. 5: 380– 386 [DOI] [PubMed] [Google Scholar]
- 20. Wu CZ, Jang JH, Woo M, Ahn JS, Kim JS, Hong YS. 2012. Enzymatic glycosylation of non-benzoquinone geldanamycin analogs via Bacillus UDP-glycosyltransferase. Appl. Environ. Microbiol. 78: 7680– 7686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pandey RP, Malla S, Simkhada D, Kim BG, Sohng JK. 2013. Production of 3-O-xylosyl quercetin in Escherichia coli. Appl. Microbiol. Biotechnol. 97: 1889– 1901 [DOI] [PubMed] [Google Scholar]
- 22. Williams GJ, Yang J, Zhang C, Thorson JS. 2011. Recombinant E. coli prototype strains for in vivo glycorandomization. ACS Chem. Biol. 6: 95– 100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.