Abstract
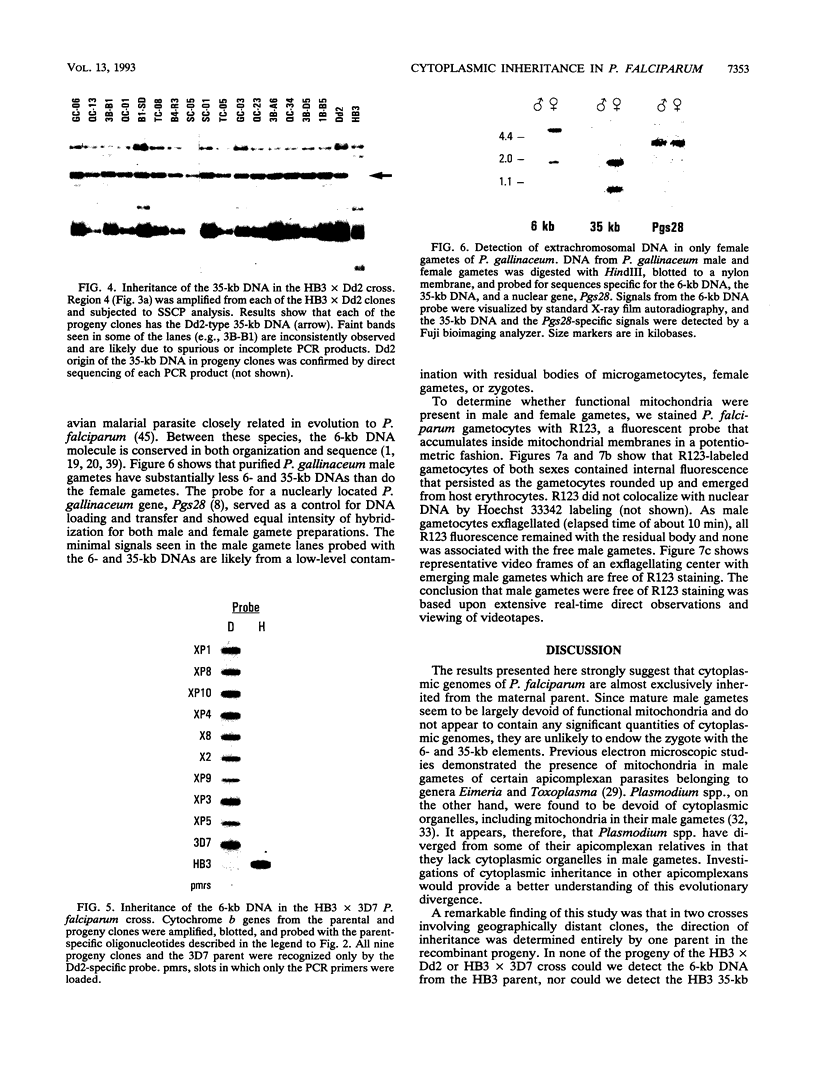
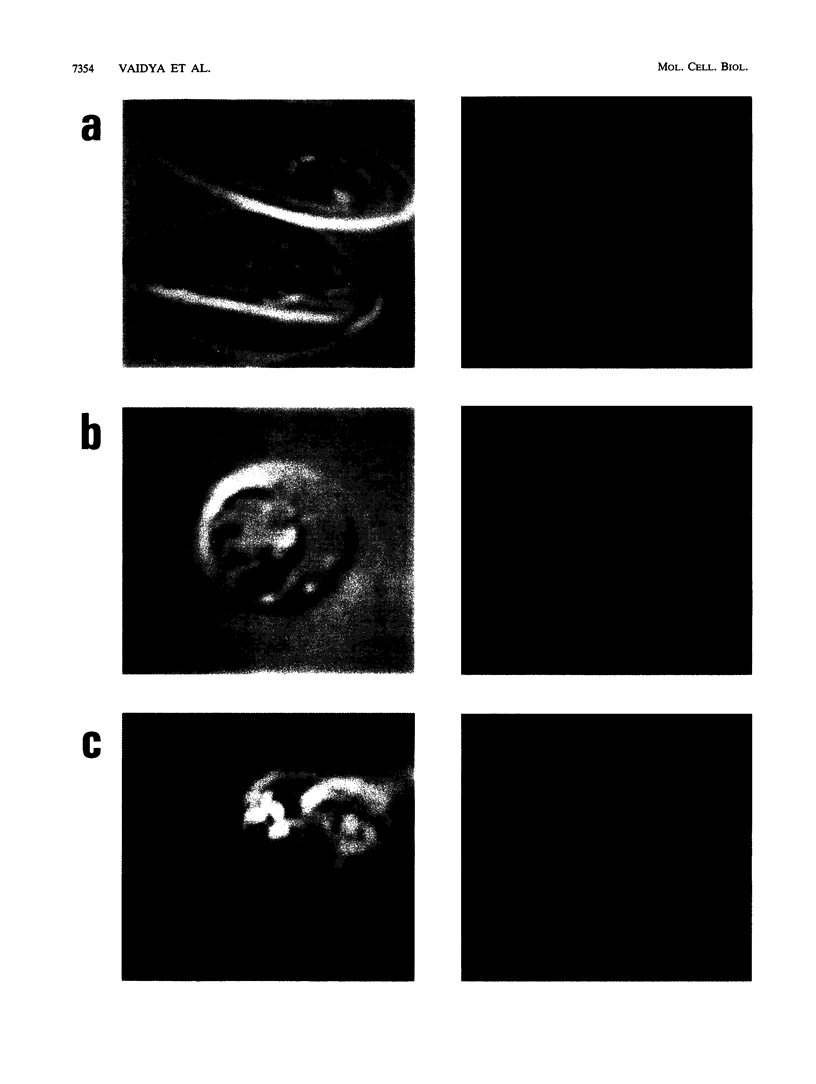
Malarial parasites have two highly conserved cytoplasmic DNA molecules: a 6-kb tandemly arrayed DNA that has characteristics of a mitochondrial genome, and a 35-kb circular DNA that encodes functions commonly found in chloroplasts. We examined the inheritance pattern of these elements in two genetic crosses of Plasmodium falciparum clones. Parent-specific oligonucleotide probes and single-strand conformation polymorphism analysis identified single nucleotide changes that distinguished the parental 6- and 35-kb DNA molecules in the progeny. In all 16 independent recombinant progeny of a cross between a Central American clone, HB3, and a Southeast Asian clone, Dd2, the 6- and 35-kb DNAs were inherited from the Dd2 parent. In all nine independent recombinant progeny of a cross between clone HB3 and a likely African clone, 3D7, the 6-kb DNA was inherited from the 3D7 parent. Inheritance of cytoplasmic genomes of the Dd2 and 3D7 parents was, therefore, dominant over that of the HB3 parent. Cytoplasmic DNA molecules were found almost exclusively in the female gametes of malarial parasites; hence, clone HB3 did not appear to have served as a maternal parent for the progeny of two crosses. Defective differentiation into male gametes by clone Dd2 is likely to be a reason for the cytoplasmic inheritance pattern seen in the HB3 x Dd2 cross. However, incompetence of male or female gametes is unlikely to explain the uniparental dominance in recombinant progeny of the HB3 x 3D7 cross, since both parents readily self-fertilized and completed the malaria life cycle on their own. Instead, the data suggest unidirectional parental incompatibility in cross-fertilization of these malarial parasites, where a usually cosexual parental clone can participate only as a male or as a female. Such an incompatibility may be speculated as indicating an early phase of reproductive isolation of P. falciparum clones from different geographical regions.
Full text
PDF






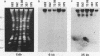
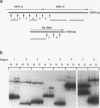
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldritt S. M., Joseph J. T., Wirth D. F. Sequence identification of cytochrome b in Plasmodium gallinaceum. Mol Cell Biol. 1989 Sep;9(9):3614–3620. doi: 10.1128/mcb.9.9.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer P. H., Gray M. W. Scrambled ribosomal RNA gene pieces in Chlamydomonas reinhardtii mitochondrial DNA. Cell. 1988 Nov 4;55(3):399–411. doi: 10.1016/0092-8674(88)90026-8. [DOI] [PubMed] [Google Scholar]
- Borst P., Nussenzweig V. Molecular parasitology at Woods Hole. Cell. 1992 Dec 11;71(6):895–899. doi: 10.1016/0092-8674(92)90386-q. [DOI] [PubMed] [Google Scholar]
- Boynton J. E., Harris E. H., Burkhart B. D., Lamerson P. M., Gillham N. W. Transmission of mitochondrial and chloroplast genomes in crosses of Chlamydomonas. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2391–2395. doi: 10.1073/pnas.84.8.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R., Gwadz R. W., McAuliffe F. M. Plasmodium gallinaceum: transmission-blocking immunity in chickens. I. Comparative immunogenicity of gametocyte- and gamete-containing preparations. Exp Parasitol. 1979 Apr;47(2):185–193. doi: 10.1016/0014-4894(79)90072-9. [DOI] [PubMed] [Google Scholar]
- Creasey A. M., Ranford-Cartwright L. C., Moore D. J., Williamson D. H., Wilson R. J., Walliker D., Carter R. Uniparental inheritance of the mitochondrial gene cytochrome b in Plasmodium falciparum. Curr Genet. 1993;23(4):360–364. doi: 10.1007/BF00310900. [DOI] [PubMed] [Google Scholar]
- Dore E., Frontali C., Forte T., Fratarcangeli S. Further studies and electron microscopic characterization of Plasmodium berghei DNA. Mol Biochem Parasitol. 1983 Aug;8(4):339–352. doi: 10.1016/0166-6851(83)90080-4. [DOI] [PubMed] [Google Scholar]
- Duffy P. E., Pimenta P., Kaslow D. C. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J Exp Med. 1993 Feb 1;177(2):505–510. doi: 10.1084/jem.177.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagin J. E., Werner E., Gardner M. J., Williamson D. H., Wilson R. J. Homologies between the contiguous and fragmented rRNAs of the two Plasmodium falciparum extrachromosomal DNAs are limited to core sequences. Nucleic Acids Res. 1992 Feb 25;20(4):879–887. doi: 10.1093/nar/20.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajadhar A. A., Marquardt W. C., Hall R., Gunderson J., Ariztia-Carmona E. V., Sogin M. L. Ribosomal RNA sequences of Sarcocystis muris, Theileria annulata and Crypthecodinium cohnii reveal evolutionary relationships among apicomplexans, dinoflagellates, and ciliates. Mol Biochem Parasitol. 1991 Mar;45(1):147–154. doi: 10.1016/0166-6851(91)90036-6. [DOI] [PubMed] [Google Scholar]
- Gardner M. J., Bates P. A., Ling I. T., Moore D. J., McCready S., Gunasekera M. B., Wilson R. J., Williamson D. H. Mitochondrial DNA of the human malarial parasite Plasmodium falciparum. Mol Biochem Parasitol. 1988 Oct;31(1):11–17. doi: 10.1016/0166-6851(88)90140-5. [DOI] [PubMed] [Google Scholar]
- Gardner M. J., Feagin J. E., Moore D. J., Spencer D. F., Gray M. W., Williamson D. H., Wilson R. J. Organisation and expression of small subunit ribosomal RNA genes encoded by a 35-kilobase circular DNA in Plasmodium falciparum. Mol Biochem Parasitol. 1991 Sep;48(1):77–88. doi: 10.1016/0166-6851(91)90166-4. [DOI] [PubMed] [Google Scholar]
- Gardner M. J., Williamson D. H., Wilson R. J. A circular DNA in malaria parasites encodes an RNA polymerase like that of prokaryotes and chloroplasts. Mol Biochem Parasitol. 1991 Jan;44(1):115–123. doi: 10.1016/0166-6851(91)90227-w. [DOI] [PubMed] [Google Scholar]
- Joseph J. T., Aldritt S. M., Unnasch T., Puijalon O., Wirth D. F. Characterization of a conserved extrachromosomal element isolated from the avian malarial parasite Plasmodium gallinaceum. Mol Cell Biol. 1989 Sep;9(9):3621–3629. doi: 10.1128/mcb.9.9.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilejian A. Circular mitochondrial DNA from the avian malarial parasite Plasmodium lophurae. Biochim Biophys Acta. 1975 May 16;390(3):276–284. doi: 10.1016/0005-2787(75)90348-2. [DOI] [PubMed] [Google Scholar]
- Nijhout M. M. Plasmodium gallinaceum: exflagellation stimulated by a mosquito factor. Exp Parasitol. 1979 Aug;48(1):75–80. doi: 10.1016/0014-4894(79)90056-0. [DOI] [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossorio P. N., Sibley L. D., Boothroyd J. C. Mitochondrial-like DNA sequences flanked by direct and inverted repeats in the nuclear genome of Toxoplasma gondii. J Mol Biol. 1991 Dec 5;222(3):525–536. doi: 10.1016/0022-2836(91)90494-q. [DOI] [PubMed] [Google Scholar]
- Peterson D. S., Walliker D., Wellems T. E. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9114–9118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtyseck E., Mehlhorn H., Hammond D. M. Electron microscope studies of microgametogenesis in Coccidia and related groups. Z Parasitenkd. 1972;38(2):95–131. doi: 10.1007/BF00329023. [DOI] [PubMed] [Google Scholar]
- Siemeister G., Buchholz C., Hachtel W. Genes for ribosomal proteins are retained on the 73 kb DNA from Astasia longa that resembles Euglena chloroplast DNA. Curr Genet. 1990 Dec;18(5):457–464. doi: 10.1007/BF00309917. [DOI] [PubMed] [Google Scholar]
- Siemeister G., Hachtel W. Organization and nucleotide sequence of ribosomal RNA genes on a circular 73 kbp DNA from the colourless flagellate Astasia longa. Curr Genet. 1990 May;17(5):433–438. doi: 10.1007/BF00334524. [DOI] [PubMed] [Google Scholar]
- Sinden R. E., Canning E. U., Bray R. S., Smalley M. E. Gametocyte and gamete development in Plasmodium falciparum. Proc R Soc Lond B Biol Sci. 1978 Jun 5;201(1145):375–399. doi: 10.1098/rspb.1978.0051. [DOI] [PubMed] [Google Scholar]
- Sinden R. E., Canning E. U., Spain B. Gametogenesis and fertilization in Plasmodium yoelii nigeriensis: a transmission electron microscope study. Proc R Soc Lond B Biol Sci. 1976 Mar 30;193(1110):55–76. doi: 10.1098/rspb.1976.0031. [DOI] [PubMed] [Google Scholar]
- Suplick K., Akella R., Saul A., Vaidya A. B. Molecular cloning and partial sequence of a 5.8 kilobase pair repetitive DNA from Plasmodium falciparum. Mol Biochem Parasitol. 1988 Sep;30(3):289–290. doi: 10.1016/0166-6851(88)90098-9. [DOI] [PubMed] [Google Scholar]
- Suplick K., Morrisey J., Vaidya A. B. Complex transcription from the extrachromosomal DNA encoding mitochondrial functions of Plasmodium yoelii. Mol Cell Biol. 1990 Dec;10(12):6381–6388. doi: 10.1128/mcb.10.12.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Orita M., Shiraishi M., Hayashi K., Sekiya T. Detection of ras gene mutations in human lung cancers by single-strand conformation polymorphism analysis of polymerase chain reaction products. Oncogene. 1990 Jul;5(7):1037–1043. [PubMed] [Google Scholar]
- Vaidya A. B., Akella R., Suplick K. Sequences similar to genes for two mitochondrial proteins and portions of ribosomal RNA in tandemly arrayed 6-kilobase-pair DNA of a malarial parasite. Mol Biochem Parasitol. 1989 Jun 15;35(2):97–107. doi: 10.1016/0166-6851(89)90112-6. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Arasu P. Tandemly arranged gene clusters of malarial parasites that are highly conserved and transcribed. Mol Biochem Parasitol. 1987 Jan 15;22(2-3):249–257. doi: 10.1016/0166-6851(87)90056-9. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Lashgari M. S., Pologe L. G., Morrisey J. Structural features of Plasmodium cytochrome b that may underlie susceptibility to 8-aminoquinolines and hydroxynaphthoquinones. Mol Biochem Parasitol. 1993 Mar;58(1):33–42. doi: 10.1016/0166-6851(93)90088-f. [DOI] [PubMed] [Google Scholar]
- Vermeulen A. N., Ponnudurai T., Beckers P. J., Verhave J. P., Smits M. A., Meuwissen J. H. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. 1985 Nov 1;162(5):1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Jonah A., Dolan S. A., Gwadz R. W., Panton L. J., Wellems T. E. An RFLP map of the Plasmodium falciparum genome, recombination rates and favored linkage groups in a genetic cross. Mol Biochem Parasitol. 1992 Apr;51(2):313–320. doi: 10.1016/0166-6851(92)90081-t. [DOI] [PubMed] [Google Scholar]
- Walliker D., Quakyi I. A., Wellems T. E., McCutchan T. F., Szarfman A., London W. T., Corcoran L. M., Burkot T. R., Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987 Jun 26;236(4809):1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- Waters A. P., Higgins D. G., McCutchan T. F. Plasmodium falciparum appears to have arisen as a result of lateral transfer between avian and human hosts. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3140–3144. doi: 10.1073/pnas.88.8.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems T. E., Panton L. J., Gluzman I. Y., do Rosario V. E., Gwadz R. W., Walker-Jonah A., Krogstad D. J. Chloroquine resistance not linked to mdr-like genes in a Plasmodium falciparum cross. Nature. 1990 May 17;345(6272):253–255. doi: 10.1038/345253a0. [DOI] [PubMed] [Google Scholar]
- Wellems T. E., Walker-Jonah A., Panton L. J. Genetic mapping of the chloroquine-resistance locus on Plasmodium falciparum chromosome 7. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3382–3386. doi: 10.1073/pnas.88.8.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Wilson R. J., Bates P. A., McCready S., Perler F., Qiang B. U. Nuclear and mitochondrial DNA of the primate malarial parasite Plasmodium knowlesi. Mol Biochem Parasitol. 1985 Feb;14(2):199–209. doi: 10.1016/0166-6851(85)90038-6. [DOI] [PubMed] [Google Scholar]
- Wilson R. J., Fry M., Gardner M. J., Feagin J. E., Williamson D. H. Subcellular fractionation of the two organelle DNAs of malaria parasites. Curr Genet. 1992 Apr;21(4-5):405–408. doi: 10.1007/BF00351702. [DOI] [PubMed] [Google Scholar]
- Wilson R. J., Gardner M. J., Feagin J. E., Williamson D. H. Have malaria parasites three genomes? Parasitol Today. 1991 Jun;7(6):134–136. doi: 10.1016/0169-4758(91)90276-t. [DOI] [PubMed] [Google Scholar]
- dePamphilis C. W., Palmer J. D. Loss of photosynthetic and chlororespiratory genes from the plastid genome of a parasitic flowering plant. Nature. 1990 Nov 22;348(6299):337–339. doi: 10.1038/348337a0. [DOI] [PubMed] [Google Scholar]